Abstract
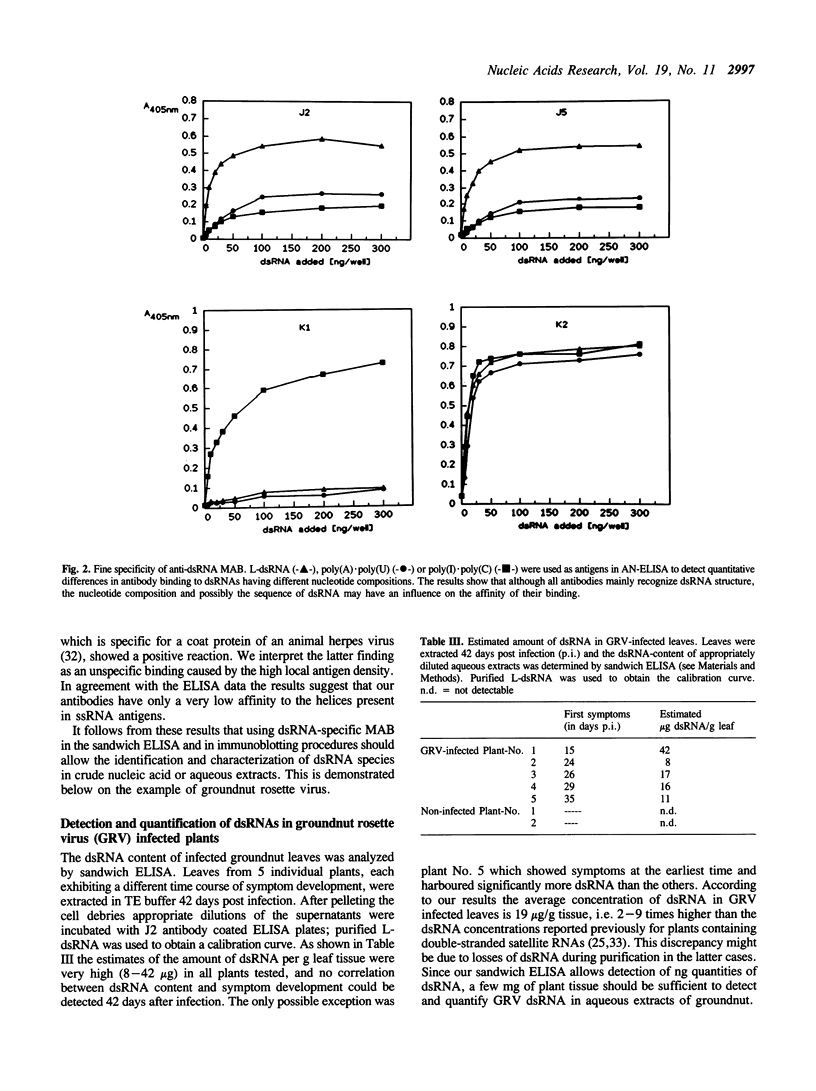
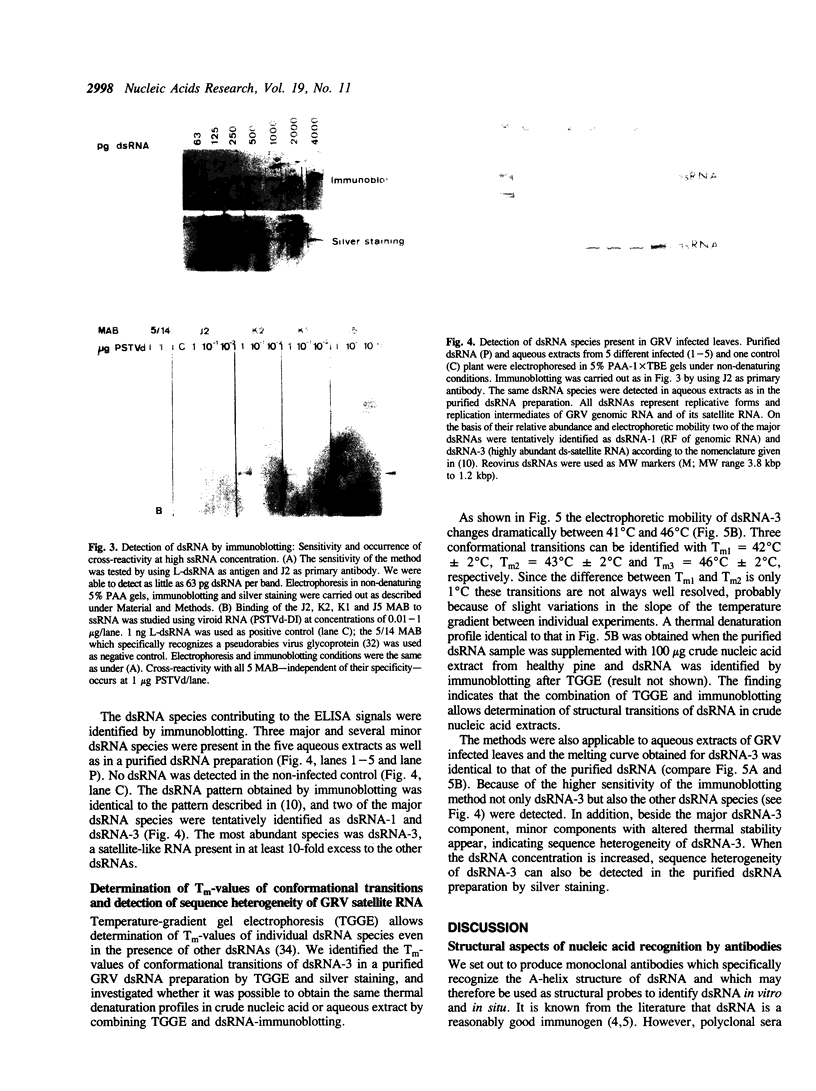
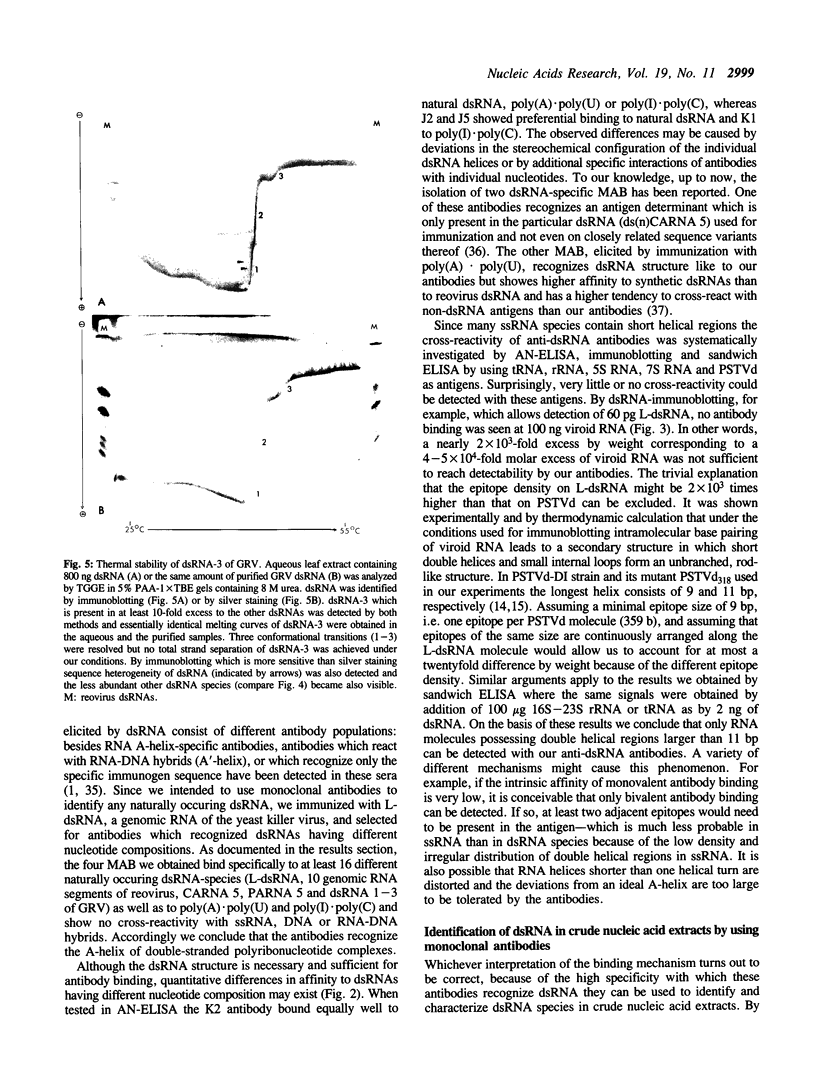
We describe four monoclonal antibodies (MAB) which specifically recognize double-stranded RNA (dsRNA) together with their use in new methods for detecting and characterizing dsRNA in unfractionated nucleic acid extracts. The specificity of the antibodies was analyzed using a panel of 27 different synthetic and naturally occurring nucleic acids. All four antibodies reacted in a highly specific manner with long dsRNA helices, irrespective of their sequence; no binding to single-stranded RNA homopolymers or to DNA or RNA-DNA hybrids was observed. The apparent affinity of the antibodies to short (less than or equal to 11 bp) RNA helices was very low in all test systems used: only background levels of binding were obtained on single-stranded RNA species which contain double-helical secondary structures (e.g. rRNA, tRNA, viroid RNA). A sandwich ELISA and a dsRNA-immunoblotting procedure have been established which allow detection and characterization of dsRNA by MAB even in the presence of a large excess of other nucleic acids. In combination with temperature-gradient gelelectrophoresis (TGGE) not only the molecular weights but also the highly characteristic Tm-values of conformational transitions of individual dsRNA