Abstract
Four flavone C-glycosides, isoorientin (1), orientin (2), vitexin (3), and isovitexin (4), were isolated from the neotropical blueberry of Anthopterus wardii, a so-called “superfruit”, using antioxidant activity-guided fractionation. A dose-response relationship of compounds 1–4 was determined for their anti-inflammatory activity against interleukin-8 (IL-8) and for the inhibition of matrix metalloproteinase-1 (MMP-1) expression, an inflammatory marker for chronic obstructive pulmonary disease. The four flavone C-glycosides exhibited inhibitory activity against IL-8 production and MMP-1 expression, with compounds 1, 3, and 4 having the most potent inhibitory activities in both assays at 100 μg/ml. The structures of compounds 1–4 were determined by spectroscopic methods. These flavone C-glycosides are reported for the first time in the Anthopterus genus.
Keywords: Anthopterus wardii, neotropical blueberry, flavone C-glycosides, orientin, ABTS, IL-8, MMP-1, COPD
1. Introduction
Chronic obstructive pulmonary disease (COPD), a major cause of death and disability, is expected to be the third leading cause of death worldwide by 2020 (Murray & Lopez, 1997). It is characterized by irreversible airflow obstruction due to chronic inflammation (Anonymous, 1995).
Acute exacerbations, common in COPD patients, increases in frequency with the severity of the disease, contributes to impaired health status, loss of lung function, and ultimately to disease progression (Donaldson, Seemungal, Bhowmik & Wedzicha, 2002; Donaldson, Seemungal, Patel, Lloyd-Owen, Wilkinson & Wedzicha, 2003). Currently, little progress has been made toward developing effective therapies for COPD and although treatments can improve symptoms, their effects are limited and they have not reduced disease progression (Calverley et al., 2007; Vestbo, Sorensen, Lange, Brix, Torre & Viskum, 1999).
Important processes in the pathogenesis of COPD include chronic airway inflammation, oxidative stress, and an imbalance of proteinases and antiproteinases. The inflammation and proteolysis in COPD is an amplification of the normal inflammatory response to cigarette smoke, which is the main etiological factor associated with the disease. (Boschetto, Quintavalle, Miotto, Lo Cascio, Zeni & Mapp, 2006; Lopez & Murray, 1998).
Matrix metalloproteinases (MMPs), a family of zinc endopeptidases, play a key role in tissue remodeling and repair and there is significant evidence that members of the MMP family may also play an important role in COPD pathology (Babusyte, Stravinskaite, Jeroch, Lotvall, Sakalauskas & Sitkauskiene, 2007; Haq et al., 2010; Omachi, Eisner, Rames, Markovtsova & Blanc, 2011).
D’Armiento et al. (1992) have demonstrated a direct role for MMPs in emphysema causation. In particular, it was demonstrated that the transgenic expression of human MMP-1 (interstitial collagenase) in the lung causes emphysema in mice, a process attributed to the digestion of type III collagen by this enzyme. They later demonstrated that MMP-1 is expressed in the lung of human patients with emphysema but not in normal control subjects (Imai et al., 2001). Therefore, it is anticipated that drugs that reduce pulmonary inflammation, inhibit MMP-1 expression and decrease oxidative stress in the lungs of patients with COPD will provide effective disease therapies. Diet may play a role in the prevention of COPD (Hirayama, Lee, Binns, Hiramatsu, Mori & Nishimura, 2010; Keranis et al., 2010; Janssens et al., 2010). The so-called Mediterranean diet, high in fruits, vegetables, fish, and whole grains, has been associated with a 50% reduction in the risk of COPD in US men (Varraso, Fung, Hu, Willett & Camargo, 2007). Epidemiological studies have shown that fruit and vegetable consumption is inversely related to incidence of a number of diseases, including cancer and COPD (Stan, Kar, Stoner & Singh, 2008). Based on these findings, some authors have hypothesized that the antioxidants present in fruits may protect lung tissue from oxidative damage caused by cigarette smoke and air pollution (Anderson, Theron & Ras, 1987; Walda et al., 2002). Thus, certain fruits have generated considerable interest recently as a rich source of phenolic antioxidants.
The focus of this study is to explore the therapeutic potential of the antioxidants of Anthopterus wardii Ball, a relative of the blueberries from the New World tropics (neotropics), in the treatment of COPD. Anthopterus wardii is a plant with dark-colored fruits belonging to the family Ericaceae, a large family with several economically important temperate species (e.g. blueberry and cranberry) and over 4,100 species (Luteyn & Pedraza-Peñalosa).
The activity of A. wardii was compared previously with that of four other neotropical blueberries and the temperate highbush blueberry (Dastmalchi, Flores, Petrova, Pedraza-Penalosa & Kennelly, 2011). Anthopterus wardii showed significantly higher antioxidant activities in all assays when compared to the highbush blueberry. For example in the DPPH assay, Anthopterus wardii is three times more active than the temperate highbush blueberry (Dastmalchi, Flores, Petrova, Pedraza-Penalosa & Kennelly, 2011). With highbush blueberry being referred to in the popular literature as a “superfruit”, Anthopterus wardii showed the potential to be even more highly promising edible fruit, on the basis of our findings.
