Summary
Programmed cell death is essential for the maintenance of lymphocyte homeostasis and immune tolerance. Dendritic cells (DCs), the most efficient antigen presenting cells, represent a small cell population in the immune system. However, DCs play major roles in the regulation of both innate and adaptive immune responses. Programmed cell death in DCs is essential for regulating DC homeostasis and consequently, the scope of immune responses. Interestingly, different DC subsets show varied turnover rates in vivo. The conventional DCs are relatively short-lived in most lymphoid organs, while plasmacytoid DCs are long-lived cells. Mitochondrion-dependent programmed cell death plays an important role in regulating spontaneous DC turnover. Antigen-specific T cells are also capable of killing DCs, thereby providing a mechanism for negative feedback regulation of immune responses. It has been shown that a surplus of DCs due to defects in programmed cell death leads to overactivation of lymphocytes and the onset of autoimmunity. Studying programmed cell death in DCs will shed light on the roles for DC turnover in the regulation of the duration and magnitude of immune responses in vivo, and in the maintenance of immune tolerance.
Keywords: dendritic cells, apoptosis, programmed cell death, immune tolerance
Introduction
Different cell types in the immune system, including T cells, B cells, and the less abundant NK cells, macrophages and DCs, are maintained in relatively constant numbers after development (1–7). Homeostasis is important for keeping a functional and balanced immune system, but the mechanisms for homeostatic regulation in the immune system is poorly understood. For a particular cell type to maintain a stable number, its rate of programmed cell death needs to be kept in balance with the rate of renewal from its precursors. DCs are the most efficient antigen presenting cells for the uptake, processing and presentation of antigens to stimulate antigen-specific lymphocytes (8). Although DCs represent a small population of cells in lymphoid and non-lymphoid organs and tissues, they are key regulators of both innate and adaptive immune responses (9, 10). DCs may also play important roles in the development and maintenance of immune tolerance (8, 11). Injection of excessive activated DCs can induce the development of systemic or tissue-specific autoimmune diseases in mice (12, 13). Either over-accumulation or depletion of DCs may disrupt immune tolerance (14–16). Maintenance of DC homeostasis by programmed cell death is likely to have major impacts on the scope of antigen-specific immune responses and immune tolerance.
Turnover rates of different DC subsets
When DCs were first identified as a cells type with characteristic protrusion of dendrites and distinct functional properties, they were found to have low proliferation potentials, yet constantly undergo rapid turnover under steady state conditions (17, 18). Many different subsets of DCs in different organs with distinct phenotypic and functional properties have been described (10, 19–21). DCs represent a heterogeneous population that can be distinguished by their cell surface markers, in vivo localizations, migratory properties, cytokine productions and other functional properties (10). The main subtypes of DCs include the conventional DCs that can be found in different organs and tissues, and plasmacytoid DCs (pDCs) (21). pDCs are a specialized DC subtype that is a major producer for interferon-α, while conventional DCs are more complex and can be further divided into migratory DCs, such as Langerhans cells in epidermal tissues, interstitial DCs and lymphoid-tissue-resident DCs (10, 19–22). Resident DCs in lymphoid tissues constitute the majority of DCs in the thymus and spleen, and about half of DCs in the lymph node. Lymphoid-tissue-resident conventional DCs can be further distinguished based on their surface expression of CD4 and CD8, including CD8+, CD4+CD8− and CD4−CD8− conventional DCs. The surface phenotype, lifespan and functions for residential DCs in different tissues are likely to be influenced by their local microenvironment (20, 22).
The lifespan of DCs in vivo has been evaluated by a series of studies that measure the kinetics for the labeling of DC with 5-bromodeoxyuridine (BrdU) (23–26). Isolated DCs generally do not proliferate in vitro (24, 26). Consistent with the low proliferative potential of differentiated DCs, pulsing with BrdU for a period of two hours gives rise to only marginal labeling of DCs (24, 26). The labeling of differentiated DCs in lymphoid organs by BrdU likely represents the newly generated DCs from precursors that have the potentials to proliferate (5). DCs in the spleen and mesenteric lymph nodes show rapid kinetics of BrdU labeling with no lagging time (26), suggesting that DCs are either derived from residential DC precursors or rapidly replenished from the blood stream. However, thymic DCs show an initial lag in BrdU labeling, followed by rapid incorporation of BrdU (26). The discontinuity in the labeling curve for the thymic DC lineage could be explained by the existence of different thymic DC lineages, or the replenish by DC precursors from different tissues that take time to migrate to the local lymphoid organs or thymus. However, the possibility of a delay for BrdU to reach the thymus cannot be ruled out.
We and others have observed that conventional DCs are labeled rapidly in vivo by BrdU (24, 26, 27). In particular, near 50% of CD11c+CD11b+ DCs in the spleens are labeled by BrdU in 48 h, suggesting that these DCs in the spleens have a half-life of approximately two days. CD8+ DCs are labeled slightly faster than CD8− DCs (24, 26). Comparing to lymphoid-tissue-resident DCs, Langerhans cells have a slower kinetics of BrdU labeling (26), indicating that Langerhans cells belong to a different cell lineage with a slower rate of cell death and self-renewal. In contrast to conventional DCs, CD11clowPDCA-1+ pDCs display significantly slower rates of BrdU labeling, with a half-life of eight to nine days (25, 27), suggesting that pDCs are long-lived cells similar to T cells in vivo. Therefore, different DC subsets have distinct kinetics of self-renewal in vivo. However, the functional significance for such differences among DC subsets is currently unknown.
Regulation of DC homeostasis
DCs are present in small numbers (usually around 1%) in lymphoid organs, and even less in non-lymphoid tissues and in the blood (28). Although the precise mechanism for the maintenance of DC homeostasis is not clear, emerging evidence suggests that cytokines may be important regulators. Fms-like tyrosine kinase receptor-3 ligand (Flt3-L) is a cytokine crucial for the development of both conventional DCs and pDCs (29–31). It has been reported that Flt3-L promotes both survival of DCs and the differentiation DCs from their precursors (31, 32). Using CD11c–DTR transgenic mice that express the receptor for diphtheria toxin (DTA) in DCs, it has been shown that depletion of DC by injection of DTA results in elevated levels of Flt3-L and increased generation of DCs from precursors (33). The level of Flt3-L in serum returns to normal when DC number is restored (33). An independent study has shown that DC expansion in response to Treg ablation also requires local production of Flt3-L (5). These results suggest that an Flt3-L-dependent feedback mechanism is involved in homeostatic control of DC numbers in vivo.
Cytokines are important regulators of cell death in DCs. It has been shown that GM-CSF synergizes with Flt3-L to maintain the total numbers of conventional DCs in vivo (34, 35). Interestingly, we have observed that withdrawal of GM-CSF from cultured bone marrow-derived DCs leads to up-regulation of pro-apoptotic Bim and accelerated cell death in DCs (36). GM-CSF may help to maintain DC homeostasis by inhibiting Bim-dependent apoptosis. In contrast to GM-CSF, IL-10 has been shown to promote cell death in DCs by inhibiting the expression of anti-apoptotic molecules Bcl-2 and Bcl-xL (37). Thymic stromal lymphopoietin (TSLP) produced by non-hematopoietic cells such as fibroblasts, epithelial cells and different types of stromal cells can promote the survival of DCs in vitro (38). Whether TSLP is indeed involved in the protection of DC viability in vivo remains to be determined. In addition, whether TSLP affects the expression of apoptosis signaling molecules in DCs should be examined. Although TGF-β1 is important for the development of Langerhans cells (39–41), TGF-β1 has been shown to induce apoptosis in monocyte-derived DCs (42). Whether TGF-β1 might induce apoptosis in certain DC subsets for immunosuppression will be interesting to investigate.
Based on their rates of BrdU labeling, we estimate that 40–50% of CD11c+CD11b+ conventional DCs in the mouse spleen undergo cell death in 48h in vivo (27). However, isolated CD11c+CD11b+ DCs undergo even faster cell death during in vitro culture (27). Interestingly, a majority (approximately 90%) of pDCs isolated from the mouse spleen undergo cell death after 24 h of in vitro culture (27). This is different from the predicted lifespan of pDCs by in vivo BrdU labeling studies that show a relatively slow rate of self-renewal for pDCs with approximately 5% of BrdU labeling over 24 h (27). This suggests that the local microenvironment of lymphoid organs plays an essential role in regulating the lifespan of DCs. Although conventional DCs have a shorter lifespan than pDCs in vivo, they survive better during in vitro culture than pDCs (27). Therefore, the local microenvironment of the spleens appears to be even more critical for the survival of pDCs. Cell-cell contact with other cell types in the spleen, as well as cytokines in the spleen environment may help to maintain the survival of DCs in vivo.
Besides the regulation by cytokines and other soluble factors, direct cell-cell contact for DCs with other cell types in the local microenvironment may also play an important role in the regulation of DC homeostasis. For example, Treg cells may inhibit Flt3-L productions to suppress novel DC generation from their precursors (5, 43). Formation of the immunological synapse between DCs and antigen-specific T cells has been shown to inhibit DC apoptosis (44). In addition, interaction with matrix in lymphoid tissues may help to sustain DC survival. Whether adhesion molecules and other cell surface receptors may induce pro-survival signaling into DCs through interactions with other cells or matrix remains to be determined.
DCs in the lymph node during cognate interaction with T effector cells
As professional antigen presenting cells, DCs are important for the activation of antigen-specific T cells. However, the interactions between DCs and T cells are not unidirectional. The activation status of T cells may determine whether T-DC interaction promotes or inhibits cell death in DCs. It has been shown that the presence of naïve T cells helps to promote the survival of DCs in vitro or in lymph nodes (38, 44). DCs have been observed to form cognate conjugates with antigen-specific T cells in the draining lymph nodes at 24 h after adoptive transfer (45). However, most of these DCs disappear from the lymph nodes 48 h post transfer (45). One possibility is that DCs migrate out of the lymph nodes after they have activated T cells. However, it is more plausible that DCs are killed by T cells that they have previously activated. Indeed, DCs can undergo rapid cell death after interaction with antigen-specific CD4+ T cells in vitro (46). CD8+ T cells have also been demonstrated to kill DCs during anti-tumor immune response and viral infections (47, 48). In addition, mouse CD62L−CCR7− effector memory T cells have been demonstrated to kill the antigen-loaded DCs in lymph nodes (49). Antigen-specific T cells may therefore function as negative feedback regulators by killing antigen-specific DCs.
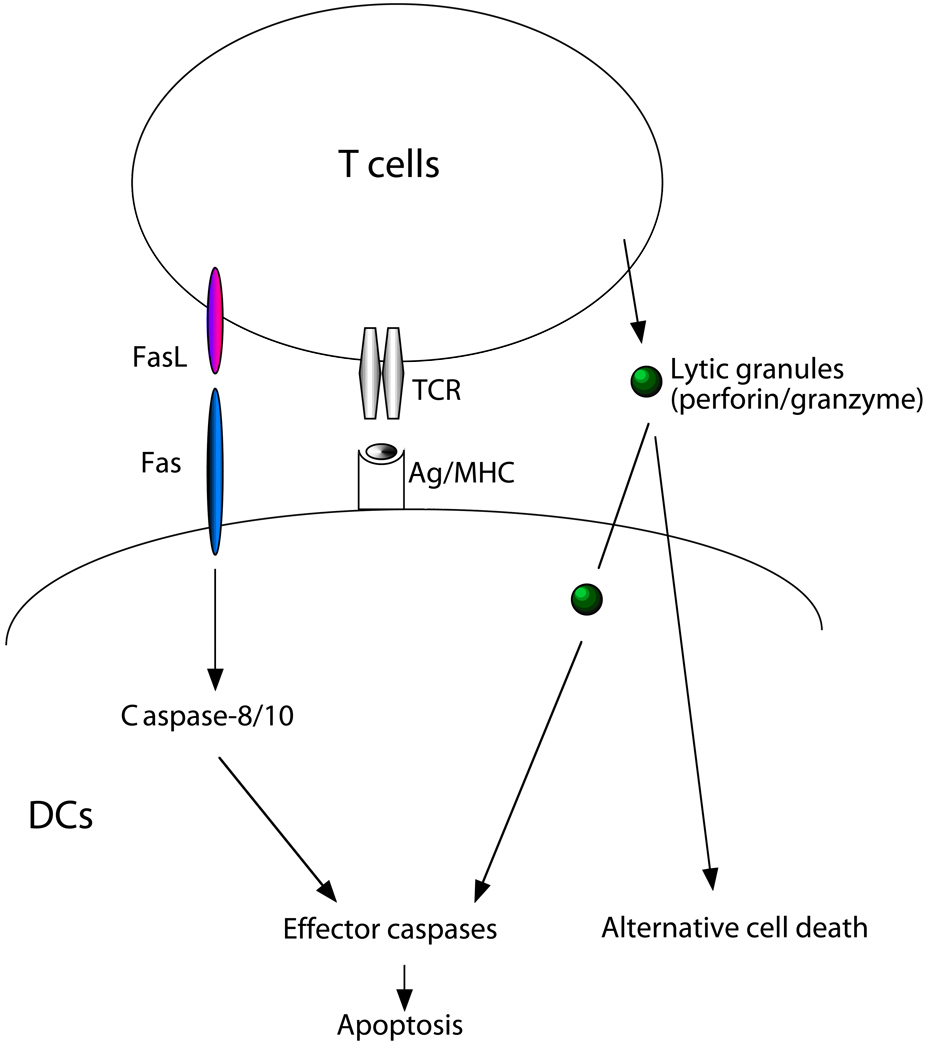
We have observed that activated T cells can kill DCs through a Fas- and perforin-dependent manner in vitro (Fig. 1) (14). Perforin-dependent elimination of DCs limits the scope of CD8+ T cell activation (50). Interestingly, memory CD8+ T cells may release TNF-α before the release of cytotoxic granules, leading to TNF-α-induced expression of an endogenous granzyme B inhibitor, PI-9, to protect DCs from CTL killing (51). Whether such a protective mechanism is unique to central memory T cells has not been determined.
