Abstract
Traditional Chinese medicine is gradually becoming a new source of anticancer drugs. One such example is wogonin, which is cytotoxic to various cancer cell lines in vitro. However, due to its low water solubility, wogonin is restricted to clinical administration. Recently, the application of drug-coated magnetic nanoparticles (MNPs) to increase water solubility of the drug and to enhance its chemotherapeutic efficiency has attracted much attention. In this study, wogonin was conjugated with the drug delivery system of MNPs by mechanical absorption polymerization to fabricate wogonin-loaded MNPs. It was demonstrated that MNPs could strengthen wogonin-induced cell inhibition, apoptosis, and cell cycle arrest in Raji cells by methylthiazol tetrazolium assay, flow cytometer assay, and nuclear 4′,6-diamidino-2-phenylindole staining. Furthermore, the molecular mechanisms of these phenomena were explored by western blot, in which the protein levels of caspase 8 and caspase 3 were increased significantly while those of survivin and cyclin E were decreased significantly in wogonin-MNPs group. These findings suggest that the combination of wogonin and MNPs provides a promising strategy for lymphoma therapy.
Keywords: wogonin, magnetic nanoparticles, Raji cell, apoptosis, cell cycle, caspase 8, caspase 3, survivin, cyclin E
Introduction
Lymphoma is a refractory malignant tumor that originates in the lymphatic system. The two main types are Hodgkin’s lymphoma and non-Hodgkin’s lymphoma (NHL). Statistics show that there were about 74,030 cases of lymphoma diagnosed in the US, including 8490 Hodgkin’s lymphoma and 65,540 NHL in 2010. With poor treatment outcomes, NHL is the eighth leading cause of cancer-related death in males and the sixth in females in the US.1 The standard chemotherapy regimen of NHL is cyclophosphamide, doxorubicin, vincristine, and prednisone, which plays an important role in the treatment of NHL. Unfortunately, a considerable number of patients undergoing cyclophosphamide, doxorubicin, vincristine, and prednisone relapse thereafter or suffer from dose-related side effects and complications. Furthermore, stem cell transplantation, as a treatment of curing NHL,2 is too expensive for patients, so curative treatment can be achieved in only a minority of NHL patients. Thus, novel therapeutic strategies are urgently needed to improve palliative treatment, prolong life expectancy, and improve quality of life in patients with lymphoma.
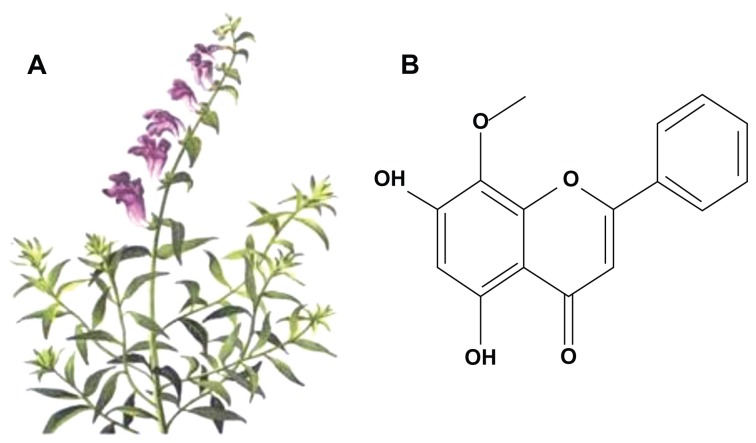
Wogonin (5,7-dihydroxy-8-methoxyflavone; Figure 1), isolated from the roots of the traditional Chinese herb Scutellaria baicalensis Georgi, has been recently recognized as a new anticancer drug that possesses cytotoxic effect against a large panel of human cancer cell lines by inducing apoptosis in vitro.3–5 Accumulating evidence demonstrates that wogonin also dramatically inhibits the development and growth of tumors in vivo.6 Notably, it has also been reported that wogonin has no or very low toxicity to nonmalignant cells.7 However, its low water solubility remains a problem, and the healing mechanisms of wogonin are still not fully known.
Figure 1.
Structure of wogonin derived from Scutellaria baicalensis Georgi. (A) S. baicalensis Georgi; (B) molecular structure of wogonin.
What’s exciting is that advances in nanotechnology have provided an innovative paradigm to overcome the problems of cancer diagnosis and therapy. Due to large surface-to-volume ratio, biodegradable nature, biocompatibility, low toxicity, and superparamagnetic property, magnetic nanoparticles (MNPs) can not only be used as a diagnostic tool,8 but also offer the capabilities of cancer therapeutics.9 Apart from improving drug solubility,10 site-specific drug delivery,11 and magnetic hyperthermia,12 it was proposed that high doses of iron oxide nanoparticles on their own may generate local oxidative stress for cancer therapy.13
In the present study, a wogonin-MNPs drug delivery system was uploaded for tumor therapy with higher efficiency, and the underlying mechanisms were further investigated.
Materials and methods
Main materials
Wogonin (Jiangsu Key Lab Carcinogenesis and Intervention, China Pharmaceutical University, Nanjing, China) was dissolved in dimethyl sulfoxide (Sigma-Aldrich Corporation, St Louis, MO), stored at −20°C, and then diluted in Gibco® Roswell Park Memorial Institute 1640 medium (Invitrogen Life Technologies, Carlsbad, CA) with 10% fetal bovine serum (Sijiqing Biological Engineering Materials Co, Ltd, Hangzhou, China); the final concentration of dimethyl sulfoxide was less than 0.15%, which did not show toxicity against Raji cells.14 Ferric chloride (FeCl3 · 6H2O), ferrous chloride (FeCl2 · 4H2O), 25% ammonia solution, and citric acid monohydrate were purchased from Sinopharm Chemical Reagent Co, Ltd, (Shanghai, China); methylthiazol tetrazolium (MTT) from Sigma-Aldrich; Annexin V-fluorescein isothiocyanate Apoptosis Detection Kit and Cell Cycle Detection Kit from KeyGen Biotech Co, Ltd, (Nanjing, China); 4, 6-diamidino-2-phenylindole (DAPI) from Beyotime Institute of Biotechnology (Haimen, China); and monoclonal antibodies, including caspase 3, caspase 8, survivin and cyclin E, were supplied by Santa Cruz Biotechnology (Santa Cruz, CA).
