Abstract
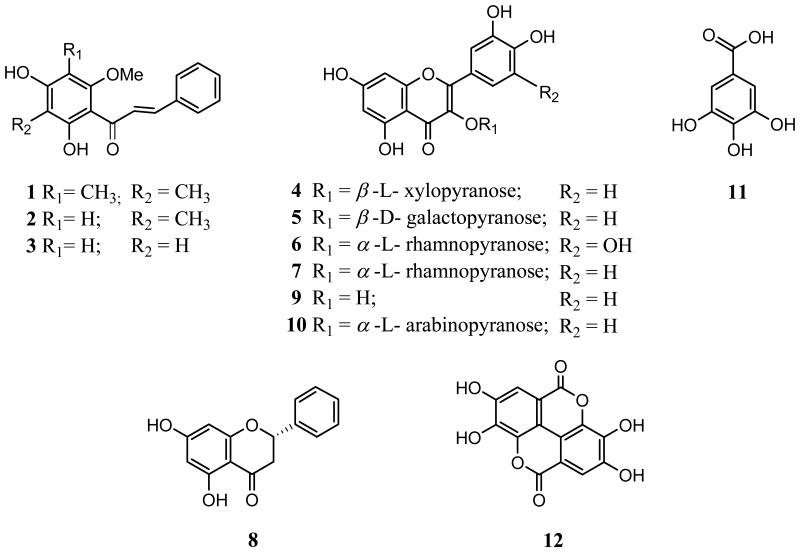
Bioassay-guided fractionation of the methanolic extracts of the pulp and seeds of the fruits of Syzygium samarangense Merr. & Perry (Blume) led to the identification of four cytotoxic compounds and eight antioxidants on the basis of HPLC-PDA analysis, MS, and various NMR spectroscopic techniques. Three C-methylated chalcones, 2′,4′-dihydroxy-3′,5′-dimethyl-6′-methoxychalcone (1), 2′,4′-dihydroxy-3′-methyl-6′-methoxychalcone (stercurensin, 2), and 2′,4′-dihydroxy-6′-methoxychalcone (cardamonin, 3), were isolated and displayed cytotoxic activity (IC50 = 10, 35, and 35 μM, respectively) against the SW-480 human colon cancer cell line. Also a number of known antioxidants were obtained including six quercetin glycosides: reynoutrin (4), hyperin (5), myricitrin (6), quercitrin (7), quercetin (9), and guaijaverin (10), one flavanone: (S)-pinocembrin (8), and two phenolic acids: gallic acid (11) and ellagic acid (12).
Keywords: Syzygium samarangense, chalcones, flavonoids, cytotoxic activity, antioxidants
1. Introduction
Syzygium samarangense (Bloom) Merr. & Perry (Myrtaceae), known commonly as wax jambu, is an evergreen tree with origins in Asia. It produces a pink fleshy fruit which is eaten fresh. Many cultivars have been developed and they are grown throughout the tropical and subtropical parts of the world. The fruit is oblong, pear-shaped, and 5 to 12 cm in length, with four fleshy calyx lobes and 1 to 4 seeds (1 to 2 cm in diameter). The tree can be grown as an ornamental, and attains a height of seven meters. Wax jambu belongs to the same genus as Syzygium aromaticum, the source of cloves, a common spice.
In Malaysia, the green fruits of wax jambu are eaten raw with salt or cooked as a sauce. The flowers, which contain tannins, desmethoxymatteucinol, 5-O-methyl-4′-desmethoxymatteucinol, oleanic acid, and β-sitosterol, are used in Taiwan to treat fever and halt diarrhea (Morton, 1987).
Previous phytochemical studies of the leaves of S. samarangense have shown the presence of ellagitannins (Nonaka, Aiko, Aritake & Nishioka, 1992), flavanones (Kuo, Yang & Lin, 2004), flavonol glycosides (Nair, Krishnan, Ravikrishna & Madhusudanan, 1999; Kuo, et al., 2004), proanthocyanidins (Nonaka et al., 1992), anthocyanidins (Nonaka et al., 1992; Kuo et al., 2004), triterpenoids (Srivastava, Shaw & Kulshreshtha, 1995), chalcones (Srivastava, Shaw & Kulshreshtha, 1995; Resurreccion-Magno, Villasenor, Harada & Monde, 2005), and volatile terpenoids (Wong & Lai, 1996).