In addition, although several of the berry-producing neotropical members have edible fruits and are used either as food or medicine in certain cultures, the phytochemistry of many of them has not been studied extensively.
In order to identify the components responsible for the antioxidant activity of the fruits of A. wardii activity-guided fractionation and purification processes were employed. The isolated compounds were tested for interleukin-8 (IL-8) and MMP-1 inhibitory activities in an in vitro model of COPD.
2. Materials and methods
2.1. General experimental procedures
1H NMR experiments were recorded with a Bruker Avance AV300 NMR in dimethyl sulfoxide-d6. Chemical shifts are expressed in δ (ppm) with reference to the solvent signals. A Waters (Milford, MA, USA) liquid chromatography system equipped with a 2695 Separation Module and a 2996 photodiode-array detector (DAD) coupled to the Waters Empower (version 5.0) for data acquisition and processing was used. Separation was carried out on a 250 × 4.6 mm, 4 μm Synergi Hydro-RP 80A column (Torrance, CA, USA). Mass spectrometry was performed on a Waters LCT Premiere XE Time of Flight (ToF) mass spectrometer (Waters MS Technologies, Manchester, UK). A Molecular Devices Versamax microplate reader (Sunnyvale, CA, USA) was used for the ABTS•+ scavenging assay. TLC analyses were carried out on RP-18 F254 plates (200–270 μm layer thickness, EMD Chemicals Inc., Gibbstown, NJ, USA), with compounds visualized under UV. Sephadex LH-20 (25–100 μm) (Pharmacia Fine Chemicals, Piscataway, NJ, USA) was used for column chromatography. Solvents for chromatography, HPLC-grade MeOH, formic acid and acetonitrile were obtained from J.T. Baker (Phillipsburg, NJ, USA). GR-grade MeOH, EtOAc, and n-BuOH were supplied by VWR Inc. (Bridgeport, PA, USA). Ultrapure water was prepared using a Millipore Milli-RO 12 plus system (Millipore Corp., Bedford, MA, USA). Trolox was purchased from Sigma Chemical-Aldrich (St. Louis, MO, USA). 2,2′-Azinobis(3-ethylbenzothiazoline-6-sulphonate) diammonium salt (ABTS) was obtained from TCI-Ace (Tokyo, Japan). Isoorientin (luteolin 6-C-glucoside), orientin (luteolin 8-C-glucoside), vitexin (apigenin 8-C-glucoside), and isovitexin (apigenin 6-C-glucoside) were purchased from Chromadex (Irvine, CA, USA).
2.2. Plant material
The fruits of A. wardii were collected in the Nolen Greenhouses of The New York Botanical Garden in July 2009 and identified by Dr. Paola Pedraza-Peñalosa.
2.3. Extraction
The freeze-dried pulp of fruits of A. wardii was extracted three times with MeOH/H2O (70:30) at room temperature with a blender for 5 min per extraction, and the combined extract was dried in vacuo. The extract was suspended in water and sequentially partitioned three times with EtOAc and n-BuOH. The combined EtOAc and n-BuOH partitions were dried in vacuo to give 1.32 g and 2.61 g of the extracts, respectively. Both extracts were tested with the ABTS assay.
2.4. Isolation and purification
The n-BuOH partition was fractionated with a Sephadex LH-20 column using MeOH (0.1 % formic acid) as eluent, and 38 fractions were collected. These fractions were combined into eight fractions on the basis of the RP-18 TLC (70:30 H2O, 5% formic acid/acetonitrile) analysis. All fractions were tested in the ABTS assay.
Fraction 8, with highest ABTS activity, was selected for further purification. HPLC was performed by using solvent A (0.1% aqueous formic acid solution) and B (0.1% aqueous formic acid solution in acetonitrile) at a constant flow of 1 ml/min to afford four pure compounds. Non-linear gradient elution was applied from the initial solvent composition A:B, 85:15 to A:B, 80:20 (v/v) in 30 min. The four peaks were collected with reference to an UV detector setting at 360 nm. The obtained fractions were evaporated under nitrogen, and the purity of the collected compounds was evaluated by HPLC analysis using the same conditions. The structure of the isolated compounds was identified using a combination of data from MS, NMR, and HPLC-PDA comparison (retention time and UV spectra) with authentic standards.
2.4.1. Isoorientin (1)
Yellowish amorphous powder. 1H NMR (DMSO- d6) ppm: 6.54 (1 H, s, H3), 6.47 (1 H, s, H8), 7.40 (1 H, d, J = 4.6 Hz, H2′), 6.63 (1 H, d, J = 8.4 Hz, H5•), 7.41 (1 H, dd, J = 8.0 Hz, 2.2 Hz, H6′), 4.57 (1 H, d, J = 9.8 Hz, H1″) ppm. MS-TOF: m/z 448.1. Data were consistent with previously published data (Krafczyk & Glomb, 2008).