Synthesis of MNPs
MNPs (magnetite Fe3O4) were prepared by coprecipitation of FeCl3 and FeCl2 (2:1 molar ratio) in an alkali ammonia solution. Briefly, 2.61 g of FeCl3·6H2O and 1.04 g of FeCl2 · 4H2O were mixed in 100 mL of demineralized water in a three-necked flask under a nitrogen atmosphere and the temperature was increased to 80°C. With constant mechanical stirring, 10 mL of ammonia solution was introduced slowly to the reaction mixture and the same temperature was maintained for another 30 minutes. The precipitates were washed several times with demineralized water by magnetic separation. Thereafter, 1.37 g of citric acid was added to magnetic fluid sample and heated to 90°C under continuous stirring for 90 minutes. The black precipitates were obtained by cooling to room temperature and then subjected to dialysis against demineralized water in an 8–14 kD cutoff cellulose membrane for 48 hours to remove excess unbound citric acid. Finally, the products were lyophilized and stored in a 4°C refrigerator.
Preparation of wogonin-MNPs as a drug delivery system
Before application in the present experiment, 10% inactivated fetal bovine serum was added to the prepared MNPs – well distributed in fresh Roswell Park Memorial Institute 1640 medium – by using ultrasound treatment to obtain a MNP colloidal suspension, as previously reported.15 Various concentrations of wogonin were then conjugated with 80 mg/L MNPs by mechanical absorption polymerization at 4°C for 48 hours, which did not show toxicity against Raji cells.
Cell line and cell culture
NHL cell line Raji was obtained from Jiangsu Institute of Hematology (Suzhou, China). The cells were cultured in Roswell Park Memorial Institute 1640 with 10% fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin at 37°C in a 5% carbon dioxide humidified environment. The cells in logarithmic growth phase were used in all experiments.
MTT assay
Raji cells (1 × 104 cells per well) were incubated with either MNPs, wogonin, or wogonin-MNPs in 96-well plates for 24, 48, and 72 hours; 20 μL of MTT solution (5 mg/mL) was added to each well at 37°C in the dark for 4 hours. The formazan crystals were solubilized in 200 μL of dimethyl sulfoxide in every well, followed by reading the absorbance at 570 nm using a plate reader (Model 550 Microplate Reader; Bio-Rad Laboratories, Tokyo, Japan). Cell viability (%) was expressed as: ODtest cells/ODcontrol cells × 100, where OD represents optical density. Cell inhibition rate (%) was determined as follows: (1–ODtest cells/ODcontrol cells) × 100. Each assay was repeated at least three independent times.
Cell cycle analysis
After treatment for 48 hours, the cells were collected by centrifugation (LD25-Z low speed auto balancing centrifuge; Beijing Medical Centrifuge Plant, Beijing, China) at 1500 rpm for 5 minutes and washed with ice-cold phosphate buffered saline, and stained with propidium iodide and ribonuclease for 30 minutes. The cell cycle was analyzed by flow cytometer (FACSCalibur flow cytometer; BD Biosciences, San Jose, CA).
Apoptosis assay
After 48 hours incubation in the medium containing different drugs at 37°C, Raji cells were collected and washed twice with phosphate buffered saline and centrifuged at 2000 rpm for 5 minutes. The cells were then suspended in 500 μL binding buffer and 5 μL Annexin V-fluorescein isothiocyanate, and incubated for 15 minutes at room temperature in the dark. The apoptotic cells were analyzed by flow cytometer with CellQuest™ software (FACSCalibur; BD Biosciences). Both early (Annexin V-positive, propidium iodide-negative) and late (Annexin V-positive, propidium iodide-negative) apoptotic cells were included in cells death determinations.
DAPI staining
Raji cells were treated for 48 hours, collected, and smeared. The cells were then fixed with methanol for 10 minutes and stained with DAPI for 15 minutes. Thereafter, the cells were observed under fluorescence microscope (B×51 Epi-Fluorescence Microscope; Olympus, Tokyo, Japan).
Western blot analysis
After experimental treatment, total protein was isolated on ice and subjected to 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis gels using modified radio immunoprecipitation assay buffer, and then transferred to a polyvinylidene difluoride membrane (65421; Pall Corporation, Port Washington, NY). The blots were stained with primary monoclonal antibodies, either anti-caspase 3, caspase 8, survivin, or cyclin E overnight at 4°C, and then with horseradish peroxidase conjugated goat anti-rabbit or mouse secondary antibody for 1 hour at room temperature. The protein bands were detected with an Amersham enhanced chemiluminescence detection system (RPN2135; GE Healthcare Life Sciences, Little Chalfont, UK). After normalization by the corresponding expression of β-actin, protein expression levels were determined by densitometry scans (Photoshop CS5 Extended, Adobe Systems Inc, San José, CA).
Statistical analysis
Data were expressed as mean ± standard deviation from triplicate experiments and analyzed with IBM SPSS (v 13.0; SPSS Inc, Chicago, IL). Differences were evaluated using one-way analysis of variance or Student’s t-test. P < 0.05 was considered statistically significant.
