Abstract
Background
Little is known about the role of guidelines for the practical management of chronic obstructive pulmonary disease (COPD) by office-based pulmonary specialists. The aim of this study was to assess their outpatient management in relation to current guideline recommendations for COPD.
Methods
A nationwide prospective cross-sectional COPD questionnaire survey in the form of a multiple-choice questionnaire was sent to 1000 office-based respiratory specialists in Germany. The product-neutral questions focused on routine COPD management and were based on current national and international COPD guideline recommendations being consistent in severity classification and treatment recommendations.
Results
A total of 590 pulmonary specialists (59%) participated in the survey. Body plethysmography was considered the standard for diagnosis (65.9%), followed by spirometry (32%). Most respondents were able to cite the correct spirometric criteria for classifying moderate (87%) to very severe COPD (77%). A quarter of the respondents equated the World Health Organization (WHO) definition of chronic bronchitis with COPD. Notably, most participants preferred the updated national COPD guidelines (51.4%) to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines (40.2%). Improvement of functional exercise capacity and quality of life were considered the two most relevant treatment goals; whereas impact on mortality was secondary. Treatment of COPD largely complied with the guidelines. However, a significant percentage of the pulmonary specialists differed in their assessment of the benefits of various therapeutic measures from evidence-based results. Referral for pulmonary rehabilitation was uncommon, regardless of the severity of COPD.
Conclusion
The findings of this large national survey suggest that most pulmonary specialists adhere to the current COPD guideline recommendations in daily practice. However, physicians’ knowledge of guidelines is not sufficient as the sole benchmark when assessing their implementation in day-to-day practice. Necessary changes in the health care system must include more effective ways to transfer knowledge to clinical practice and to give access to interventions of proven clinical benefit.
Keywords: pulmonary rehabilitation, survey, GOLD, clinical outcomes, therapy, diagnosis
Introduction
Chronic obstructive pulmonary disease (COPD) is one of the most significant chronic conditions worldwide and is now the fourth most common cause of death, resulting in an enormous, steadily increasing economic and social burden.1–3 COPD is regarded as a preventable and treatable disease. Consequently, greater focus on early diagnosis and appropriate treatment may prevent and improve symptoms, reduce the rate and severity of exacerbations, improve quality of life, improve exercise capacity and physical activity, and prolong survival.4 In recent years, evidence-based clinical guidelines have been developed at both the national and international level in an effort to help doctors diagnose, treat, and prevent this condition.5–7 The diagnostic and therapeutic recommendations of the current German COPD guidelines are based on the international Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines.7
A number of studies suggest that there are substantial gaps between recommended and real life management of COPD patients in primary care practice.8–13 The extent to which guidelines are accepted and implemented differs widely from one group of doctors to another.14 We hypothesized that pulmonary specialists, who generally have a better knowledge of lung diseases, are more likely to adhere to COPD guidelines.15 In general, little is known about the current practice patterns of respiratory specialists in outpatient care. A recent survey studied the guideline conformity of COPD management among pulmonary specialists and general practitioners, revealing deficiencies with regard to the diagnosis, treatment, and implementation of educational measures in COPD.16 However, this study was based on the outdated German COPD guidelines from 200217 which differed from the current German and GOLD guidelines in essential points, such as the classification of COPD severity and treatment. The aim of this nationwide survey with 1000 office-based pulmonary specialists in Germany was to investigate their COPD management and how this compares with current national and international guideline recommendations.
Methods
Anonymized questionnaires were sent with a stamped addressed envelope to 1000 office-based pulmonary specialists (or pulmonary group practices) in Germany. Physicians were selected from a representative national physicians’ register. There was a total of 1088 office-based pulmonary specialists at the time of the investigation (OneKey database, Cegedim, Bensheim, Germany). Academic institutions or hospitals were not included in the survey.
The self-administered postal questionnaire survey was based on current national COPD guidelines.5,7 It had 43 multiple choice questions, focusing on the following items:
Epidemiology and diagnosis of COPD
Patient education
Treatment
Knowledge and acceptance of cur rent COPD guidelines
Questions regarding the physician’s practice.