2.4.2. Orientin (2)
Yellowish amorphous powder. 1H NMR (DMSO-d6) ppm: 6.49 (1 H, s, H3), 6.18 (1 H, s, H6), 7.41 (1 H, d, J = 7.6 Hz, H2′), 6.85 (1 H, d, J = 8.4 Hz, H5′), 7.42 (1 H, dd, J = 6.2 Hz, 2.2 Hz, H6′), 4.66 (1 H, d, J = 10.0 Hz, H1″). MS-TOF: m/z 448.1. Data were consistent with previously published data (Krafczyk et al., 2008).
2.4.3. Vitexin (3)
Yellowish amorphous powder. 1H NMR (DMSO-d6) ppm: 6.77 (1 H, s, H3), 6.28 (1 H, s, H6), 8.01 (2 H, d, J = 8.7 Hz, H2′, H6′), 6.92 (2 H, d, J = 8.7 Hz, H3′, H5′), 4.82 (1 H, d, J = 9.8 Hz, H1″). MS-TOF: m/z 432.1. Data were consistent with previously published data (Krafczyk et al., 2008).
2.4.4. Isovitexin (4)
Yellowish amorphous powder. 1H NMR (DMSO-d6) ppm: 6.77 (1 H, s, H3), 6.48 (1 H, s, H 8), 7.91 (2 H, d, J = 8.8 Hz, H2′, H6′), 6.86 (2 H, d, J = 8.8 Hz, H3′, H53), 4.57 (1 H, d, J = 9.9 Hz, H1″) ppm. MS-TOF: m/z 432.1. Data were consistent with previously published data (Krafczyk et al., 2008).
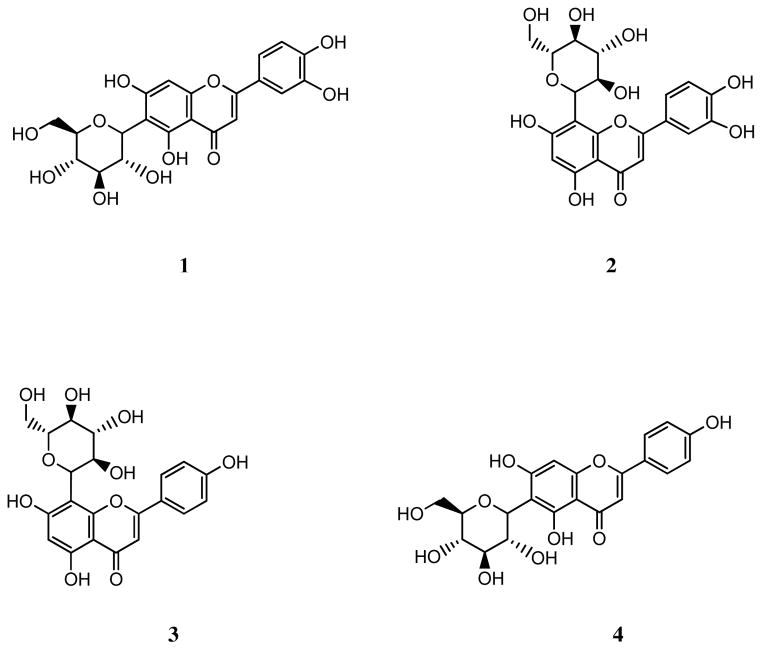
Fig. 2 shows the structures of compounds 1–4.
Fig. 2.
Chemical structures of flavone C-glycosides isolated from A. wardii. Isoorientin (1), orientin (2), vitexin (3), and isovitexin (4).
2.5. ABTS assay
The antioxidant activity the EtOAc and the n-BuOH partitions and of the fractions were measured by the ABTS•+ scavenging assay (Re, Pellegrini, Proteggente, Pannala, Yang & Rice-Evans, 1999). This assay is based on the formation of the free radical cation ABTS•+ by reacting of ABTS aqueous solution (7mM) with K2S2O8 (2.45 mM, final concentration) at ambient temperature in the dark for 12–16 h. Before use, this solution was diluted with ethanol to an absorbance of 0.700 ± 0.020 at 734 nm. In a final volume of 200 μl, the reaction mixture compromised 198 μl of ABTS•+ solution and 2 μl of the sample at different concentrations. Absorbances at 734 nm were measured at 5 min intervals during 40 min. Similarly, the reaction mixture of standard group was obtained by mixing 198 μl of ABTS•+ solution and 2 μl of Trolox. ABTS scavenging ability was expressed as the Trolox equivalent antioxidant capacity (TEAC, mmole Trolox/g of the sample) at different time intervals.
2.6. IL-8 Immunoassay
Human SAE cells were cultured according to supplier instructions (Lonza, Walkersville, MD, USA) and maintained in a controlled atmosphere of air-5% CO2 at 37°C. 80 % confluent SAE cells at passages 2 to 5 were used for experiments.
CSE was prepared using a modified protocol (Laurent, Janoff & Kagan, 1983). Briefly, a Barnet vacuum pump operating at constant flow was used to draw the smoke of one 3R4F research grade cigarette (University of Kentucky, Lexington, KY, USA) through 25 ml of Dulbecco’s phosphate-buffered saline. This solution (100% CSE) was adjusted to pH 7.4, filtered, diluted with small airway growth medium to a final concentration of 5%, and added to the cells immediately.