Ethanolic leaf extract exhibited immunostimulant activity (Srivastava, Shaw & Kulshreshtha, 1995), the hexane extract was found to relax the hypermotility of the gut (Ghayur, Gilani, Khan, Amor, Villasenor & Choudhary, 2006), while the alcoholic extract of the stem bark showed antibacterial activity (Chattopadhayay, Sinha & Vaid, 1998).
The immunomodulatory (Kuo et al., 2004), antihyperglycaemic (Resurreccion-Magno et al., 2005), spasmolitic (Amor, Villasenor, Ghayur, Gilani & Choudhary, 2005), and prolyl endopeptidase inhibitor effects of chalcones 1 and 2 and the flavanone 5-O-methyl-4-desmethoxymatteucinol isolated from the leaves have also been reported (Amor, Villasenor, Yasin & Choudhary, 2004).
Chalcones are a group of plant-derived polyphenolic compounds that are intermediates in the biosynthesis of flavonoids and are associated with several biological activities, including antiviral (Kiat, Pippen, Yusof, Ibrahim, Khalid & Rahman, 2006), antifungal (Svetaz et al., 2004) anti-inflammatory (Lespagnol et al., 1972) and antioxidant (Han, Kang, Windono, Lee & Seo, 2006). They also displayed anticancer and cytotoxic activity (Go, Wu & Liu, 2005).
As part of a programme to find novel antioxidant and cytotoxic compounds from plants, (Yang et al., 2005), we have investigated the anticancer activity and antioxidant properties of wax jambu, and we report here the bioassay-guided identification of polyphenolic constituents and cytotoxic chalcones from the pulp and seeds of the fruits of S. samarangense.
2. Materials and methods
2.1. General methods
Optical rotation was measured on an Autopol III Automatic Polarimeter (Rudolph Research Analytical, Newburgh Hackttstown, NJ, USA); 1H and 13C NMR spectra were recorded using a Bruker Avance 300, operating at 300 and 75 MHz, respectively. Spectra were obtained in CD3OD or CDCl3, with chemical shifts expressed in δ and coupling constant (J) in Hertz (Hz). Electrospray ionization mass spectrometry (ESI-MS) was performed with a ThermoFinnigan LCQ instrument (San Jose, CA, USA), equipped with Xcalibur software. Samples were dissolved in HPLC grade MeOH and introduced by direct injection. Nitrogen was used as both an auxiliary and sheath gas, the capillary voltage was 10 V, the spray voltage was 4.5 kV, the capillary temperature was 230 °C and the tube lens offset was 0 V. HPLC analyses were carried out on a Waters 2695 Separations Module (Milford, MA, USA) equipped with a model 996 photodiode array detector and Empower software, using a 250 × 4.6 mm i.d., 5 μm, Aqua C-18 column (Phenomenex, Torrance, CA, USA). Preparative HPLC was carried out using a Waters 600 controller with a Waters 486 tunable absorbance detector and Waters Empower software with a 250 × 21.20 mm i.d., 10 μm Luna C-18 column (Phenomenex Torrance, CA, USA) and the mobile phase consisted of 10 % aqueous formic acid (A) and HPLC grade acetonitrile (B). The flow rate was 20 ml/min, with a linear gradient consisting of 40 % A to 30 % A in 20 min run time and the column at room temperature.
A Molecular Devices (Sunnyvale, CA, USA) Versamax tunable absorbance detector was used for the 1,1-diphenyl-2-picrylhydrazyl (DPPH) antiradical assay, total flavonoid content (TFC), and total phenolic content measurements (TPC).
