Abstract
Dendritic cells (DCs) harbor an active mitochondrion-dependent cell death pathway regulated by Bcl-2 family members and undergo rapid turnover in vivo. However, the functions for mitochondrion-dependent cell death of DCs in immune regulation remain to be elucidated. Here we show that DC-specific knockout of pro-apoptotic Bcl-2 family members, Bax and Bak, induced spontaneous T cell activation and autoimmunity in mice. In addition to a defect in spontaneous cell death, Bax−/−Bak−/− DCs were resistant to killing by CD4+FoxP3+ T regulatory (Treg) cells compared to wild type DCs. Treg cells inhibited the activation of T effector cells by wild type, but not Bax−/−Bak−/− DCs. Bax−/−Bak−/− DCs showed increased propensity for inducing autoantibodies. Moreover, the autoimmune potential of Bax−/−Bak−/− DCs was resistant to suppression by Treg cells. Our data suggest that Bax and Bak not only mediate intrinsic spontaneous cell death in DCs, but also regulate DC killing triggered by Treg cells. Bax- and Bak-dependent cell death mechanisms help to maintain DC homeostasis, and contribute to the regulation of T cell activation and the suppression of autoimmunity.
INTRODUCTION
During T cell development in the thymus, T cells that recognize self MHC molecules presenting autoantigens can survive by positive selection, while highly self-reactive T cells are subsequently deleted by negative selection (1–4). However, T cells that undergo successful maturation and eventually populate the peripheral lymphoid organs carry certain degree of autoreactivity. It is essential to keep these mature T cells in check to maintain peripheral tolerance. Programmed cell death of mature lymphocytes is a major mechanism for the maintenance of lymphocyte homeostasis and peripheral tolerance (5–7). In addition, natural Treg cells that express FoxP3 have been established to play an essential role in the protection of immune tolerance (8–14).
DCs, the most efficient antigen presenting cells, are important regulators of both innate and adaptive immune responses (15–19). DCs may also play important roles in the maintenance of immune tolerance (20, 21). We have previously observed that DC-specific expression of the baculoviral caspase inhibitor, p35, leads to inhibition of Fas-mediated apoptosis in DCs and the development of systemic autoimmune symptoms (22). Consistently, knockout of Fas in DCs also induces the onset of autoimmunity in mice (23). Interestingly, interactions of antigen-pulsed DCs with the antigen-specific T cells may lead to accelerated loss of DCs in vivo (24). It is possible that Fas-dependent killing of DCs by activated T cells provides a negative feedback mechanism that helps to terminate the activation of lymphocytes by antigen-bearing DCs (25).
It has been recognized that DCs have a short lifespan since the original discovery of this cell type (26). The short half-life of DCs in vivo has been linked to an active mitochondrion-dependent cell death pathway regulated by Bcl-2 family members (27–29). The Bcl-2 family members are upstream regulators of mitochondrion-dependent apoptosis pathway (30, 31). They share the Bcl-2 homology (BH) domains and are divided into three subfamilies (30, 31), including pro-apoptotic Bax and Bak; the anti-apoptotic subfamily proteins, such as Bcl-2, Bcl-xL and Mcl-1; and the pro-apoptotic BH3-only subfamily, such as Bim, Bid and Bad. BH3-only proteins are the upstream sensors for different apoptosis signaling in specific cell types (32). BH3-only proteins initiate mitochondrion-dependent apoptosis by either inhibiting the anti-apoptotic molecules, or directly activating pro-apoptotic Bax or Bak to induce apoptosis (32, 33). While deficiency in either Bax or Bak has no salient phenotypes, knockout of both Bax and Bak abolishes mitochondrion-dependent apoptosis (34), indicating that Bax and Bak are essential for mitochondrion-dependent apoptosis but functionally redundant of each other. Both negative selection of developing T cells in the thymus and apoptosis of mature T cells are defective in Bax−/−Bak−/− mice (35), suggesting that Bax- and Bak-dependent mitochondrial apoptosis in T cells is involved in the regulation of lymphocyte homeostasis and immune tolerance.
The rapid turnover rates of DCs in vivo could be attributed to the active mitochondrion-dependent cell death pathway in DCs (27). Indeed, transgenic expression of anti-apoptotic Bcl-2 or deletion of pro-apoptotic Bim can inhibit spontaneous cell death in DCs (28, 29). In contrast, Fas-mediated signaling is not required for spontaneous cell death in DCs (29). It is therefore possible that mitochondrion-dependent and Fas-mediated cell death pathways regulate DC turnover at different phases of immune responses. However, the function for the active mitochondrion-dependent cell death pathway of DCs in immune regulation has not been well characterized. Furthermore, whether mitochondrion-dependent cell death in DCs plays a role in the regulation of immune tolerance is not known.
Treg cells can interact with DCs to inhibit the activation of antigen-specific T cells in vivo (36–42). Treg cells may down-regulate important co-stimulatory molecules on DCs (40, 43–48). Interestingly, one report has suggested that Treg cells may cause the disappearance of DCs in the draining lymph nodes (38). Whether Treg cells can induce cell death in DCs, and whether such interactions help to protect immune tolerance have not be determined. Using mice with deficiencies of Bax and Bak in DCs, we show that CD4+FoxP3+ Treg cells efficiently induce mitochondrion-dependent cell death in DCs. Different from the killing of DCs by T effector cells through Fas, our current study suggests that Treg cells exploit the active mitochondrion-dependent apoptosis pathway in DCs for immune regulation. Such interactions between Treg cells and DCs potentially play a fundamental role in the regulation of initiation and expansion of antigen-specific immune responses, and in the protection of immune tolerance.
Materials and Methods
Mice
Bak−/−, Baxflox, Bim−/−, perforin−/−, lpr, CD11c-cre transgenic, OVA-specific-OT1 or -OT2 transgenic mice were obtained from the Jackson Laboratory and maintained on the C57BL/6 background. Granzyme A−/−granzyme B−/− mice and wild type controls on the 129X1/SvJ background were also obtained from the Jackson Laboratory. FoxP3GFP knock-in mice were provided by Dr. Alexander Rudensky (49). FoxP3GFP mice were crossed with perforin−/− mice to generate perforin−/−FoxP3GFP mice, and with OT2 mice to generate OT2-FoxP3GFP mice. OT2 mice were also crossed with CD45.1 congenic mice. The mice were maintained in a specific-pathogen-free facility and used with the approval of the Institutional Animal Care and Use Committee at Baylor College of Medicine.
Flow cytometry, preparation of DCs, measurement of autoantibodies, cell death assays and histochemistry
Flow cytometry analyses of different cell types, preparation of bone marrow derived DCs (BMDCs) and splenic DCs, histochemistry, measurements of spontaneous cell death in DCs, DC turnover in vivo by bromodeoxyuridine (BrdU) labeling, and detection of autoantibodies in mice were preformed as described (22, 29).
Proliferation assays
Mice were immunized with ovalbumin (OVA; 50 μg/mouse) emulsified in complete Freud adjuvant (CFA) at the footpad. Ten days later, total cells (2×105/well) from the draining popliteal lymph nodes were cultured in 96-well plates with various concentrations of OVA for 72 h. The cells were pulsed with 1 μCi/well [3H]-thymidine for the last 12 h and harvested to measure [3H]-thymidine incorporation.
CD4+FoxP3+ Treg cells were sorted from FoxP3GFP mice and expanded in vitro with anti-CD3-and anti-CD28-coated Dynabeads (Invitrogen; 10 μl beads/106 cells) in the presence of 1000 U/ml IL-2 for 3 days. Wild type or Bax−/−Bak−/− DCs were either unpulsed or pulsed with OVA323–339 peptide. DCs (103/well) were mixed with freshly sorted CD4+CD25− T cells from OT2 mice (104/well) in 96-well U-bottom plates, in the presence of different numbers of expanded Treg cells. The proliferation of OT2 T cells was measured by [3H]-thymidine incorporation 4 days later. Alternatively, CD4+CD25− Teff cells were sorted from wild type mice by flow cytometry. Different numbers of Treg cells expanded as above were added to CD4+CD25− Teff cells (5×104/well) at different ratios in the presence of 0.25 μg/ml soluble anti-CD3 (2C11; BD Biosciences) and irradiated (3000 rads) T cell- and DC-depleted syngeneic splenocytes (5×104/well) in 96-well U-bottom plates. [3H]-thymidine incorporation was measured 3 days later.
CD4+ T cells from OT2 mice (CD45.1 × CD45.2) were either sorted and labeled with 5 μM carboxyfluorescein diacetate succinimidyl ester (CFSE, Invitrogen) at room temperature for 10 min. The CFSE-labeled OT2 T cells were injected into C57BL/6 mice retro-orbitally (2×106/mouse). DCs (2×105) with or without pulsing with OVA323–339 peptide were injected intradermally at footpad 24 h later with or without Treg cells. Four days later, popliteal lymph nodes were collected and CD4+CD45.1+ T cells were analyzed by flow cytometry to determine CFSE dilution.