Cells were treated with 5% CSE or pure compounds or pretreated with pure compounds 1 h prior to 5% CSE exposure. Cell viability was accessed following CSE exposure using the alamarBlue kit (Invitrogen, Carlsbad, CA, USA) according to manufacturer’s specifications. After 24 h, measurement of human IL-8 in cell culture supernatants was performed by ELISA (R&D Systems Inc., Minneapolis, MN, USA).
2.7. MMP-1 mRNA expression
The cells were cultured as described above. After 24 h of treatment, total RNA from the human SAE cells was isolated (RNeasy kit, Qiagen, Valencia, CA, USA) and converted into cDNA (high capacity cDNA kit, Applied Biosystems, Carlsbad, CA, USA). Relative expression of MMP-1 was measured using real-time quantitative PCR and Taqman probes with GAPDH as an endogenous control (Applied Biosystems, Carlsbad, CA, USA).
2.8. Statistical analysis
ABTS data were expressed as the means of eight experiments ± standard deviations (SD). Each experimental set was compared with one-way analysis of variance (ANOVA) with significant differences between means determined by the Student’s t-test using JMP Statistics software package (SAS Institute Inc., NC).
3. Results and discussion
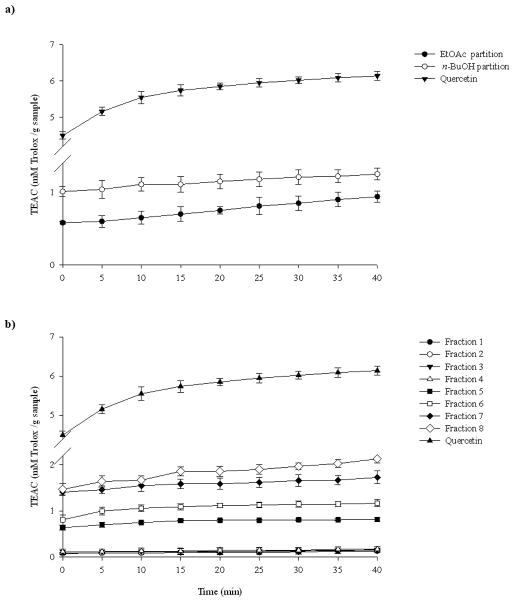
The ABTS antioxidant activity of the EtOAc and the n-BuOH partitions from A. wardii was determined (Fig. 3a), with quercetin as positive control. This method allows monitoring of the activity of the sample over a specified period of time, and thus samples having different rates of antioxidant activity can be distinguished. The scavenging activity for the EtOAc significantly increased up to 40 min (P < 0.05), but since the n-BuOH partition was slightly more effective it was separated by Sephadex LH-20 to afford 8 fractions. All these fractions were also evaluated for their ABTS•+ scavenging activity (Fig. 3b), and quercetin was used as positive control. All the fractions demonstrated a wide range of ABTS•+ scavenging activities. At 0 min the order of activity was fractions 8 and 7 (significantly not different P > 0.05) > fractions 6 and 5 (significantly not different P > 0.05) > fractions 4, 3, 2, and 1 (significantly not different P > 0.05). The order of activity changed up to 15 min which was fraction 8 > fraction 7 > fraction 6 > fraction 5 > fraction 4, 3, 2, and 1 (significantly not different P > 0.05). After 15 min of reaction the observed differences in activities among all the fractions was maintained through the remaining reaction time. In this assay, quercetin showed higher inhibitory activity than the partitions and the fractions.
Fig. 3.
ABTS.+ scavenging activity of A. wardii n-BuOH and EtOAc partition (a) and of A. wardii fractions 1–8. Quercetin was used as positive control. Values are expressed as means ± 95% confidence intervals (n=8) of Trolox equivalent antioxidant capacity (TEAC) (concentration of Trolox in mmol/l having the ABTS.+ scavenging activity equal to a 1.0 mg/ml sample solution).
Subsequently, the most potent in the ABTS assay, fraction 8, underwent HPLC purification to give four flavone C-glycosides (1–4) for the first time from Anthopterus species. The scheme of the extraction, fractionation, and isolation of these compounds is indicated in Fig.1.
Fig. 1.
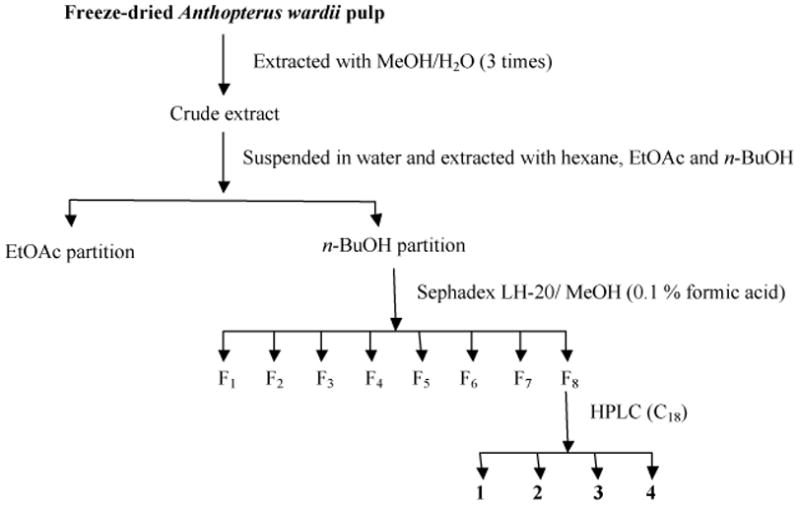
Activity-guided fractionation scheme for the isolation of compounds 1–4 from A. wardii. HPLC conditions of C18 are indicated in Section 2.