TLC analyses were performed on Si gel 60 F254 (1 mm layer thickness, EM Science, Darmstadt, Germany) and RP-18 F254 plates (1 mm layer thickness, EM Science, Darmstadt, Germany), with compounds visualized by spraying with a vanillin solution (1.0 g of vanillin in 10 ml of concentrated H2SO4 and 90 ml of EtOH) and heating at 50 °C. Sephadex LH-20 (25-100 μm) (Pharmacia Fine Chemicals, Piscataway, NJ, USA), silica gel (230-400 mesh) (EM Science, Darmstadt, Germany), Diaion HP-20 (HP-20) (Supelco, Bellefonte, PA, USA), and C-18 reversed-phase Si gel (40 μm) (J. T. Baker, Phillipsburg, NJ, USA) were used for column chromatography. Solvents for chromatography, HPLC-grade acetonitrile, MeOH, formic acid, and HPLC-grade water, were obtained from J. T. Baker (Phillipsburg, NJ, USA), and GR-grade MeOH, EtOAc and acetone from VWR Inc (Bridgeport, PA, USA). AlCl3, DMSO, FeCl3, anhydrous Na2CO3, NaNO2, NaOAc, NaOH, trolox, 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical, 2,4,6-tri-pyridiyl-s-triazine, and the Folin-Ciocalteu phenol reagent were purchased from Sigma-Aldrich (St. Louis, MO, USA). Ellagic acid, gallic acid, myricitrin, quercetin, and quercitrin were also obtained from Sigma-Aldrich (St. Louis, MO, USA).
2.2. Plant material
Fruits of S. samarangense were collected from the Fruit and Spice Park (Homestead, FL, USA) in June 2001. Fresh fruits were shipped to New York City by overnight courier and stored at −20 °C until extracted. A voucher specimen (Reynertson 17) was prepared, identified, and deposited at the Steere Herbarium of The New York Botanical Garden (Bronx, NY).
2.3. Extraction
The fresh frozen pulp (3.2 kg) of the fruits of S. samarangense were extracted twice with MeOH (5 l) at room temperature for 1 h per extraction. After the MeOH was removed in vacuo, the resulting dark extract (27.0 g) was suspended in water and sequentially partitioned with hexane (1l, × 3), EtOAc (1l, × 3), and n-BuOH (1l, × 3), respectively. The EtOAc and n-BuOH partitions were concentrated in vacuo to give 3.6 g and 18.0 g of dark brown extracts, respectively.
The seeds (400.0 g) of S. samarangense were processed in the same way as the pulp, and 15.0 g of a dark brown extract were obtained, which were suspended in water and sequentially partitioned with hexane (300 ml, × 3), EtOAc (300 ml, × 3), and n-BuOH (300 ml, × 3). The EtOAc and n-BuOH partitions were concentrated in vacuo to give 1.7 g and 10.4 g of two dark brown residues, respectively.
Each selected fraction obtained was screened for free radical-scavenging capacity (Wu, Tung, Wang, Shyur, Kuo & Chang, 2005). Briefly, subfractions were loaded individually on a baseline of the RP-18 TLC. The TLC plate was developed using a 1:1 MeOH/H2O solvent system and the layer was dried and stained with 0.2 % DPPH· (w/v) solution in EtOH. The appearance of yellow colour in the spots indicates free radical-scavenging capacity of the test samples. The antioxidant activity of each active fraction was then assessed by the standard DPPH· assay.
2.4. Isolation and purification
The EtOAc partition (15.0 g) of the seeds was subjected to repeated column chromatography over Sephadex LH-20 using MeOH as eluent and eight subfractions (SEA-1 to 8) were collected. Fraction SEA-5 (28.0 mg, IC50 = 18.3 μg/ml in the DPPH· assay and IC50 = 10.0 μg/ml in MTT assay) was subjected to preparative C-18 HPLC to obtain 2′,4′-dihydroxy-3′,5′-dimethyl-6′-methoxychalcone (1, 14.0 mg), 2′,4′-dihydroxy-3′-methyl-6′-methoxychalcone (2, 6.0 mg), and 2′,4′-dihydroxy-6′-methoxychalcone (3, 0.6 mg).
The n-BuOH partition (10.4 g, IC50 =16.8 μg/ml in the DPPH· assay and IC50 = 55.0 μg/ml in MTT assay) was processed using a similar procedure of EtOAc partition and 15.7 mg of gallic acid (11) and 12.9 mg of ellagic acid (12) were isolated.