species could be determined by immunoblotting. An example of the general use of these methods for the detection of plant virus infections is demonstrated with groundnut rosette virus (GRV) dsRNAs. We were able to estimate the dsRNA content of infected leaves, identify the dsRNA species present in crude extracts and to determine the Tm- values of GRV dsRNA-3.
Full text
PDF

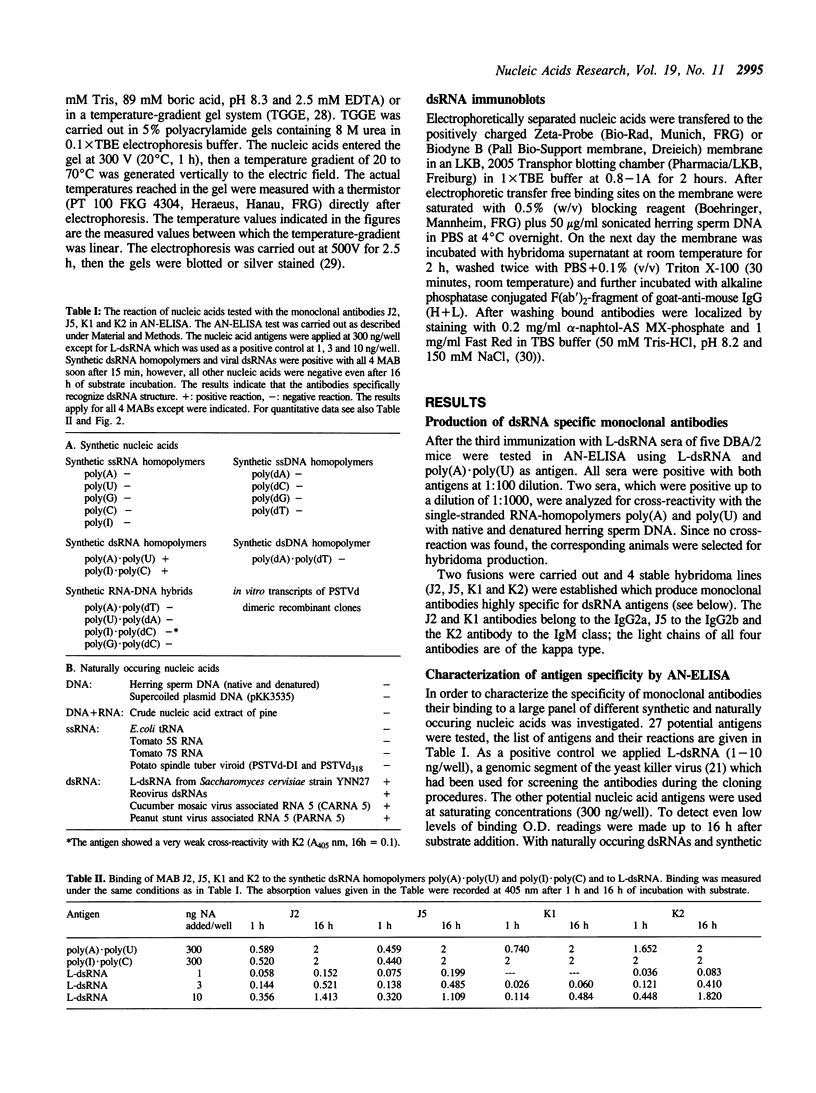
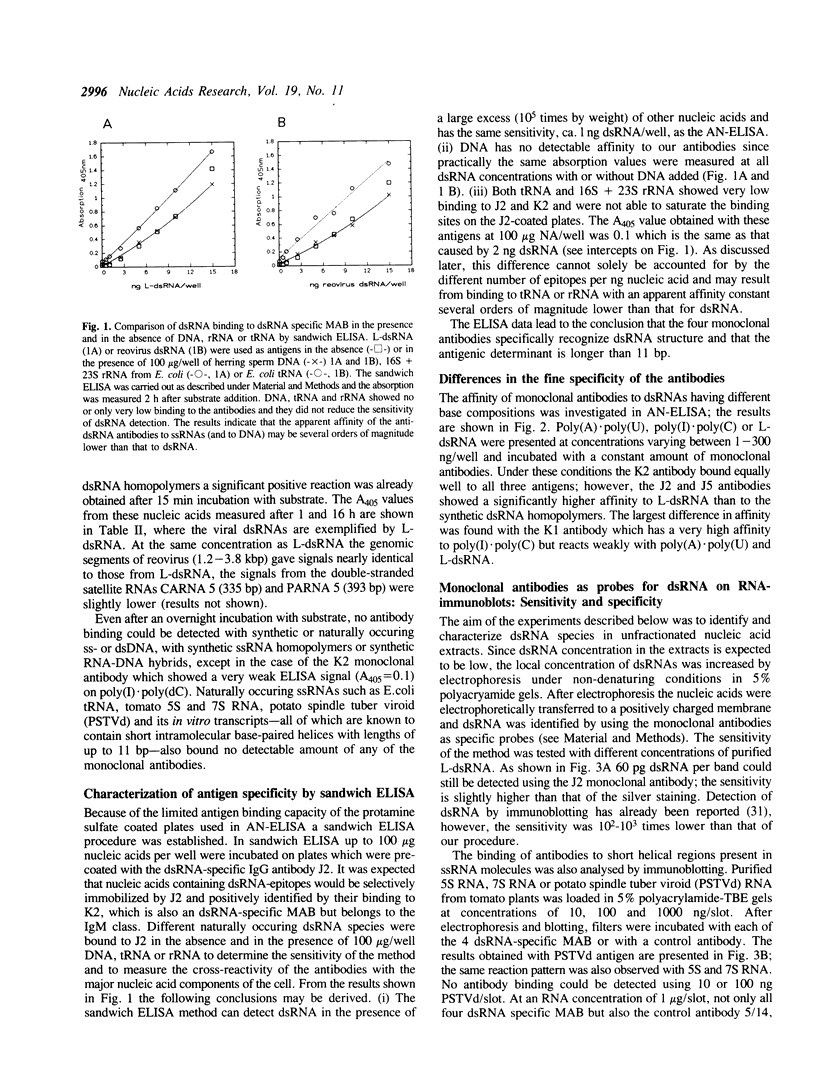

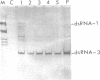
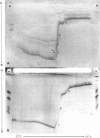
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson W. F., Cygler M., Braun R. P., Lee J. S. Antibodies to DNA. Bioessays. 1988 Feb-Mar;8(2):69–74. doi: 10.1002/bies.950080206. [DOI] [PubMed] [Google Scholar]
- Brosius J., Dull T. J., Sleeter D. D., Noller H. F. Gene organization and primary structure of a ribosomal RNA operon from Escherichia coli. J Mol Biol. 1981 May 15;148(2):107–127. doi: 10.1016/0022-2836(81)90508-8. [DOI] [PubMed] [Google Scholar]
- Cashdollar L. W., Chmelo R., Esparza J., Hudson G. R., Joklik W. K. Molecular cloning of the complete genome of reovirus serotype 3. Virology. 1984 Feb;133(1):191–196. doi: 10.1016/0042-6822(84)90438-0. [DOI] [PubMed] [Google Scholar]