Spontaneous cell death in DCs and Killing of DCs or other target cells by Treg cells
CD11c+I-Ab+ DCs were sorted from the spleens of DC-DKO or control mice. DCs were cultured in RPMI complete medium for 0 or 24 h. Spontaneous cell death of DCs were quantitated as described (29). BMDCs or CD11c+CD11b+ splenic DCs from wild type, DC-DKO or Bim−/− mice were labeled with 1 μM CFSE (Invitrogen) for 10 min at 37 °C. The killing of DCs was analyzed similar to the previously described protocol (50). Treg cells were expanded as above. In some experiments, CD4+CD25high Treg cells were sorted from granzyme A−/−granzyme B−/− mice or wild type controls and expanded as above. Treg cells were mixed with DCs (2×104/well) at different ratios in 96-well U-bottomed plates for 6 h. the cells were incubated with 5 ng/ml 7-amino-actinomycin D (7-AAD; BD Biosciences) at room temperature for 10 min, followed by flow cytometry. Induction of cell death in DCs was quantified essentially as described (50) with the following formula: percentages of killing of DCs by T cells = 100% × (DCcontrol−DCT)/DCcontrol, with DCcontrol and DCT representing CFSE+7-AAD− DCs in the absence or presence of T cells, respectively. In some experiments, 10 μg/ml blocking antibody to LAG3 (clone C9B7W; BD Biosciences), LFA-1 (clone M17/4; BD Biosciences) or I-A/I-E (clone M5/114.15.2; Biolegend), or rat IgG were added to the culture.
To measure mitochondrial membrane potential, DCs with or without incubation with Treg cells above were incubated with 10 μM etramethylrhodamine ethyl ester (TMRE; Invitrogen) at 37 °C for 20 min, followed by flow cytometry. In some experiments, DCs were incubated with 10μg/ml agonist anti-I-A/I-E (clone 2G9; BD Bioscience) or control rat IgG for 6 h, followed with TMRE staining and flow cytometry.
Splenic CD11c+ DCs were purified with anti-CD11c-MACS beads (Miltenyi Biotec). Activated CD4+ T cells were generated by stimulating sorted CD4+CD25− cells anti-CD3- and anti-CD28-coated Dynabeads in the presence of 100 U/ml IL-2 for 3 days. B cells were purified with anti-CD19 MACS beads and stimulated with 1 μg/ml LPS and 100 μM CpG for 48 h. Treg-mediated killing of splenic DCs and activated T or B cells were measured as the killing of BMDCs above.
CD4+FoxP3+ Treg cells or CD4+FoxP3− Teff cells sorted from OT2-FoxP3GFP mice were stimulated with OVA323–339 peptide-pulsed DCs (T:DC=2:1) for 3 days. OT2 Treg or Teff cells were then isolated by removing DCs with anti-CD11c-MACS beads (Miltenyi Biotec) and used for killing of DCs with or without pulsing with OVA323–339 peptide. Percentages of killing of DCs by T cells were measured as above.
Analyses of interaction between Treg cells and DCs
To detect interactions between Treg cells and DCs in vivo, DCs and Treg cells were labeled at 37 °C for 10 min with 10 μM 5-(and -6)-(((4-chloromethyl) benzoyl) amino) tetramethylrhodamine (CMTMR) and CFSE (Invitrogen), respectively. Wild type or DKO DCs (1×106) were injected into the footpad of recipient mice. Treg cells (0.5×106) were also injected into the footpad of some recipient mice. Draining (popliteal) lymph nodes were harvested 24 h later and frozen sections were analyzed using a LSM 510 confocal microscope (Zeiss).
To determine the conjugate formation between DCs and Treg cells in vitro, DCs and Treg cells were labeled at 37 °C for 10 min with 0.3 μM CMTMR and CFSE, respectively. The cells were mixed at a ratio of 1:1 and centrifuged at 500 g for 5 min. The cells were incubated at 37 °C for 0, 1, 2 or 3 h in the absence or presence of 10 μg/ml anti-LAG3, anti-LFA-1 (BD Bioscience) or rat IgG. The cells were washed with PBS and analyzed by flow cytometry.
Adoptive transfer of DCs
To determine autoantibody production after adoptive transfer, wild type or Bax−/−Bak−/− BMDCs (106 or 5×106/mouse) were injected into 8-week-old C57BL/6 mice (6 mice/group) with or without Treg cells (with Treg:DC at 0.5:1) intraperitoneally essentially as described (29, 51). The mice were then injected with LPS (30 μg/mouse) intraperitoneally one day later. Sera were collected from the recipient mice 1 week after DC transfer. ANAs, anti-ssDNA and anti-dsDNA were measured as above. In parallel experiments, CFSE-labeled wild type or Bax−/−Bak−/−BMDCs (106 or 5×106/mouse) were injected into 8-week-old C57BL/6 mice (6 mice/group) with or without Treg cells (with Treg:DC at 0.5:1) at the footpad. Twenty four hours later, draining (popliteal) lymph nodes were collected. Total cell numbers were counted and percentages of CFSE+ DCs were determined by flow cytometry. Total CFSE+ DCs in the draining lymph nodes were calculated.
Western blot
To determine Treg-mediated signaling in DCs, wild type or DKO DCs were incubated with Treg cells at a ratio of 2:1 for 6 h at 37 °C. The cells were incubated with anti-CD11c-MACS beads (Miltenyi Biotec) to isolate DCs. DCs were then lysed for Western blot analyses. The following primary antibodies were used: polyclonal rabbit antibodies to caspase-8, caspase-3, Bcl-xL, Bad (Cell Signaling), Bax (Santa Cruz Biotechnology), Bcl-2, Bak (Upstate Biotechnology), Mcl-1 (Fitzgerald), Bim (Stressgen), Bid (Imgenex), Blk or Bmf (Biovision), or mouse monoclonal antibody to caspase-9 (MBL), Noxa (Imgenex) or XIAP (BD Bioscience). The blots were then probed with HRP-conjugated secondary antibodies and developed using the chemiluminescent method (Pierce). CD11c+CD11b+ DCs, CD3+ T cells or CD19+ B cells sorted from the spleen of DC-DKO and control mice were also used for Western blot analyses of Bax and Bak as above. The blots were also probed with anti-α-tubulin (Santa Cruz Biotechnology) to ensure equal loading.
Intracellular staining for cytokines
CD11c+ bone marrow–derived DCs (106/ml) were cultured in the absence or presence of 1 μg/ml LPS (Sigma) or 0.5 μM phosphorothioate-stabilized CpG oligonucleotide (TCCATGACGTTCCTGATGCT) for 24 hours. Brefeldin A (1 μg/mL) and monensin (2 μM) were added during the last 6 hours to inhibit cytokine secretion. Cells were stained with FITC-anti-CD11c, followed by fixation and permeabilization with Cytofix/Cytoperm solution (BD Biosciences) and staining with PE-conjugated anti-IL-12p40/p70 (BD Biosciences), PE-conjugated IL-6 (BD Biosciences) or PE-conjugated rat IgG1 as isotype control. The cells were than analyzed by flow cytometry.
Statistical analysis
Data were presented as mean ± SD. P values were determined by two-tailed Student’s t-test using GraphPad Prism software version 4.0 for Macintosh. P<0.05 is considered to be statistically significant.
RESULTS
Accumulation of DCs due to deficiencies in Bax and Bak
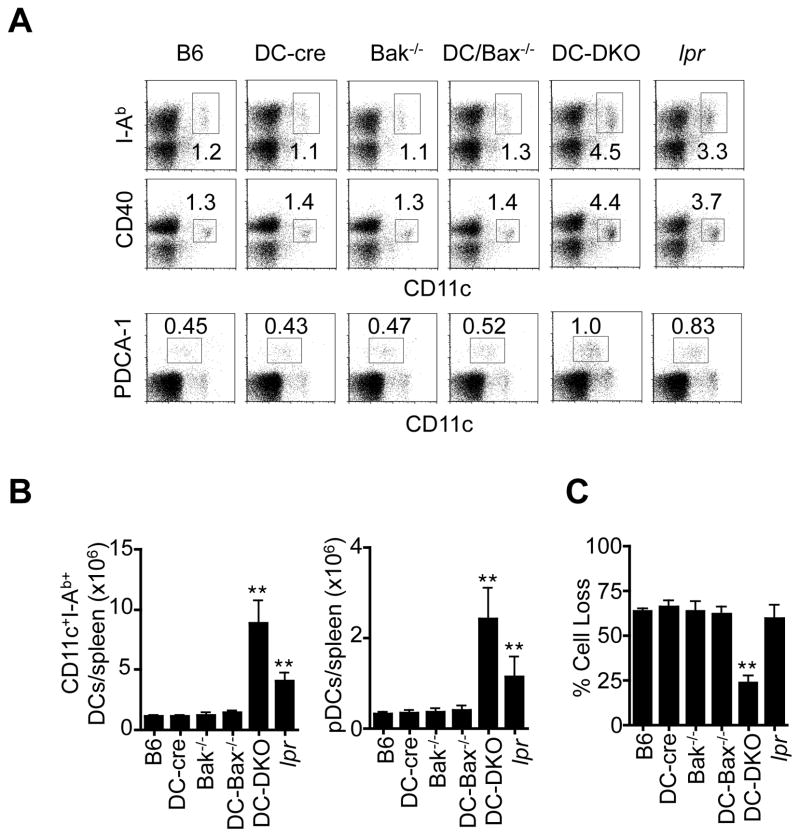
To determine the functions of mitochondrion-dependent apoptosis in immune regulation, we generated double knockouts of Bax and Bak in DCs (DC-DKO) by crossing CD11c-cre mice with Baxflox and Bak−/− mice. Specific deletion of Bax in DCs were observed in DC-DKO mice (Supplemental Fig. 1A). Because Bax and Bak are functionally redundant of each other (34), deletion of Bak alone is not expected to affect apoptosis in other cell types. DC-DKO, but not Bak−/− or DC-Bax−/− mice, displayed enlargement of the spleen and lymph nodes (Supplemental Fig. 1B). Terminally differentiated DCs have low proliferative potentials, pulsing with bromodeoxyuridine (BrdU) in vivo can be used to measure the rate of DC turnover by the appearance of newly generated BrdU+ DCs (52). We found that DCs were labeled more slowly with BrdU in DC-DKO mice (Supplemental Fig. 1C), indicating a reduced DC turnover rate for DCs in these mice. Consistently, the percentages and total numbers of CD11chighI-Ab+ or CD11chighCD40+ conventional DCs were increased in DC-DKO mice (Fig. 1A and B). CD11clowPDCA-1+ plasmacytoid DCs increased to a lesser extent in DC-DKO mice (Fig. 1A and B). The percentages of T, B or NK cells were not elevated in DC-DKO mice (Supplemental Fig. 1D). DCs from DC-DKO, but not Bak−/−, DC-Bax−/−, Fas-deficient lpr, CD11c-cre or C57BL/6 control mice, showed defects in spontaneous cell death (Fig. 1C). However, splenic DCs from DC-DKO mice and wild type controls express similar levels of CD40, MHC-II, B7.1, B7.2 and ICAM-1 (Supplemental Fig. 1E), suggesting that deficiencies of Bax and Bak cause DC accumulation but not abnormal DC activation.