Results
Characterization of MNPs
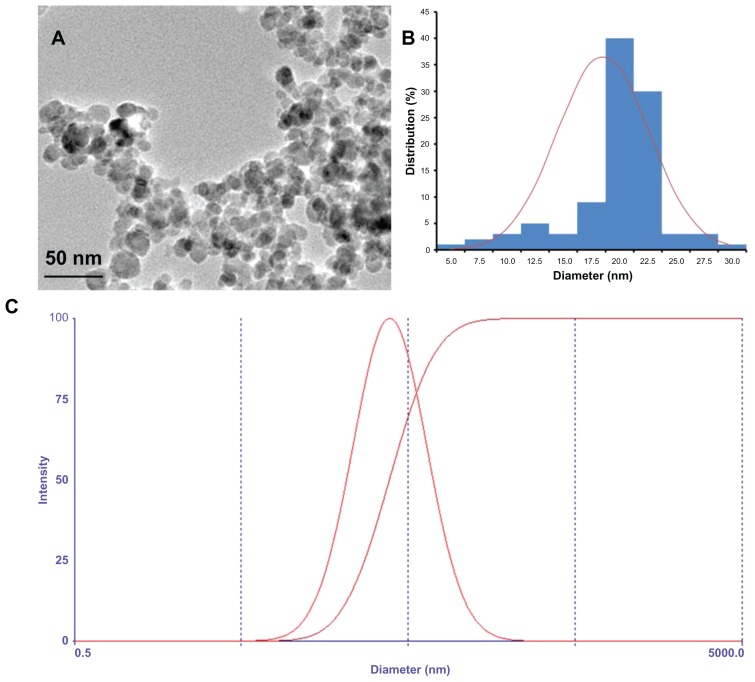
The magnetic iron oxide nanoparticles with a spherical shape had an average size of 18 nm determined by transmission electron microscope (Figure 2A). As shown in Figure 2B, the diameter of MNPs ranged from 5 nm to 30 nm. The hydrodynamic diameter of the citric acid-coated magnetic particles was 38.9 ± 1.2 nm with a narrow polydispersity (0.293 ± 0.007) based on photon correlation spectroscopy (Nicomp 380; Particle Sizing Systems, Santa Barbara, CA) (Figure 2C). In the present study, citric acid-coated MNPs did not settle down under the influence of the magnet, suggesting that the colloidal solution was stable. It was also observed that wogonin did not precipitate in the colloidal suspension of the wogonin-MNPs drug delivery system after 2 months of storage, which indicates that the solubility of wogonin was improved by MNPs.
Figure 2.
Physical characteristic of the citric acid-coated magnetic particles. (A) Transmission electron microscopic images of magnetic nanoparticles; (B) size distribution histogram of magnetic nanoparticles; (C) hydrodynamic particle size distribution of citric acid-coated magnetic particles.
Cell growth inhibition
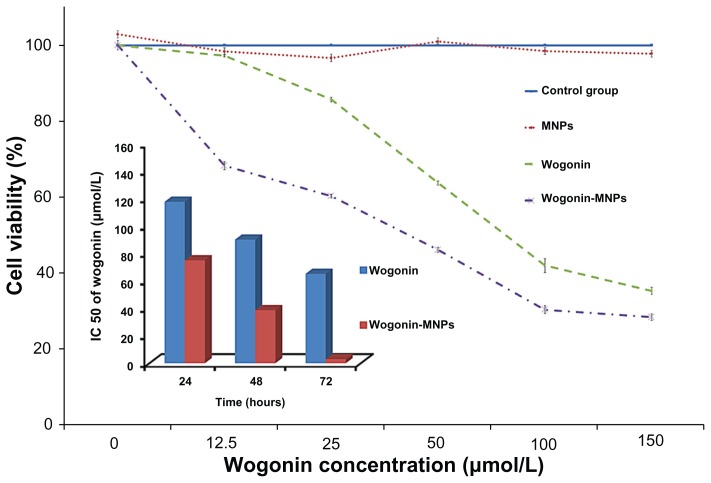
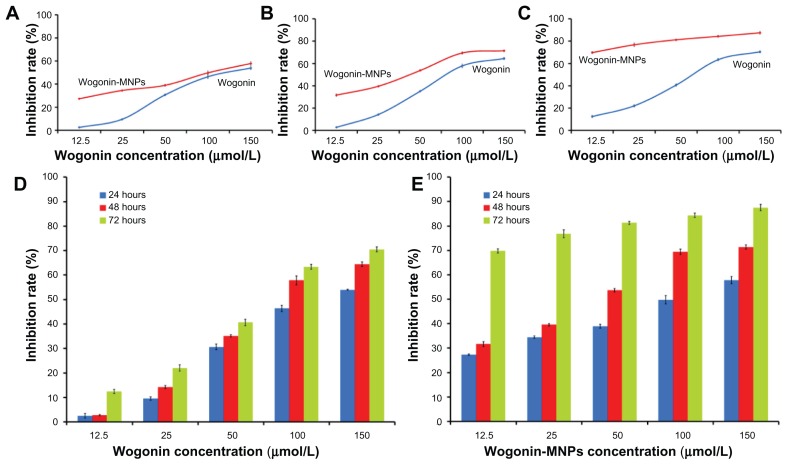
MTT assays were carried out to explore cell viability and inhibition rate of lymphoma cancer cells after being coincubated with different concentrations of wogonin or wogonin-MNPs with equivalent wogonin concentration for 24, 48, and 72 hours. As shown in Figures 3 and 4, results suggest that wogonin-MNPs could inhibit growth of Raji cells more effectively than wogonin alone (P < 0.05). Notably, there was no significant difference between MNPs alone and control group (P > 0.05), which confirmed their low cytotoxicity.