Figure 1. T cell-mediated killing of DCs.
Activated T cells produce FasL and also secrete cytolytic granules containing perforin and granzyme B. DCs can be killed by antigen-specific T cells through Fas- and perforin-dependent manners. Triggering of Fas can lead to the activation of caspase-8 and caspase-10 (in humans) and trigger a downstream caspase cascade to induce apoptosis. Cytolytic components secreted by T cells depend on perforin to enter DCs. Granzyme B may direct cleave effector caspases to induce apoptosis. Other components may also induce other forms of programmed cell death. However, alternative cell death pathways in DCs have not been well characterized.
Induction of cell death in antigen-presenting DCs by activated T effector cells provides an important mechanism for feedback regulation of antigen-specific immune responses. This many help to restrict the scope of an immune response to a specific antigen by preventing excessive T cell activation. However, such a negative feedback mechanism should not take place too quickly. Elimination of antigen-presenting DCs by activated antigen-specific T cells too early in an immune response could result in poor immune responses. Indeed, rapid deletion of DCs by activated viral-specific T cells has been shown to cause immunosuppression after infection by measles virus or lymphocytic choriomeningitis virus (47, 52). On the other hand, inhibition of DC apoptosis by T cells after formation of immunological synapse may be essential for efficient immune responses (44). It will be interesting to investigate the temporal relation of T cell activation by DCs and T cell-mediated killing of DCs, and how this regulates the magnitude of primary, secondary and memory responses to antigens presented by DCs. Studying the regulation of cell death ion DCs may help to improve the strategies for successful DC-based vaccination.
Interaction of Treg cells with DCs
It has been well established that CD4+FoxP3+ Treg cells can inhibit the activation of T effector cells (53–55). Treg cells may inhibit T effector cells through direct cell-cell contact or secretion of soluble factors. Intriguingly, co-transfer of Treg cells with DCs leads to decrease of DCs in draining lymph nodes (56). Treg cells may lead to a decrease in the lifespan of DCs in this case. However, a direct effect of Treg cells on the regulation of DC lifespan has yet to be demonstrated. An in vitro study shows that Treg cells can employ a perforin/granzyme-dependent mechanism to kill DCs (57). Treg-mediated killing of DCs could provide an alternative mechanism for immunosuppression by Treg cells. It will be interesting to determine whether Treg cells could directly induce cell death in DCs in vitro and in lymphoid organs to inhibit DC-dependent immune responses.
Loss of Treg cells has been shown to induce the increases in total DCs, especially the conventional CD11c+CD11b+ DCs (43). Treg cells could also indirectly regulate the lifespan of DCs through other cell types. Increased Flt3-L and accelerated generation of DCs from the committed precursors of conventional DCs (pre-cDCs) have been observed after Treg depletion (5). This suggests that Treg cells may have a suppressive role in restricting the generation of DCs from their precursors by inhibiting Flt3-L production from an unidentified cell type (43).
Although temporary deletion of DCs through injection of DTA into CD11c-DTR transgenic mice leads to weakened immune responses (58), constitutive ablation of DCs by DC-specific expression of DTA in CD11c-DTA transgenic mice has been shown to break immune tolerance (16). DCs are important for supporting homeostatic expansion of Treg cells in the periphery (59). In particular, it has been shown that certain DC subsets are capable of inducing the development of Treg cells (60). The expression of indoleamine 2,3 dioxygenase, an enzyme important for the degradation of tryptophan, in pDCs, allows for direct activation of Treg cells (61). Local microenvironment or engulfment of apoptotic cells may also confer tolerogenic properties to DCs to induce the generation of Treg cells (62, 63). We also observed a small but reproducible increase of CD4+FoxP3+ Treg cells in autoimmune mice harboring apoptosis deficiency in DCs (14). How apoptosis-deficient DCs overcome the inhibition by Treg cells to over-activate T effector cells still needs to be elucidated. A potential direct interaction between DCs and Treg cells in immune regulation will be a very interesting topic to study.
DC apoptosis in the regulation of antigen-specific immune responses
Increased survival of DCs with the expression of Bcl-2 transgene has been shown to enhance antigen-specific T cell activation and antibody production (64, 65). Increased total DC numbers could allow more DCs to trigger the activation of T cells simultaneously. Increased survival may also prolong the duration for DCs to stimulate T cells. Conversely, temporary ablation of DCs may limit antigen-specific immune responses to infections (58). Cell death in DCs may inhibit the induction of tumor-specific immune responses (66), while suppressing apoptosis in DCs can enhance the potency of DC-based vaccine to trigger tumor-specific immunity (67–70). These data suggest that the lifespan of DCs indeed affects the magnitude of immune responses to antigens.
We have observed that inhibition of apoptosis in DCs leads to accumulation of DCs, over-activation of lymphocytes and the breakdown of immune tolerance (14). Consistently, it has been shown that conditional deletion of Fas on DCs induces the development of systemic autoimmune responses (15). Deficiency in Bim, a Bcl-2 family member involved in mitochondria-dependent apoptosis, has also been shown to contribute to DC accumulation, enhanced T cell activation and the development of autoimmune diseases (36). Therefore, perturbation of programmed cell death in DCs can have a profound effect in the disruption of antigen-specific immune responses and immune tolerance.
Types of programmed cell death
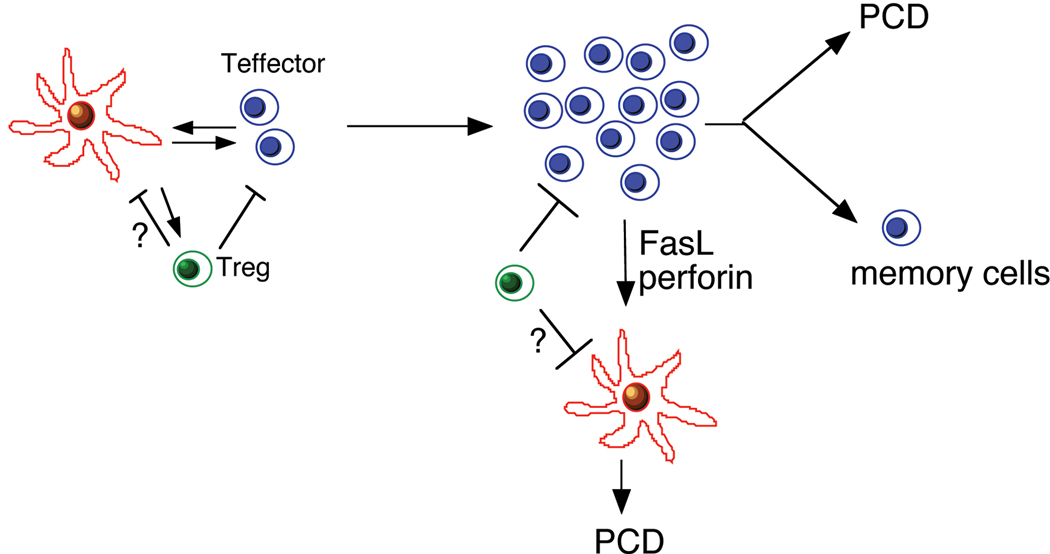
DCs are generated from their precursors that have intermediate potentials for proliferation (5). Because DCs no longer proliferate after differentiation, the homeostasis of DCs must be maintained through the balance between their regeneration from precursors and programmed cell death. Regulation of the intrinsic apoptosis pathway likely regulates the rate of spontaneous cell death in DCs. Interaction with other cell types in the immune system, especially T effector cells and Treg cells, may also play crucial roles in regulating the survival and functions of DCs (Fig. 2). Here we will focus on the regulation of DC homeostasis by programmed cell death.
Figure 2. Interactions of DCs with T effector cells and Treg cells in immune responses.
DCs process and present antigens to activation antigen-specific T effector cells, while T effector cells may provide survival signals to DCs. Treg cells inhibit DCs potentially through suppression of DC renewal. After activation, however, T effector cells may kill antigen-presenting DCs through Fas- and perforin-dependent manner. Treg cells may induce cell death in DCs, however, a molecular mechanism for Treg-mediated suppression of DC is unknown. Most activation T cells undergo programmed cell death (PCD) after the clearance of antigens, while some will survive as memory cells. Whether DC influence PCD in activated T cells and the generation will be interesting to investigate.
Death receptor-mediated apoptosis
Apoptosis is the best-characterized form of programmed cell death. Necrosis and autophagic cell death may also play important roles in cell death when apoptosis pathways are suppressed (71–75). After significant expansion of antigen-specific lymphocytes, programmed cell death is important for the contraction of these expanded cell types (3, 7, 76). Two major apoptosis pathways, including the death receptor-mediated and mitochondrion-dependent apoptosis, have been intensively studied in the immune system. The tumor necrosis factor receptor (TNFR) superfamily members contain characteristic cysteine-rich repeats in the extracellular domains. Some of the TNFR family members that contain the conserved death domain (DD) in the intracellular region are called death receptors, including Fas, TNFR I, DR3, TRAIL-R1/DR4, TRAIL-R2/DR5, and DR6 (77). After engagement by their ligands, Fas and TRAIL receptors, bind to an adaptor, FADD, through homotypic interactions of their DDs (78–80). FADD then recruit caspase-8 to the death receptor through another homotypic interactions of their death effector domains (DEDs), resulting in the formation of the death-inducing signaling complex (DISC) to initiate caspase activation (80–82). Caspase-10 is a conserved caspase homologous to caspase-8. Caspase-10 gene has been reported to be in many species, such as sea squirt, zebra fish, xenopus, chicken, rabbit, rhesus monkey and human (NCBI Genebank), but it is not found in mice (83). Mice may have lost caspase-10 during evolution. Caspase-10 is also recruited to DISC and mediates death receptor-induced apoptosis (84, 85). Caspase-8 and caspase-10, the two caspases may have overlapping and distinct functions (86, 87). Caspase-8 and caspase-10 mutations have been identified in human autoimmune patients that contribute to autoimmunity or immunodeficiency (88, 89). Deletion of caspase-8 in mice causes early embryonic lethality (90), suggesting that caspase-8 is a non-redundant caspase in mice. Other death receptors use similar but more complicated signaling modules to initiate cell death signaling (91). Death receptor-dependent apoptosis are usually triggered by the ligands that belong to the TNF superfamily (77). DCs have been shown to express death receptors such as Fas, TNF receptors and TRAIL receptors, and are susceptible to FasL, TNF and TRAIL-mediated apoptosis (14, 92, 93).
Mitochondrion-dependent apoptosis
Induction of DNA damage or inhibition of DNA repair, loss of growth factors, as well as other factors triggering cellular stress that are sensed by the BH3-only proteins of the Bcl-2 family, can induce the intrinsic mitochondrion-dependent apoptosis pathway, resulting in the release of cytochrome C from mitochondrion into the cytosol (94–96). Cytochrome c interacts with Apaf-1 and caspase-9, leading to caspase-9 activation and subsequent activation of downstream effector caspases and apoptosis (95). Smac/Diablo is also released from mitochondrion to promote caspase activation by neutralizing the inhibitors of apoptosis (IAPs) (97, 98). In addition, endonuclease G and apoptosis inducing factor (AIF) are released from mitochondrion and may cause cell death in a caspase-independent manner (99, 100). Accumulating evidence suggests that DCs are sensitive to mitochondrion-dependent apoptosis and Bcl-2 family members are important for the regulation of programmed cell death in DCs (3, 27, 36, 64).
Alternative cell death pathways
It has been shown that Receptor Interacting Protein 1 (RIP1) and RIP3 kinases mediate necrotic signaling when caspases are inhibited (72–74). In the absence of caspase activation, cells under stress may also attempt to rescue themselves through autophagy, a process of degradation of self-components by autophagosomes (101, 102). However, excessive autophagy will lead to cell demise (71, 103). It appears that apoptosis involving caspase activation is a rapid and predominant form of programmed cell death. When apoptosis pathways are inhibited, necrotic or autophagic cell death may take place. Interestingly, autophagy has been shown to be important for antigen processing in DCs (104). Whether autophagic or necrotic cell death also play important roles in the regulation of programmed cell death in DCs remains to be determined.
Fas-dependent apoptosis in DCs
It has been well established that lymphocytes are susceptible to Fas-mediated apoptosis. Several studies have demonstrated that DCs can be induced to undergo apoptosis through Fas (46, 105, 106). However, other studies have shown resistance to Fas-dependent cell death in DCs (107–109). Such variations could be due to different sources of DCs used in the assays. In autoimmune lpr mice, both lymphocytes and DCs undergo significant expansion (110). If DCs are refractory to Fas-mediated apoptosis, however, the expansion of DCs in Fas-deficient lpr mice would have to be attributed to the effects other than the intrinsic apoptosis defects in DCs. We have therefore performed a screening for the signaling molecules of the Fas signaling pathway in DCs (14). Because activated T cells are known to harbor an active Fas-signaling pathway, we compared Fas signaling pathway in DCs and T cells. Interestingly, we observed no significant difference between DCs and T cells in the expression of Fas, FADD, caspase-8 and FLIP that are important components for the formation of the death-inducing signaling complex (14). This suggests that the Fas signaling pathway is active in DCs.
It has been observed that T cells are capable of killing DCs (46–48). We have therefore determined whether DCs are susceptible to T cell-mediated killing through Fas. Using allo-specific T cells derived from mixed lymphocyte reaction, we demonstrate that both CD4+ T cells and CD8+ T cells are capable of killing DCs (14). Perforin−/− T cells killed DCs less efficiently than wild type T cells, suggesting the involvement of perforin in T cell-mediated killing of DCs (14, 50). Using soluble Fas or TRAIL receptors, we observed that killing of DCs by perforin−/− T cells were completely inhibited by Fas-Fc, but not TRAIL-R-Fc, suggesting that Fas is the dominant death receptor employed in T cell-mediated killing of DCs in addition to perforin (Fig. 1). These in vitro data suggest that T cells kill DCs through a Fas- and perforin-dependent mechanism.