Figure 1. Reduced spontaneous cell death of DCs, DC accumulation and spontaneous lymphocyte activation in DC-DKO mice.
(A) Splenocytes from 6-month-old DC-DKO and control mice were stained with antibodies to different markers. The cells were analyzed by flow cytometry of CD11chighI-Ab+ or CD11chighCD40+ conventional DCs and CD11clowPDCA-1+ pDCs. (B) CD11chighI-Ab+ DCs in the spleen of 6-month-old wild type C57BL/6 (B6), CD11c-cre (DC-cre), Bak−/−, DC-Bax−/−, DC- Bax−/−Bak−/− (DC-DKO) and lpr mice. **P<0.01, n=5. (C) Splenic CD11c+CD11b+ DCs from DC-DKO, lpr or control mice were cultured in vitro for 0 or 24 h. Cell death after 24 h of culture was determined. **P<0.01.
Spontaneous T cell activation and systemic autoimmunity in DC-DKO mice
T cells from DC-DKO mice showed increased expression of an activation marker, CD69 (Supplemental Fig. 2A). In particular, more than half of CD4+ T cells were CD69+ (Supplemental Fig. 2A). CD69 was also increased on CD8+ T cells and CD19+ B cells in DC-DKO mice (Supplemental Fig. 2A). In addition, similar to lpr mice, T cells with the CD44+CD62L− activated/memory phenotype were increased in DC-DKO mice compared to controls (Supplemental Fig. 2A). These data suggest that deficiencies of Bax and Bak in DCs lead to DC expansion and abnormal lymphocyte activation.
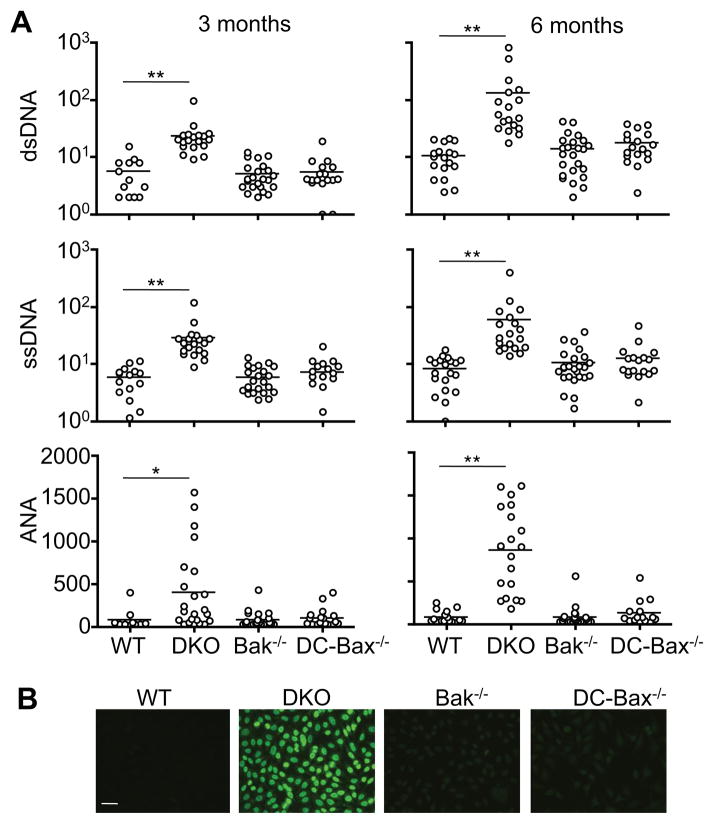
We determined whether deficiencies in mitochondrion-dependent cell death induce autoimmune responses in DC-DKO mice. DC expansion and enlargement of lymphocyte areas were observed in spleen sections of 6-month-old DC-DKO mice (Supplemental Fig. 1F). Severe perivascular lymphocyte infiltration was found in the liver, lung and kidney of DC-DKO mice, but not DC-Bax−/−, Bak−/− or wild type controls (Supplemental Fig. 1G). IgG deposits were also observed in the glomeruli of kidneys in DC-DKO mice (Supplemental Fig. 1H). We also detected autoantibodies, including anti-dsDNA, anti-ssDNA and antinuclear antibodies (ANAs) in the sera of 3- and 6-month-old DC-DKO mice, but not in controls by ELISA (Fig. 2A). Consistent with the production of ANAs, sera from DC-DKO mice showed nuclear staining of Hep2 cells (Fig. 2B). Together, these observations suggest that deficiencies of Bax and Bak in DCs lead to the development of systemic autoimmunity. Normal levels of CD4+FoxP3+ natural Treg cells were detected in DC-DKO mice (Supplemental Fig. 2A). Moreover, natural Treg cells from DC-DKO and control mice showed comparable activities in inhibiting the proliferation of T effector (Teff) cells (Supplemental Fig. 2B), indicating that there is no intrinsic defect in natural Treg cells in DC-DKO mice.
Figure 2. Systemic autoimmunity in DC-DKO mice.
(A) ELISA for autoantibodies in the sera of 3- or 6-month-old DC-cre mice as wild type (WT) control, DC-DKO, Bak−/−, DC-Bax−/−mice. *P<0.05, **P<0.01. (B) Hep2 cell slides were incubated with sera (1:640 dilution) from 6-month-old DC-DKO or control mice, followed by staining with FITC-anti-IgG. Scale bar: 30 μm. Data are representative results of 5 mice per group analyzed.
Increased immunogenicity of Bax−/−Bak−/− DCs
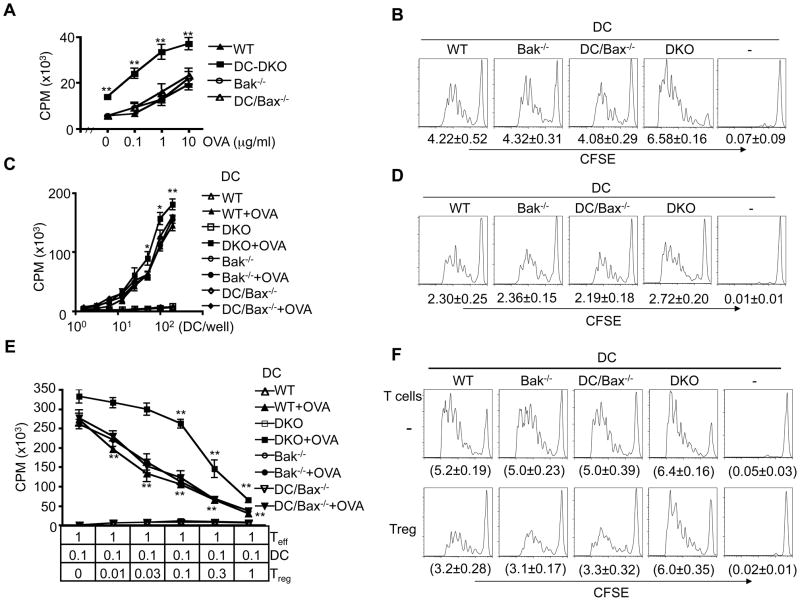
We found that cells from the draining lymph nodes of immunized DC-DKO mice responded better to antigen re-stimulation than those of wild type, Bak−/− or DC-Bax−/− mice (Fig. 3A). Consistently, Bax−/−Bak−/− DCs induced more robust proliferation of antigen-specific T cells than control DCs in vivo as assayed by CFSE dilution (Fig. 3B). This suggests that Bax−/−Bak−/−DCs are more efficient in priming antigen-specific T cells in vivo. However, Bax−/−Bak−/− DCs did not produce more IL-6 or IL-12 (Supplemental Fig. 2F), suggesting that Bax−/−Bak−/− DCs do not over-produce these cytokines to induce more activation of Teff cells. Interestingly, the increase in the capacity for Bax−/−Bak−/− DCs to stimulate antigen-specific Teff cells in vitro was detectable but less dramatic (Fig. 3C, D; Supplemental Fig. 2D, E). This is reminiscent of previous observations that cell death in DCs has a more profound effect on the immunogenicity of DCs in vivo than in vitro (53). Such differences between in vivo and in vitro observations could be due to the presence of other cell types that regulate the interactions between DCs and T cells in vivo.
Figure 3. Increased immunogenicity of Bax−/−Bak−/− DC.