The questionnaire had been applied in a similar format in a previous survey.16 Non-responders were not followed up and no reminders were sent during the 4-month term of the survey. Physicians were paid 75 euro as compensation for participation in the survey.
Statistics
DCAS (DCAS Software Solutions Inc, Plano, TX) software (Medidata GmbH, Konstanz, Germany) and SAS software (v 9.1.3; SAS Institute Inc, Cary, NC) were used for statistical analysis of the anonymous, machine-readable questionnaires. The frequencies for each category, in relation to the total number of responses, are given in text results and Tables 1–3. The anonymized data were analyzed by Medidata.
Table 1.
Demographic characteristics of respondents (n = 590)
| Proportion (n and %) | |
|---|---|
| Age group | |
| 30–40 years | 46 (7.8) |
| 41–50 years | 240 (40.7) |
| 51–60 years | 225 (38.1) |
| >60 years | 70 (11.9) |
| Missing data | 9 (1.5) |
| Sex | |
| Men | 447 (75.8) |
| Women | 132 (22.4) |
| Missing data | 11 (1.9) |
| Specialty | |
| Pulmonologist | 565 (95.8) |
| Internist | 15 (2.5) |
| Missing data | 10 (1.7) |
| Location of practice | |
| Urban | 285 (48.3) |
| Rural | 289 (49.0) |
| Missing data | 17 (2.9) |
| Practice type | |
| Single practice | 269 (45.6) |
| Single-speciality group practice | 214 (36.3) |
| Multi-speciality group practice | 95 (16.1) |
| Missing data | 13 (2.2) |
| Length of time in practice | |
| <5 years | 152 (25.8) |
| 5–10 years | 123 (20.8) |
| 11–20 years | 209 (35.4) |
| >20 years | 96 (16.3) |
| Missing data | 10 (1.7) |
Table 3.
Evaluation of the clinical benefits of various treatments
| Proportion of respondents (%) | |||||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Slows disease progression | Prolongs life | Improves physical capacity | Reduces exacerbation rate | Improves symptoms | Improves quality of life | No benefits | |
| No smoking | 94.9 | 86.1 | 79.5 | 76.3 | 75.1 | 74.2 | 0 |
| SABD | 2.4 | 3.7 | 68.8 | 9.2 | 91.7 | 48.5 | 1.4 |
| LAMA | 45.6 | 27.8 | 91.0 | 79.7 | 87.6 | 88.8 | 0.2 |
| LABA | 26.4 | 15.3 | 89.7 | 49.3 | 91.4 | 83.9 | 0.5 |
| ICS | 21.5 | 9.7 | 27.5 | 88.6 | 61.2 | 49.5 | 3.1 |
| ICS + LABA | 33.2 | 16.3 | 80.8 | 87.5 | 86.6 | 81.0 | 1.7 |
| OCS | 6.3 | 6.9 | 39.2 | 24.9 | 77.5 | 40.8 | 10.8 |
| PR | 28.6 | 32.4 | 92.5 | 45.8 | 75.6 | 89.5 | 1.7 |
| LTOT | 4.2 | 77.1 | 78.8 | 8.5 | 71.2 | 84.9 | 0.2 |
| Theophylline | 4.1 | 1.7 | 41.2 | 11.9 | 75.6 | 30.3 | 17.8 |
Note: Respondents (n = 590) could give more than one answer for any of the interventions.
Abbreviations: ICS, inhaled corticosteroids; LABA, long-acting β2-agonists; LAMA, long-acting muscarinic agonists; LTOT, long-term oxygen therapy given for 16–24 hours/day; OCS, oral corticosteroid; PR, pulmonary rehabilitation; SABD, short-acting bronchodilators.