Figure 3.
Viability of Raji cells treated with magnetic nanoparticles, wogonin, or wogonin-magnetic nanoparticles for 48 hours by methylthiazol tetrazolium assay. Inset: Comparison of the half maximal inhibitory concentration of wogonin and wogonin-magnetic nanoparticles at different culture times for Raji cells.
Abbreviations: MNPs, magnetic nanoparticles; IC50, half maximal (50%) inhibitory concentration.
Figure 4.
Inhibition rates of Raji cells treated with different concentrations of wogonin or wogonin-magnetic nanoparticles for 24, 48, and 72 hours. (A) Inhibition rates of Raji cells treated with different concentrations of wogonin or wogonin-magnetic nanoparticles for 24 hours; (B) inhibition rates of Raji cells treated with different concentrations of wogonin or wogonin-magnetic nanoparticles for 48 hours; (C) inhibition rates of Raji cells treated with different concentrations of wogonin or wogonin-magnetic nanoparticles for 72 hours; (D) inhibition rates of Raji cells treated with different concentrations of wogonin for 24, 48, and 72 hours; (E) inhibition rates of Raji cells treated with different concentrations of wogonin-magnetic nanoparticles for 24, 48, and 72 hours.
Abbreviation: MNPs, magnetic nanoparticles.
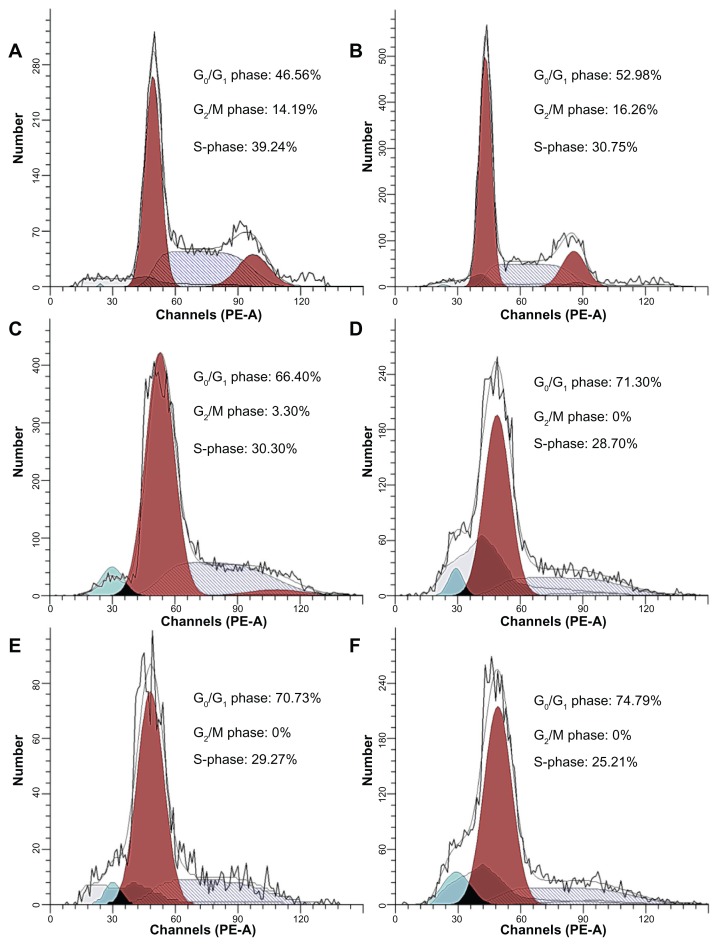
Cell cycle arrest
The effects of MNPs, wogonin, and wogonin-MNPs on the cell cycle were investigated by flow cytometry at 48 hours. Results showed an increased amount of the cell population in G0/G1 phase. Notably, the shift of cell distribution into the G0/G1 phase was increased in a dose-dependent manner and augmented by MNPs (Figure 5; Table 1). There was no significant difference between MNPs alone and control group (P > 0.05).
Figure 5.
Cell cycle distributions of Raji cells treated with wogonin or wogonin-magnetic nanoparticles for 48 hours. (A) Control group; (B) 80 mg/L magnetic nanoparticles; (C) 12.5 μmol/L wogonin; (D) 12.5 μmol/L wogonin-magnetic nanoparticles; (E) 50 μmol/L wogonin; (F) 50 μmol/L wogonin-magnetic nanoparticles.
Abbreviation: MNPs, magnetic nanoparticles.
Table 1.
Cell cycle distributions of Raji cells
| Group | Cell cycle phase | ||
|---|---|---|---|
|
|
|||
| G0/G1 | G2/M | S | |
| Control group | 46.27 ± 1.36 | 14.88 ± 0.61 | 38.85 ± 1.52 |
| 80 mg/L MNPs | 52.69 ± 2.16 | 16.15 ± 0.28 | 31.16 ± 1.91 |
| 12.5 μmol/L wogonin | 66.90 ± 2.32 | 2.37 ± 0.93 | 30.73 ± 1.87 |
| 50 μmol/L wogonin | 70.58 ± 0.98 | 0 | 29.41 ± 0.98 |
| 12.5 μmol/L wogonin-MNPs | 71.58 ± 0.77 | 0 | 28.42 ± 0.77 |
| 50 μmol/L wogonin-MNPs | 75.12 ± 1.63 | 0 | 24.88 ± 1.63 |
Note: Mean ± standard deviation, %.
Abbreviation: MNPs, magnetic nanoparticles.