The EtOAc fraction (1.8 g, IC50 = 54.7 μg/ml in the DPPH· assay and IC50 = 20.0 μg/ml in MTT assay) of the pulp was subjected to column chromatography over Sephadex LH-20 and eluted with an isocratic system using MeOH and 13 fractions (100 ml each) were collected. These fractions were combined into five fractions (EA-A to E) on the basis of RP-18 TLC (7:3 MeOH/H2O) analysis. All fractions were tested in the MTT and DPPH· assays; fraction EA-C (1.1 g, IC50 = 45.0 μg/ml in the MTT assay and 25.0 μg/ml in the DPPH· assay) and fraction EA-E (80 mg, 20.4 μg/ml in the DPPH assay) showed cytotoxic and antioxidant activities.
Fraction EA-C was chromatographed by Sephadex LH-20 (100% MeOH) and repeated RP-18 CC, eluting with a gradient of 3:7 MeOH/H2O to 9:1 MeOH/H2O (10% increments) to yield (S)-pinocembrin (8) (7.2 mg) and quercetin (9) (2.0 mg).
Fraction EA-E was chromatographed using the same procedure as described above to afford reynoutrin (4) (6.3 mg), hyperin (1.6 mg) (5), myricitrin (6) (0.5 mg), quercitrin (1.6 mg) (7), and guaijaverin (10) (4.5 mg). The n-BuOH partition (18.0 g, IC50= 87.8 μg/ml in the DPPH· assay) was processed using a similar procedure and 12.0 mg of gallic acid (11) and 11.6 mg of ellagic acid (12) were isolated.
Those nine compounds from the pulp and five compounds from the seeds of S. samarangense were identified by the spectroscopic methods and HPLC-PDA analysis with authentic standards (Figure 1):
Figure 1.
Structure of compounds (1-12) isolated from S. samarangense.
2′,4′-Dihydroxy-3′,5′-dimethyl-6′-methoxychalcone (1): yellow-orange crystals (14.0 mg) (Table 1), the yield was 35.0 mg/kg fresh weight from the seeds. Negative ESIMS: m/z 297 [M - H]−; 1H and 13C NMR data are consistent with previously published data (Resurreccion-Magno et al., 2005).
Table 1.
Results of cytotoxic activity on the SW-480 human colon cancer cell line, antiradical DPPH assay, ferric reducing antioxidant power, total flavonoid content, and total phenolic content of methanol extracts and compounds 1-3 and 8 from the pulp and seeds of the fruits of S. samarangense
| Compounds/Methanol extract | Cytotoxic activity a, IC50 | DPPHa, IC50 | FRAP c | TFC d | TPC e |
|---|---|---|---|---|---|
| 1 | 10 | 205 ± 1.2 b | 196 ± 0.0 | NT | NT |
| 2 | 35 | 141 ± 2.3 | 191 ± 0.1 | NT | NT |
| 3 | 35 | 124 ± 3.4 | 173 ± 0.0 | NT | NT |
| 8 | 60 | 199 ± 0.8 | 196 ± 0.5 | NT | NT |
| Pulp methanol extract | 60 | 72.9 ± 0.0 | 14.8 ± 0.2 | 294 ± 0.0 | 460 ± 0.0 |
| Seeds methanol extract | NS | 78.4 ± 0.0 | 31.3 ± 0.2 | 19.9 ± 0.0 | 1278 ± 0.1 |
| EGCG | 50 | ||||
| Gallic acid | 25.0 ± 0.1 |
Cytotoxic and antiradical DPPH activity are expressed as IC50 in μM for pure compound and μg/ml for extracts.
Mean ± S.D. (n = 3);
Expressed as μM trolox equivalents/500 μmol for pure compound and μmol trolox/g fresh weight for extracts;
Total flavonoid content (TFC) expressed as mg quercetin/100 g fresh weight;
Total phenolic content (TPC) expressed as mg gallic acid/100 g fresh weight; NS: Not completely soluble. NT: Not tested.