Results
Demographics
Fifty-nine percent of the 1000 questionnaires sent out were returned (n = 590 physicians). Ninety-six percent of the physicians were pulmonary specialists. The data quality was very good, as reflected by the low frequency of missing data (≤3.1%). The demographic data of the participants are shown in Table 1. Forty-eight percent of pulmonary specialists reported that they treat 101–200 COPD patients per month. Thirty-six percent of the physicians reported treating >200 COPD patients per month, primarily patients with moderate COPD. Fifty-four percent of the respondents reported that 5%–15% of their patients showed an overlap of asthma and COPD.
Epidemiology and risk factors
The incidence of COPD is increasing according to 90% of the physicians and 94% of the physicians regard COPD as a big or very big “public health” problem in Germany. The two most important risk factors for COPD were smoking (99% of the physicians) and air pollution (37%), followed by bacterial and/or viral infections (29%) and genetic predisposition (26%). Almost half of the respondents (49%) reported that 16%–30% of their patients experienced an exacerbation per year requiring therapy with systemic steroids and/or antibiotics. Eighteen percent of the physicians stated that 31%–50% of their COPD patients had at least one moderate exacerbation per year. Hospitalizations due to exacerbations were reported less frequently: 61% of the physicians stated that <5% of their patients had at least one exacerbation-related hospitalization per year.
Diagnosis of COPD
Table 2 illustrates the respondents’ criteria for diagnosis of COPD. Ninety-one percent of the physicians stated that a diagnosis of “suspected COPD” is reasonable in patients showing symptoms such as cough, expectoration, shortness of breath, and/or risk factors for COPD. The most relevant test for patients with suspected COPD was considered to be body plethysmography (66% of pulmonary specialists), followed by spirometry (32%).
Table 2.
Criteria used by respondents to diagnose COPD
| Proportion (n and %) | |
|---|---|
| Diagnostic criteria for COPD | |
| FEV1/VC ratio of <70% predicted | 358 (60.7) |
| Cough and sputum for 3 months in 2 consecutive years | 157 (26.6) |
| FEV1 of <80% predicted | 53 (9.0) |
| FEV1 of <1.5 L | 7 (1.2) |
| Signs of pulmonary emphysema on chest X-ray | 5 (0.8) |
| Hypoxemia and/or hypercapnia | 4 (0.7) |
| Missing data | 12 (2.0) |
| Definition of moderate COPD | |
| FEV1/VC ratio of <70% + FEV1 of <80% but ≥50% predicted | 504 (85.4) |
| FEV1/VC ratio of <70% + FEV1 of <50% predicted | 63 (10.7) |
| Clinical symptoms (dyspnoea, reduced physical capacity) | 9 (1.5) |
| FEV1/VC ratio of <50% | 5 (0.8) |
| FEV1 of >1 L | 1 (0.2) |
| Missing data | 10 (1.7) |
| Definition of severe COPD | |
| FEV1/VC ratio of <70% + FEV1 of <50% but ≥30% predicted | 514 (87.1) |
| FEV1/VC ratio of <50% | 51 (8.6) |
| Clinical symptoms (severe dyspnea at rest) | 15 (2.5) |
| FEV1 of <1 L | 5 (0.8) |
| FEV1/VC ratio of <70% + FEV1 of <80% predicted | 2 (0.3) |
| Missing data | 10 (1.7) |
| Definition of very severe COPD | |
| FEV1/VC ratio of <70% + FEV1 of <30% predicted | 452 (76.6) |
| FEV1/VC ratio of <70% + FEV1 of <50% predicted + chronic respiratory failure (signs of right-sided heart failure)1 | 327 (55.4) |
| FEV1/VC ratio of <30% | 70 (11.9) |
| Clinical symptoms (severe dyspnea at rest, frequent exacerbations) | 30 (5.1) |
| FEV1 of <1 L | 20 (3.4) |
| Missing data | 11 (1.9) |
Note: Respondents (n = 590) could give more than one answer to all questions.
Abbreviations: FEV1, forced expiratory volume in 1 second; VC, vital capacity.