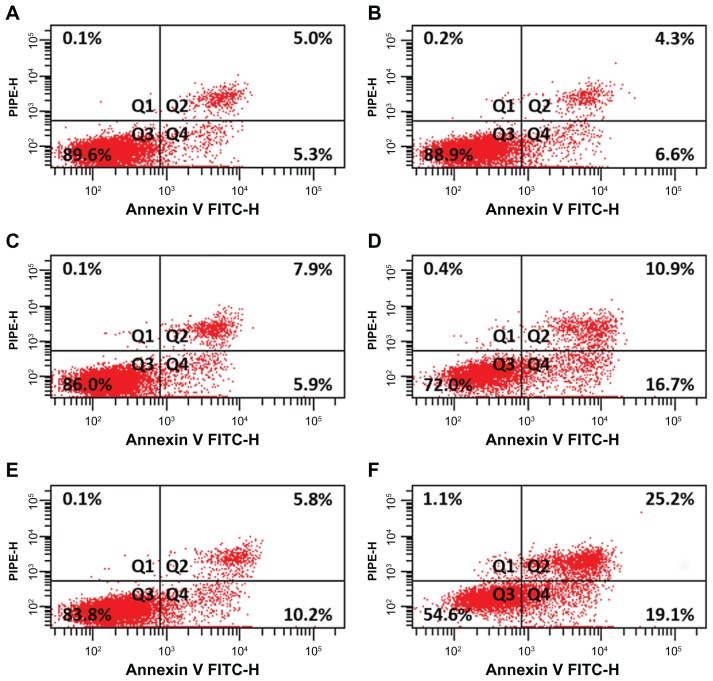
Enhancement of apoptosis
Apoptotic rates of Raji cells were examined with Annexin V propidium iodide double-staining assay. Flow cytometry results show that the proportion of apoptotic cells in wogonin-MNPs group increased significantly compared with that in wogonin group (P < 0.05). However, there was no significant difference between MNPs alone and control group (P > 0.05) (Figure 6). Notably, apoptotic rates in Raji cells treated with wogonin increased in a dose-dependent manner, which were higher when combined with MNPs. All these results further strengthen the possibility that MNPs could strengthen the effect of wogonin on cell apoptosis.
Figure 6.
Apoptotic rates of Raji cells treated with magnetic nanoparticles, wogonin, or wogonin-magnetic nanoparticles for 48 hours. (A) Control group; (B) 80 mg/L magnetic nanoparticles; (C) 12.5 μmol/L wogonin; (D) 12.5 μmol/L wogonin-magnetic nanoparticles; (E) 50 μmol/L wogonin; (F) 50 μmol/L wogonin-magnetic nanoparticles.
Abbreviations: FITC-H, peak height of fluorescein isothiocyanate channel; MNPs, magnetic nanoparticles; PI, propidium iodide; PE-H, peak height of phycoerythrin channel; Q1, necrosis; Q2, late apoptosis; Q3, healthy cells; Q4, early apoptosis.
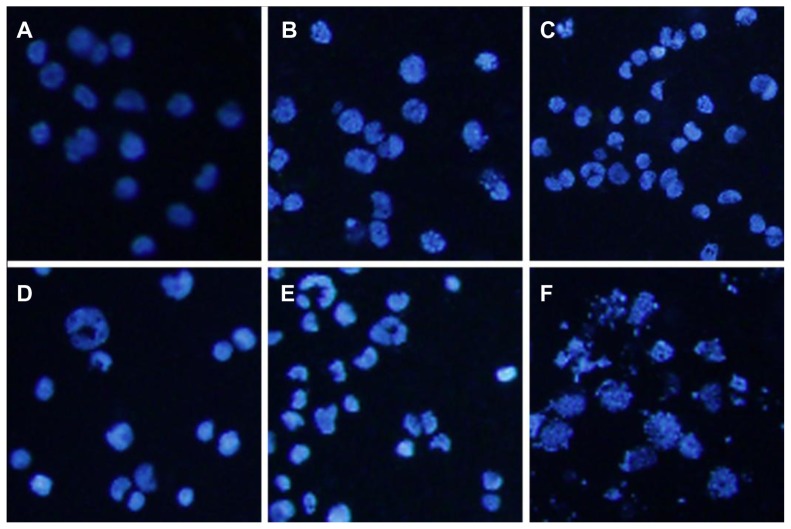
Morphologic characterization of apoptosis
Nucleolus changes of Raji cells under fluorescence microscope are shown in Figure 7. Raji cells with equably blue fluorescence in MNPs alone and control group demonstrated that chromatin equably distributed in the nucleolus. However, after treatment with various concentrations of wogonin and wogonin-MNPs, Raji cells displayed the typical features of apoptosis, such as chromatin condensation, nucleolus pyknosis, nuclear fragmentation, and apoptotic bodies. Notably, it was more evident that the features of apoptosis in the cells incubated with wogonin-MNPs were observed compared to wogonin alone.
Figure 7.
Morphologic characterization of Raji cells after different treatments for 48 hours under inverted fluorescence micrographs (100×, 4′,6-diamidino-2-phenylindole staining). (A) Control group; (B) 80 mg/L magnetic nanoparticles; (C) 12.5 μmol/L wogonin; (D) 12.5 μmol/L wogonin-magnetic nanoparticles; (E) 50 μmol/L wogonin; (F) 50 μmol/L wogonin-magnetic nanoparticles.