- Delage G., Nahon E., Huynh T., Jeusset J., Lacour F. A monoclonal antibody to the double-stranded polyribonucleotide complex poly(A) X poly(U). Mol Immunol. 1984 Oct;21(10):939–944. doi: 10.1016/0161-5890(84)90150-0. [DOI] [PubMed] [Google Scholar]
- Diaz-Ruiz J. R., Kaper J. M. Cucumber mosaic virus-associated RNA 5. III. Little nucleotide sequence homology between CARNA 5 and helper RNA. Virology. 1977 Jul 1;80(1):204–213. doi: 10.1016/0042-6822(77)90393-2. [DOI] [PubMed] [Google Scholar]
- Gabriel C. J. Detection of double-stranded RNA by immunoblot electrophoresis. J Virol Methods. 1986 Jul;13(4):279–283. doi: 10.1016/0166-0934(86)90052-2. [DOI] [PubMed] [Google Scholar]
- Garcia-Luque I., Brieva A., Diaz-Ruiz J. R., Rubio N. Isolation and partial characterization of a monoclonal antibody specific for a naturally occurring double-stranded RNA. Virology. 1986 Jul 15;152(1):252–255. doi: 10.1016/0042-6822(86)90389-2. [DOI] [PubMed] [Google Scholar]
- Gould A. R., Francki R. I. Immunochemical detection of ds-RNA in healthy and virus-infected plants and specific detection of viral ds-RNA by hybridization to labelled complementary DNA. J Virol Methods. 1981 Apr;2(5):277–286. doi: 10.1016/0166-0934(81)90026-4. [DOI] [PubMed] [Google Scholar]
- Hecker R., Wang Z. M., Steger G., Riesner D. Analysis of RNA structures by temperature-gradient gel electrophoresis: viroid replication and processing. Gene. 1988 Dec 10;72(1-2):59–74. doi: 10.1016/0378-1119(88)90128-x. [DOI] [PubMed] [Google Scholar]