As reported by the physicians, diagnosis of COPD is primarily based on a forced expiratory volume in 1 second/(forced) vital capacity (FEV1/VC) index of <70% (61% of the physicians). On the other hand, 27% of the physicians used the WHO criteria for chronic bronchitis instead. Of the respondents, 85% applied the correct spirometric criteria to assess the severity of moderate (GOLD II) COPD, with 87% and 77% of respondents using the criteria to assess the severity of severe (GOLD III) and very severe (GOLD IV) COPD, respectively.
Patient education
Ninety-seven percent of the physicians stated that they or their staff regularly instructed patients in the correct use of inhalers. Most physicians advised their patients to stop smoking at every visit: (moderate COPD 78%, severe/very severe COPD 83%) or several times a year (moderate COPD 20% severe/very severe COPD 14%). A total of 60% of the respondents considered it difficult to implement measures to help patients give up smoking.
Treatment
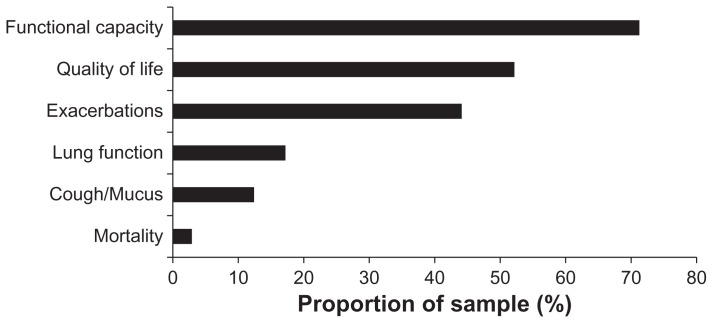
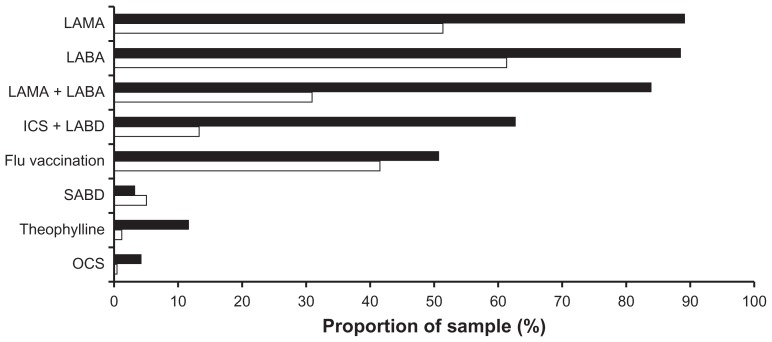
The two most important indicators of successful treatment as stated by pulmonary specialists were improved functional exercise capacity (72%) and quality of life (52%), followed by the prevention of exacerbations (Figure 1). The treatments prescribed by doctors for at least 50% of their patients with moderate to severe COPD based on the GOLD classification are summarized in Figure 2. Long- acting bronchodilators (β2-agonists and/or anticholinergics) were the most commonly prescribed treatments. Short- acting bronchodilators (β2-agonists or anticholinergics) and inhaled steroids, on the other hand, were rarely prescribed on their own. A combination of long-acting β2-agonists and long-acting anticholinergics is primarily prescribed for severe COPD. The percentage of patients with moderate COPD treated with theophylline was low, but was markedly higher in severe COPD. Long-term treatment (>3 months) with inhaled steroids in combination with long-acting bronchodilators was used primarily for patients with severe to very severe COPD. A relatively low portion of patients with moderate and severe to very severe COPD were given a yearly influenza vaccination by pulmonary specialists (Figure 1).
Figure 1.
The most relevant treatment goals for COPD as seen by pulmonary specialists.
Notes: Improvements in functional (exercise) capacity (72%) and quality of life (52%) were rated highest by the physicians, followed by a reduction of COPD exacerbations (44%). Effects on lung function (17%), cough/sputum production (12%) and, in particular, on mortality (3%) were seen as less important indicators of success. Two answers were required for this question; n = 590.
Figure 2.
Treatment given by pulmonary specialists to ≥50% of their patients.
Notes: Moderate COPD (white columns) or severe to very severe COPD (black columns) according to the GOLD severity classification.5 Several answers were possible for this question; n = 590.