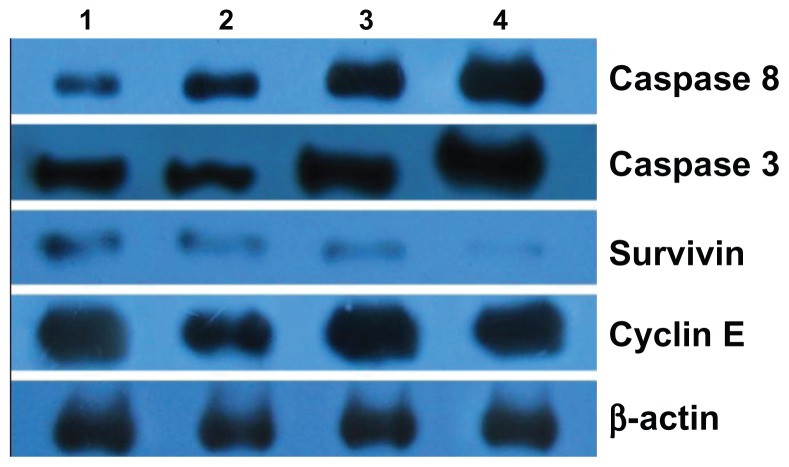
Expression of caspase 3, caspase 8, survivin, and cyclin E
Based on computer-assisted image analysis, it was demonstrated that survivin, caspase 3, and caspase 8 proteins in Raji cells treated with 80 mg/L MNPs for 48 hours had no significant changes when compared with control group (P > 0.05). However, caspase 3 and caspase 8 expression in Raji cells treated with either wogonin or wogonin-MNPs for 48 hours were upregulated compared with MNPs alone (P < 0.05). The level of survivin was downregulated in wogonin group, and this downregulation was further augmented in wogonin-MNPs group. In addition, the expression of cyclin E, which did not significantly change when Raji cells were treated with wogonin alone (P > 0.05), was dramatically downregulated in Raji cells treated with wogonin-MNPs compared with other groups (P < 0.05) (Figure 8).
Figure 8.
Expression of caspase 3, caspase 8, survivin, and cyclin E protein in Raji cells by western blot after treatment of wogonin with or without magnetic nanoparticles for 48 hours. Lane 1, control group; Lane 2, 80 mg/L magnetic nanoparticles; Lane 3, 12.5 μmol/L wogonin; Lane 4, 12.5 μmol/L wogonin-magnetic nanoparticles.
Discussion
The high toxicity of anticancer drugs to normal tissues is the biggest obstacle of cancer therapy, which restricts the application of antineoplastics.16 Enhancing the efficacy of chemotherapy and ameliorating the side effects of cancer chemotherapies are the major goals in the development of tumor treatment.17 With negligible toxicity to nonmalignant cells,7 wogonin, a remedy in traditional Chinese medicine, is becoming an attractive candidate for the development of a new anticancer drug. It has been demonstrated that wogonin possesses a direct cytotoxicity to a large panel of human malignant cell lines in vitro3–5 and suppresses tumor growth in vivo.6 Outcomes in the present study indicate that wogonin can inhibit the growth of Raji cells in a dose- and time-dependent manner. However, the current barrier to clinical application of wogonin is its low water solubility (<100 μmol/L), which was observed in the present experiment.
In order to overcome such limitation, many strategies, such as delivery of drugs by nanocarriers, have been designed to solve problems exhibited by conventional “free” drugs, including limited stability, poor solubility, rapid clearing, and, in particular, lack of selectivity.18 With satisfactory chemical stability, favorable toxicity profile, and superparamagnetic property, the use of MNPs has emerged as an innovative theranostic.19–21 However, large surface-to-volume ratio and magnetic property of iron oxide nanoparticles in nature make them aggregate in aqueous media, seriously impeding their biomedical application.22 Citric acid, as a stabilizer, not only makes MNPs stable in biological liquids but also provides the uncoordinated carboxyl groups for further functionalization.23 Thus, citric acid-modified MNPs were fabricated. Under the influence of a magnet, MNPs were not observed to settle down, which confirmed the stability of the colloidal solution. One of the most important factors that determines biomedical applications of MNPs is hydrodynamic size. MNPs <10 nm are subject to tissue extravasation and renal clearance, whereas those >100 nm are quickly opsoninized and eliminated from circulation via the reticuloendothelial system.19 Gupta and Wells24 reported that the hydrodynamic diameter of well-designed MNPs ranges from 10 nm to 100 nm, which is optimal for in vivo applications. In the present study, the fabricated citric acid-functionalized MNPs, 18 nm in size, were up to grade and suitable for drug delivery. It was noteworthy that MNPs could not only improve water solubility but also substantially augment the anticancer efficacy of wogonin, especially with a low dose of wogonin or long incubation time; even when the concentration of MNPs was up to 80 mg/L, they showed no obvious toxicity to Raji cells. These results suggest that MNPs are a promising and simple drug carrier for cancer therapy, which is reinforced by previous studies that demonstrated that intracellular drug concentration could increase via nanoparticle-mediated endocytosis25 and that fluctuations in drug levels could be avoided by providing sustained release of drugs to enhance the anticancer efficacy.10
The cell cycle is a series of events that take place in a cell leading to its division and duplication. Regulation of the cell cycle involves processes crucial to the survival of a cell. The G1/S checkpoint and G2/M checkpoint monitor and regulate the progress of the cell cycle, so regulation of the cell cycle is favorable to the treatment of cancer. Recently, Min reported that wogonin exerted potential induction of cycle arrest at G0/G1 phase in several cancer cell lines.17 In the present study, an accumulation of cells in G0/G1 phase was similarly observed in wogonin-treated Raji cells. Notably, the shift of cell distribution into the G0/G1 phase was augmented by MNPs. Further studies should be investigated to elucidate the mechanism of the shift.
Cell cycle progress is the result of an orchestrated interaction between several proteins, such as cyclins and cyclin-dependent kinases, which determine a cell’s progress through the cell cycle. It is well known that cyclin D-CDK4/6 complex and cyclin E-CDK2 complex are crucial for G1 checkpoint control. Exposure of different cancer cell lines to wogonin was previously shown to decline cyclin D protein levels, resulting in growth inhibition at G0/G1 phase.17 Consistent with this notion, an accumulation of cells in the G0/G1 phase was observed in wogonin-treated Raji cells in the present study. Notably, reduced cyclin E protein level was observed in Raji cells treated with wogonin-MNPs. These differing results suggest that there may be fundamental differences between cell types. Therefore, wogonin-MNPs possess an enormous potential to evoke cell cycle arrest at G0/G1 phase via downregulation of cyclin E.