Other researchers have investigated the biological activities of flavone C-glycosides. For example, compound 4 has exhibited high antioxidant activity in macrophages RAW264.7 stimulated with lipopolysaccharide (LPS) (Lin et al., 2005). Compounds 1, 2, and 3 have shown inhibitory effects on low-density lipoprotein (LDL) and displayed about 50% of scavenging activity against hydroxyl radicals at concentrations below 10 μM (Kim, Jun, Jeong & Chung, 2005). Snijman et al. (2009) have evaluated the antioxidant activities of these flavone C-glycosides using ABTS•+ radical cation scavenging, metal chelation, and Fe (II)-induced microsomal lipid peroxidation assay. The antioxidant activity of these four compounds has also been reported previously. For example, compounds 1 and 2 had stronger 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging than Trolox, and were found to be most potent antioxidants in the superoxide anion radical assay (Leong, Kinjo, Tako, Iwasaki, Oku & Tamaki). Several researchers have also studied anti-inflammatory properties of these four flavone C-glycosides. The ability to suppress inducible nitric oxide synthase (iNOS) via inhibition of NF-Kappa B has been described for compound 4 (Lin et al., 2005). Huang et al. (2005) reported that compound 4 inhibited the release of TNF-α, upon LPS activation with an IC50 of 78.6 μM. Compound 4 also reduced the LPS-stimulated PGE2 production in a dose-dependent manner, with an IC50 of 80.0 μM. De Melo et al. (2005) have described that compound 3 causes an important decrease of lung neutrophils influx in mice exposed to LPS. In a study published by Kupeli et al. (2004) compound 1 demonstrated significant anti-inflammatory activity.
However, there are no studies that consider the effect of flavone C-glycosides in a COPD model. Since COPD is considered steroid resistant it has been proposed that non-steroidal anti-inflammatory agents that target chemokine pathways are needed as new therapies (Biswas, McClure, Jimenez, Megson & Rahman, 2005; De Boer, 2002). The potential of the flavone C-glycosides 1–4 as a therapy for COPD, was explored by evaluating their efficacy in inhibition of IL-8 and MMP-1 production in SAE cells before and after treatment with CSE (Mercer, Kolesnikova, Sonett & D’Armiento, 2004; Reynertson et al., 2006). Concentrations of 10, 25, 50, and 100 μg/ml were used. First, the effect of the flavone C-glycosides on SAE cell viability was examined at various concentrations. The treatment of flavone C-glycosides (10–100 μg/ml, for 24 h) did not show any significant cytotoxic effect in the present experiments (data not shown).
In non-treated cells the four flavone C-glycosides were capable of decreasing the basal production of IL-8 at all the concentrations tested (Fig. 4). When the control cells were exposed to CSE, the amount of IL-8 in the cells increased by threefold. The production of IL-8 in treated cells decreased with the addition of compounds 1–4. All the compounds were capable of inhibiting IL-8 production in the CSE treated cells. Compounds 1 and 4 demonstrated IL-8 inhibition in the CSE treated cells in a dose dependent manner. Compounds 1, 3, and 4 showed highest inhibitory activities in the CSE treated cells at 100 μg/ml. In case of compound 2 the highest inhibition was observed at 50 μg/ml.
Fig. 4.
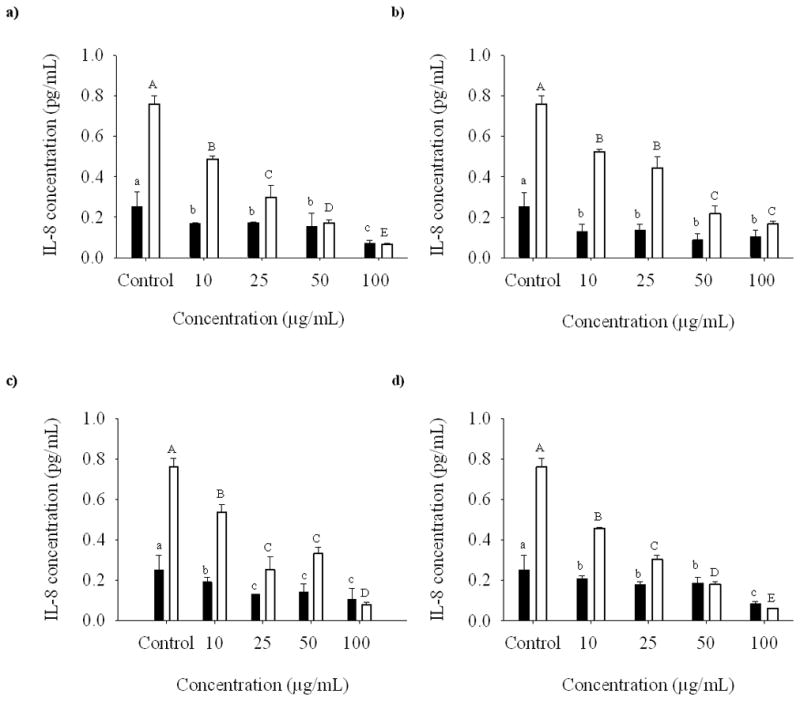
Dose-response relationship of isoorientin (a), orientin (b), vitexin (c), and isovitexin (d) at 10, 25, 50, and 100 μg/ml on the expression of IL-8 in SAE cells untreated (bold bars) and treated (open bars) with CSE.