Abbreviations: LAMA, long-acting muscarinic agonist; LABA, long-acting beta-agonists; ICS, inhaled corticosteroids; LABD, long-acting bronchodilators (LABA and/or LAMA); SABD, short-acting bronchodilators (SABA and/or SAMA); OCS, oral corticosteroids.
The most important criteria for long-term therapy with inhaled glucocorticosteroids in addition to long-acting bronchodilators were COPD with FEV1 of <50% and at least one exacerbation in the past year requiring treatment with systemic steroids and/or antibiotics (73% of the physicians) as well as improved symptoms due to inhaled glucocorticosteroids (71%). Of the pulmonary specialists, 85% said they prescribed oral glucocorticosteroids for a short time only in the case of exacerbations. Of the physicians, 62% felt pulmonary rehabilitation was indicated in the case of moderate COPD (GOLD II), while 31% believed this was only necessary in severe forms of COPD (GOLD III 29%, GOLD IV 2%). In practice, only a small percentage of patients with moderate (2%) or severe/very severe COPD (16%) actually received pulmonary rehabilitation.
Assessment of the benefits of therapeutic measures
The results of an assessment of the benefits of various treatments are given in Table 3. Nicotine abstinence was seen as the most effective measure for prolonging life expectancy and slowing down the progression of the disease. The major advantages of pulmonary rehabilitation were seen in an improvement in exercise capacity, quality of life, and symptoms.
The assessment of the benefits of short-acting bronchodilators (β2-agonists, anticholinergics) was based primarily on the improvement in clinical symptoms, exercise capacity, and quality of life. Compared with the benef its attributed to long-acting bronchodilators (β2-agonists, anticholinergics) – including the positive impact on symptoms, exercise capacity, quality of life, and exacerbations – theophylline was felt to be of limited clinical benefit. Reductions in exacerbations were quoted in connection with long-acting bronchodilators, inhaled corticosteroids, and the combination of these drugs. Long- term oxygen therapy, when used, was seen as an effective measure for improving quality of life, exercise capacity, symptoms, and mortality. Contrary to the evidence from clinical trials and guideline recommendations, long-acting bronchodilators, inhaled corticosteroids, and pulmonary rehabilitation were found to have some impact on disease progression and mortality.
Knowledge and acceptance of current COPD guidelines
Fifty-three percent of the participants regarded the recommendations contained in the national and international guidelines for the diagnosis and treatment of COPD as very useful while 46% found them suitable for guidance. The most relevant guideline on diagnosis and therapy of COPD was the current national COPD guidelines (51%) followed by the international GOLD guidelines (40%).
Discussion
Evidence-based guidelines aim to improve the medical care of patients and support physicians in making the appropriate diagnosis and initiating adequate measures for prevention and therapy. This local survey investigated the outpatient management of practicing pulmonary specialists in relation to current guideline recommendations for COPD.
The updated German COPD Guidelines7 now conform to the international GOLD guidelines5,6 regarding severity classification and treatment recommendations. In the present survey, this realignment has led to improvements in COPD severity classification and treatment compared with a previous survey based on the former national COPD Guidelines of 2002.17 However, it must be pointed out that the national COPD guidelines, like the American Thoracic Society/European Respiratory Society (ATS/ERS) consensus on clinical pulmonary function testing, defines obstruction on the basis of the FEV1/VC ratio.7,18 In borderline cases, this could mean discrepancies compared with GOLD. Acceptance was high among the physicians questioned and in contrast to a previous survey there was a preference for the national COPD guidelines.16 This may be a reflection of the fact that the GOLD guidelines are aimed at an international readership with a wide range of access to health care and treatment, while the recommendations of the national COPD guidelines are tailored to the specifics of the German health care system.