To determine a potential role for Fas in regulating apoptosis in DCs, we generated transgenic mice expressing the baculoviral p35, a potent inhibitor for caspase-8 and several downstream caspases, but not caspase-9, in DCs (DC-p35) (111). DCs from DC-p35 transgenic mice show reduced Fas-dependent apoptosis, leading to the accumulation of conventional DCs and pDCs. This supports a role for Fas-mediated apoptosis in regulating DC homeostasis in vivo. Consistent with our studies, conditional knockout of Fas in DCs also lead to significant expansion of DCs (15). These in vivo data shows that Fas-mediated apoptosis indeed plays an important role in regulating DC homeostasis in vivo. It has been reported that deficiency in perforin exacerbates lymphoproliferation and autoimmunity in Fas-deficient lpr mice (112). It will be interesting to test whether perforin and Fas synergize to maintain immune tolerance through induction of cell death in DCs.
Other TNFR family members in the regulation of cell death in DCs
Decoy receptor 3 (DcR3), also known as TR6 or M68, is a soluble member in the TNF receptor superfamily (35, 113, 114). It can bind to both FasL and LIGHT, and inhibit apoptosis by interfering with FasL-Fas interaction or suppressing the interaction between LIGHT and lymphotoxin β receptor (LTβR). The up-regulation of DcR3 in cancer patients is associated with DcR3-mediated inhibition of cell death (113, 115). Following stimulation of Toll like receptors, myeloid derived DCs have been shown to release DcR3 (116). Interestingly, however, it has been shown that DcR3 can bind to heparan sulfate proteoglycans on DCs to promote cell death by up-regulation of TRAIL R2/DR5 (117). In addition, DcR3 has also been shown to interfere with DC differentiation and maturation (118). The potential roles for DcR3 in the promotion or inhibition of cell death, and in DC development in vivo remain to be elucidated.
Death receptors such as Fas, TNF receptors and TRAIL receptors are expressed on DCs (14, 92, 93). Knockout studies show that the TRAIL receptor (TRAIL-R) in mice has no obvious apoptosis functions for apoptosis signaling in DCs, but rather inhibits DC functions through suppression of NF-κB activation (92). Intriguingly, TRAIL-R2/DR5-induced activation of caspase-8 can promote the immunogenicity of DCs through the generation of apoptotic bodies (119). Whether rapid cell death in DCs might promote antigen presentation will also be very interesting to study. It has been reported that microfilariae, the parasite stage of Brugia malayi in the blood, can trigger FasL- and TRAIL-induced cell death in DCs (120). This may be responsible for immune suppression during filarial infections. The involvement of different death receptors in DCs in vivo remains to be clarified. How microbes and viruses exploit the cell death pathways in DCs to escape the surveillance by the immune system will be an interesting area to explore.
Signals from CD40 may boost the survival of DCs through activation of NF-κB and AP-1 (121). It has been reported that the effect for CD40 to promote DC survival is less potent than that of TSLP (38). RANKL is another member of the TNF superfamily that is expressed on T cells or DCs (122–124). RANKL interacts with RANK of the TNFR superfamily expressed on DCs to promote the survival and cytokine production by DCs (122–124). This may be related to the ability for RANK to induce NF-κB that promotes the expansion of anti-apoptotic molecules in DCs (122, 124). Indeed, DCs in p50/RelA double knockout mice do not survive and up-regulate anti-apoptotic molecules in response to stimulation by RANKL or LPS (125), suggesting an important role for p50/RelA in the maintenance of DC survival. Osteoprotegerin, a decoy soluble receptor for RANKL that interferes with RANKL-RANK interaction, may suppress the anti-apoptotic effects of RANK to promote cell death in DCs (126). Although RANKL can promote DC survival (122, 124), it can also induce the expression of Fas on DCs to promote Fas-dependent apoptosis in DCs (127). Therefore, the effect on RANKL in promoting or inhibiting the survival of DCs may vary depending on whether FasL-expressing T cells are present.
Bcl-2 family members in the regulation of cell death in DCs
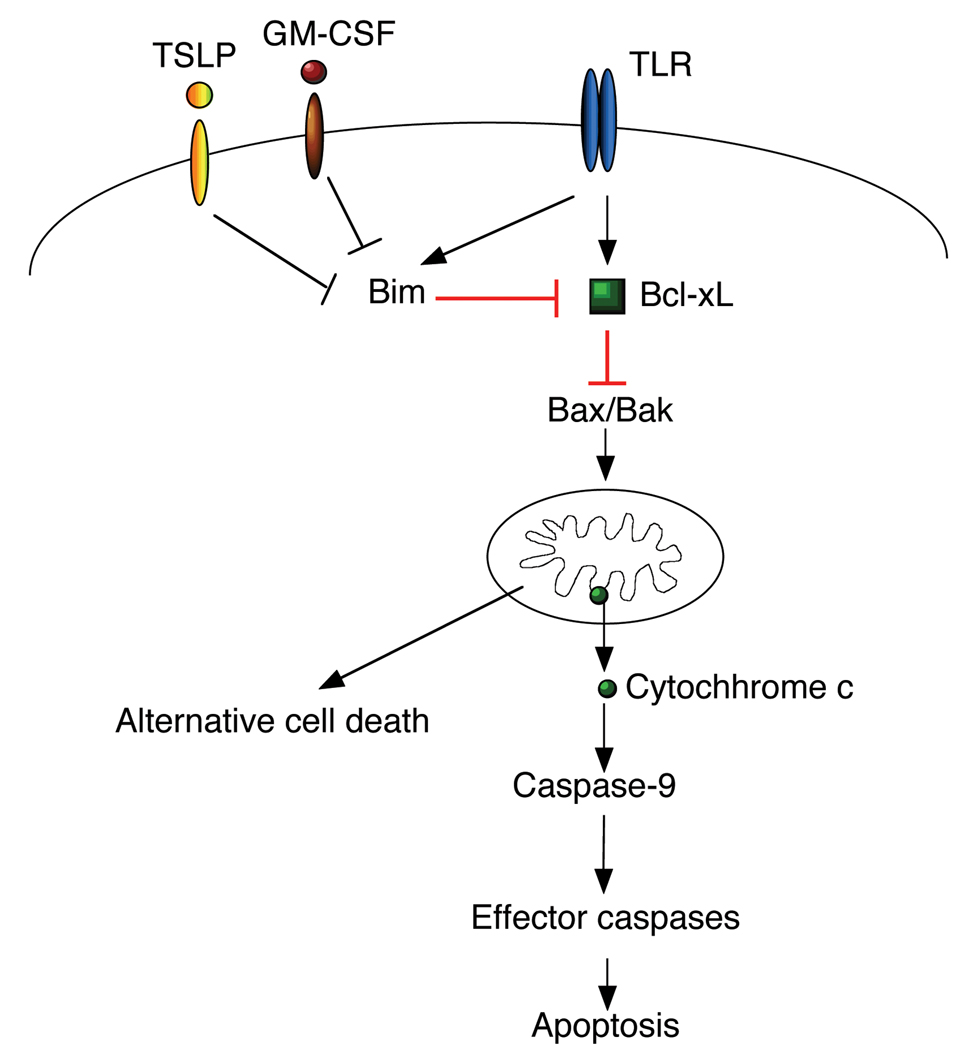
The Bcl-2 family proteins are upstream regulators for mitochondrion-dependent apoptosis (128, 129). Bcl-2 family members share one or more conserved Bcl-2 homology (BH) domains and can be divided into three subfamilies. The anti-apoptotic subfamily proteins, the pro-apoptotic Bax- and Bak-like proteins and the pro-apoptotic BH3-only subfamily. Pro-apoptotic Bax and Bak can oligomerize to form pores on the outer mitochondrial membrane that cause mitochondrial membrane permeabilization. The anti-apoptotic Bcl-2 family proteins can bind to pro-apoptotic Bax or Bak to sequester and inhibit these molecules (76). BH3-only proteins, on the other hand, are the upstream sensors for different apoptosis signaling (130). BH3-only proteins can function as “de-repressors” to inhibit the anti-apoptotic molecules, resulting in the aggregation of Bax or Bak. Alternatively, BH3-only proteins may directly activate pro-apoptotic Bax or Bak to trigger mitochondrial membrane permeabilization. Aggregation of Bax and Bak then leads to the release of cytochrome c from the mitochondrial intermembrane space to the cytosol and activation of caspases (128–130). We have found that knockout of anti-apoptotic Bcl-2 accelerates the rates of renewal of DCs by BrdU labeling (27), while deletion of pro-apoptotic Bcl-2 family member, Bim, inhibits spontaneous apoptosis in DCs (36). This suggests that Bcl-2 family members that are important modulators for mitochondrion-dependent apoptosis regulate the lifespan of DCs in vivo (Fig. 3).
Figure 3. Regulation of mitochondrion-dependent cell death in DCs.
Conventional DCs have limited expression of Bcl-2 that is correlated with high rate of spontaneous cell death. Signaling from TLRs can induce the expression of Bcl-xL to promote DC survival.
Anti-apoptotic Bcl-2 and Bcl-xL
Transgenic expression of Bcl-2 in DCs suggests that inhibition of cell death in DCs promotes antigen-specific immune responses in vivo (64, 65). We have found that transfection with Bcl-2 prolongs the survival of primary bone marrow-derived DCs in vitro (27). In addition, knockout of Bcl-2 leads to faster DC turnover in vivo (27). However, we have observed that DCs only express very limited levels of Bcl-2 (14, 27). Moreover, we have found that Bcl-2, different from Bcl-xL, is not readily inducible after stimulation via TLRs or CD40 (14, 36). Therefore, we propose that the limited expression of Bcl-2 contributes to rapid cell death in DCs, however, regulation of DC survival is more likely achieved through regulated expression of Bcl-2 family members other than Bcl-2.
In contrast to Bcl-2, we have found that Bcl-xL is readily induced in conventional DCs by different stimuli from CD40 or TLRs (14, 36). This suggests that Bcl-xL, rather than Bcl-2, is important for promoting DC survival in response to different stimulations. In pDCs that express TLR9, CpG also leads to significant up-regulation of Bcl-xL (27). Therefore, Bcl-xL is an important molecular target for various stimuli to promote the survival of different DC subsets. Based on the levels of steady state expression and inducibility in DCs, we postulate that limited expression of Bcl-2 contributes to an active mitochondrial apoptosis pathway in DCs, whereas Bcl-xL is a molecular target for various stimuli to inhibit programmed cell death in DCs.
BH3-only proteins in DCs
While comparing the expression of apoptosis signaling molecules in T cells and DCs, we observed that the expression of several BH3-only molecules was limited in DCs, including Bim, BAD, Bnip3L/Nix and PUMA (14). Interestingly, several of these BH3-only molecules, including Bim, BAD and Nix, were inducible after stimulation of TLRs (14, 36). However, further analyses showed that Bad was constitutively phosphorylated at Serine 112 and serine 136 residues (36). Moreover, TLR stimulation significantly increased the phosphorylation of Bad at serine 155 (36). It has been shown that phosphorylation suppresses the pro-apoptotic activity of BAD by inhibiting its binding to Bcl-xL (131). Signals that induce dephosphorylation of BAD may promote cell death in DCs.
We have also generated Nix/Bnip3L-deficient mice with the intention of testing the roles for Nix in regulating apoptosis in DCs. However, we have not found accumulation of DCs in Nix−/− mice (132, 133). We have also detected no defect in DC apoptosis in response to a variety of stimuli (132, 133). Moreover, Nix−/− mice do not display autoimmune symptoms (our unpublished data). Rather, Nix−/− mice display abnormalities in mitochondrial autophagy during terminal erythroid cell differentiation (133). However, TLR stimulation can induce up-regulation of Nix (14). Whether Nix might regulate DC functions when it is induced, such as during infections, remains to be determined.
Bim is a pro-apoptotic BH3-only protein in the Bcl-2 family that has been shown to play a important role in regulating apoptosis and homeostasis of lymphocytes (134). Bim can be rapidly induced in DCs by different stimuli, such as CD40 ligation, TLR stimulation and cytokine withdrawal (36). Moreover, deficiency in Bim causes significant expansion of lymphocytes and autoimmunity in mice (134). In Bim−/− mice, defective negative selection for autoreactive T cells and B cells likely contributes to the development of autoimmune diseases (135, 136).
Bim is an important mediator for spontaneous cell death in DCs. We have observed that deficiency in Bim reduces spontaneous cell death in both conventional DCs and pDCs derived from mouse spleens, as well as in bone marrow-derived conventional DCs (36). Withdrawal of GM-CSF also leads to significant up-regulation of Bim in DCs (36), suggesting that up-regulation of Bim may be responsible for increased spontaneous cell death in DCs in the absence of GM-CSF. It has been reported that activation of MAP kinases and induction of FOXO1 are involved in the expression of Bim (137–139). It will be interesting to determine whether GM-CSF receptor signaling suppresses the expression of Bim through regulation of MAP kinase signaling.
Stimulation of TLRs leads to up-regulation of Bim in both CD11c+CD11b+ conventional DCs and CD11clowPDCA-1+ pDCs (36). Simultaneously, anti-apoptotic Bcl-xL is also up-regulated in DCs by TLR stimulation (36). Interestingly, TLR stimulation enhances the survival of DCs during in vitro culture (36). The increased expression of the pro-survival Bcl-xL may therefore have a dominant effect to antagonize the action of induced Bim, resulting in improved survival of TLR-stimulated DCs. The balance between pro-apoptotic (such as Bim) and anti-apoptotic (such as Bcl-xL) Bcl-2 family proteins, as well as their temporal expression patterns, may determine the survival and lifespan of DCs.