Before treatment, SAE cells demonstrated MMP-1 expression which increased by sevenfold after 24 h of CSE exposure (Fig. 5). The addition of compounds 1–4 reduced the production of MMP-1 in non-treated cells. In the case of compounds 1, 3, and 4 the decrease in MMP-1 expression was observed only at 25 μg/ml and higher concentrations. When the cells were treated with CSE, compounds 1–4 decreased MMP-1 expression. The inhibitory effect of compound 1 on the gene expression of MMP-1 followed a dose response manner. The highest degree of inhibitory activity was observed when 100 μg/ml of compounds 1, 3, and 4 were added to the cells.
Fig. 5.
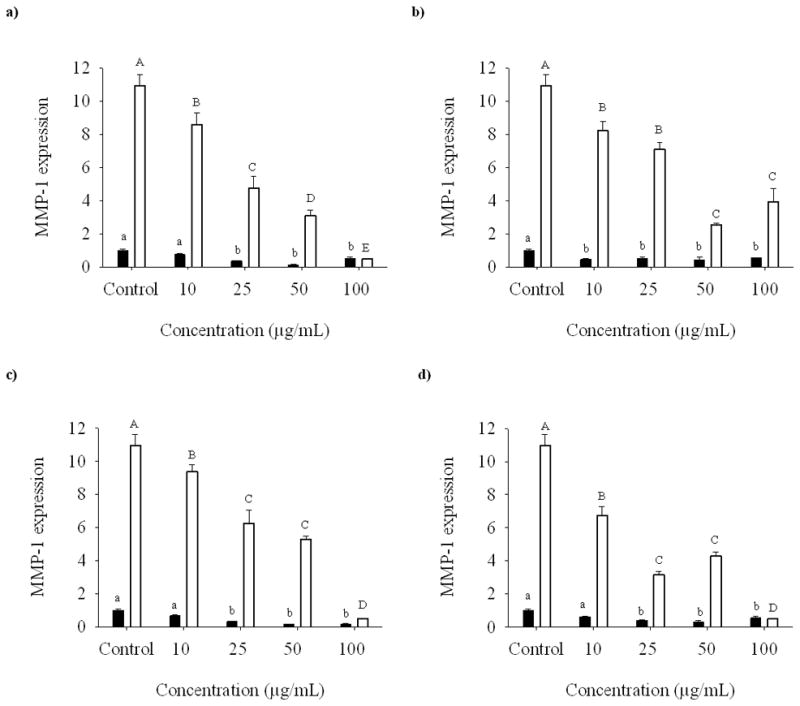
Dose-response relationship of isoorientin (a), orientin (b), vitexin (c), and isovitexin (d) at 10, 25, 50, and 100 μg/ml on the expression of MMP-1 mRNA in SAE cells untreated (bold bars) and treated (open bars) with CSE.
4. Conclusions
The ability of compounds 1–4 to reduce IL-8 production demonstrates their potential as anti-inflammatory agents. In addition their inhibition of MMP-1 expression may be relevant to COPD therapy by preventing the pathological disruption of the lung extracellular matrix observed in this disease. In this study, we examined the dose-response relationship of the four compounds to the production of IL-8 and MMP-1. We observed that compounds 1, 3, and 4 displayed the highest inhibition in both the assays at 100 μg/ml. Therefore, based on its IL-8 and MMP-1 inhibitory activities, compounds 1, 3, and 4 could play a therapeutic role in the treatment of COPD, and we are further investigating these lead compounds in vivo.
Highlights.
Four flavones C-glycosides were isolated for the first time from the neotropical superfruit Anthopterus wardii.
Dose-response relationships of compounds 1–4 was determined for IL-8 and MMP-1
Compounds 1, 3, and 4 showed the most potent inhibitory activity in the IL-8 and MMP-1 assays at 100 μg/mL.