(A) Draining lymph node cells from 6-week-old DC-cre mice as wild type (WT) control, DC-DKO, Bak−/−, DC-Bax−/− mice immunized with OVA (5 mice per group) were re-stimulated in vitro with OVA. Cell proliferation was measured by 3H-thymidine incorporation 4 days later. WT versus DKO: **P<0.01. (B) CD4+CD25− OT2 T cells from OT2 transgenic mice crossed with CD45.1 congenic mice (CD45.1×CD45.2 OT2 F1) were sorted, labeled with CFSE and injected intravenously into recipient C57BL6 mice. Recipient mice (5 mice/group) were then immunized with bone marrow-derived DCs (BMDCs) from DC-DKO or control mice pulsed with OVA323–339 peptide the next day. Proliferation of transferred CD4+CD45.1+ T cells by CFSE dilution in draining lymph nodes was analyzed 4 days later. The numbers of cell cycle (average ± SD) are shown. WT versus DKO: P<0.01, with 65% increased in cell cycles induced by DKO DCs. (C) BMDCs from DC-DKO or control mice with or without OVA323–339 pulsing were incubated with CD4+CD25− OT2 T cells. 3H-thymidine incorporation was measured on day 4. WT versus DKO: *P<0.05, **P<0.01. (D) CFSE-labeled OT2 T cells (5×104/well) were incubated with BMDCs from DC-DKO or control mice pulsed with OVA323–339 peptide (300 DCs/well) in 96-well plates. Cell proliferation was measured by CFSE dilution 4 days later. The numbers of cell cycle (average ± SD) are shown. WT versus DKO: P<0.05 (n=4), with 13.9% increase in cell cycles induced by DKO DCs. (E) BMDCs from DC-DKO or control mice pulsed with OVA323–339 peptide were cultured with CD4+CD25− T cells from OT2 mice, in the absence or presence of Treg cells sorted and expanded from FoxP3GFP mice. Proliferation of OT2 T cells was measured by 3H-thymidine incorporation 4 days later. Statistic comparison with control group containing no Treg cells: **P<0.01 (n=3). (F) CD4+CD25− OT2 T cells as in Fig. 3B were labeled with CFSE and injected intravenously into recipient C57BL6 mice. Recipient mice were then immunized at the footpad with BMDCs from DC-DKO or control mice pulsed with OVA323–339 peptide together with Treg cells stimulated with anti-CD3- anti-CD28-coated Dynabeads in the presence of IL-2 for 3 days (Treg:DC: 0.3:1). The proliferation of CD45.1+ OT2 T cells in the draining popliteal lymph nodes was quantitated by CFSE dilution 4 days later. The numbers of cell cycle (average ± SD) are shown (n=4). Statistic comparison between groups with or without Treg cells: P<0.01 (WT DC), not significant (DKO DC).
Deficiencies in Bax and Bak enable DCs to overcome the suppression by Treg cells
Although natural FoxP3+ Treg cells were not reduced or dysfunctional in DC-DKO mice (Supplemental Fig. 2A, Supplemental Fig. 2B), it remains possible that Bax−/−Bak−/− DCs might be refractory to suppression by Treg cells to induce uncontrolled T cell activation. We investigated whether deficiencies of Bax and Bak might enable DCs to overcome the inhibitory effect of Treg cells. We sorted and expanded CD4+FoxP3+ natural Treg cells from FoxP3GFP knock-in mice (49). To the culture of antigen-pulsed DCs and ovalbumin (OVA)-specific OT2 Teff cells (with DCs:Teff at a fixed ratio of 0.1:1), we added varied numbers of the expanded Treg cells (with Treg:Teff ratios ranging from 0.01:1 to 1:1). When Foxp3+ Treg cell numbers were low (Treg:Teff from 0.01:1 to 0.1:1), we detected significant suppression of antigen-specific T cell proliferation induced by wild type, Bax−/−, and Bak−/− but not Bax−/−Bak−/− DCs (Fig. 3E). When Treg:Teff ratios were higher (0.1:1 to 1:1), significant suppression of Bax−/−Bak−/− DC-induced proliferation of OT2 Teff cells was detected (Fig. 3E). However, this could at least be partially attributed to a direct suppression of Teff cells by Treg cells at higher Treg: Teff ratios (Supplemental Fig. 2C).
Consistent with in vitro observations (Fig. 3E), CD4+FoxP3+ Treg cells suppressed the proliferation of OT2 Teff cells induced by wild type, Bax−/− or Bak−/− DCs in vivo as measured by CFSE dilution (Fig. 3F). In contrast, Bax−/−Bak−/− DC-induced T cell proliferation was less susceptible to inhibition by Treg cells in vivo (Fig. 3F). This supports the conclusion that Bax−/−Bak−/− DCs are refractory to suppression by Treg cells.
Induction of Bax- and Bak-dependent cell death in DCs by Treg cells
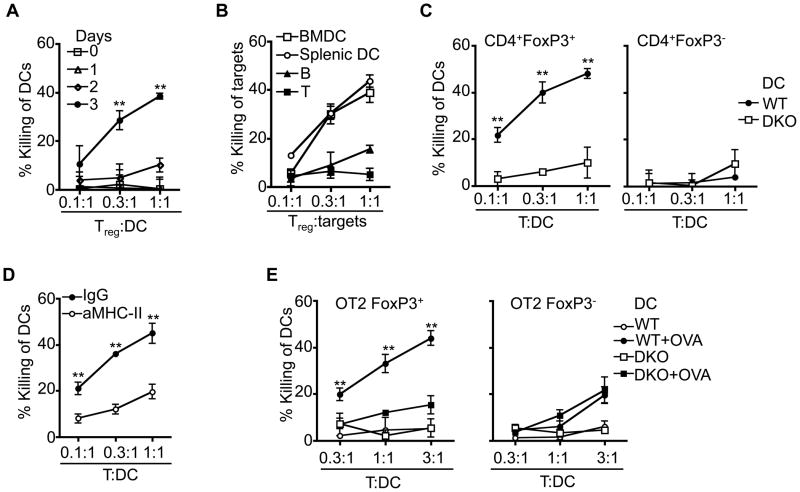
We found that activated Treg cells acquired cytotoxicity against DCs (Fig. 4A). We examined whether activated Treg cells could directly induce cell death in DCs in a Bax- and Bak-dependent manner. We observed that DCs were more susceptible than activated B or T cells to killing by CD4+FoxP3+ Treg cells (Fig. 4B). Wild type, but not Bax−/−Bak−/− DCs were sensitive to killing by Treg cells (Fig. 4C; Supplemental Fig. 3A, B), suggesting that Treg cells induce DC cell death in a Bax- and Bak-dependent manner. Treg-mediated killing of DCs is not affected by treating DCs with LPS (Supplemental Fig. 3C), suggesting that Treg-mediated killing of DCs is not affected by DC maturation. It has been shown that Treg cells can negatively regulate DCs by restricting DC development (54), inhibiting the expression of co-stimulatory molecules on DCs or competing with Teff cells in the interaction with DCs (36–38, 40). Apoptotic DCs may promote further induction of Treg cells (55). Inducing Bax- and Bak-dependent cell death in DCs may provide another mechanism for DC regulation by Treg cells.
Figure 4. Susceptibility of DCs to killing by Treg cells.
(A) Sorted CD4+FoxP3+ Treg cells were cultured in vitro with anti-CD3- anti-CD28-coated Dynabeads in the presence of IL-2 for 0, 1, 2 or 3 days. BMDCs were labeled with CFSE and incubated with Treg cells for 6 h. Killing of DCs by Treg cells was determined as described in Materials and Methods. Day 3 versus day 0 at 0.3: and 1:1 Treg: DC ratios: **P<0.01 (n=3). (B) CD4+FoxP3+ Treg cells were sorted and stimulated with anti-CD3- anti-CD28-coated Dynabeads in the presence of IL-2 for 3 days. Treg-mediated killing (n=5) of BMDCs, splenic DCs, or activated B or T cells were was determined as described in Materials and Methods. (C) CD4+FoxP3+ Treg cells or CD4+FoxP3−Teff cells sorted and expanded as in Fig. 3 were incubated with wild type or Bax−/−Bak−/− (DKO) BMDCs for 6 h, followed by staining with 7-AAD and analyses by flow cytometry. Loss of 7-AAD− DCs was quantitated. WT versus DKO: **P<0.01 (n=3). (D) Killing of DCs by CD4+FoxP3+ Treg cells as in (A) in the presence of anti-MHC-II or control IgG. **P<0.01 (n=3). (E) CD4+FoxP3+ Treg cells or CD4+FoxP3− Teff cells were sorted from OT2/FoxP3GFP mice and expanded with OVA323–339 peptide-pulsed BMDCs for 3 days. OT2 Treg or Teff cells were then isolated by removing DCs with anti-CD11c-MACS beads (Miltenyi Biotec) and used for killing of BMDCs with or without pulsing with OVA323–339 peptide. WT versus DKO: **P<0.01 (n=3).
Blocking MHC-II inhibited the killing of DCs by polyclonal Treg cells (Fig. 4D). This suggests that the polyclonal Treg cells are potentially autoreactive. By using OVA-specific OT2 FoxP3+ Treg cells, we found that FoxP3+ Treg cells killed antigen-pulsed, but not unpulsed DCs (Fig. 4E). Using FoxP3+ Treg cells expressing a transgenic TCR specific for a foreign antigen may not recuperate the action of natural Treg cells carrying TCRs that tend to be autoreactive. Nevertheless, our data obtained by using OT2 FoxP3+ Treg cells suggest that recognition of antigens on DCs is important for killing by Treg cells. In contrast to Treg cells, polyclonal Teff cells did not efficiently kill DCs in the absence of anti-CD3 (Fig. 4C), while OT2 Teff cells showed killing of DCs in the presence of OVA antigen at a higher T:DC ratio (3:1; Fig. 4E).