In adoptive transfer studies, we found that Bim−/− DCs survived better than their wild type counterparts after transfer into recipient mice (36). This suggests that Bim−/− DCs undergo reduced spontaneous cell death in vivo. Interestingly, co-transfer with antigen-specific T cells reduced the total numbers of DCs in the draining lymph nodes, supporting the findings that antigen-specific T cells promotes the loss of antigen-bearing DCs in vivo (45). Similar degrees of reduction of wild type and Bim−/− DCs in the draining lymph nodes were observed in the presence of antigen-specific T cells (36). This is consistent with the possibility that Bim does not affect the susceptibility of DCs to killing by antigen-specific T cells in vivo.
Regulation of the lifespan in different DC subsets by Bcl-2 family members
The susceptibility for DCs to apoptosis may play an important role in determining the lifespan of DCs in vivo. The lifespan of different DC subsets vary significantly in vivo. We have therefore investigated the molecular mechanisms that account for the differences in the lifespan between conventional DCs and pDCs. We enriched CD11clowPDCA-1+ pDCs and CD11c+CD11b+ conventional DCs from mouse spleens and examined the expression of apoptosis signaling molecules by Western blot. We detected less anti-apoptotic Bcl-2 and Bcl-xL in conventional DCs than in pDCs or T cells (27). We also transfected Bcl-2 and Bcl-xL into primary DCs derived from mouse bone marrow. We observed that both Bcl-2 and Bcl-xL can inhibit spontaneous cell death in DCs (27). It has been reported that transgenic overexpression of Bcl-2 in DCs slows down DC replacement in the mouse spleen by BrdU labeling (64). Consistently, we also observed that conventional DCs and pDCs in the spleens of bcl-2−/− mice were labeled faster with BrdU than in wild type controls. These data suggest that Bcl-2 and Bcl-xL helps to prolong the lifespan of both conventional DCs and pDCs in vivo. Interestingly, conventional DCs expressed significantly more pro-apoptotic Bax than pDCs or T cells (27). The relatively lower ratios of between pro-apoptotic and anti-apoptotic molecules are correlated with the faster rates of spontaneous turnover in conventional DCs in vivo.
It has been well established that stimulation with TLR stimuli induces the activation and maturation of DCs (8, 140). DC maturation is characterized by higher expression of co-stimulatory molecules on DCs and improved capacities for DCs to activate antigen-specific T cells (8, 141). We observed that stimulation with TLR stimuli increased the expression of Bcl-xL and promoted the survival of conventional DCs and pDCs (27). It has been reported that pDCs express TLR9, a receptor for CpG, but lack most other TLRs (10). CpG, but not ligands for other TLRs, induced up-regulation of Bcl-xL in pDCs. In addition to the up-regulation of co-stimulatory molecules, prolonged survival of DCs is another potential mechanism for increases in the immunogenicity of DCs induced by TLR stimulation or other maturation signals. A basic helix-loop-helix transcription factor, E2-2, has been identified as the key factor in determining the lineage of pDCs (142). E2-2 is also expressed in B cells (142). It will be interesting to determine whether E2-2 directs the expression of similar apoptosis signaling molecules in pDCs and B cells, and whether these genes are indeed responsible for the slower turnover in pDCs and in B cells.
Other factors in regulating DC cell death
An increasing number of receptors and cytokines are implicated in the regulation of cell death in DCs. It has been reported that stimulation of CD14 by LPS induces apoptosis in terminally differentiated DCs through activation of NFAT (143). CCR7 may promote DC survival through inhibition of death signaling (144), while IFN-β may induce apoptosis in mature DCs through activation of caspase-11 and caspase-3 (145). After injection of LPS into mice, such IFN-β-induced cell death in DCs may help to ameliorate LPS-induced inflammatory responses (145). Therefore, cell death in DCs is likely to be regulated by an array of cytokines and chemokines during immune responses in vivo.
Mechanisms for the establishment and maintenance of immune tolerance
Thymic development of T effector and Treg cells
T cell maturation in the thymus involves both positive and negative selections (146–149). After somatic rearrangement of the genes coding for the TCRs, the TCR-expressing cells need to interact with the MHC molecules on the epithelial cells in the thymus. Most of these MHC molecules are occupied by self-peptide. Only the cells expressing TCRs with sufficient affinity for the self-MHC/peptide complexes can survive. This is followed by negative selection to delete T cells expressing TCR with high affinity for self-MHC/peptide complexes, resulting in the elimination of the highly self-reactive T cells. However, thymic selection is not fool proof in eliminating autoreactive T cells. All T cells that mature and exit the thymus are somewhat self-reactive. Different mechanisms for peripheral tolerance are critical for keeping T cells in check to prevent autoimmunity. FoxP3+ natural Treg cells developed in the thymus also populate the peripheral lymphoid organs to inhibit the scope of T cell activation (150, 151). FoxP3+ Treg cells provide a dominant suppression mechanism to help to maintain peripheral tolerance.
Apoptosis in the protection of peripheral tolerance
During an immune response, antigen-specific T cells can undergo significant clonal expansion. After the clearance of antigens, however, these expanded antigen-specific T cells need to be cleared during the contraction phase of the immune response. It has been well established that apoptosis in the immune system plays a critical role in the maintenance of immune tolerance (1, 7, 152). Different genes in the apoptosis pathway has been linked to the development of autoimmune diseases in mice and humans. lpr mice, which lack Fas expression due to a retroviral insertion in the intron of Fas gene (153), develop spontaneous autoimmune and lymphoproliferative diseases (152). gld mice with similar autoimmune manifestations harbor a point mutation in the extracellular region of FasL that abolishes its interaction with Fas (154). Consistent with the studies in mice, mutations in Fas or FasL gene have also been identified in the human Autoimmune Lymphoproliferative Syndrome (ALPS) (7, 155–158). These patients display various degrees of autoimmune symptoms, accumulation of lymphocytes and the accumulation of the unusual TCRαβ+CD4−CD8− DNT T cells (7). Similar to lpr mice, ALPS patients display characteristic apoptosis deficiency in Fas-mediated apoptosis in T cells (7). It has been shown that thymic negative selection is normal in lpr mice (159). Therefore, Fas mutation likely causes the breakdown of peripheral tolerance.
Inhibition of apoptosis in the T cell compartment in transgenic and conditional knockout models
Mutations of Fas gene lead to the development of lymphoproliferation and autoimmune symptoms in both humans and mice (152, 155, 156), supporting an essential role for Fas-dependent apoptosis in the protection of immune tolerance. Because Fas plays an important role in T cell apoptosis, it is logical to postulate that defective apoptosis in T cells is the main culprit in the breakdown of immune tolerance. Several lines of transgenic mice have been generated to inhibit apoptosis in T cells. However, these transgenic mice do not display the salient autoimmune phenotypes observed in lpr mice (160–166). The expression of Fas is important for limiting the gene expression of FasL genes (167), T cell-specific knockout of Fas results in up-regulation of FasL on activated T cells, leading to accelerated killing of Fas-expressing cells (168). T/Fas−/− mice develop severe lymphopenia and succumb to a fatal wasting syndrome caused by massive leukocyte infiltration in the lungs, characterized by chronic inflammation resembling idiopathic pulmonary fibrosis (IPF) in humans (168). Nevertheless, mild lymphoproliferation was observed when T/Fas−/− mice were crossed once to the autoimmune prone MRL strain. Autoantibody production was observed in T and B cell-specific Fas knockout mouse models from another study (15). However, it is clear that non-lymphoid cells are critically important for the development of autoimmune and lymphoproliferative symptoms in Fas-deficient mice (168).
Programmed cell death of DCs in the protection of immune tolerance
DC accumulation due to apoptosis deficiency
In the human Autoimmune Lymphoproliferative Syndrome, we have detected apoptosis deficiency in DCs in some patients (88). We hypothesized that DCs with apoptosis deficiency play an important role in the development of autoimmunity (88). DCs have been suggested to be can important regulator for immune tolerance (8, 11). Immunization with excessive activated DCs has been shown to induce systemic and tissue-specific autoimmune responses (12, 13). To test whether apoptosis in DCs might affect immune responses and self tolerance, we generated transgenic mice expressing a baculoviral caspase inhibitor, p35, under the control of a DC-specific CD11c promoter (14). DC-p35 mice display accumulation of DCs with aging (14). However, the expression of co-stimulatory molecules on DCs was not changed (our unpublished data). We have also observed that apoptosis deficiency in DCs leads to increases in spontaneous T cell activation, but not T cell expansion in DC-p35 mice (14). Therefore, we postulate that decreased cell death in DCs results in uncontrolled T cell activation, but not T cell expansion. In DC-p35 mice, we have failed to detect the unusually TCRαβ+B220+CD4−CD8− double negative T cells (DNT) that are abundant in Fas-deficient lpr mice. This suggests that the accumulation of DNT in lpr mice is not caused by apoptosis-deficient DCs.
Normal negative selection and Treg cell development in DC-p35 mice
We have also determined the effects of DC accumulation on thymic negative selection. C57BL/6 mice carry an endogenous superantigen, mtv-9, that deletes approximately 60% of Vβ5+ T cells during thymic development (169, 170). We observed similar deletion of CD4+Vβ5+ T cells in DC-p35 and control mice on the C57BL/6 background (14). This indicates that negative selection of autoreactive T cells is likely to be normal in DC-p35 mice. Therefore, apoptosis deficiency in DCs preferentially affects peripheral tolerance to induce autoimmunity. We detected normal levels of Treg cells in the thymus and spleen of DC-p35 mice (14). Moreover, Treg cells isolated from DC-p35 were normal in suppressing the proliferation of antigen-specific T cells. This suggests that autoimmunity in DC-p35 mice is not due to a reduction in Treg cells or the loss of Treg cell activity. Therefore, DC accumulation in DC-p35 mice leads to selective overactivation of T effector cells without affecting Treg cell development. Whether apoptosis-deficient DCs might disrupt the functional balance between T effector and Treg cells remains to be determined.
Induction of systemic autoimmunity by apoptosis deficiency in DCs
DC-p35 mice produce antinuclear autoantibodies and display lymphocyte infiltration in multiple tissues (14). Consistent with our studies, Stranger et al. showed that conditional knockout of Fas in DCs also leads to the development of systemic autoimmunity, including the development of antinuclear antibodies, increases in serum immunoglobulins, splenomegaly and leukocytes infiltrations in different tissues (15). We have consistently observed that DCs in the spleen and lymph nodes express high levels of CD40, MHC-II, B7.1, B7.2 and ICAM-1 in wild type DCs. Although significant DC accumulation and spontaneous T cell activation are observed in DC-p35 mice, these surface markers are not increased on DCs (our unpublished data). This suggests that the increased survival, but not increased activation status, are important for the development of autoimmune diseases in these mice. However, genetic background also plays an important role in determining the onset and severity of autoimmunity in DC-p35 mice. The effects for apoptosis deficiency in causing autoimmune responses are more prominent in the autoimmune-prone MRL strain than the C57BL/6 background (14). Therefore, both apoptosis deficiency in DCs and other genetic or environmental risk factors are likely to be important for the development of severe autoimmune diseases.
Mechanisms for apoptosis deficient DCs in the over-activation of lymphocytes
What is the main source of FasL to induce Fas-dependent apoptosis in DCs? It is well known that activated T cells produce high levels of FasL that can cause autocrine cell death in T cells (171). Other cell types that do not produce significant levels of FasL, such as B cells, may depend on FasL-producing T cells to undergo Fas-dependent apoptosis (172). Although differentiated DCs express high levels of Fas on the cell surface, the expression of FasL at the protein and mRNA levels was relatively low in DCs (our unpublished data). Therefore, DCs likely dependent on other cell types, most likely the activated T cells, as the main donor for FasL to induce Fas-dependent cell death (Fig. 1). We have found that the conventional CD11c+CD11b+ DCs express low levels of FasL (14). The CD11clowPDCA-1+ pDCs do not have detectable levels of FasL on the cell surface (our unpublished observation). Using allo-specific T cells, we observed that activated T cells are capable of killing DCs in Fas- and perforin-dependent manner (14). However, Fas deficiency dose not affect spontaneous cell death in DCs (36). It therefore appears that Fas-dependent apoptosis is not critical for regulating spontaneous cell death, but is involved in T cell-induced cell death in DCs.
Upon activation by antigen-presenting DCs, T cells up-regulate Fas on the cell surface but remain insensitive to Fas-mediated apoptosis at the beginning. IL-2 sensitizes T cells to Fas-mediated apoptosis and activation-induced cell death after re-engagement of TCRs (173, 174). FasL is significantly induced in activated T cells (171). This may allow activated T cells to kill DCs that have activated them. We observed that activated T cells are indeed capable of killing DCs in a Fas- and perforin-dependent manner in vitro (14). This could provide an interesting negative feedback mechanism for activated T cells to quench immune responses by deleting antigen-bearing DCs. Defective Fas-mediated cell death in DCs may abolish such a feedback mechanism, resulting in uncontrolled T cell activation. It will be important to determine whether T cells employ Fas- and perforin-dependent mechanisms to kill DCs and restrict the scope of immune responses in vivo.
Deficiency of Bim in DCs in the induction of autoimmunity
Bim in spontaneous cell death of DCs
Bim−/− mice display systemic autoimmune symptoms with aging. We have observed that Bim−/− DCs, but not Fas-deficient lpr DCs, are defective in spontaneous cell death (36). This suggests that Bim, but not Fas, regulates spontaneous cell death in DCs (36). Therefore, Bim-deficient DCs and Fas-deficient DCs may display survival advantages at different phases of immune responses. During the contraction phase of immune responses, T cells and DCs may also undergo spontaneous cell death. This could be due to the disappearance of the cytokines that are required to sustain cell survival. Interestingly, we observed that withdrawal of GM-CSF from in vitro DC culture induced Bim expression and increased spontaneous cell death in DCs (36). It will be interesting to determine whether other cytokines important for prolonging the survival of DCs can also suppress Bim expression in DCs.