Pulmonary specialists were well aware of the epidemiological and health–economic dimensions of the disease. Questions about diagnosis criteria for COPD prompted different responses. More than half of the pulmonary specialists used the fixed FEV1/VC ratio as a criterion for diagnosing COPD. On the other hand, 26.6% of the participants considered the classical WHO criterion of chronic bronchitis (former GOLD class 0) as an indicator for diagnosis. However, this was an 11.6% decrease compared with a previous study in the same country.16
There were slight deficiencies in terms of grading COPD according to spirometric criteria. About 85%–87% of participants applied appropriate lung function criteria for the classification of moderate to severe COPD. There was greater disparity when describing very severe COPD – defined as either FEV1 < 30% or FEV1 < 50% predicted plus the presence of chronic respiratory failure – and this may indicate a particular degree of uncertainty with regard to the additional criteria of ventilatory failure or right heart insufficiency. Pulmonary specialists felt the most important aims of therapy were improvement in functional exercise capacity and quality of life, followed by prevention of exacerbations. This is well in line with the leading symptoms of patients with COPD, who primarily complain of shortness of breath upon exercise and the lasting impact of exacerbations. However, this survey did not explore how physicians’ views might differ from patients’ views. Interestingly, the positive impact on mortality was not of particular significance to physicians in terms of treatment objectives. This probably reflects the minor effect of symptomatic medical treatment and the – at best – modest effect of interventions, such as oxygen therapy, on mortality. Given the practical relevance of physical activity to address disease progression and the impact of pharmacotherapy in COPD, the development of valid and practicable instruments to measure physical activity is urgently required.19–21
Two-thirds of the respondents prescribed a combination of a long-acting β2-agonist and an anticholinergic for more than half of their patients with severe COPD. Inhaled steroids and theophylline were also prescribed considerably more often in this patient group than for patients with moderate COPD. In line with the recommendations of current national and international guidelines, most pulmonary specialists prescribed systemic steroids for exacerbations only.5–7 The relatively low number of influenza vaccinations prescribed by pulmonary specialists contrasts with guideline recommendations. This might be explained by the fact that flu vaccinations are mainly performed by general practitioners in Germany.
Guidelines consider pulmonary rehabilitation to be standard care for those with at least moderate COPD (GOLD II) or at any stage in the presence of symptoms and disability.5–7 However, pulmonary rehabilitation was not prescribed as often as it should have been despite the known benefits, even in the advanced stages of the disease. There is now strong support for pulmonary rehabilitation in the event of COPD following recent hospitalization for an exacerbation.22–25 Pulmonary rehabilitation could thus already be organized during hospitalization for an exacerbation. However, there are issues surrounding specific aspects of pulmonary rehabilitation in the local health system – for instance, the motivation of physicians and patients, a significant administrative burden, financial incentives, or the limited availability of qualified and certified centers – that are likely to have prevented more widespread implementation of pulmonary rehabilitation in Germany so far.
In some cases, assessment of the clinical benefit of various pharmacological and non-pharmacological procedures, as offered by the office-based clinicians surveyed, conflicted with the clinical evidence from randomized clinical trials. For instance, so far no data are available from studies that corroborate the effect of pulmonary rehabilitation, bronchodilators, or inhaled steroids on mortality and the progression of the disease.5,6 We also found certain discrepancies between COPD guidelines and routine treatments. Some treatments such as inhaled corticosteroids (ICS) were overused in moderate COPD, whereas rehabilitation was not prescribed in a substantial number of cases in which it would have been indicated. Finally, a significant percentage of respondents still used the WHO definition of chronic bronchitis to define COPD.
As is the case with all self-reported surveys, the results of this study can be neither generalized nor confirmed. It is also unclear to what extent there was any positive selection bias, recall bias, or if respondents’ answers differ from actual practice and knowledge or from those of non-pulmonary experts and non-participants. In addition, it remains unclear to what extent the treatment prescribed by physicians with a sound knowledge of the guidelines differs from that suggested by physicians who are less familiar with the current recommendations.