2′,4′-Dihydroxy-3′-methyl-6′-methoxychalcone (stercurensin) (2): yellow-orange crystals (3.9 mg) (Table 1); the yield was 9.7 mg/kg fresh weight from the seeds. Negative ESIMS: m/z 283 [M - H]−; 1H and 13C NMR data are consistent with previously published data (Resurreccion-Magno et al., 2005).
2′,4′-Dihydroxy-6′-methoxychalcone (cardamonin) (3): yellow-orange crystals (0.8 mg) (Table 1); the yield was 2.0 mg/kg fresh weight from the seeds. Negative ESIMS: m/z 269 [M - H]−; 1H and 13C NMR data are consistent with previously published data (Jaipetch et al., 1982).
Reynoutrin (4): yellow powder (6.3 mg); the yield was 2.37 mg/kg fresh weight from the fruits. Negative ESIMS: m/z 433 [M - H]−; 1H and 13C NMR data are consistent with previously published data (Lu & Foo, 1997).
Hyperin (5): yellow powder (1.6 mg); the yield was 0.5 mg/kg fresh weight from the fruits. Negative ESIMS: m/z 463 [M - H]−; 1H and 13C NMR data are consistent with previously published data (Lu et al., 1997).
Myricitrin (6). yellow powder (0.5 mg); the yield was 0.2 mg/kg fresh weight from the fruits. The compound was identified by HPLC-PDA analysis (retention time and UV spectrum) with authentic standard.
Quercitrin (7): yellow powder (1.6 mg); the yield was 1.4 mg/kg fresh weight from the fruits. The compound was identified by HPLC-PDA analysis (retention time and UV spectrum) with authentic standard.
(S)-Pinocembrin (8): white powder (7.2 mg); the yield was 2.2 mg/kg fresh weight from the fruits. Positive ESIMS: m/z 255 [M + H]+; 1H and 13C NMR data are consistent with previously published data (Kuroyanagi, et al. 1983). The specific rotation of 8 was measured as [α]D29.0 = −25.9 (c, 0.004 in MeOH); therefore, 8 was identified as (S)-pinocembrin (Kuroyanagi et al. 1983).
Quercetin (9): yellow powder (2.0 mg); the yield was 0.6 mg/kg fresh weight from the fruits. The compound was identified by HPLC-PDA analysis (retention time and UV spectrum) with authentic standard.
Guaijaverin (10): yellow powder (4.5 mg); the yield was 1.5 mg/kg fresh weight from the fruits. Negative ESIMS: m/z 433 [M - H]−; 1H and 13C NMR data are consistent with previously published data (Stark, Bareuther & Hofmann, 2005).
Gallic acid (11): white powder (29.7 mg); the yield was 3.8 mg/kg fresh weight from the fruits and 39.8 mg/kg fresh weight from the seeds. Negative ESIMS: m/z 169 [M - 1]−; 1H and 13C NMR data are consistent with previously published data (Khac, Tran-Van, Campos, Lallemand & Fetizon, 1990). The compound was also identified by HPLC-PDA analysis (retention time and UV spectrum) with authentic standard.
Ellagic acid (12): brown powder (27.5 mg); the yield was 3.6 mg/kg fresh weight from the fruits and 44.3 mg/kg fresh weight from the seeds. Negative ESIMS: m/z 301 [M - 1]−; 1H and 13C NMR data are consistent with previously published data (Khac et al., 1990). The compound was also identified by HPLC-PDA analysis (retention time and UV spectrum) with authentic standard.
The above mentioned twelve compounds and MeOH extracts of the pulp and seeds were tested for antioxidant activity in the DPPH· and FRAP assays and cytotoxic activity against SW-480 cell lines in the MTT assay (Table 1).