Bim−/− DCs have prolonged survival and are more efficient in inducing the proliferation of antigen-specific T cells in vitro and in vivo (36). Although we detected increased production of IL-12 by Bim−/− DCs, intracellular staining of IL-12p40/p70 showed that the production of IL-12 was similar between wild type and Bim−/− DCs on a per cell basis. Therefore, increased T cell activation and cytokine production is potentially due to the availability of more live DCs in Bim−/− mice.
Autoimmunity in Bim−/− mice
Defective negative selection in the thymus has been detected in Bim−/− mice (135). This suggests that autoreactive T cells may escape thymic selection and induce autoimmune responses in Bim−/− mice. However, defective thymic negative selection most likely can be attributed to an intrinsic defect of apoptosis in T cells of these mice (135). Compared with control mice, Bim−/− mice contains slightly increased CD4+FoxP3+ Treg cells in both the spleen and the lymph nodes (36). This implies that the development of autoimmunity in Bim−/− mice is not due to a loss of Treg cells. In adoptive transfer experiments, we observed that Bim−/− DCs have the propensity for inducing autoantibodies in recipient mice (36). This is consistent with the possibility that increased lifespan of DCs promotes the breakdown of immune tolerance.
We have consistently observed that DCs in the spleens and lymph node express high levels of the surface markers, including CD40, MHC-II, B7.1, B7.2 and ICAM-1 (36). Although DCs are accumulated in Bim−/− mice and show the propensity for the over-activation of lymphocytes and induction of autoimmune responses, DCs do not display increased levels of MHC-II co-stimulatory molecules, including CD40, B7.1, B7.2 and ICAM-1 (36). This suggests that the increased survival, but not increased activation status, are important for the development of autoimmune diseases in these mice.
Bax−/−Bak−/− mice
Deletion of Bax and Bak in mice has shown that Bax and Bak are essential for carrying out all mitochondrion-dependent apoptosis (175, 176). Although born at lower than expected rates, some Bax−/−Bax−/− mice can survive to adulthood (175). However, these mice show growth retardation and develop autoimmune symptoms. Interestingly, Bax−/−Bak−/− mice display distinct defects in thymic negative selection for autoreactive T cells (170). We have observed that Bax- and Bak-dependent apoptosis in DCs helps to regulate DC survival and functions in vivo (our unpublished observations). Further studies will shed light on whether Bax- and Bak-dependent mitochondrial apoptosis in DCs is involved in the regulation of antigen-specific immune responses and immune tolerance.
Potential physiological functions for rapid DC turnover
Naïve T cells are relatively long-lived cells that undergo slow self-renewal (177). After antigen-specific stimulation and clonal expansion, however, activated T lymphocytes undergo significant programmed cell death (3, 7, 76, 177). This is important for the clearance of expanded lymphocytes after immune responses to maintain lymphocyte homeostasis. Different from lymphocytes, however, fully differentiated DCs do not proliferate. Although the lifespan of different subsets of DCs varies considerably, conventional DCs are mostly short-lived. Why conventional DCs that no longer proliferate need to harbor active cell death pathways is still a mystery.
In the absence of antigen stimulation, resting T cells likely undergo slow rates of homeostatic proliferation and cell death. DCs, on the other hand, are the sentinels that may be constantly undertaking the task of sensing potential antigens that they encounter. DCs may uptake apoptotic cells in the body, and the relatively harmless microorganisms that each individual is likely to be exposed to, such as those from the environment and gut flora. It has been shown that DCs constitutively present self antigens (178). Most of antigen processing and presentation by DCs would be futile without inducing immune responses. Whether such constant routine as antigen-presenting cells represents a molecular wearing and tearing for DCs is an interesting possibility that should be investigated.
Activated T cells can potentially induce Fas- and perforin-dependent cell death in DCs (14). This suggests an important negative feedback mechanism in shutting down the immune responses by inducing cell death in DCs. In our in vitro studies, we usually observe rapid decrease in the number of live DCs in culture. However, apoptotic DCs are difficult to detect (our unpublished observations). Apoptotic DCs are presumably taken up rapidly by live DCs in the culture. It could be envisioned that apoptotic DCs are also rapidly engulfed by neighboring DCs in vivo. Whether this process promotes presentation of antigens that have been processed in apoptotic DCs will be interesting to study.
Concluding remarks
DCs play important roles in the regulation of multiple aspects of innate and adaptive immune responses. Programmed cell death is important for maintaining the homeostasis of DCs in vivo. Dysregulated programmed cell death in DCs may change the lifespan of DCs in vivo and lead to the breakdown of immune tolerance. However, molecular mechanisms for apoptosis and other forms of programmed cell death in DCs have not been as well characterized as in lymphocytes. The effects of various receptors, cytokines and chemokines may affect the lifespan of DCs at various stages of immune responses. The interplays between different cell types and cytokines in regulating programmed cell death in DCs will be a challenging but important area for investigation.
Currently, most of our understanding of programmed cell death in DCs has come from studies of CD11c+CD11b+ conventional DCs derived from mouse spleen and lymph nodes, or monocyte-derived DCs cultured from mouse bone marrow or human peripheral blood. We have used purified TLR ligands to stimulate pattern recognition receptors on DCs. The in vivo effects of viral or bacterial infections on the survival and turnover of DCs should be studied. This will enable us to determine how DCs behave in response to various sources of antigens, such as infections, cancers and allergens, and how various antigen sources affect the lifespan of DCs. With the delineation of DC development from different precursors (5), it is now feasible to trace the development of individual DC subsets in different tissues. The relations between homeostatic proliferation of DC precursors, differentiation and programmed cell death of DCs in different tissues should be studied.
The precise mechanisms for apoptosis-deficient DCs in the overactivation of lymphocytes and the breakdown of immune tolerance remain to be elucidated. DCs deficient in Fas-mediated apoptosis may be resistant to killing by the antigen-specific T cells that they have activated. Although these DCs do not have a steady state survival advantage over DCs that have a functional Fas pathway, they potentially survive better during cognate T-DC interactions. This is particularly relevant for interactions between DCs and T cells that express high levels of FasL. How various T cells types, including Th1, Th2, Th17, Treg and the recently defined follicular T help cells (Tfh) (179) influence the survival and immunogenicity of DCs under various settings of immune responses will be of great interest to investigate.
We have observed that stimulation of DCs via TLRs leads to simultaneous up-regulation of anti-apoptotic Bcl-xL and pro-apoptotic Bim and BAD (14). However, DCs survive better after TLR stimulation, suggesting that anti-apoptotic molecules may overpower the effects of pro-apoptotic molecules to promote DC survival. What happens after stimulatory signals disappear has not been studied. Will anti-apoptotic molecules continue to show an advantage over the pro-apoptotic molecules? Simultaneous induction of both pro-apoptotic and anti-apoptotic machineries may program DCs for cell death if anti-apoptotic molecules undergo faster turnover after stimulation signals are removed. Will dephosphorylation of BAD promote cell death in DCs? Investigating the turnover and posttranslational modification of cell death molecules may provide a clear picture of the regulation of programmed cell death in DCs after apoptosis signaling molecules are induced.
It is relatively straightforward to define the molecular mechanisms for rapid cell death in conventional DCs. However, the functional significance for such rapid DC death remains unknown. As the sentinel of the immune system, DCs may be constantly encountered with antigens or stimulated by infections agents that trigger their pattern recognition receptors. Apoptotic DCs could be rapid engulfed by other DCs. Will this facilitate antigen processing and presentation if apoptotic DCs contain processed antigens? Does engulfment of apoptotic DCs promote cross presentation of extracellular antigens to the MHC-I pathway for stimulation of cytotoxic T cells? Addressing these questions will undoubtedly shed light on the functions of programmed cell death of DCs in immune regulation.
Reference
- 1.Rathmell JC, Thompson CB. The central effectors of cell death in the immune system. Annu Rev Immunol. 1999;17:781–828. doi: 10.1146/annurev.immunol.17.1.781. [DOI] [PubMed] [Google Scholar]
- 2.Gaudin E, Rosado M, Agenes F, McLean A, Freitas AA. B-cell homeostasis, competition, resources, and positive selection by self-antigens. Immunol Rev. 2004;197:102–115. doi: 10.1111/j.0105-2896.2004.0095.x. [DOI] [PubMed] [Google Scholar]
- 3.Hildeman D, Jorgensen T, Kappler J, Marrack P. Apoptosis and the homeostatic control of immune responses. Curr Opin Immunol. 2007;19:516–521. doi: 10.1016/j.coi.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takada K, Jameson SC. Naive T cell homeostasis: from awareness of space to a sense of place. Nat Rev Immunol. 2009;9:823–832. doi: 10.1038/nri2657. [DOI] [PubMed] [Google Scholar]
- 5.Liu K, Victora GD, Schwickert TA, Guermonprez P, Meredith MM, et al. In vivo analysis of dendritic cell development and homeostasis. Science. 2009;324:392–397. doi: 10.1126/science.1170540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Becker T, Loch G, Beyer M, Zinke I, Aschenbrenner AC, et al. FOXO-dependent regulation of innate immune homeostasis. Nature. 2010;463:369–373. doi: 10.1038/nature08698. [DOI] [PubMed] [Google Scholar]
- 7.Lenardo M, Chan KM, Hornung F, McFarland H, Siegel R, et al. Mature T lymphocyte apoptosis--immune regulation in a dynamic and unpredictable antigenic environment. Annu Rev Immunol. 1999;17:221–253. doi: 10.1146/annurev.immunol.17.1.221. [DOI] [PubMed] [Google Scholar]
- 8.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 9.Steinman RM, Hemmi H. Dendritic cells: translating innate to adaptive immunity. Curr Top Microbiol Immunol. 2006;311:17–58. doi: 10.1007/3-540-32636-7_2. [DOI] [PubMed] [Google Scholar]
- 10.Liu YJ. Dendritic cell subsets and lineages, and their functions in innate and adaptive immunity. Cell. 2001;106:259–262. doi: 10.1016/s0092-8674(01)00456-1. [DOI] [PubMed] [Google Scholar]
- 11.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 12.Roskrow MA, Dilloo D, Suzuki N, Zhong W, Rooney CM, Brenner MK. Autoimmune disease induced by dendritic cell immunization against leukemia. Leuk Res. 1999;23:549–557. doi: 10.1016/s0145-2126(99)00045-4. [DOI] [PubMed] [Google Scholar]
- 13.Ludewig B, Odermatt B, Landmann S, Hengartner H, Zinkernagel RM. Dendritic cells induce autoimmune diabetes and maintain disease via de novo formation of local lymphoid tissue. J Exp Med. 1998;188:1493–1501. doi: 10.1084/jem.188.8.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen M, Wang YH, Wang Y, Huang L, Sandoval H, et al. Dendritic cell apoptosis in the maintenance of immune tolerance. Science. 2006;311:1160–1164. doi: 10.1126/science.1122545. [DOI] [PubMed] [Google Scholar]
- 15.Stranges PB, Watson J, Cooper CJ, Choisy-Rossi CM, Stonebraker AC, et al. Elimination of antigen-presenting cells and autoreactive T cells by Fas contributes to prevention of autoimmunity. Immunity. 2007;26:629–641. doi: 10.1016/j.immuni.2007.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ohnmacht C, Pullner A, King SB, Drexler I, Meier S, et al. Constitutive ablation of dendritic cells breaks self tolerance of CD4 T cells and results in spontaneous fatal autoimmunity. J Exp Med. 2009;206:549–559. doi: 10.1084/jem.20082394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J Exp Med. 1973;137:1142–1162. doi: 10.1084/jem.137.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. II. Functional properties in vitro. J Exp Med. 1974;139:380–397. doi: 10.1084/jem.139.2.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shortman K, Liu YJ. Mouse and human dendritic cell subtypes. Nat Rev Immunol. 2002;2:151–161. doi: 10.1038/nri746. [DOI] [PubMed] [Google Scholar]