Bax- and Bak-dependent clearance of DCs in the draining lymph nodes after adoptive transfer
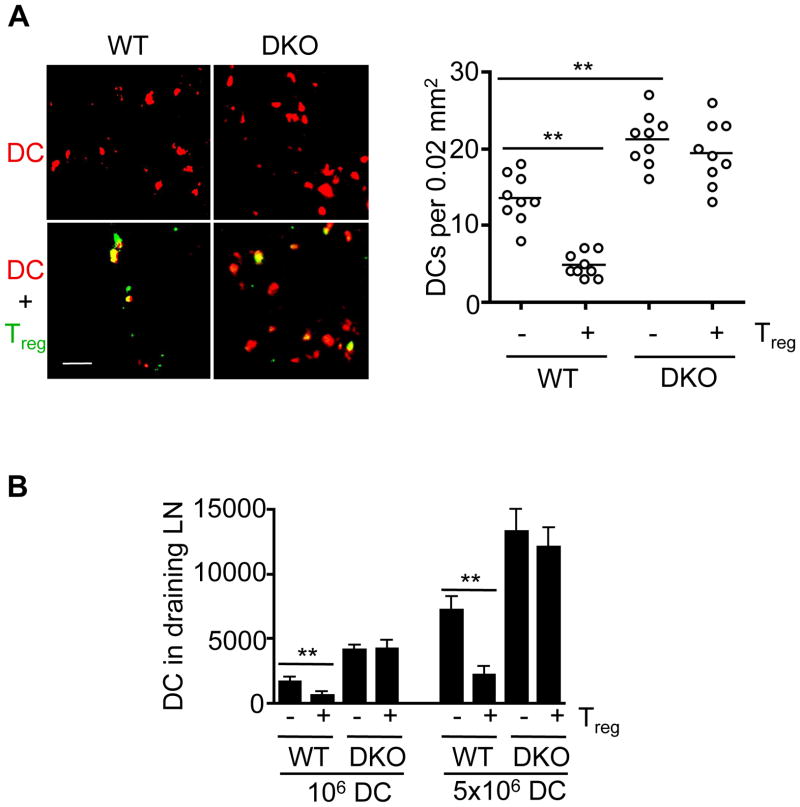
We consistently detect more Bax−/−Bak−/− DCs in the draining lymph nodes after adoptive transfer (Fig. 5A). Moreover, co-transfer of Treg cells led to the loss of wild type but not Bax−/−Bak−/− DCs in the draining lymph nodes (Fig. 5A). This is consistent with the conclusion that Bax and Bak regulate spontaneous cell death of DCs, as well as Treg-dependent DC killing. Treg cells can induce Bax- and Bak-dependent cell death in DCs. In addition, we also observed that Treg cells formed conjugates with both wild type and Bax−/−Bak−/− DCs in the draining lymph nodes (Fig. 5A). Conjugate formation between Treg cells and DCs was also detected in vitro (Supplemental Fig. 3D–H). Wild type and DKO DCs were similar in forming conjugates with Treg cells after 1 hour of incubation in vitro (Supplemental Fig. 3E, H). Consistent with confocal microscopy analyses, more Bax−/−Bak−/− DCs were found in the draining lymphocytes than wild type DCs as determined by flow cytometry (Fig. 5B). Co-transfer of Treg cells also promoted the clearance of wild type but not Bax−/−Bak−/− DCs (Fig. 5B). These data suggest that Bax and Bak do not affect conjugate formation between Treg cells and DCs. Rather, Bax and Bak regulate spontaneous cell death of DCs, as well as Treg-mediated clearance of DCs in vivo.
Figure 5. Clearance of DCs by Treg cells after adoptive transfer.
(A) Wild type and Bax−/−Bak−/− BMDCs were labeled with CMTMR (red) and injected subcutaneously into footpad. In some groups, CFSE (green)-labeled Treg cells were also injected into footpad. Draining (popliteal) lymph nodes were harvested 24 h later. Frozen sections were prepared and analyzed by confocal microscopy. Scale bar: 20 μm. Quantification of DCs per focal section field (0.02 mm2) in the draining lymph nodes is shown (right panel). **P<0.01 (n=9). (B) CD11c+ WT or DKO BMDCs (106 or 5×106) labeled with CFSE were injected with or without Treg cells (Treg:DC=0.5:1) into syngenic C57BL/6 recipient mice at the footpad. Twenty-four hours later, the draining (popliteal) lymph nodes (LN) were harvested. CFSE+ DCs were analyzed by flow cytometry and total CFSE+ DCs in the draining LN were calculated. **P<0.01 (n=6).
Autoimmune potential of Bax−/−Bak−/− DCs in adoptive transfer
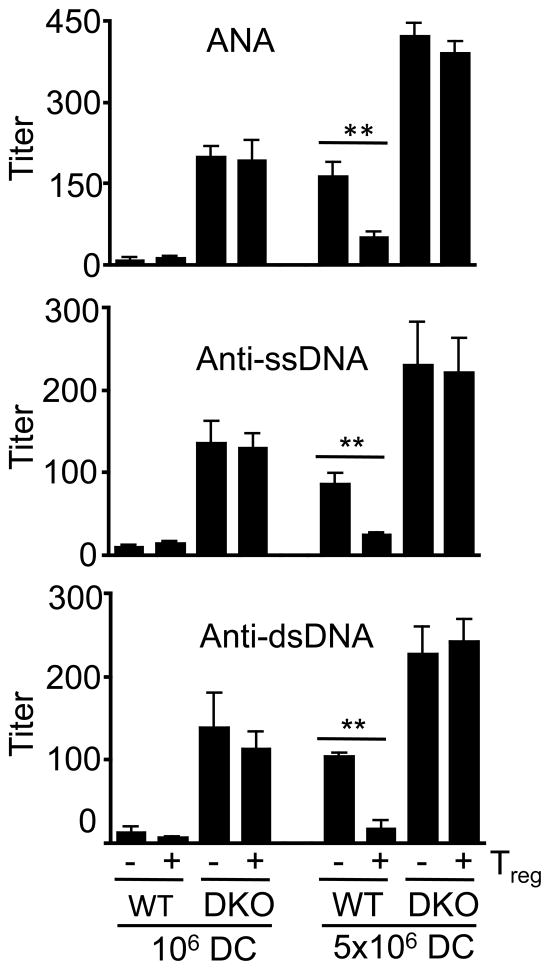
To directly test whether Bax−/−Bak−/− DCs can induce autoimmune responses, we performed adoptive transfer of wild type and Bax−/−Bak−/− DCs with or without Treg cells. It has been shown that transfer of excessive activated DCs can trigger the production of autoantibodies in recipient mice (51, 56). Adoptive transfer of Bax−/−Bak−/− DCs at a low dose (1×106 cells/mouse) induced the production of anti-dsDNA, anti-ssDNA and ANAs, while a higher dose (5×106 cells/mouse) triggered more autoantibody production (Fig. 6). Co-transfer of Treg cells did not significantly suppress autoantibody production induced by Bax−/−Bak−/− DCs (Fig. 6). In contrast, wild type DCs induced detectable levels of autoantibody production only at the high dose (5×106 cells/mouse), which was efficiently suppressed by co-transfer of Treg cells (Fig. 6). These data provide direct evidence to support the conclusion that Bax−/−Bak−/− DCs have the propensity for triggering autoantibody production. Moreover, Bax−/−Bak−/−, but not wild type DCs, are resistant to suppression by Treg cells in the induction of autoimmune responses.
Figure 6. Suppression of DC-induced autoantibody production by Treg cells after adoptive transfer.
CD11c+ WT or DKO BMDCs (106 or 5×106) were injected with or without Treg cells (Treg:DC=0.5:1) into syngenic C57BL/6 recipient mice intraperitoneally (6 mice/group), followed by injection of LPS intraperitoneally (30 μg/mouse) 24 hours later. Sera were collected from recipient mice 1 week later for quantitation of ANA, anti-ssDNA, and anti-dsDNA by ELISA. **P<0.01 (n=6).
Molecules involved in the killing of DCs by activated Treg cells
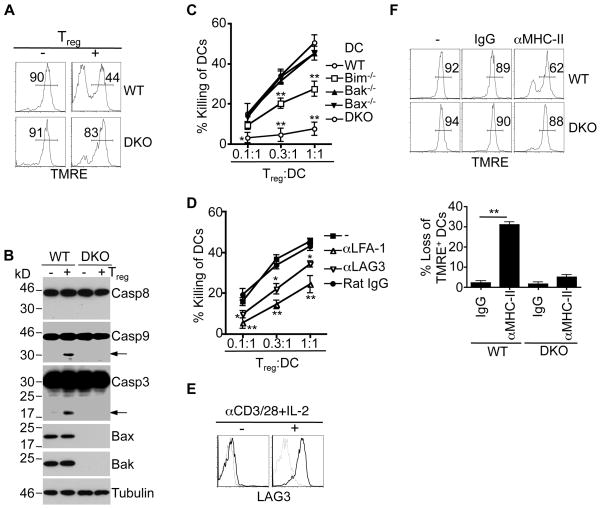
We next investigated apoptosis signaling in DCs induced by Treg cells. After incubation with Treg cells, wild type DCs lost mitochondrial membrane potential (ΔΨm), while DKO DCs were relatively resistant to the loss of ΔΨm (Fig. 7A). Consistent with the activation of mitochondrion-dependent cell death in wild type DCs, Treg cells induced the activation of caspase-9 and caspase-3 in wild type, but not Bax−/−Bak−/− DCs (Fig. 7B). It has been shown that cleavage of Bid into active tBid by caspases can trigger mitochondrial apoptosis (57). Caspase-dependent cleavage may inactivate anti-apoptotic Bcl-2 family proteins to promote mitochondrion-dependent cell death (58). However, we did not find processing of Bcl-2 family proteins in DCs after incubation with Treg cells (Supplemental Fig. 4A). Treg cells also did not change the expression of Bcl-2 family proteins in DCs (Fig. 7B, Supplemental Fig. 4A). Interestingly, Bim−/− DCs showed resistance to killing by Treg cells compared to wild type DCs, while deletion of either Bax or Bak in DCs did not affect their killing by Treg cells (Fig. 7C). This suggests that Bim serves as a mediator to activate Bax and Bak in DCs after encountering Treg cells. Although the precise mechanism for the activation of Bim is not resolved, Bim potentially transmits cell death signaling by sequestering anti-apoptotic molecules or directly activating Bax and Bak (59, 60).