- Kaper J. M., Tousignant M. E. Cucumber mosaic virus-associated RNA 5. V. Extensive nucleotide sequence homology among CARNA 5 preparations of different CMV strains. Virology. 1978 Mar;85(1):323–327. doi: 10.1016/0042-6822(78)90438-5. [DOI] [PubMed] [Google Scholar]
- Kaper J. M., Tousignant M. E., Diaz-Ruiz J. R., Tolin S. A. Peanut stunt virus-associated RNA 5: second tripartite genome virus with an associated satellite-like replicating RNA. Virology. 1978 Jul 1;88(1):166–170. doi: 10.1016/0042-6822(78)90119-8. [DOI] [PubMed] [Google Scholar]
- Kitagawa Y., Okuhara E. The separation of three antibody populations from anti-poly(A).poly(U) antibodies elicited in mice or rabbits and antigenic features of poly(A).poly(U)). Mol Immunol. 1982 Feb;19(2):257–266. doi: 10.1016/0161-5890(82)90339-x. [DOI] [PubMed] [Google Scholar]
- Klotz J. L., Minami R. M., Teplitz R. L. An enzyme-linked immunosorbent assay for antibodies to native and denatured DNA. J Immunol Methods. 1979;29(2):155–165. doi: 10.1016/0022-1759(79)90065-6. [DOI] [PubMed] [Google Scholar]
- Lacour F., Nahon-Merlin E., Michelson M. Immunological recognition of polynucleotide structure. Curr Top Microbiol Immunol. 1973;62:1–39. doi: 10.1007/978-3-642-65772-6_1. [DOI] [PubMed] [Google Scholar]