- 20.Shortman K, Naik SH. Steady-state and inflammatory dendritic-cell development. Nat Rev Immunol. 2007;7:19–30. doi: 10.1038/nri1996. [DOI] [PubMed] [Google Scholar]
- 21.Wu L, Liu YJ. Development of dendritic-cell lineages. Immunity. 2007;26:741–750. doi: 10.1016/j.immuni.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 22.Villadangos JA, Schnorrer P. Intrinsic and cooperative antigen-presenting functions of dendritic-cell subsets in vivo. Nat Rev Immunol. 2007;7:543–555. doi: 10.1038/nri2103. [DOI] [PubMed] [Google Scholar]
- 23.Ruedl C, Koebel P, Bachmann M, Hess M, Karjalainen K. Anatomical origin of dendritic cells determines their life span in peripheral lymph nodes. J Immunol. 2000;165:4910–4916. doi: 10.4049/jimmunol.165.9.4910. [DOI] [PubMed] [Google Scholar]
- 24.Kamath AT, Pooley J, O'Keeffe MA, Vremec D, Zhan Y, et al. The development, maturation, and turnover rate of mouse spleen dendritic cell populations. J Immunol. 2000;165:6762–6770. doi: 10.4049/jimmunol.165.12.6762. [DOI] [PubMed] [Google Scholar]
- 25.O'Keeffe M, Hochrein H, Vremec D, Caminschi I, Miller JL, et al. Mouse plasmacytoid cells: long-lived cells, heterogeneous in surface phenotype and function, that differentiate into CD8(+) dendritic cells only after microbial stimulus. J Exp Med. 2002;196:1307–1319. doi: 10.1084/jem.20021031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kamath AT, Henri S, Battye F, Tough DF, Shortman K. Developmental kinetics and lifespan of dendritic cells in mouse lymphoid organs. Blood. 2002;100:1734–1741. [PubMed] [Google Scholar]
- 27.Chen M, Huang L, Shabier Z, Wang J. Regulation of the lifespan in dendritic cell subsets. Mol Immunol. 2007;44:2558–2565. doi: 10.1016/j.molimm.2006.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 29.McKenna HJ, Stocking KL, Miller RE, Brasel K, De Smedt T, et al. Mice lacking flt3 ligand have deficient hematopoiesis affecting hematopoietic progenitor cells, dendritic cells, and natural killer cells. Blood. 2000;95:3489–3497. [PubMed] [Google Scholar]
- 30.Karsunky H, Merad M, Cozzio A, Weissman IL, Manz MG. Flt3 ligand regulates dendritic cell development from Flt3+ lymphoid and myeloid-committed progenitors to Flt3+ dendritic cells in vivo. J Exp Med. 2003;198:305–313. doi: 10.1084/jem.20030323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Waskow C, Liu K, Darrasse-Jeze G, Guermonprez P, Ginhoux F, et al. The receptor tyrosine kinase Flt3 is required for dendritic cell development in peripheral lymphoid tissues. Nat Immunol. 2008;9:676–683. doi: 10.1038/ni.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Whartenby KA, Calabresi PA, McCadden E, Nguyen B, Kardian D, et al. Inhibition of FLT3 signaling targets DCs to ameliorate autoimmune disease. Proc Natl Acad Sci U S A. 2005;102:16741–16746. doi: 10.1073/pnas.0506088102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hochweller K, Miloud T, Striegler J, Naik S, Hammerling GJ, Garbi N. Homeostasis of dendritic cells in lymphoid organs is controlled by regulation of their precursors via a feedback loop. Blood. 2009;114:4411–4421. doi: 10.1182/blood-2008-11-188045. [DOI] [PubMed] [Google Scholar]
- 34.Kingston D, Schmid MA, Onai N, Obata-Onai A, Baumjohann D, Manz MG. The concerted action of GM-CSF and Flt3-ligand on in vivo dendritic cell homeostasis. Blood. 2009;114:835–843. doi: 10.1182/blood-2009-02-206318. [DOI] [PubMed] [Google Scholar]
- 35.Gilliet M, Boonstra A, Paturel C, Antonenko S, Xu XL, et al. The development of murine plasmacytoid dendritic cell precursors is differentially regulated by FLT3-ligand and granulocyte/macrophage colony-stimulating factor. J Exp Med. 2002;195:953–958. doi: 10.1084/jem.20020045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen M, Huang L, Wang J. Deficiency of Bim in dendritic cells contributes to overactivation of lymphocytes and autoimmunity. Blood. 2007;109:4360–4367. doi: 10.1182/blood-2006-11-056424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chang WL, Baumgarth N, Eberhardt MK, Lee CY, Baron CA, et al. Exposure of myeloid dendritic cells to exogenous or endogenous IL-10 during maturation determines their longevity. J Immunol. 2007;178:7794–7804. doi: 10.4049/jimmunol.178.12.7794. [DOI] [PubMed] [Google Scholar]
- 38.Watanabe N, Hanabuchi S, Soumelis V, Yuan W, Ho S, et al. Human thymic stromal lymphopoietin promotes dendritic cell-mediated CD4+ T cell homeostatic expansion. Nat Immunol. 2004;5:426–434. doi: 10.1038/ni1048. [DOI] [PubMed] [Google Scholar]
- 39.Borkowski TA, Letterio JJ, Farr AG, Udey MC. A role for endogenous transforming growth factor beta 1 in Langerhans cell biology: the skin of transforming growth factor beta 1 null mice is devoid of epidermal Langerhans cells. J Exp Med. 1996;184:2417–2422. doi: 10.1084/jem.184.6.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Borkowski TA, Letterio JJ, Mackall CL, Saitoh A, Farr AG, et al. Langerhans cells in the TGF beta 1 null mouse. Adv Exp Med Biol. 1997;417:307–310. [PubMed] [Google Scholar]
- 41.Hacker C, Kirsch RD, Ju XS, Hieronymus T, Gust TC, et al. Transcriptional profiling identifies Id2 function in dendritic cell development. Nat Immunol. 2003;4:380–386. doi: 10.1038/ni903. [DOI] [PubMed] [Google Scholar]
- 42.Ito M, Minamiya Y, Kawai H, Saito S, Saito H, et al. Tumor-derived TGFbeta-1 induces dendritic cell apoptosis in the sentinel lymph node. J Immunol. 2006;176:5637–5643. doi: 10.4049/jimmunol.176.9.5637. [DOI] [PubMed] [Google Scholar]
- 43.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 44.Riol-Blanco L, Delgado-Martin C, Sanchez-Sanchez N, Alonso CL, Gutierrez-Lopez MD, et al. Immunological synapse formation inhibits, via NF-kappaB and FOXO1, the apoptosis of dendritic cells. Nat Immunol. 2009;10:753–760. doi: 10.1038/ni.1750. [DOI] [PubMed] [Google Scholar]
- 45.Ingulli E, Mondino A, Khoruts A, Jenkins MK. In vivo detection of dendritic cell antigen presentation to CD4(+) T cells. J Exp Med. 1997;185:2133–2141. doi: 10.1084/jem.185.12.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matsue H, Edelbaum D, Hartmann AC, Morita A, Bergstresser PR, et al. Dendritic cells undergo rapid apoptosis in vitro during antigen-specific interaction with CD4+ T cells. J Immunol. 1999;162:5287–5298. [PubMed] [Google Scholar]
- 47.Borrow P, Evans CF, Oldstone MB. Virus-induced immunosuppression: immune system-mediated destruction of virus-infected dendritic cells results in generalized immune suppression. J Virol. 1995;69:1059–1070. doi: 10.1128/jvi.69.2.1059-1070.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hermans IF, Ritchie DS, Yang J, Roberts JM, Ronchese F. CD8+ T cell-dependent elimination of dendritic cells in vivo limits the induction of antitumor immunity. J Immunol. 2000;164:3095–3101. doi: 10.4049/jimmunol.164.6.3095. [DOI] [PubMed] [Google Scholar]
- 49.Guarda G, Hons M, Soriano SF, Huang AY, Polley R, et al. L-selectin-negative CCR7- effector and memory CD8+ T cells enter reactive lymph nodes and kill dendritic cells. Nat Immunol. 2007;8:743–752. doi: 10.1038/ni1469. [DOI] [PubMed] [Google Scholar]
- 50.Yang J, Huck SP, McHugh RS, Hermans IF, Ronchese F. Perforin-dependent elimination of dendritic cells regulates the expansion of antigen-specific CD8+ T cells in vivo. Proc Natl Acad Sci U S A. 2006;103:147–152. doi: 10.1073/pnas.0509054103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Watchmaker PB, Urban JA, Berk E, Nakamura Y, Mailliard RB, et al. Memory CD8+ T cells protect dendritic cells from CTL killing. J Immunol. 2008;180:3857–3865. doi: 10.4049/jimmunol.180.6.3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fugier-Vivier I, Servet-Delprat C, Rivailler P, Rissoan MC, Liu YJ, Rabourdin-Combe C. Measles virus suppresses cell-mediated immunity by interfering with the survival and functions of dendritic and T cells. J Exp Med. 1997;186:813–823. doi: 10.1084/jem.186.6.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bluestone JA, Tang Q. How do CD4+CD25+ regulatory T cells control autoimmunity? Curr Opin Immunol. 2005;17:638–642. doi: 10.1016/j.coi.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 54.Hori S, Takahashi T, Sakaguchi S. Control of autoimmunity by naturally arising regulatory CD4+ T cells. Adv Immunol. 2003;81:331–371. doi: 10.1016/s0065-2776(03)81008-8. [DOI] [PubMed] [Google Scholar]
- 55.Lu LF, Rudensky A. Molecular orchestration of differentiation and function of regulatory T cells. Genes Dev. 2009;23:1270–1282. doi: 10.1101/gad.1791009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tadokoro CE, Shakhar G, Shen S, Ding Y, Lino AC, et al. Regulatory T cells inhibit stable contacts between CD4+ T cells and dendritic cells in vivo. J Exp Med. 2006;203:505–511. doi: 10.1084/jem.20050783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grossman WJ, Verbsky JW, Barchet W, Colonna M, Atkinson JP, Ley TJ. Human T regulatory cells can use the perforin pathway to cause autologous target cell death. Immunity. 2004;21:589–601. doi: 10.1016/j.immuni.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 58.Jung S, Unutmaz D, Wong P, Sano G, De los Santos K, et al. In vivo depletion of CD11c(+) dendritic cells abrogates priming of CD8(+) T cells by exogenous cell-associated antigens. Immunity. 2002;17:211–220. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Suffner J, Hochweller K, Kuhnle MC, Li X, Kroczek RA, et al. Dendritic Cells Support Homeostatic Expansion of Foxp3+ Regulatory T Cells in Foxp3.LuciDTR Mice. J Immunol. 2010 doi: 10.4049/jimmunol.0902420. [DOI] [PubMed] [Google Scholar]
- 60.Yamazaki S, Dudziak D, Heidkamp GF, Fiorese C, Bonito AJ, et al. CD8+ CD205+ splenic dendritic cells are specialized to induce Foxp3+ regulatory T cells. J Immunol. 2008;181:6923–6933. doi: 10.4049/jimmunol.181.10.6923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sharma MD, Baban B, Chandler P, Hou DY, Singh N, et al. Plasmacytoid dendritic cells from mouse tumor-draining lymph nodes directly activate mature Tregs via indoleamine 2,3-dioxygenase. J Clin Invest. 2007;117:2570–2582. doi: 10.1172/JCI31911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Belkaid Y, Oldenhove G. Tuning microenvironments: induction of regulatory T cells by dendritic cells. Immunity. 2008;29:362–371. doi: 10.1016/j.immuni.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kushwah R, Wu J, Oliver JR, Jiang G, Zhang J, et al. Uptake of apoptotic DC converts immature DC into tolerogenic DC, which induce differentiation of Foxp3+ regulatory T cells. Eur J Immunol. 2010 doi: 10.1002/eji.200939782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nopora A, Brocker T. Bcl-2 controls dendritic cell longevity in vivo. J Immunol. 2002;169:3006–3014. doi: 10.4049/jimmunol.169.6.3006. [DOI] [PubMed] [Google Scholar]
- 65.Gautier EL, Huby T, Saint-Charles F, Ouzilleau B, Pirault J, et al. Conventional dendritic cells at the crossroads between immunity and cholesterol homeostasis in atherosclerosis. Circulation. 2009;119:2367–2375. doi: 10.1161/CIRCULATIONAHA.108.807537. [DOI] [PubMed] [Google Scholar]
- 66.Hayakawa Y, Screpanti V, Yagita H, Grandien A, Ljunggren HG, et al. NK cell TRAIL eliminates immature dendritic cells in vivo and limits dendritic cell vaccination efficacy. J Immunol. 2004;172:123–129. doi: 10.4049/jimmunol.172.1.123. [DOI] [PubMed] [Google Scholar]
- 67.Kang TH, Lee JH, Noh KH, Han HD, Shin BC, et al. Enhancing dendritic cell vaccine potency by combining a BAK/BAX siRNA-mediated antiapoptotic strategy to prolong dendritic cell life with an intracellular strategy to target antigen to lysosomal compartments. Int J Cancer. 2007;120:1696–1703. doi: 10.1002/ijc.22377. [DOI] [PubMed] [Google Scholar]
- 68.Kim TW, Lee JH, He L, Boyd DA, Hardwick JM, et al. Modification of professional antigen-presenting cells with small interfering RNA in vivo to enhance cancer vaccine potency. Cancer Res. 2005;65:309–316. [PubMed] [Google Scholar]
- 69.Peng S, Kim TW, Lee JH, Yang M, He L, et al. Vaccination with dendritic cells transfected with BAK and BAX siRNA enhances antigen-specific immune responses by prolonging dendritic cell life. Hum Gene Ther. 2005;16:584–593. doi: 10.1089/hum.2005.16.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pirtskhalaishvili G, Shurin GV, Gambotto A, Esche C, Wahl M, et al. Transduction of dendritic cells with Bcl-xL increases their resistance to prostate cancer-induced apoptosis and antitumor effect in mice. J Immunol. 2000;165:1956–1964. doi: 10.4049/jimmunol.165.4.1956. [DOI] [PubMed] [Google Scholar]