2.5. Total phenolic and total flavonoid contents (TPC and TFC)
The TPCs were determined by the Folin and Ciocalteu reagent method (Yildirim, Mavi & Kara, 2001). Briefly, 2.0 g of freeze-dried samples of the fruits and seeds were extracted twice with MeOH for 1 h at room temperature. Filtered MeOH extract (100 μl) and 10 % Folin and Ciocalteu reagent (1.0 ml) in ethanol were placed in a test tube and incubated for 5 min at room temperature (22 °C). The mixture was left to stand for 5 min. and 1.0 ml of 10 % sodium carbonate was added. After 90 min of incubation at room temperature, the resulting absorbance was measured at 765 nm. The calibration curve was performed with gallic acid, (concentrations ranging from 31.3 μg/ml to 500.0 μg/ml) and the results were expressed as μg of gallic acid equivalents per 100 g of fresh material. (Liu, Li, Weber, Lee, Brown & Liu, 2002). The total flavonoid contents of the extracts were determined. Briefly, 250 μl of the sample (0.6 mg of the freeze dry extract per ml) was diluted with 1.25 ml of water. Then 75 μl of 5% NaNO2 solution were added to the mixture. After 5 min, 150 μl of 10% AlCl3 .6 H2O were added and the mixture was allowed to stand for 5 min. Then 500 μl of 1M NaOH solution and 275 μl of distilled water were added to make a total of 2.5 ml. The absorbance was measured immediately against the prepared blank at 510 nm. The results were expressed as milligrammes of quercetin equivalents per 100 g of fresh material. All data are reported as means ± SD for at least three replications.
2.6. Cell culture
The SW-480 human colon cell cancer line was purchased from The American Type Culture Collection. The cell line was maintained in Dulbecco's modified Eagle's medium (Gibco-BRL, Grand Island, NY, USA) with 10 % fetal bovine serum (Gibco) at 5 % CO2 and 37°C. The culture was passaged weekly, and the medium was changed three times per week. No antibiotics were added at any time during the experiments. In all experiments, chalcones and plant extracts were dissolved in DMSO and added to the medium at the start of the incubation. The incubation time was 72 h for the MTT experiment.
2.7. Microtetrazolium (MTT) assay
The MTT assay (Boeringher-Mannheim, Indianapolis, IN, USA) was carried out according to the manufacturer's instructions. In brief, about 3000 cells were plated in 96-well flat-bottom plates in 100 μl of medium. When cells reached 40% confluence, the medium was changed and cells were exposed to the plant extracts or isolates. After 72 h, cells were washed once with PBS followed by the addition of 100 μl of Dulbecco's modified Eagle medium, and 10 μl of 5 mg/ml MTT solution in PBS were added to each well for 6 h. Finally, 100 μl of MTT solubilization solution were added to each well to dissolve the formazan crystals. The absorbance at 575 nm was determined using a Biokinetics plate reader (Bio-Tek Instruments Inc., Winooski, VT, USA). Octuplicate wells were assayed for each condition, and mean as well as standard deviations were determined. The IC50 values were determined by linear regression analysis. Epigallocatechin gallate (EGCG) was used as a positive control (IC50 = 50 μM).
2.8. Antioxidant properties
Antioxidant properties of the methanol extracts and pure compounds isolated from the fruits and seeds of S. samarangense were determined by the DPPH free-radical-scavenging and the ferric reducing antioxidant power (FRAP) assays. The DPPH· assay was performed on fractions and purified isolates as previously described (Smith, Reeves, Dage & Schnettler, 1987). Gallic acid was used as the positive control and percent inhibition by sample treatment was determined by comparison with a DMSO-treated control group. FRAP assay (Benzie & Strain, 1996) was adapted for use in a 96 well microplate spectrophotometer (Versamax Molecular Devices, Sunnyvale, CA, USA). FRAP values were expressed in μmol trolox equivalents (TE) per g of fresh material for the extracts and μmol TE per 500 μmol of pure compounds.