- Lukàcs N., Thiel H. J., Mettenleiter T. C., Rziha H. J. Demonstration of three major species of pseudorabies virus glycoproteins and identification of a disulfide-linked glycoprotein complex. J Virol. 1985 Jan;53(1):166–173. doi: 10.1128/jvi.53.1.166-173.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver S. G., McCREADY S. J., Holm C., Sutherland P. A., McLaughlin C. S., Cox B. S. Biochemical and physiological studies of the yeast virus-like particle. J Bacteriol. 1977 Jun;130(3):1303–1309. doi: 10.1128/jb.130.3.1303-1309.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Po T., Steger G., Rosenbaum V., Kaper J., Riesner D. Double-stranded cucumovirus associated RNA 5: experimental analysis of necrogenic and non-necrogenic variants by temperature-gradient gel electrophoresis. Nucleic Acids Res. 1987 Jul 10;15(13):5069–5083. doi: 10.1093/nar/15.13.5069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riesner D., Gross H. J. Viroids. Annu Rev Biochem. 1985;54:531–564. doi: 10.1146/annurev.bi.54.070185.002531. [DOI] [PubMed] [Google Scholar]
- Riesner D., Steger G., Zimmat R., Owens R. A., Wagenhöfer M., Hillen W., Vollbach S., Henco K. Temperature-gradient gel electrophoresis of nucleic acids: analysis of conformational transitions, sequence variations, and protein-nucleic acid interactions. Electrophoresis. 1989 May-Jun;10(5-6):377–389. doi: 10.1002/elps.1150100516. [DOI] [PubMed] [Google Scholar]