- 71.Yu L, Alva A, Su H, Dutt P, Freundt E, et al. Regulation of an ATG7-beclin 1 program of autophagic cell death by caspase-8. Science. 2004;304:1500–1502. doi: 10.1126/science.1096645. [DOI] [PubMed] [Google Scholar]
- 72.Cho YS, Challa S, Moquin D, Genga R, Ray TD, et al. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 2009;137:1112–1123. doi: 10.1016/j.cell.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.He S, Wang L, Miao L, Wang T, Du F, et al. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell. 2009;137:1100–1111. doi: 10.1016/j.cell.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 74.Hitomi J, Christofferson DE, Ng A, Yao J, Degterev A, et al. Identification of a molecular signaling network that regulates a cellular necrotic cell death pathway. Cell. 2008;135:1311–1323. doi: 10.1016/j.cell.2008.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang DW, Shao J, Lin J, Zhang N, Lu BJ, et al. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science. 2009;325:332–336. doi: 10.1126/science.1172308. [DOI] [PubMed] [Google Scholar]
- 76.Rathmell JC, Thompson CB. Pathways of apoptosis in lymphocyte development, homeostasis, and disease. Cell. 2002;109 Suppl:S97–S107. doi: 10.1016/s0092-8674(02)00704-3. [DOI] [PubMed] [Google Scholar]
- 77.Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001;104:487–501. doi: 10.1016/s0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- 78.Boldin MP, Varfolomeev EE, Pancer Z, Mett IL, Camonis JH, Wallach D. A novel protein that interacts with the death domain of Fas/APO1 contains a sequence motif related to the death domain. J Biol Chem. 1995;270:7795–7798. doi: 10.1074/jbc.270.14.7795. [DOI] [PubMed] [Google Scholar]
- 79.Chinnaiyan AM, O'Rourke K, Tewari M, Dixit VM. FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell. 1995;81:505–512. doi: 10.1016/0092-8674(95)90071-3. [DOI] [PubMed] [Google Scholar]
- 80.Kischkel FC, Hellbardt S, Behrmann I, Germer M, Pawlita M, et al. Cytotoxicity-dependent APO-1 (Fas/CD95)-associated proteins form a death-inducing signaling complex (DISC) with the receptor. EMBO J. 1995;14:5579–5588. doi: 10.1002/j.1460-2075.1995.tb00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Boldin MP, Goncharov TM, Goltsev YV, Wallach D. Involvement of MACH, a novel MORT1/FADD-interacting protease, in Fas/APO-1- and TNF receptor-induced cell death. Cell. 1996;85:803–815. doi: 10.1016/s0092-8674(00)81265-9. [DOI] [PubMed] [Google Scholar]
- 82.Muzio M, Chinnaiyan AM, Kischkel FC, O'Rourke K, Shevchenko A, et al. FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death--inducing signaling complex. Cell. 1996;85:817–827. doi: 10.1016/s0092-8674(00)81266-0. [DOI] [PubMed] [Google Scholar]
- 83.Reed JC, Doctor K, Rojas A, Zapata JM, Stehlik C, et al. Comparative analysis of apoptosis and inflammation genes of mice and humans. Genome Res. 2003;13:1376–1388. doi: 10.1101/gr.1053803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang J, Chun HJ, Wong W, Spencer DM, Lenardo MJ. Caspase-10 is an initiator caspase in death receptor signaling. Proc Natl Acad Sci U S A. 2001;98:13884–13888. doi: 10.1073/pnas.241358198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kischkel FC, Lawrence DA, Tinel A, LeBlanc H, Virmani A, et al. Death receptor recruitment of endogenous caspase-10 and apoptosis initiation in the absence of caspase-8. J Biol Chem. 2001;276:46639–46646. doi: 10.1074/jbc.M105102200. [DOI] [PubMed] [Google Scholar]
- 86.Fischer U, Stroh C, Schulze-Osthoff K. Unique and overlapping substrate specificities of caspase-8 and caspase-10. Oncogene. 2006;25:152–159. doi: 10.1038/sj.onc.1209015. [DOI] [PubMed] [Google Scholar]
- 87.Bae S, Ha TS, Yoon Y, Lee J, Cha HJ, et al. Genome-wide screening and identification of novel proteolytic cleavage targets of caspase-8 and −10 in vitro. Int J Mol Med. 2008;21:381–386. [PubMed] [Google Scholar]
- 88.Wang J, Zheng L, Lobito A, Chan FK, Dale J, et al. Inherited human Caspase 10 mutations underlie defective lymphocyte and dendritic cell apoptosis in autoimmune lymphoproliferative syndrome type II. Cell. 1999;98:47–58. doi: 10.1016/S0092-8674(00)80605-4. [DOI] [PubMed] [Google Scholar]
- 89.Chun HJ, Zheng L, Ahmad M, Wang J, Speirs CK, et al. Pleiotropic defects in lymphocyte activation caused by caspase-8 mutations lead to human immunodeficiency. Nature. 2002;419:395–399. doi: 10.1038/nature01063. [DOI] [PubMed] [Google Scholar]
- 90.Varfolomeev EE, Schuchmann M, Luria V, Chiannilkulchai N, Beckmann JS, et al. Targeted disruption of the mouse Caspase 8 gene ablates cell death induction by the TNF receptors, Fas/Apo1, and DR3 and is lethal prenatally. Immunity. 1998;9:267–276. doi: 10.1016/s1074-7613(00)80609-3. [DOI] [PubMed] [Google Scholar]
- 91.Wallach D, Varfolomeev EE, Malinin NL, Goltsev YV, Kovalenko AV, Boldin MP. Tumor necrosis factor receptor and Fas signaling mechanisms. Annu Rev Immunol. 1999;17:331–367. doi: 10.1146/annurev.immunol.17.1.331. [DOI] [PubMed] [Google Scholar]
- 92.Diehl GE, Yue HH, Hsieh K, Kuang AA, Ho M, et al. TRAIL-R as a negative regulator of innate immune cell responses. Immunity. 2004;21:877–889. doi: 10.1016/j.immuni.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 93.Funk JO, Walczak H, Voigtlander C, Berchtold S, Baumeister T, et al. Cutting edge: resistance to apoptosis and continuous proliferation of dendritic cells deficient for TNF receptor-1. J Immunol. 2000;165:4792–4796. doi: 10.4049/jimmunol.165.9.4792. [DOI] [PubMed] [Google Scholar]
- 94.Zou H, Henzel WJ, Liu X, Lutschg A, Wang X. Apaf-1 a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3. Cell. 1997;90:405–413. doi: 10.1016/s0092-8674(00)80501-2. [DOI] [PubMed] [Google Scholar]
- 95.Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, et al. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 96.Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 1997;275:1132–1136. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- 97.Du C, Fang M, Li Y, Li L, Wang X. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell. 2000;102:33–42. doi: 10.1016/s0092-8674(00)00008-8. [DOI] [PubMed] [Google Scholar]
- 98.Verhagen AM, Ekert PG, Pakusch M, Silke J, Connolly LM, et al. Identification of DIABLO, a mammalian protein that promotes apoptosis by binding to and antagonizing IAP proteins. Cell. 2000;102:43–53. doi: 10.1016/s0092-8674(00)00009-x. [DOI] [PubMed] [Google Scholar]
- 99.Li LY, Luo X, Wang X. Endonuclease G is an apoptotic DNase when released from mitochondria. Nature. 2001;412:95–99. doi: 10.1038/35083620. [DOI] [PubMed] [Google Scholar]
- 100.Daugas E, Nochy D, Ravagnan L, Loeffler M, Susin SA, et al. Apoptosis-inducing factor (AIF): a ubiquitous mitochondrial oxidoreductase involved in apoptosis. FEBS Lett. 2000;476:118–123. doi: 10.1016/s0014-5793(00)01731-2. [DOI] [PubMed] [Google Scholar]
- 101.Levine B. Eating oneself and uninvited guests: autophagy-related pathways in cellular defense. Cell. 2005;120:159–162. doi: 10.1016/j.cell.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 102.Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shimizu S, Kanaseki T, Mizushima N, Mizuta T, Arakawa-Kobayashi S, et al. Role of Bcl-2 family proteins in a non-apoptotic programmed cell death dependent on autophagy genes. Nat Cell Biol. 2004;6:1221–1228. doi: 10.1038/ncb1192. [DOI] [PubMed] [Google Scholar]
- 104.Schmid D, Pypaert M, Munz C. Antigen-loading compartments for major histocompatibility complex class II molecules continuously receive input from autophagosomes. Immunity. 2007;26:79–92. doi: 10.1016/j.immuni.2006.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Koppi TA, Tough-Bement T, Lewinsohn DM, Lynch DH, Alderson MR. CD40 ligand inhibits Fas/CD95-mediated apoptosis of human blood-derived dendritic cells. Eur J Immunol. 1997;27:3161–3165. doi: 10.1002/eji.1830271212. [DOI] [PubMed] [Google Scholar]
- 106.Bjorck P, Banchereau J, Flores-Romo L. CD40 ligation counteracts Fas-induced apoptosis of human dendritic cells. Int Immunol. 1997;9:365–372. doi: 10.1093/intimm/9.3.365. [DOI] [PubMed] [Google Scholar]
- 107.Ashany D, Savir A, Bhardwaj N, Elkon KB. Dendritic cells are resistant to apoptosis through the Fas (CD95/APO-1) pathway. J Immunol. 1999;163:5303–5311. [PubMed] [Google Scholar]
- 108.Willems F, Amraoui Z, Vanderheyde N, Verhasselt V, Aksoy E, et al. Expression of c-FLIP(L) and resistance to CD95-mediated apoptosis of monocyte-derived dendritic cells: inhibition by bisindolylmaleimide. Blood. 2000;95:3478–3482. [PubMed] [Google Scholar]
- 109.Lundqvist A, Nagata T, Kiessling R, Pisa P. Mature dendritic cells are protected from Fas/CD95-mediated apoptosis by upregulation of Bcl-X(L) Cancer Immunol Immunother. 2002;51:139–144. doi: 10.1007/s00262-002-0265-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fields ML, Sokol CL, Eaton-Bassiri A, Seo S, Madaio MP, Erikson J. Fas/Fas ligand deficiency results in altered localization of anti-double-stranded DNA B cells and dendritic cells. J Immunol. 2001;167:2370–2378. doi: 10.4049/jimmunol.167.4.2370. [DOI] [PubMed] [Google Scholar]
- 111.Ryan CA, Stennicke HR, Nava VE, Burch JB, Hardwick JM, Salvesen GS. Inhibitor specificity of recombinant and endogenous caspase-9. Biochem J. 2002;366:595–601. doi: 10.1042/BJ20020863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Peng SL, Moslehi J, Robert ME, Craft J. Perforin protects against autoimmunity in lupus-prone mice. J Immunol. 1998;160:652–660. [PubMed] [Google Scholar]
- 113.Bai C, Connolly B, Metzker ML, Hilliard CA, Liu X, et al. Overexpression of M68/DcR3 in human gastrointestinal tract tumors independent of gene amplification and its location in a four-gene cluster. Proc Natl Acad Sci U S A. 2000;97:1230–1235. doi: 10.1073/pnas.97.3.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yu KY, Kwon B, Ni J, Zhai Y, Ebner R, Kwon BS. A newly identified member of tumor necrosis factor receptor superfamily (TR6) suppresses LIGHT-mediated apoptosis. J Biol Chem. 1999;274:13733–13736. doi: 10.1074/jbc.274.20.13733. [DOI] [PubMed] [Google Scholar]
- 115.Pitti RM, Marsters SA, Lawrence DA, Roy M, Kischkel FC, et al. Genomic amplification of a decoy receptor for Fas ligand in lung and colon cancer. Nature. 1998;396:699–703. doi: 10.1038/25387. [DOI] [PubMed] [Google Scholar]
- 116.Kim S, McAuliffe WJ, Zaritskaya LS, Moore PA, Zhang L, Nardelli B. Selective induction of tumor necrosis receptor factor 6/decoy receptor 3 release by bacterial antigens in human monocytes and myeloid dendritic cells. Infect Immun. 2004;72:89–93. doi: 10.1128/IAI.72.1.89-93.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.You RI, Chang YC, Chen PM, Wang WS, Hsu TL, et al. Apoptosis of dendritic cells induced by decoy receptor 3 (DcR3) Blood. 2008;111:1480–1488. doi: 10.1182/blood-2007-09-114850. [DOI] [PubMed] [Google Scholar]
- 118.Hsu TL, Chang YC, Chen SJ, Liu YJ, Chiu AW, et al. Modulation of dendritic cell differentiation and maturation by decoy receptor 3. J Immunol. 2002;168:4846–4853. doi: 10.4049/jimmunol.168.10.4846. [DOI] [PubMed] [Google Scholar]
- 119.Chattergoon MA, Muthumani K, Tamura Y, Ramanathan M, Shames JP, et al. DR5 activation of caspase-8 induces DC maturation and immune enhancement in vivo. Mol Ther. 2008;16:419–426. doi: 10.1038/sj.mt.6300373. [DOI] [PubMed] [Google Scholar]
- 120.Semnani RT, Venugopal PG, Mahapatra L, Skinner JA, Meylan F, et al. Induction of TRAIL- and TNF-alpha-dependent apoptosis in human monocyte-derived dendritic cells by microfilariae of Brugia malayi. J Immunol. 2008;181:7081–7089. doi: 10.4049/jimmunol.181.10.7081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kriehuber E, Bauer W, Charbonnier AS, Winter D, Amatschek S, et al. Balance between NF-kappaB and JNK/AP-1 activity controls dendritic cell life and death. Blood. 2005;106:175–183. doi: 10.1182/blood-2004-08-3072. [DOI] [PubMed] [Google Scholar]
- 122.Wong BR, Josien R, Lee SY, Sauter B, Li HL, et al. TRANCE (tumor necrosis factor [TNF]-related activation-induced cytokine), a new TNF family member predominantly expressed in T cells, is a dendritic cell-specific survival factor. J Exp Med. 1997;186:2075–2080. doi: 10.1084/jem.186.12.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Castera L, Hatzfeld-Charbonnier AS, Ballot C, Charbonnel F, Dhuiege E, et al. Apoptosis-related mitochondrial dysfunction defines human monocyte-derived dendritic cells with impaired immuno-stimulatory capacities. J Cell Mol Med. 2009;13:1321–1335. doi: 10.1111/j.1582-4934.2008.00358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cremer I, Dieu-Nosjean MC, Marechal S, Dezutter-Dambuyant C, Goddard S, et al. Long-lived immature dendritic cells mediated by TRANCE-RANK interaction. Blood. 2002;100:3646–3655. doi: 10.1182/blood-2002-01-0312. [DOI] [PubMed] [Google Scholar]