Apoptosis or programmed cell death, which occurs after sufficient cellular damage, is a normal component of the development and health of multicellular organisms. Dysregulation of apoptosis can disrupt the balance between cell growth and cell death and is an important step in the development of cancer.26 Evasion of apoptosis has been considered as a hallmark of cancer.27 Therefore, activation of apoptosis is more widely used as a potential anticancer strategy. Results in the present study showed that the apoptotic rate of Raji cells treated with wogonin-MNPs increased significantly compared with wogonin alone, suggesting that MNPs synergistically enhance wogonin-induced cell apoptosis. Results were also confirmed by the typical morphological features of apoptosis in Raji cells stained with DAPI after treatment with wogonin or wogonin-MNPs.
Apoptotic cell death can be executed by two pathways: the death-receptor pathway (extrinsic pathway) and the mitochondrial pathway (intrinsic pathway). In both pathways, apoptotic death stimulus results in activation of caspases, which are the central executioners of the apoptotic pathway.28,29 Initiator caspases, such as caspase 2, 8, 9, 10, 11, and 12 initiate disassembly in response to extracellular apoptosis-inducing ligands and are activated in a complex associated with the cytoplasmic death domain of many cell-surface ligand receptors. On the other hand, effector caspases, such as caspase 3, 6, and 7 are then activated by these active initiator caspases through proteolytic cleavage and carry out the cell death program. Thus, caspase 8 and caspase 3 are situated at pivotal junctions in apoptotic pathways. In the present study, a significant increase in the expression of active caspase 8 and caspase 3 protein was observed in Raji cells treated with wogonin and wogonin-MNPs, indicating that the extrinsic pathway is involved in wogonin-induced apoptotic pathways. A recent report suggesting that wogonin could sensitize cancer cells toward TNFα-induced apoptosis (extrinsic pathway)30 also strengthens the present findings. Moreover, apoptosis is tightly regulated by a fine-tuned balance between proapoptotic and antiapoptotic factors. Survivin, as an inhibitor of apoptosis protein, is not limited to apoptosis inhibition in cancer pathogenesis but is also involved in the regulation of the mitotic spindle checkpoint and the promotion of angiogenesis and chemoresistance. 31 In the present study, either wogonin alone or wogonin-MNPs increased apoptosis of Raji cells and this was accompanied by the downregulation of survivin protein. In general, wogonin-MNPs induced cell apoptosis through various pathways.
Conclusion
The present study demonstrates that MNPs not only increase the water solubility of wogonin but also enhance its therapeutic efficiency via blocking the cell cycle at G0/G1 phase and inducing apoptosis of Raji cells. These findings indicate that the combination of wogonin and MNPs provides a promising strategy for lymphoma therapy.
Acknowledgments
This work was financially supported by National Key Basic Research Program 973 of China (No 2010CB732404), National Natural Science Funds of People’s Republic of China (No 81170492), and Key Medical Disciplines of Jiangsu Province.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.van Besien K. Current status of allogeneic transplantation for aggressive non-Hodgkin lymphoma. Curr Opin Oncol. 2011;23(6):681–691. doi: 10.1097/CCO.0b013e32834bb88e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dong P, Zhang Y, Gu J, et al. Wogonin, an active ingredient of Chinese herb medicine Scutellaria baicalensis, inhibits the mobility and invasion of human gallbladder carcinoma GBC-SD cells by inducing the expression of maspin. J Ethnopharmacol. 2011;137(3):1373–1380. doi: 10.1016/j.jep.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 4.Chow SE, Chang YL, Chuang SF, Wang JS. Wogonin induced apoptosis in human nasopharyngeal carcinoma cells by targeting GSK-3β and ΔNp63. Cancer Chemother Pharmacol. 2011;68(4):835–845. doi: 10.1007/s00280-010-1552-1. [DOI] [PubMed] [Google Scholar]
- 5.Gao J, Morgan WA, Sanchez-Medina A, Corcoran O. The ethanol extract of Scutellaria baicalensis and the active compounds induce cell cycle arrest and apoptosis including upregulation of p53 and Bax in human lung cancer cells. Toxicol Appl Pharmacol. 2011;254(3):221–228. doi: 10.1016/j.taap.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 6.Yu J, Liu H, Lei J, Tan W, Hu X, Zou G. Antitumor activity of chloroform fraction of Scutellaria barbata and its active constituents. Phytother Res. 2007;21(9):817–822. doi: 10.1002/ptr.2062. [DOI] [PubMed] [Google Scholar]
- 7.Lee DH, Kim C, Zhang K, Lee YJ. Role of p53, PUMA, and Bax in wogonin-induced apoptosis in human cancer cells. Biochem Pharmacol. 2008;75(10):2020–2033. doi: 10.1016/j.bcp.2008.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu H, Cheng L, Wang C, Ma X, Li Y, Liu Z. Polymer encapsulated upconversion nanoparticle/iron oxide nanocomposites for multi-modal imaging and magnetic targeted drug delivery. Biomaterials. 2011;32(35):9364–9373. doi: 10.1016/j.biomaterials.2011.08.053. [DOI] [PubMed] [Google Scholar]