Figure 7. Induction of mitochondrion-dependent cell death in DCs by Treg cells.
(A) Wild type or DKO BMDCs with or without incubation with Treg cells for 6 h were labeled with TMRE, followed by flow cytometry. Percentages of TMRE+ cells (mean ± SD): wild type, 89.4 ± 2.1 (no Treg), 44.9 ± 5.0 (with Treg), P=0.0001; DKO, 91.2 ± 1.5 (no Treg), 83.2 ± 5.8 (with Treg), P=0.081 (n=3). (B) Wild type or DKO BMDCs with or without incubation with Treg cells for 6 h were lysed for Western blotting of caspase-8 (Casp8), Casp9, Casp3, Bax or Bak. (C) Treg cells were incubated with wild type, Bak−/−, Bax−/−, Bim−/− or DKO BMDCs, followed by analysis of killing of DCs as in Fig. 4. Bim−/− or DKO versus WT DCs: *P<0.05, **P<0.01 (n=3). (D) Treg cells were incubated with wild type BMDCs for 6 h in the presence of various antibodies of control IgG, followed by quantification of killing of DCs as in Fig. 6A. Antibody treatments versus IgG control: *P<0.05; **P<0.01 (n=3). (E) CD4+FoxP3+ Treg cells with or without stimulation with anti-CD3/anti-CD28 Dynal beads plus IL-2 for 3 days were stained with PE-conjugated anti-LAG3 (solid line) or an isotype control (dashed line), followed by analyses by flow cytometry. (F) WT or DKO DCs were incubated with anti-I-Ab or control IgG for 6 h, followed by incubation with TMRE. The cells were analyzed by flow cytometry and the loss of TMRE+ cells were calculated. **P<0.01 (n=3).
We then investigated which effector molecules in Treg cells that could induce cell death in DCs. The killing of DCs by Teff cells involves Fas and perforin (22, 61, 62), but does not appear to require Bax and Bak in DCs (Supplemental Fig. 4B and C), suggesting that Treg and Teff cells use different mechanism to kill DCs. Interestingly, Treg cells in tumor tissues can kill DCs in a perforin/granzyme-dependent manner (41), while Treg cell-induced cell death in Teff cells is independent of Fas or perforin (63). We found that Treg-mediated killing of DCs did not involve Fas but required prior activation of Treg cells (Fig. 4A, Supplemental Fig. 4D). Also, Treg cells did not induce proteolytic activation of caspase-8 in DCs (Fig. 7B), consistent with the possibility that Treg cells do not engage Fas on DCs to activate caspase-8. In addition, deficiencies in perforin or granzymes A/B in Treg cells did not affect the killing of DCs (Supplemental Fig. 4E), suggesting that these molecules are not required for the killing of DCs by natural Treg cells.
We observed that blocking LFA-1 inhibited Treg-mediated killing of DCs (Fig. 7D). The conjugate formation between Treg cells and DCs was inhibited by blocking LFA-1 (Supplemental Fig. 4G, H), suggesting that cell adhesion promotes the killing of DCs by Treg cells. Molecules highly expressed by Treg cells have been suggested to be critical for Treg functions (11, 40, 64, 65). We observed that inhibition of LAG3 partially suppressed the killing of DCs by Treg cells (Fig. 7D), while neutralization antibodies to soluble factors, including IL-10 and TGF-β, have no effect (data not shown). LAG3 is a potent ligand for MHC II molecules and can trigger negative signaling in DCs to suppress maturation and immunostimulatory capacity of DCs (66). On un-stimulated Treg cells, the expression of LAG3 was low (Fig. 7E). Treg cells acquired cytotoxicity against DCs after activation (Fig. 4A), while LAG3 was also significantly up-regulated on activated Treg cells (Fig. 7E). Increased LAG3 on activated Treg cells may enable Treg cells to trigger cell death in DCs by engaging MHC-II. Indeed, we observed that engagement of MHC-II on DCs with an agonist antibody triggered the loss of ΔΨm in wild type, but not Bax−/−Bak−/− DCs (Fig. 7F). Engagement of MHC-II or other molecules on DCs by activated Treg cells may transmit signals into DCs through BH3-only molecules, such as Bim, to induce the activation of Bax and Bak, leading to mitochondrial disruption and cell death.
DISCUSSION
DCs harbor an active mitochondrion-dependent apoptosis pathway regulated by Bcl-2 family members (27–29). In this study, we determined the functions of mitochondrion-dependent cell death in DCs in immune regulation using mice with DC-specific knockout of Bax and Bak. Deficiencies of Bax and Bak in DCs resulted in DC expansion, spontaneous T cell activation and development of systemic autoimmunity. In addition to regulating spontaneous cell death by Bax and Bak, our data suggest another level of regulation of Treg-induced cell death in DCs through Bax and Bak. Expression of LAG3 may enable Treg cells to trigger mitochondrion-dependent cell death in DCs. Adoptive transfer experiments provide direct evidence to show that Bax- and Bak-deficient DCs have increased propensity for inducing the production of autoantibodies. Moreover, Treg cells inhibited wild type, but not Bax- and Bak-deficient DCs in the induction of autoantibodies after adoptive transfer. Our data suggest that Bax- and Bak-dependent pathway is involved in both spontaneous cell death and Treg-mediated killing of DCs, and both of these mechanisms are important for maintaining DC homeostasis and preventing autoimmunity.
Bax- and Bak-dependent spontaneous cell death in DCs may occur throughout the courses of immune responses. In contrast, only pre-activated Treg cells expressed higher levels of LAG3 (Fig. 7E) and showed the capacity to kill DCs (Fig. 4A), suggesting that Bax- and Bak-dependent killing of DCs may happen only after Treg cells are activated. We and others have previously demonstrated that DCs are susceptible to Fas-mediated apoptosis (22, 23). Fas-dependent killing of DCs may take place when activated T cells that express high levels of Fas ligand are present. This potentially provide a negative feedback mechanism for the suppression of DC-dependent activation of T cells at late stages of immune responses, possibly after significant T cell activation induced by DCs has taken place. It has been reported that Treg cells can kill autologous CD8+ T cells or LPS-induced monocytes through FasL/Fas interactions (67, 68). Other studies have shown that the suppressive effect of Treg cells is not inhibited by neutralization of FasL or using FasL-deficient Treg cells (69, 70). We also observed that the killing of DCs by Treg cells was independent of Fas (Supplemental Fig. 4). Such differences in the involvement of FasL/Fas interactions could be due to different target cells used in these studies.
Fas-mediated killing is usually dependent on activated T cells that express high levels of FasL. Spontaneous cell death in DCs through mitochondrion-dependent intrinsic apoptosis pathway is regulated by Bcl-2 family members (28, 71). Different from Fas-dependent cell death, Bax- and Bak-dependent spontaneous cell death in DCs may have a more broad influence at the initiation, expansion and contraction phases of immune responses. On the other hand, killing of DCs by activated Treg cells through Bax and Bak may only function at the contraction phase of immune responses.
Recognition of antigens by TCRs on Treg cells and LFA-1-dependent Treg-DC conjugate formation may be critical for Treg cells to kill DCs. Interestingly, activated Treg cells expressed elevated LAG3 and acquired killing activities towards DCs, and blocking of LAG3 partially inhibited the killing of DCs by Treg cells (Fig. 7). It has been shown that LAG3 can trigger negative signaling to inhibit the maturation and immunostimulatory capacity of DCs (66). Interestingly, crosslinking of MHC-II triggered the loss of ΔΨm in DCs in a Bax- and Bak-dependent manner (Fig. 7H). Our data suggest that LAG3 on activated Treg cells can engage MHC-II on DCs to induce mitochondrial disruption and cell death signaling in DCs.
Certain DC subsets can induce the generation of Treg cells in vitro and in vivo (20, 72–77). On the other hand, Treg cells also restrict the development of DCs in vivo (10, 54). Our data suggest that Treg cells can restrict DC expansion through Bax- and Bak-dependent killing, and deficiency in the killing of DCs by Treg cells contributes to DC accumulation and induction of autoimmunity in DC-Bax−/−Bak−/− mice. Therefore, dynamic interplays between DCs and Treg cells are important for the maintenance of a balanced immune system. Induction of mitochondrion-dependent cell death in DCs by Treg cells may serve as one important mechanism for immune regulation. Promoting the interactions between Treg cells and DCs may provide effective avenues to prevent and treat autoimmune diseases, whereas inhibiting the killing of DCs by Treg cells may help to boost immune responses to infections and cancer.