- 125.Ouaaz F, Arron J, Zheng Y, Choi Y, Beg AA. Dendritic cell development and survival require distinct NF-kappaB subunits. Immunity. 2002;16:257–270. doi: 10.1016/s1074-7613(02)00272-8. [DOI] [PubMed] [Google Scholar]
- 126.Chino T, Draves KE, Clark EA. Regulation of dendritic cell survival and cytokine production by osteoprotegerin. J Leukoc Biol. 2009;86:933–940. doi: 10.1189/jlb.0708419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Izawa T, Ishimaru N, Moriyama K, Kohashi M, Arakaki R, Hayashi Y. Crosstalk between RANKL and Fas signaling in dendritic cells controls immune tolerance. Blood. 2007;110:242–250. doi: 10.1182/blood-2006-11-059980. [DOI] [PubMed] [Google Scholar]
- 128.Adams JM, Huang DC, Puthalakath H, Bouillet P, Vairo G, et al. Control of apoptosis in hematopoietic cells by the Bcl-2 family of proteins. Cold Spring Harb Symp Quant Biol. 1999;64:351–358. doi: 10.1101/sqb.1999.64.351. [DOI] [PubMed] [Google Scholar]
- 129.Gross A, McDonnell JM, Korsmeyer SJ. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999;13:1899–1911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- 130.Strasser A. The role of BH3-only proteins in the immune system. Nat Rev Immunol. 2005;5:189–200. doi: 10.1038/nri1568. [DOI] [PubMed] [Google Scholar]
- 131.Puthalakath H, Strasser A. Keeping killers on a tight leash: transcriptional and post-translational control of the pro-apoptotic activity of BH3-only proteins. Cell Death Differ. 2002;9:505–512. doi: 10.1038/sj.cdd.4400998. [DOI] [PubMed] [Google Scholar]
- 132.Chen M, Sandoval H, Wang J. Selective mitochondrial autophagy during erythroid maturation. Autophagy. 2008;4:926–928. doi: 10.4161/auto.6716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sandoval H, Thiagarajan P, Dasgupta SK, Schumacher A, Prchal JT, et al. Essential role for Nix in autophagic maturation of erythroid cells. Nature. 2008;454:232–235. doi: 10.1038/nature07006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bouillet P, Metcalf D, Huang DC, Tarlinton DM, Kay TW, et al. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science. 1999;286:1735–1738. doi: 10.1126/science.286.5445.1735. [DOI] [PubMed] [Google Scholar]
- 135.Bouillet P, Purton JF, Godfrey DI, Zhang LC, Coultas L, et al. BH3-only Bcl-2 family member Bim is required for apoptosis of autoreactive thymocytes. Nature. 2002;415:922–926. doi: 10.1038/415922a. [DOI] [PubMed] [Google Scholar]
- 136.Enders A, Bouillet P, Puthalakath H, Xu Y, Tarlinton DM, Strasser A. Loss of the pro-apoptotic BH3-only Bcl-2 family member Bim inhibits BCR stimulation-induced apoptosis and deletion of autoreactive B cells. J Exp Med. 2003;198:1119–1126. doi: 10.1084/jem.20030411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lu J, Quearry B, Harada H. p38-MAP kinase activation followed by BIM induction is essential for glucocorticoid-induced apoptosis in lymphoblastic leukemia cells. FEBS Lett. 2006;580:3539–3544. doi: 10.1016/j.febslet.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 138.Sunters A, Fernandez de Mattos S, Stahl M, Brosens JJ, Zoumpoulidou G, et al. FoxO3a transcriptional regulation of Bim controls apoptosis in paclitaxel-treated breast cancer cell lines. J Biol Chem. 2003;278:49795–49805. doi: 10.1074/jbc.M309523200. [DOI] [PubMed] [Google Scholar]
- 139.Gilley J, Coffer PJ, Ham J. FOXO transcription factors directly activate bim gene expression and promote apoptosis in sympathetic neurons. J Cell Biol. 2003;162:613–622. doi: 10.1083/jcb.200303026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lanzavecchia A, Sallusto F. Regulation of T cell immunity by dendritic cells. Cell. 2001;106:263–266. doi: 10.1016/s0092-8674(01)00455-x. [DOI] [PubMed] [Google Scholar]
- 141.Blander JM, Medzhitov R. Toll-dependent selection of microbial antigens for presentation by dendritic cells. Nature. 2006;440:808–812. doi: 10.1038/nature04596. [DOI] [PubMed] [Google Scholar]
- 142.Cisse B, Caton ML, Lehner M, Maeda T, Scheu S, et al. Transcription factor E2-2 is an essential and specific regulator of plasmacytoid dendritic cell development. Cell. 2008;135:37–48. doi: 10.1016/j.cell.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zanoni I, Ostuni R, Capuano G, Collini M, Caccia M, et al. CD14 regulates the dendritic cell life cycle after LPS exposure through NFAT activation. Nature. 2009;460:264–268. doi: 10.1038/nature08118. [DOI] [PubMed] [Google Scholar]
- 144.Escribano C, Delgado-Martin C, Rodriguez-Fernandez JL. CCR7-dependent stimulation of survival in dendritic cells involves inhibition of GSK3beta. J Immunol. 2009;183:6282–6295. doi: 10.4049/jimmunol.0804093. [DOI] [PubMed] [Google Scholar]
- 145.Yen JH, Ganea D. Interferon beta induces mature dendritic cell apoptosis through caspase-11/caspase-3 activation. Blood. 2009;114:1344–1354. doi: 10.1182/blood-2008-12-196592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Marrack P, Kappler J. Positive selection of thymocytes bearing alpha beta T cell receptors. Curr Opin Immunol. 1997;9:250–255. doi: 10.1016/s0952-7915(97)80144-6. [DOI] [PubMed] [Google Scholar]
- 147.von Boehmer H, Swat W, Kisielow P. Positive selection of immature alpha beta T cells. Immunol Rev. 1993;135:67–79. doi: 10.1111/j.1600-065x.1993.tb00644.x. [DOI] [PubMed] [Google Scholar]
- 148.Nossal GJ. Negative selection of lymphocytes. Cell. 1994;76:229–239. doi: 10.1016/0092-8674(94)90331-x. [DOI] [PubMed] [Google Scholar]
- 149.Werlen G, Hausmann B, Naeher D, Palmer E. Signaling life and death in the thymus: timing is everything. Science. 2003;299:1859–1863. doi: 10.1126/science.1067833. [DOI] [PubMed] [Google Scholar]
- 150.Josefowicz SZ, Rudensky A. Control of regulatory T cell lineage commitment and maintenance. Immunity. 2009;30:616–625. doi: 10.1016/j.immuni.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30:636–645. doi: 10.1016/j.immuni.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 152.Nagata S, Suda T. Fas and Fas ligand: lpr and gld mutations. Immunol Today. 1995;16:39–43. doi: 10.1016/0167-5699(95)80069-7. [DOI] [PubMed] [Google Scholar]
- 153.Adachi M, Watanabe-Fukunaga R, Nagata S. Aberrant transcription caused by the insertion of an early transposable element in an intron of the Fas antigen gene of lpr mice. Proc Natl Acad Sci U S A. 1993;90:1756–1760. doi: 10.1073/pnas.90.5.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Takahashi T, Tanaka M, Brannan CI, Jenkins NA, Copeland NG, et al. Generalized lymphoproliferative disease in mice, caused by a point mutation in the Fas ligand. Cell. 1994;76:969–976. doi: 10.1016/0092-8674(94)90375-1. [DOI] [PubMed] [Google Scholar]
- 155.Rieux-Laucat F, Le Deist F, Hivroz C, Roberts IA, Debatin KM, et al. Mutations in Fas associated with human lymphoproliferative syndrome and autoimmunity. Science. 1995;268:1347–1349. doi: 10.1126/science.7539157. [DOI] [PubMed] [Google Scholar]
- 156.Fisher GH, Rosenberg FJ, Straus SE, Dale JK, Middleton LA, et al. Dominant interfering Fas gene mutations impair apoptosis in a human autoimmune lymphoproliferative syndrome. Cell. 1995;81:935–946. doi: 10.1016/0092-8674(95)90013-6. [DOI] [PubMed] [Google Scholar]
- 157.Wu J, Wilson J, He J, Xiang L, Schur PH, Mountz JD. Fas ligand mutation in a patient with systemic lupus erythematosus and lymphoproliferative disease. J Clin Invest. 1996;98:1107–1113. doi: 10.1172/JCI118892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bidere N, Su HC, Lenardo MJ. Genetic disorders of programmed cell death in the immune system. Annu Rev Immunol. 2006;24:321–352. doi: 10.1146/annurev.immunol.24.021605.090513. [DOI] [PubMed] [Google Scholar]
- 159.Singer GG, Abbas AK. The fas antigen is involved in peripheral but not thymic deletion of T lymphocytes in T cell receptor transgenic mice. Immunity. 1994;1:365–371. doi: 10.1016/1074-7613(94)90067-1. [DOI] [PubMed] [Google Scholar]
- 160.Smith KG, Strasser A, Vaux DL. CrmA expression in T lymphocytes of transgenic mice inhibits CD95 (Fas/APO-1)-transduced apoptosis, but does not cause lymphadenopathy or autoimmune disease. Embo J. 1996;15:5167–5176. [PMC free article] [PubMed] [Google Scholar]
- 161.Wu Z, Roberts M, Porter M, Walker F, Wherry EJ, et al. Viral FLIP impairs survival of activated T cells and generation of CD8+ T cell memory. J Immunol. 2004;172:6313–6323. doi: 10.4049/jimmunol.172.10.6313. [DOI] [PubMed] [Google Scholar]
- 162.Walsh CM, Wen BG, Chinnaiyan AM, O'Rourke K, Dixit VM, Hedrick SM. A role for FADD in T cell activation and development. Immunity. 1998;8:439–449. doi: 10.1016/s1074-7613(00)80549-x. [DOI] [PubMed] [Google Scholar]
- 163.Doerfler P, Forbush KA, Perlmutter RM. Caspase enzyme activity is not essential for apoptosis during thymocyte development. J Immunol. 2000;164:4071–4079. doi: 10.4049/jimmunol.164.8.4071. [DOI] [PubMed] [Google Scholar]
- 164.Izquierdo M, Grandien A, Criado LM, Robles S, Leonardo E, et al. Blocked negative selection of developing T cells in mice expressing the baculovirus p35 caspase inhibitor. Embo J. 1999;18:156–166. doi: 10.1093/emboj/18.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Newton K, Harris AW, Bath ML, Smith KG, Strasser A. A dominant interfering mutant of FADD/MORT1 enhances deletion of autoreactive thymocytes and inhibits proliferation of mature T lymphocytes. Embo J. 1998;17:706–718. doi: 10.1093/emboj/17.3.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zornig M, Hueber AO, Evan G. p53-dependent impairment of T-cell proliferation in FADD dominant-negative transgenic mice. Curr Biol. 1998;8:467–470. doi: 10.1016/s0960-9822(98)70182-4. [DOI] [PubMed] [Google Scholar]
- 167.Watanabe D, Suda T, Hashimoto H, Nagata S. Constitutive activation of the Fas ligand gene in mouse lymphoproliferative disorders. EMBO J. 1995;14:12–18. doi: 10.1002/j.1460-2075.1995.tb06970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hao Z, Hampel B, Yagita H, Rajewsky K. T cell-specific ablation of Fas leads to Fas ligand-mediated lymphocyte depletion and inflammatory pulmonary fibrosis. J Exp Med. 2004;199:1355–1365. doi: 10.1084/jem.20032196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Gollob KJ, Palmer E. Divergent viral superantigens delete V beta 5+ T lymphocytes. Proc Natl Acad Sci U S A. 1992;89:5138–5141. doi: 10.1073/pnas.89.11.5138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Rathmell JC, Lindsten T, Zong WX, Cinalli RM, Thompson CB. Deficiency in Bak and Bax perturbs thymic selection and lymphoid homeostasis. Nat Immunol. 2002;3:932–939. doi: 10.1038/ni834. [DOI] [PubMed] [Google Scholar]
- 171.Suda T, Okazaki T, Naito Y, Yokota T, Arai N, et al. Expression of the Fas ligand in cells of T cell lineage. J Immunol. 1995;154:3806–3813. [PubMed] [Google Scholar]
- 172.Wang J, Lenardo MJ. Essential lymphocyte function associated 1 (LFA-1): intercellular adhesion molecule interactions for T cell-mediated B cell apoptosis by Fas/APO-1/CD95. J Exp Med. 1997;186:1171–1176. doi: 10.1084/jem.186.7.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lenardo MJ. Interleukin-2 programs mouse alpha beta T lymphocytes for apoptosis. Nature. 1991;353:858–861. doi: 10.1038/353858a0. [DOI] [PubMed] [Google Scholar]
- 174.Zheng L, Trageser CL, Willerford DM, Lenardo MJ. T cell growth cytokines cause the superinduction of molecules mediating antigen-induced T lymphocyte death. J Immunol. 1998;160:763–769. [PubMed] [Google Scholar]
- 175.Lindsten T, Ross AJ, King A, Zong WX, Rathmell JC, et al. The combined functions of proapoptotic Bcl-2 family members bak and bax are essential for normal development of multiple tissues. Mol Cell. 2000;6:1389–1399. doi: 10.1016/s1097-2765(00)00136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, et al. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Tough DF, Sprent J. Turnover of naive- and memory-phenotype T cells. J Exp Med. 1994;179:1127–1135. doi: 10.1084/jem.179.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wilson NS, El-Sukkari D, Villadangos JA. Dendritic cells constitutively present self antigens in their immature state in vivo and regulate antigen presentation by controlling the rates of MHC class II synthesis and endocytosis. Blood. 2004;103:2187–2195. doi: 10.1182/blood-2003-08-2729. [DOI] [PubMed] [Google Scholar]
- 179.Nurieva RI, Chung Y, Hwang D, Yang XO, Kang HS, et al. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity. 2008;29:138–149. doi: 10.1016/j.immuni.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]