- 9.Xia G, Chen B, Ding J, et al. Effect of magnetic Fe3O4 nanoparticles with 2-methoxyestradiol on the cell-cycle progression and apoptosis of myelodysplastic syndrome cells. Int J Nanomedicine. 2011;6:1921–1927. doi: 10.2147/IJN.S24078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang C, Zhang H, Chen B, Yin H, Wang W. Study of the enhanced anticancer efficacy of gambogic acid on Capan-1 pancreatic cancer cells when mediated via magnetic Fe3O4 nanoparticles. Int J Nanomedicine. 2011;6:1929–1935. doi: 10.2147/IJN.S24707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.von Maltzahn G, Park JH, Lin KY, et al. Nanoparticles that communicate in vivo to amplify tumour targeting. Nat Mater. 2011;10(7):545–552. doi: 10.1038/nmat3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pradhan P, Giri J, Rieken F, et al. Targeted temperature sensitive magnetic liposomes for thermo-chemotherapy. J Control Release. 2010;142(1):108–121. doi: 10.1016/j.jconrel.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 13.Foy SP, Labhasetwar V. Oh the irony: iron as a cancer cause or cure? Biomaterials. 2011;32(35):9155–9158. doi: 10.1016/j.biomaterials.2011.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guevara AP, Vargas C, Sakurai H, et al. An antitumor promoter from Moringa oleifera Lam. Mutat Res. 1999;440(2):181–188. doi: 10.1016/s1383-5718(99)00025-x. [DOI] [PubMed] [Google Scholar]
- 15.Jiang Z, Chen BA, Xia GH, et al. The reversal effect of magnetic Fe3O4 nanoparticles loaded with cisplatin on SKOV3/DDP ovarian carcinoma cells. Int J Nanomedicine. 2009;4:107–114. doi: 10.2147/ijn.s5393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeVita VT. Dose-response is alive and well. J Clin Oncol. 1986;4(8):1157–1159. doi: 10.1200/JCO.1986.4.8.1157. [DOI] [PubMed] [Google Scholar]
- 17.Min LW. New therapeutic aspects of flavones: the anticancer properties of Scutellaria and its main active constituents Wogonin, Baicalein and Baicalin. Cancer Treat Rev. 2009;35(1):57–68. doi: 10.1016/j.ctrv.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 18.Ashley CE, Carnes EC, Phillips GK, et al. The targeted delivery of multicomponent cargos to cancer cells by nanoporous particle-supported lipid bilayers. Nat Mater. 2011;10(5):389–397. doi: 10.1038/nmat2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cole AJ, Yang VC, David AE. Cancer theranostics: the rise of targeted magnetic nanoparticles. Trends Biotechnol. 2011;29(7):323–332. doi: 10.1016/j.tibtech.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jang JT, Nah H, Lee JH, Moon SH, Kim MG, Cheon J. Critical enhancements of MRI contrast and hyperthermic effects by dopant-controlled magnetic nanoparticles. Angew Chem Int Ed Engl. 2009;48(7):1234–1238. doi: 10.1002/anie.200805149. [DOI] [PubMed] [Google Scholar]
- 21.Comes Franchini M, Baldi G, Bonacchi D, et al. Bovine serum albumin-based magnetic nanocarrier for MRI diagnosis and hyperthermic therapy: a potential theranostic approach against cancer. Small. 2010;6(3):366–370. doi: 10.1002/smll.200901689. [DOI] [PubMed] [Google Scholar]
- 22.Goodarzi A, Sahoo Y, Swihart MT, Prasad PN. Aqueous ferrofluid of citric acid coated magnetite particles. Mater Res Soc Symp Proc. 2004;789:661–666. [Google Scholar]
- 23.Nigam S, Barick KC, Bahadur D. Development of citrate-stabilized Fe3O4 nanoparticles: conjugation and release of doxorubicin for therapeutic applications. J Magn Magn Mater. 2011;323(2):237–243. [Google Scholar]
- 24.Gupta AK, Wells S. Surface-modified superparamagnetic nanoparticles for drug delivery: preparation, characterization, and cytotoxicity studies. IEEE Trans Nanobioscience. 2004;3(1):66–73. doi: 10.1109/tnb.2003.820277. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y, Kohler N, Zhang M. Surface modification of superpara-magnetic magnetite nanoparticles and their intracellular uptake. Biomaterials. 2002;23(7):1553–1561. doi: 10.1016/s0142-9612(01)00267-8. [DOI] [PubMed] [Google Scholar]
- 26.Kerr JF, Winterford CM, Harmon BV. Apoptosis: its significance in cancer and cancer therapy. Cancer. 1994;73(8):2013–2026. doi: 10.1002/1097-0142(19940415)73:8<2013::aid-cncr2820730802>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 27.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 28.Nicholson DW. Caspase structure, proteolytic substrates, and function during apoptotic cell death. Cell Death Differ. 1999;6(11):1028–1042. doi: 10.1038/sj.cdd.4400598. [DOI] [PubMed] [Google Scholar]
- 29.Chen B, Liang Y, Wu W, et al. Synergistic effect of magnetic nano-particles of Fe3O4 with gambogic acid on apoptosis of K562 leukemia cells. Int J Nanomedicine. 2009;4:251–259. doi: 10.2147/ijn.s7932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fas SC, Baumann S, Zhu JY, et al. Wogonin sensitizes resistant malignant cells to TNFalpha- and TRAIL-induced apoptosis. Blood. 2006;108(12):3700–3706. doi: 10.1182/blood-2006-03-011973. [DOI] [PubMed] [Google Scholar]
- 31.Mita AC, Mita MM, Nawrocki ST, Giles FJ. Survivin: key regulator of mitosis and apoptosis and novel target for cancer therapeutics. Clin Cancer Res. 2008;14(16):5000–5005. doi: 10.1158/1078-0432.CCR-08-0746. [DOI] [PubMed] [Google Scholar]