3. Results and discussion
The methanol extract of the pulp and seeds of the fruits of S. samarangense (Table 1) were each partitioned with hexane, EtOAc and n-BuOH. The EtOAc partitions were each subjected to bioassay-guided fractionation with an initial separation by Sephadex LH-20 column chromatography. Subsequent purification of active fractions led to the isolation of four cytotoxic compounds against the SW-480 human colon cancer cell line: 2′,4′-dihydroxy-3′,5′-dimethyl-6′-methoxychalcone (1), stercurensin (2), cardamonin (3), and (S)-pinocembrin (8) (Table 1, Figure 1), and eight well known antioxidants: reynoutrin (4), hyperin (5), myricitrin (6), quercitrin (7), quercetin (9), guaijaverin (10), gallic acid (11) and ellagic acid (12) (Figure 1). Compounds 1-3, 11, and 12 were isolated from the seeds, and compounds 4-12 were obtained from the pulp. Compounds were identified using a combination of data from MS, NMR and HPLC-PDA comparison (retention time and UV spectra) with authentic standards. The yields of the 12 polyphenols isolated ranged from 0.2 to 3.8 mg per kg fresh weight from the pulp and 1.5 to 39.7 mg per kg fresh weight from the seeds, respectively. The total phenolic content, total flavonoid content, DPPH·-scavenging activity and FRAP results for the extracts are shown in Table 1.
Chalcones are flavonoids that lack the C ring. The presence of a 4-OH group, and an α, β-unsaturated double bond are essential features for the cytotoxic activity of this class of compound (Cai, Sun, X., Luo & Corke, 2006). In a report on structure activity relationships of chalcones, a series of O-methylated chalcones showed activity against human tumor cell replication (KB, KB-VCR and A 549 cell lines) (Tatsuzaki, Bastow, Nakagawa-Goto, Nakamura, Itokawa & Lee, 2006). Another investigation showed that several ring-A methoxylated chalcones exhibited cytotoxic activity by inhibiting tubulin polymerization against a variety of tumor cell lines in the low micromolar range (IC50< 50 μM) (Go, Wu & Liu, 2005).
Compounds 1, 2, and 3 are chalcones that have a 2′-hydroxy, a 4′-hydroxy and 6′-methoxy group substituting the A-ring. An uncommon feature is the additional C-methylation in 1 and 2; these compounds have been previously isolated from the leaves of this plant and reported to have antidiabetic properties (Resurreccion-Magno et al., 2005), antibacterial (Gafner, Wolfender, Mavi & Hostettmann, 1996), spasmolitic (Amor et al., 2005; Ghayur et al., 2006) properties, and inhibited prolyl endopeptidase (Amor, et al., 2004).
This is the first report of the isolation of chalcone 3 from S. samarangense. This chalcone, cardamonin (2′,4′-dihydroxy-6′-methoxychalcone), is one of the main constituents from the seeds of Alpinia katsumadai, and was first isolated from the seeds of Amomum subulatum (Bheemasankara, Namosiva & Suryaprakasam, 1976). It has anti-inflammatory activity (Lee, Jung, Giang, Jin, Lee, Son et al., 2006) and anti-mutagenic effects upon activation of heterocyclic amines (Trakoontivakorn et al., 2001), and inhibits lipopolysaccharide-induced expression of inducible nitric oxide synthase and tumor necrosis factor-α in RAW 264.7 cells (Ban et al., 2004) and proinflammatory mediators (Syahida et al., 2006).
The flavanone (S)-pinocembrin (8), and the chalcones 1, 2, and 3, displayed cytotoxic and weak antioxidant activity (Table 1). Compounds 4-7 and 9-10 had values higher than 100 μM in the MTT assay, and compounds 11 and 12 were not tested in this assay. The lower cytotoxic activity of the flavanone (S)-pinocembrin (8, IC50 = 60 μM in the MTT assay) in relation to the chalcones isolated could be related to the rigidity of the flavanone structure (Figure 1). The 5′-methyl group appears to be an important structural requirement for cytotoxic activity among the chalcones 1, 2, and 3, as seen from the fall in activity when the structure loses this methyl group (10 μM for compound 1 and 35 μM for compounds 2 and 3). The weak antioxidant activity displayed by the isolated chalcones could be attributed to the lack of hydroxyl groups in ring-B (Cai et al., 2006). There is no apparent correlation between cytotoxic and antioxidant activity.
The edible fruits of Syzygium samarangense represent potential benefits for human health because they are a rich dietary source of polyphenolic antioxidants. In addition, the seeds are a rich source of the cytotoxic chalcones 1-3, especially true for compound 1, since it is present in high concentration (35.0 mg per kg fresh weight).