Acknowledgments
Support for this study was provided by NIH-NHLBI grant 5SC1HL096016 and by the Spanish Ministry of Science and Innovation postdoctoral fellowship (G.F.). The authors thank the staff of the Nolen Greenhouses at The New York Botanical Garden, and especially the Manager Marc Hachadourian. The authors also thank Dr. Mario Figueroa, Lehman College (CUNY), for his assistance in the 1H NMR experiments and Dr. Chunhui Ma, Lehman College (CUNY) for his help in the LC-MS analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson R, Theron AJ, Ras GJ. Regulation by the antioxidants ascorbate, cysteine, and dapsone of the increased extracellular and intracellular generation of reactive oxidants by activated phagocytes from cigarette smokers. Am Rev Respir Dis. 1987;135(5):1027–1032. doi: 10.1164/arrd.1987.135.5.1027. [DOI] [PubMed] [Google Scholar]
- Anonymous. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease. American Thoracic Society. Am J Respir Crit Care Med. 1995;152(5):S77–121. [PubMed] [Google Scholar]
- Babusyte A, Stravinskaite K, Jeroch J, Lotvall J, Sakalauskas R, Sitkauskiene B. Patterns of airway inflammation and MMP-12 expression in smokers and ex-smokers with COPD. Respir Res. 2007;8:81. doi: 10.1186/1465-9921-8-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas SK, McClure D, Jimenez LA, Megson IL, Rahman I. Curcumin induces glutathione biosynthesis and inhibits NF-kappaB activation and interleukin-8 release in alveolar epithelial cells: mechanism of free radical scavenging activity. Antioxid Redox Signal. 2005;7(1–2):32–41. doi: 10.1089/ars.2005.7.32. [DOI] [PubMed] [Google Scholar]
- Boschetto P, Quintavalle S, Miotto D, Lo Cascio N, Zeni E, Mapp CE. Chronic obstructive pulmonary disease (COPD) and occupational exposures. J Occup Med Toxicol. 2006;1:11. doi: 10.1186/1745-6673-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, Yates JC, Vestbo J. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356(8):775–789. doi: 10.1056/NEJMoa063070. [DOI] [PubMed] [Google Scholar]
- Dastmalchi K, Flores G, Petrova V, Pedraza-Penalosa P, Kennelly EJ. Edible Neotropical Blueberries: Antioxidant and Compositional Fingerprint Analysis. J Agric Food Chem. 2011;59(7):3020–3026. doi: 10.1021/jf200367j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Boer WI. Cytokines and therapy in COPD: a promising combination? Chest. 2002;121(5):209S–218S. doi: 10.1378/chest.121.5_suppl.209s. [DOI] [PubMed] [Google Scholar]
- Donaldson GC, Seemungal TA, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax. 2002;57(10):847–852. doi: 10.1136/thorax.57.10.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson GC, Seemungal TA, Patel IS, Lloyd-Owen SJ, Wilkinson TM, Wedzicha JA. Longitudinal changes in the nature, severity and frequency of COPD exacerbations. Eur Respir J. 2003;22(6):931–936. doi: 10.1183/09031936.03.00038303. [DOI] [PubMed] [Google Scholar]
- Haq I, Chappell S, Johnson SR, Lotya J, Daly L, Morgan K, Guetta-Baranes T, Roca J, Rabinovich R, Millar AB, Donnelly SC, Keatings V, MacNee W, Stolk J, Hiemstra PS, Miniati M, Monti S, O’Connor CM, Kalsheker N. Association of MMP-2 polymorphisms with severe and very severe COPD: a case control study of MMPs-1, 9 and 12 in a European population. BMC Med Genet. 2010;11:7. doi: 10.1186/1471-2350-11-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirayama F, Lee AH, Binns CW, Hiramatsu N, Mori M, Nishimura K. Dietary intake of isoflavones and polyunsaturated fatty acids associated with lung function, breathlessness and the prevalence of chronic obstructive pulmonary disease: possible protective effect of traditional Japanese diet. Mol Nutr Food Res. 2010;54(7):909–917. doi: 10.1002/mnfr.200900316. [DOI] [PubMed] [Google Scholar]
- Huang ST, Chen CT, Chieng KT, Huang SH, Chiang BH, Wang LF, Kuo HS, Lin CM. Inhibitory effects of a rice hull constituent on tumor necrosis factor alpha, prostaglandin E2, and cyclooxygenase-2 production in lipopolysaccharide-activated mouse macrophages. Ann N Y Acad Sci. 2005;1042:387–395. doi: 10.1196/annals.1338.059. [DOI] [PubMed] [Google Scholar]
- Imai K, Dalal SS, Chen ES, Downey R, Schulman LL, Ginsburg M, D’Armiento J. Human collagenase (matrix metalloproteinase-1) expression in the lungs of patients with emphysema. Am J Respir Crit Care Med. 2001;163(3):786–791. doi: 10.1164/ajrccm.163.3.2001073. [DOI] [PubMed] [Google Scholar]