Overall, we found considerably greater conformity with guideline recommendations in terms of outpatient COPD management by pulmonary specialists compared to a previous survey in 2005.16 This suggests a high level of knowledge and significant progress in the implementation of COPD guidelines in Germany. However, even among pulmonary specialists there were certain deficits as regards the diagnosis and the pharmacological and non-pharmacological treatment of COPD. These gaps can be closed by using COPD guidelines for tailored education and training or as the foundation of national disease management programs for COPD. However, physicians’ knowledge of the guidelines is not sufficient as the sole benchmark when assessing implementation of the guidelines in day-to-day practice. Necessary changes in the health care system must include more effective ways to transfer knowledge to clinical practice and to give access to interventions of proven clinical benefit.
Acknowledgments
The study was run and sponsored by Boehringer Ingelheim and Pfizer in cooperation with the Bundesverband der Pneumologen (BDP, Federal Association of Pneumologists). We thank Dr Heike Rau-Berger for her excellent technical assistance.
Footnotes
Disclosure
TG was an employee of Boehringer Ingelheim at the time of manuscript submission. CV received consulting fees and travel support from Boehringer Ingelheim; payment for board membership from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Mundipharma, Novartis, and Nycomed; fees for expert testimony and grant support from Talecris Biotherapeutics; and lecture fees from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Janssen, Merck, Novartis, Nycomed, and Talecris Biotherapeutics. AH declared no conflict of interest. RB has received reimbursement for attending scientific conferences, and/or fees for speaking and/or consulting from AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Novartis, Nycomed, and Pfizer. The Pulmonary Department at Mainz University Hospital received financial compensation for services performed during participation in clinical trials organized by various pharmaceutical companies.
References
- 1.Lopez AD, Murray CC. The global burden of disease, 1990–2020. Nat Med. 1998;4(11):1241–1243. doi: 10.1038/3218. [DOI] [PubMed] [Google Scholar]
- 2.Chapman KR, Mannino DM, Soriano JB, et al. Epidemiology and costs of chronic obstructive pulmonary disease. Eur Respir J. 2006;27(1):188–207. doi: 10.1183/09031936.06.00024505. [DOI] [PubMed] [Google Scholar]
- 3.Nowak D, Berger K, Lippert B, Kilgert K, Caeser M, Sandtmann R. Epidemiology and health economics of COPD across Europe: a critical analysis. Treat Respir Med. 2005;4(6):381–395. doi: 10.2165/00151829-200504060-00003. [DOI] [PubMed] [Google Scholar]
- 4.Celli BR, MacNee W ATS/ERS Task Force. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23(6):932–946. doi: 10.1183/09031936.04.00014304. [DOI] [PubMed] [Google Scholar]
- 5.Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of COPD. [Accessed August 25, 2010]. 2009 update. Available from: http://www.goldcopd.org.
- 6.National Institute for Health and Clinical Excellence. Chronic obstructive pulmonary disease: Management of chronic obstructive pulmonary disease in adults in primary and secondary care. National Clinical Guideline Centre; 2010. [Accessed May 14, 2011]. Available from: http://guidance.nice.org.uk/CG101/Guidance/pdf/English. [Google Scholar]
- 7.Vogelmeier C, Buhl R, Criée CP, et al. Guidelines for the diagnosis and therapy of COPD issued by Deutsche Atemwegsliga and Deutsche Gesellschaft für Pneumologie und Beatmungsmedizin. Pneumologie. 2007;61(5):e1–e40. doi: 10.1055/s-2007-959200. German. [DOI] [PubMed] [Google Scholar]
- 8.Soler N, Ballester E, Martín A, Gobartt E, Miravitlles M, Torres A. Changes in management of chronic obstructive pulmonary disease (COPD) in primary care: EMMEPOC study. Respir Med. 2010;104(1):67–75. doi: 10.1016/j.rmed.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 9.Albers M, Schermer T, Molema J, et al. Do family physicians’ records fit guideline diagnosed COPD? Fam Pract. 2009;26(2):81–87. doi: 10.1093/fampra/cmp005. [DOI] [PubMed] [Google Scholar]