Supplementary Material
Acknowledgments
We thank Yaming Liang, Yiqing Zhang, Jie Huang and Shanshan Bai for technical assistance, David Corry and John Rodgers for discussions. M.C. and J.W. designed and performed experiments, and wrote the manuscript. K.F. assisted with histochemistry and performed confocal microscopy analyses.
This work was supported by National Institute of Health Grants R01AI074949 and R01 GM087710 (to J.W.), and R01DK083164 (to M.C.).
Abbreviations used
- 7-AAD
7-amino-actinomycin D
- ANA
antinuclear antibodies
- BMDCs
bone marrow-derived DCs
- CMTMR
5-(and -6)-(((4-chloromethyl) benzoyl) amino) tetramethylrhodamine
- TMRE
etramethylrhodamine ethyl ester
- Teff
T effector
- Treg
T regulatory
- ΔΨm
mitochondrial membrane potential
References
- 1.von Boehmer H, Aifantis I, Gounari F, Azogui O, et al. Thymic selection revisited: how essential is it? Immunol Rev. 2003;191:62–78. doi: 10.1034/j.1600-065x.2003.00010.x. [DOI] [PubMed] [Google Scholar]
- 2.Marrack P, Kappler J. Positive selection of thymocytes bearing alpha beta T cell receptors. Curr Opin Immunol. 1997;9:250–255. doi: 10.1016/s0952-7915(97)80144-6. [DOI] [PubMed] [Google Scholar]
- 3.Nossal GJ. Negative selection of lymphocytes. Cell. 1994;76:229–239. doi: 10.1016/0092-8674(94)90331-x. [DOI] [PubMed] [Google Scholar]
- 4.Mathis D, Benoist C. Aire. Annu Rev Immunol. 2009;27:287–312. doi: 10.1146/annurev.immunol.25.022106.141532. [DOI] [PubMed] [Google Scholar]
- 5.Rathmell JC, Thompson CB. Pathways of apoptosis in lymphocyte development, homeostasis, and disease. Cell. 2002;109(Suppl):S97–107. doi: 10.1016/s0092-8674(02)00704-3. [DOI] [PubMed] [Google Scholar]
- 6.Lenardo M, Chan KM, Hornung F, McFarland H, et al. Mature T lymphocyte apoptosis--immune regulation in a dynamic and unpredictable antigenic environment. Annu Rev Immunol. 1999;17:221–253. doi: 10.1146/annurev.immunol.17.1.221. [DOI] [PubMed] [Google Scholar]
- 7.Nagata S, Suda T. Fas and Fas ligand: lpr and gld mutations. Immunol Today. 1995;16:39–43. doi: 10.1016/0167-5699(95)80069-7. [DOI] [PubMed] [Google Scholar]
- 8.Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, et al. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 9.Wing K, Sakaguchi S. Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nat Immunol. 2010;11:7–13. doi: 10.1038/ni.1818. [DOI] [PubMed] [Google Scholar]
- 10.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 11.Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30:636–645. doi: 10.1016/j.immuni.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 12.Tang Q, Bluestone JA. The Foxp3+ regulatory T cell: a jack of all trades, master of regulation. Nat Immunol. 2008;9:239–244. doi: 10.1038/ni1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwartz RH. Natural regulatory T cells and self-tolerance. Nat Immunol. 2005;6:327–330. doi: 10.1038/ni1184. [DOI] [PubMed] [Google Scholar]
- 14.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 15.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 16.Liu YJ. Dendritic cell subsets and lineages, and their functions in innate and adaptive immunity. Cell. 2001;106:259–262. doi: 10.1016/s0092-8674(01)00456-1. [DOI] [PubMed] [Google Scholar]
- 17.Lanzavecchia A, Sallusto F. Regulation of T cell immunity by dendritic cells. Cell. 2001;106:263–266. doi: 10.1016/s0092-8674(01)00455-x. [DOI] [PubMed] [Google Scholar]
- 18.Pulendran B. Variegation of the immune response with dendritic cells and pathogen recognition receptors. J Immunol. 2005;174:2457–2465. doi: 10.4049/jimmunol.174.5.2457. [DOI] [PubMed] [Google Scholar]
- 19.Trombetta ES, Mellman I. Cell biology of antigen processing in vitro and in vivo. Annu Rev Immunol. 2005;23:975–1028. doi: 10.1146/annurev.immunol.22.012703.104538. [DOI] [PubMed] [Google Scholar]
- 20.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 21.Banchereau J, Pascual V, Palucka AK. Autoimmunity through cytokine-induced dendritic cell activation. Immunity. 2004;20:539–550. doi: 10.1016/s1074-7613(04)00108-6. [DOI] [PubMed] [Google Scholar]
- 22.Chen M, Wang YH, Wang Y, Huang L, et al. Dendritic cell apoptosis in the maintenance of immune tolerance. Science. 2006;311:1160–1164. doi: 10.1126/science.1122545. [DOI] [PubMed] [Google Scholar]
- 23.Stranges PB, Watson J, Cooper CJ, Choisy-Rossi CM, et al. Elimination of antigen-presenting cells and autoreactive T cells by Fas contributes to prevention of autoimmunity. Immunity. 2007;26:629–641. doi: 10.1016/j.immuni.2007.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ingulli E, Mondino A, Khoruts A, Jenkins MK. In vivo detection of dendritic cell antigen presentation to CD4(+) T cells. J Exp Med. 1997;185:2133–2141. doi: 10.1084/jem.185.12.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen M, Wang J. Programmed cell death of dendritic cells in immune regulation. Immunol Rev. 2010;236:11–27. doi: 10.1111/j.1600-065X.2010.00916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. II. Functional properties in vitro. J Exp Med. 1974;139:380–397. doi: 10.1084/jem.139.2.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hou WS, Van Parijs L. A Bcl-2-dependent molecular timer regulates the lifespan and immunogenicity of dendritic cells. Nat Immunol. 2004;5:583–589. doi: 10.1038/ni1071. [DOI] [PubMed] [Google Scholar]
- 28.Nopora A, Brocker T. Bcl-2 controls dendritic cell longevity in vivo. J Immunol. 2002;169:3006–3014. doi: 10.4049/jimmunol.169.6.3006. [DOI] [PubMed] [Google Scholar]
- 29.Chen M, Huang L, Wang J. Deficiency of Bim in dendritic cells contributes to overactivation of lymphocytes and autoimmunity. Blood. 2007;109:4360–4367. doi: 10.1182/blood-2006-11-056424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adams JM, Huang DC, Puthalakath H, Bouillet P, et al. Control of apoptosis in hematopoietic cells by the Bcl-2 family of proteins. Cold Spring Harb Symp Quant Biol. 1999;64:351–358. doi: 10.1101/sqb.1999.64.351. [DOI] [PubMed] [Google Scholar]
- 31.Gross A, McDonnell JM, Korsmeyer SJ. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999;13:1899–1911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- 32.Strasser A. The role of BH3-only proteins in the immune system. Nat Rev Immunol. 2005;5:189–200. doi: 10.1038/nri1568. [DOI] [PubMed] [Google Scholar]
- 33.Willis SN, Adams JM. Life in the balance: how BH3-only proteins induce apoptosis. Curr Opin Cell Biol. 2005;17:617–625. doi: 10.1016/j.ceb.2005.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lindsten T, Ross AJ, King A, Zong WX, et al. The combined functions of proapoptotic Bcl-2 family members bak and bax are essential for normal development of multiple tissues. Mol Cell. 2000;6:1389–1399. doi: 10.1016/s1097-2765(00)00136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rathmell JC, Lindsten T, Zong WX, Cinalli RM, Thompson CB. Deficiency in Bak and Bax perturbs thymic selection and lymphoid homeostasis. Nat Immunol. 2002;3:932–939. doi: 10.1038/ni834. [DOI] [PubMed] [Google Scholar]
- 36.Oldenhove G, de Heusch M, Urbain-Vansanten G, Urbain J, et al. CD4+ CD25+ regulatory T cells control T helper cell type 1 responses to foreign antigens induced by mature dendritic cells in vivo. J Exp Med. 2003;198:259–266. doi: 10.1084/jem.20030654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tang Q, Adams JY, Tooley AJ, Bi M, et al. Visualizing regulatory T cell control of autoimmune responses in nonobese diabetic mice. Nat Immunol. 2006;7:83–92. doi: 10.1038/ni1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tadokoro CE, Shakhar G, Shen S, Ding Y, et al. Regulatory T cells inhibit stable contacts between CD4+ T cells and dendritic cells in vivo. J Exp Med. 2006;203:505–511. doi: 10.1084/jem.20050783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.DiPaolo RJ, Brinster C, Davidson TS, Andersson J, Glass D, Shevach EM. Autoantigen-specific TGFbeta-induced Foxp3+ regulatory T cells prevent autoimmunity by inhibiting dendritic cells from activating autoreactive T cells. J Immunol. 2007;179:4685–4693. doi: 10.4049/jimmunol.179.7.4685. [DOI] [PubMed] [Google Scholar]