- Janssens W, Bouillon R, Claes B, Carremans C, Lehouck A, Buysschaert I, Coolen J, Mathieu C, Decramer M, Lambrechts D. Vitamin D deficiency is highly prevalent in COPD and correlates with variants in the vitamin D-binding gene. Thorax. 2010;65(3):215–220. doi: 10.1136/thx.2009.120659. [DOI] [PubMed] [Google Scholar]
- Keranis E, Makris D, Rodopoulou P, Martinou H, Papamakarios G, Daniil Z, Zintzaras E, Gourgoulianis KI. Impact of dietary shift to higher-antioxidant foods in COPD: a randomised trial. Eur Respir J. 2010;36(4):774–780. doi: 10.1183/09031936.00113809. [DOI] [PubMed] [Google Scholar]
- Kim YC, Jun M, Jeong WS, Chung SK. Antioxidant properties of flavone C-glycosides from Atractylodes japonica leaves in human low-density lipoprotein oxidation. J Food Sci. 2005;70(9):S575–S580. [Google Scholar]
- Krafczyk N, Glomb MA. Characterization of phenolic compounds in rooibos tea. J Agric Food Chem. 2008;56(9):3368–3376. doi: 10.1021/jf703701n. [DOI] [PubMed] [Google Scholar]
- Laurent P, Janoff A, Kagan HM. Cigarette smoke blocks cross-linking of elastin in vitro. Am Rev Respir Dis. 1983;127(2):189–192. doi: 10.1164/arrd.1983.127.2.189. [DOI] [PubMed] [Google Scholar]
- Leong AC-N, Kinjo Y, Tako M, Iwasaki H, Oku H, Tamaki H. Flavonoid glycosides in the shoot system of Okinawa Taumu (Colocasia esculenta S.) Food Chemistry. 119(2):630–635. [Google Scholar]
- Lin CM, Huang ST, Liang YC, Lin MS, Shih CM, Chang YC, Chen TY, Chen CT. Isovitexin suppresses lipopolysaccharide-mediated inducible nitric oxide synthase through inhibition of NF-kappa B in mouse macrophages. Planta Med. 2005;71(8):748–753. doi: 10.1055/s-2005-871287. [DOI] [PubMed] [Google Scholar]
- Lopez AD, Murray CC. The global burden of disease, 1990–2020. Nat Med. 1998;4(11):1241–1243. doi: 10.1038/3218. [DOI] [PubMed] [Google Scholar]
- Luteyn JL, Pedraza-Peñalosa P. [accessed 10/1/10];Neotropical Blueberries: The Plant Family Ericaceae. < http://www.nybg.org/bsci/res/lut2>.
- Mercer BA, Kolesnikova N, Sonett J, D’Armiento J. Extracellular regulated kinase/mitogen activated protein kinase is up-regulated in pulmonary emphysema and mediates matrix metalloproteinase-1 induction by cigarette smoke. J Biol Chem. 2004;279(17):17690–17696. doi: 10.1074/jbc.M313842200. [DOI] [PubMed] [Google Scholar]
- Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990–2020: Global Burden of Disease Study. Lancet. 1997;349(9064):1498–1504. doi: 10.1016/S0140-6736(96)07492-2. [DOI] [PubMed] [Google Scholar]
- Omachi TA, Eisner MD, Rames A, Markovtsova L, Blanc PD. Matrix metalloproteinase-9 predicts pulmonary status declines in alpha1-antitrypsin deficiency. Respir Res. 2011;12:35. doi: 10.1186/1465-9921-12-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26(9–10):1231–1237. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Reynertson KA, Wallace AM, Adachi S, Gil RR, Yang H, Basile MJ, D’Armiento J, Weinstein IB, Kennelly EJ. Bioactive depsides and anthocyanins from jaboticaba (Myrciaria cauliflora) J Nat Prod. 2006;69(8):1228–1230. doi: 10.1021/np0600999. [DOI] [PubMed] [Google Scholar]
- Snijman PW, Joubert E, Ferreira D, Li XC, Ding Y, Green IR, Gelderblom WC. Antioxidant activity of the dihydrochalcones Aspalathin and Nothofagin and their corresponding flavones in relation to other Rooibos (Aspalathus linearis) Flavonoids, Epigallocatechin Gallate, and Trolox. J Agric Food Chem. 2009;57(15):6678–6684. doi: 10.1021/jf901417k. [DOI] [PubMed] [Google Scholar]
- Stan SD, Kar S, Stoner GD, Singh SV. Bioactive food components and cancer risk reduction. J Cell Biochem. 2008;104(1):339–356. doi: 10.1002/jcb.21623. [DOI] [PubMed] [Google Scholar]
- Varraso R, Fung TT, Hu FB, Willett W, Camargo CA. Prospective study of dietary patterns and chronic obstructive pulmonary disease among US men. Thorax. 2007;62(9):786–791. doi: 10.1136/thx.2006.074534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestbo J, Sorensen T, Lange P, Brix A, Torre P, Viskum K. Long-term effect of inhaled budesonide in mild and moderate chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 1999;353(9167):1819–1823. doi: 10.1016/s0140-6736(98)10019-3. [DOI] [PubMed] [Google Scholar]
- Walda IC, Tabak C, Smit HA, Rasanen L, Fidanza F, Menotti A, Nissinen A, Feskens EJ, Kromhout D. Diet and 20-year chronic obstructive pulmonary disease mortality in middle-aged men from three European countries. Eur J Clin Nutr. 2002;56(7):638–643. doi: 10.1038/sj.ejcn.1601370. [DOI] [PubMed] [Google Scholar]