- Rosenbaum V., Riesner D. Temperature-gradient gel electrophoresis. Thermodynamic analysis of nucleic acids and proteins in purified form and in cellular extracts. Biophys Chem. 1987 May 9;26(2-3):235–246. doi: 10.1016/0301-4622(87)80026-1. [DOI] [PubMed] [Google Scholar]
- Schumacher J., Randles J. W., Riesner D. A two-dimensional electrophoretic technique for the detection of circular viroids and virusoids. Anal Biochem. 1983 Dec;135(2):288–295. doi: 10.1016/0003-2697(83)90685-1. [DOI] [PubMed] [Google Scholar]
- Schwartz E. F., Stollar B. D. Antibodies to polyadenylate-polyuridylate copolymers as reagents for double strand RNA and DNA-RNA hybrid complexes. Biochem Biophys Res Commun. 1969 Apr 10;35(1):115–120. doi: 10.1016/0006-291x(69)90490-2. [DOI] [PubMed] [Google Scholar]
- Stollar B. D. Antibodies to DNA. CRC Crit Rev Biochem. 1986;20(1):1–36. doi: 10.3109/10409238609115899. [DOI] [PubMed] [Google Scholar]
- Stollar B. D., Koo R., Stollar V. Immunofluorescent detection of nuclear double-stranded RNA in situ in Vero and mosquito cells. Science. 1978 Jun 23;200(4348):1381–1383. doi: 10.1126/science.26972. [DOI] [PubMed] [Google Scholar]
- Stollar B. D. The specificity and applications of antibodies to helical nucleic acids. CRC Crit Rev Biochem. 1975 May;3(1):45–69. doi: 10.3109/10409237509102552. [DOI] [PubMed] [Google Scholar]