- 40.Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 41.Boissonnas A, Scholer-Dahirel A, Simon-Blancal V, Pace L, et al. Foxp3+ T cells induce perforin-dependent dendritic cell death in tumor-draining lymph nodes. Immunity. 2010;32:266–278. doi: 10.1016/j.immuni.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 42.Tran DQ, Glass DD, Uzel G, Darnell DA, et al. Analysis of adhesion molecules, target cells, and role of IL-2 in human FOXP3+ regulatory T cell suppressor function. J Immunol. 2009;182:2929–2938. doi: 10.4049/jimmunol.0803827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Misra N, Bayry J, Lacroix-Desmazes S, Kazatchkine MD, Kaveri SV. Cutting edge: human CD4+CD25+ T cells restrain the maturation and antigen-presenting function of dendritic cells. J Immunol. 2004;172:4676–4680. doi: 10.4049/jimmunol.172.8.4676. [DOI] [PubMed] [Google Scholar]
- 44.Houot R, Perrot I, Garcia E, Durand I, Lebecque S. Human CD4+CD25high regulatory T cells modulate myeloid but not plasmacytoid dendritic cells activation. J Immunol. 2006;176:5293–5298. doi: 10.4049/jimmunol.176.9.5293. [DOI] [PubMed] [Google Scholar]
- 45.Bayry J, Triebel F, Kaveri SV, Tough DF. Human dendritic cells acquire a semimature phenotype and lymph node homing potential through interaction with CD4+CD25+ regulatory T cells. J Immunol. 2007;178:4184–4193. doi: 10.4049/jimmunol.178.7.4184. [DOI] [PubMed] [Google Scholar]
- 46.Oderup C, Cederbom L, Makowska A, Cilio CM, Ivars F. Cytotoxic T lymphocyte antigen-4-dependent down-modulation of costimulatory molecules on dendritic cells in CD4+ CD25+ regulatory T-cell-mediated suppression. Immunology. 2006;118:240–249. doi: 10.1111/j.1365-2567.2006.02362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Veldhoen M, Moncrieffe H, Hocking RJ, Atkins CJ, Stockinger B. Modulation of dendritic cell function by naive and regulatory CD4+ T cells. J Immunol. 2006;176:6202–6210. doi: 10.4049/jimmunol.176.10.6202. [DOI] [PubMed] [Google Scholar]
- 48.Hanig J, Lutz MB. Suppression of mature dendritic cell function by regulatory T cells in vivo is abrogated by CD40 licensing. J Immunol. 2008;180:1405–1413. doi: 10.4049/jimmunol.180.3.1405. [DOI] [PubMed] [Google Scholar]
- 49.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 50.Grossman WJ, Verbsky JW, Barchet W, Colonna M, Atkinson JP, Ley TJ. Human T regulatory cells can use the perforin pathway to cause autologous target cell death. Immunity. 2004;21:589–601. doi: 10.1016/j.immuni.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 51.Hanada T, Yoshida H, Kato S, Tanaka K, et al. Suppressor of cytokine signaling-1 is essential for suppressing dendritic cell activation and systemic autoimmunity. Immunity. 2003;19:437–450. doi: 10.1016/s1074-7613(03)00240-1. [DOI] [PubMed] [Google Scholar]
- 52.Kamath AT, Henri S, Battye F, Tough DF, Shortman K. Developmental kinetics and lifespan of dendritic cells in mouse lymphoid organs. Blood. 2002;100:1734–1741. [PubMed] [Google Scholar]
- 53.Choi BK, Kim YH, Kwon PM, Lee SC, et al. 4-1BB functions as a survival factor in dendritic cells. J Immunol. 2009;182:4107–4115. doi: 10.4049/jimmunol.0800459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu K, Victora GD, Schwickert TA, Guermonprez P, et al. In vivo analysis of dendritic cell development and homeostasis. Science. 2009;324:392–397. doi: 10.1126/science.1170540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kushwah R, Oliver JR, Zhang J, Siminovitch KA, Hu J. Apoptotic dendritic cells induce tolerance in mice through suppression of dendritic cell maturation and induction of antigen-specific regulatory T cells. J Immunol. 2009;183:7104–7118. doi: 10.4049/jimmunol.0900824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Roskrow MA, Dilloo D, Suzuki N, Zhong W, Rooney CM, Brenner MK. Autoimmune disease induced by dendritic cell immunization against leukemia. Leuk Res. 1999;23:549–557. doi: 10.1016/s0145-2126(99)00045-4. [DOI] [PubMed] [Google Scholar]
- 57.Li H, Zhu H, Xu CJ, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- 58.Cheng EH, Kirsch DG, Clem RJ, Ravi R, et al. Conversion of Bcl-2 to a Bax-like death effector by caspases. Science. 1997;278:1966–1968. doi: 10.1126/science.278.5345.1966. [DOI] [PubMed] [Google Scholar]
- 59.Puthalakath H, Huang DC, O’Reilly LA, King SM, Strasser A. The proapoptotic activity of the Bcl-2 family member Bim is regulated by interaction with the dynein motor complex. Mol Cell. 1999;3:287–296. doi: 10.1016/s1097-2765(00)80456-6. [DOI] [PubMed] [Google Scholar]
- 60.Zhu Y, Swanson BJ, Wang M, Hildeman DA, et al. Constitutive association of the proapoptotic protein Bim with Bcl-2-related proteins on mitochondria in T cells. Proc Natl Acad Sci U S A. 2004;101:7681–7686. doi: 10.1073/pnas.0402293101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Matsue H, Edelbaum D, Hartmann AC, Morita A, et al. Dendritic cells undergo rapid apoptosis in vitro during antigen-specific interaction with CD4+ T cells. J Immunol. 1999;162:5287–5298. [PubMed] [Google Scholar]
- 62.Yang J, Huck SP, McHugh RS, Hermans IF, Ronchese F. Perforin-dependent elimination of dendritic cells regulates the expansion of antigen-specific CD8+ T cells in vivo. Proc Natl Acad Sci U S A. 2006;103:147–152. doi: 10.1073/pnas.0509054103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pandiyan P, Zheng L, Ishihara S, Reed J, Lenardo MJ. CD4+CD25+Foxp3+ regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4+ T cells. Nat Immunol. 2007;8:1353–1362. doi: 10.1038/ni1536. [DOI] [PubMed] [Google Scholar]
- 64.Josefowicz SZ, Rudensky A. Control of regulatory T cell lineage commitment and maintenance. Immunity. 2009;30:616–625. doi: 10.1016/j.immuni.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8:523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liang B, Workman C, Lee J, Chew C, et al. Regulatory T cells inhibit dendritic cells by lymphocyte activation gene-3 engagement of MHC class II. J Immunol. 2008;180:5916–5926. doi: 10.4049/jimmunol.180.9.5916. [DOI] [PubMed] [Google Scholar]
- 67.Venet F, Pachot A, Debard AL, Bohe J, et al. Human CD4+CD25+ regulatory T lymphocytes inhibit lipopolysaccharide-induced monocyte survival through a Fas/Fas ligand-dependent mechanism. J Immunol. 2006;177:6540–6547. doi: 10.4049/jimmunol.177.9.6540. [DOI] [PubMed] [Google Scholar]
- 68.Strauss L, Bergmann C, Whiteside TL. Human circulating CD4+CD25highFoxp3+ regulatory T cells kill autologous CD8+ but not CD4+ responder cells by Fas-mediated apoptosis. J Immunol. 2009;182:1469–1480. doi: 10.4049/jimmunol.182.3.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Takahashi T, Kuniyasu Y, Toda M, Sakaguchi N, et al. Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int Immunol. 1998;10:1969–1980. doi: 10.1093/intimm/10.12.1969. [DOI] [PubMed] [Google Scholar]
- 70.Mohamood AS, Trujillo CJ, Zheng D, Jie C, et al. Gld mutation of Fas ligand increases the frequency and up-regulates cell survival genes in CD25+CD4+ TR cells. Int Immunol. 2006;18:1265–1277. doi: 10.1093/intimm/dxl057. [DOI] [PubMed] [Google Scholar]
- 71.Hon H, Rucker EB, 3rd, Hennighausen L, Jacob J. bcl-xL is critical for dendritic cell survival in vivo. J Immunol. 2004;173:4425–4432. doi: 10.4049/jimmunol.173.7.4425. [DOI] [PubMed] [Google Scholar]
- 72.Wakkach A, Fournier N, Brun V, Breittmayer JP, Cottrez F, Groux H. Characterization of dendritic cells that induce tolerance and T regulatory 1 cell differentiation in vivo. Immunity. 2003;18:605–617. doi: 10.1016/s1074-7613(03)00113-4. [DOI] [PubMed] [Google Scholar]
- 73.Sato K, Yamashita N, Baba M, Matsuyama T. Modified myeloid dendritic cells act as regulatory dendritic cells to induce anergic and regulatory T cells. Blood. 2003;101:3581–3589. doi: 10.1182/blood-2002-09-2712. [DOI] [PubMed] [Google Scholar]
- 74.Watanabe N, Wang YH, Lee HK, Ito T, Cao W, Liu YJ. Hassall’s corpuscles instruct dendritic cells to induce CD4+CD25+ regulatory T cells in human thymus. Nature. 2005;436:1181–1185. doi: 10.1038/nature03886. [DOI] [PubMed] [Google Scholar]
- 75.Yamazaki S, Dudziak D, Heidkamp GF, Fiorese C, et al. CD8+ CD205+ splenic dendritic cells are specialized to induce Foxp3+ regulatory T cells. J Immunol. 2008;181:6923–6933. doi: 10.4049/jimmunol.181.10.6923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Travis MA, Reizis B, Melton AC, Masteller E, et al. Loss of integrin alpha(v)beta8 on dendritic cells causes autoimmunity and colitis in mice. Nature. 2007;449:361–365. doi: 10.1038/nature06110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sharma MD, Baban B, Chandler P, Hou DY, et al. Plasmacytoid dendritic cells from mouse tumor-draining lymph nodes directly activate mature Tregs via indoleamine 2,3-dioxygenase. J Clin Invest. 2007;117:2570–2582. doi: 10.1172/JCI31911. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.