- 10.Löfdahl CG, Tilling B, Ekström T, Jörgensen L, Johansson G, Larsson K. COPD health care in Sweden – A study in primary and secondary care. Respir Med. 2010;104(3):404–411. doi: 10.1016/j.rmed.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 11.Bourbeau J, Sebaldt RJ, Day A, et al. Practice patterns in the management of chronic obstructive pulmonary disease in primary practice: the CAGE study. Can Respir J. 2008;15(1):13–19. doi: 10.1155/2008/173904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rutschmann OT, Janssens JP, Vermeulen B, Sarasin FP. Knowledge of guidelines for the management of COPD: a survey of primary care physicians. Respir Med. 2004;98(10):932–937. doi: 10.1016/j.rmed.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 13.Lange P, Andersen KK, Munch E, et al. Quality of COPD care in hospital outpatient clinics in Denmark: The KOLIBRI study. Respir Med. 2009;103(11):1657–1662. doi: 10.1016/j.rmed.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 14.Harrold L, Field T, Gurwitz J. Knowledge, patterns of care, and outcomes of care for generalists and specialists. J Gen Intern Med. 1999;14(8):499–511. doi: 10.1046/j.1525-1497.1999.08168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garcia-Aymerich J, Escarrabil J, Marrades RM, et al. Differences in COPD care among doctors who control the disease: general practitioner vs pneumologist. Respir Med. 2006;100(2):332–339. doi: 10.1016/j.rmed.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 16.Glaab T, Banik N, Rutschmann OT, Wencker M. National survey of guideline-compliant COPD management among pneumologists and primary care physicians. COPD. 2006;3(3):141–148. doi: 10.1080/15412550600829299. [DOI] [PubMed] [Google Scholar]
- 17.Worth H, Buhl R, Cegla U, et al. Guidelines for the diagnosis and treatment chronic obstructive bronchitis and pulmonary emphysema issued by Deutsche Atemwegsliga and Deutsche Gesellschaft für Pneumologie. Pneumologie. 2002;56(11):704–738. doi: 10.1055/s-2002-35553. [DOI] [PubMed] [Google Scholar]
- 18.Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26(5):948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 19.Watz H, Waschki C, Boehme M, et al. Extrapulmonary effects of chronic obstructive pulmonary disease on physical activity: a cross-sectional study. Am J Respir Crit Care Med. 2008;177(7):743–751. doi: 10.1164/rccm.200707-1011OC. [DOI] [PubMed] [Google Scholar]
- 20.Pitta F, Troosters T, Probst V, Langer D, Decramer M, Gosselink R. Are patients with COPD more active after pulmonary rehabilitation? Chest. 2008;134(2):273–280. doi: 10.1378/chest.07-2655. [DOI] [PubMed] [Google Scholar]
- 21.Glaab T, Vogelmeier C, Buhl R. Outcome measures in chronic obstructive pulmonary disease (COPD): strengths and limitations. Respir Res. 2010;11:79. doi: 10.1186/1465-9921-11-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Riario-Sforza GG, Yacoub MR, Incorvaia C. Pulmonary rehabilitation as evaluated by clinical trials: an overview. Rev Recent Clin Trials. 2010;5(2):76–84. doi: 10.2174/157488710791233617. [DOI] [PubMed] [Google Scholar]
- 23.Casaburi R, ZuWallack R. Pulmonary rehabilitation for management of chronic obstructive pulmonary disease. N Engl J Med. 2009;360(13):1329–1335. doi: 10.1056/NEJMct0804632. [DOI] [PubMed] [Google Scholar]
- 24.Seymour JM, Moore L, Jolley CJ, et al. Outpatient pulmonary rehabilitation following acute exacerbations of COPD. Thorax. 2010;65(5):423–428. doi: 10.1136/thx.2009.124164. [DOI] [PubMed] [Google Scholar]
- 25.Puhan MA, Scharplatz M, Troosters T, Steurer J. Respiratory rehabilitation after acute exacerbation of COPD may reduce risk for readmission and mortality – a systematic review. Respir Res. 2005;6:54. doi: 10.1186/1465-9921-6-54. [DOI] [PMC free article] [PubMed] [Google Scholar]