Acknowledgments
We would like to thank Chris Rollins, Director of the Fruit and Spice Park in Homestead, FL, and Larry Schokman, Director of the Kampong in Coconut Grove, FL for providing the fruits in this study. This research was supported by the funds from the National Institutes of Health-National Institute of General Medical Sciences SCORE award S06GM08225 and from the United States Department of Agriculture.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amor EC, Villasenor IM, Yasin A, Choudhary MI. Prolyl endopeptidase inhibitors from Syzygium samarangense (Blume) merr. & l. M. Perry. Zeitschrift fuer Naturforschung, C: Journal of Biosciences. 2004;59(1/2):86–92. doi: 10.1515/znc-2004-1-218. [DOI] [PubMed] [Google Scholar]
- Amor EC, Villasenor IM, Ghayur MN, Gilani AH, Choudhary MI. Spasmolytic flavonoids from Syzygium samarangense (Blume) merr. & l.M. Perry. Zeitschrift fuer Naturforschung, C: Journal of Biosciences. 2005;60(1/2):67–71. doi: 10.1515/znc-2005-1-213. [DOI] [PubMed] [Google Scholar]
- Ban HS, Suzuki K, Lim SS, Jung SH, Lee S, Ji J, et al. Inhibition of lipopolysaccharide-induced expression of inducible nitric oxide synthase and tumor necrosis factor-alpha by 2′-hydroxychalcone derivatives in raw 264.7 cells. Biochemical pharmacology. 2004;67(8):1549–57. doi: 10.1016/j.bcp.2003.12.016. [DOI] [PubMed] [Google Scholar]
- Benzie IFF, Strain JJ. The ferric reducing ability of plasma (frap) as a measure of “antioxidant power”: The frap assay. Analyticla Biochemistry. 1996;239(1):70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Bheemasankara RC, Namosiva Rao T, Suryaprakasam S. Cardamonin and alpinetin from the seeds of Amomum subulatum. Planta Medica. 1976;29(4):391–392. doi: 10.1055/s-0028-1097682. [DOI] [PubMed] [Google Scholar]
- Cai YZ, Sun M, Jie X, Luo Q, Corke H. Structure-radical scavenging activity relationships of phenolic compounds from traditional Chinese medicinal plants. Life Sciences. 2006;78(25):2872–2888. doi: 10.1016/j.lfs.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Chattopadhayay ED, Sinha BK, Vaid LK. Antibacterial activity of syzygium species. Fitoterapia. 1998;119(4):365–367. [Google Scholar]
- Gafner S, Wolfender JL, Mavi S, Hostettmann K. Antifungal and antibacterial chalcones from Myrica serrata. Planta Medica. 1996;62(1):67–69. doi: 10.1055/s-2006-957804. [DOI] [PubMed] [Google Scholar]
- Ghayur MN, Gilani AH, Khan A, Amor EC, Villasenor IM, Choudhary MI. Presence of calcium antagonist activity explains the use of Syzygium samarangense in diarrhoea. Phytotherapy Research. 2006;20(1):49–52. doi: 10.1002/ptr.1801. [DOI] [PubMed] [Google Scholar]
- Go ML, Wu X, Liu XL. Chalcones: An update on cytotoxic and chemoprotective properties. Current Medicinal Chemistry. 2005;12(4):483–499. doi: 10.2174/0929867053363153. [DOI] [PubMed] [Google Scholar]
- Han AR, Kang YJ, Windono T, Lee SK, Seo EK. Prenylated flavonoids from the heartwood of Artocarpus communis with inhibitory activity on lipopolysaccharide-induced nitric oxide production. Journal of Natural Products. 2006;69(4):719–721. doi: 10.1021/np0600346. [DOI] [PubMed] [Google Scholar]
- Jaipetch T, Kanghae S, Pancharoen O, Patrick VA, Reutrakul V, Tuntiwachwuttikul P, et al. Constituents of Boesenbergia pandurata (syn. Kaempferia pandurata) isolation, crystal structure and synthesis of (+-)-boesenbergin a. Australian Journal of Chemeistry. 1982;35:351–361. [Google Scholar]
- Khac D, Tran-Van S, Campos MA, Lallemand Y, Fetizon M. Ellagic compounds from Diplopanax stachyanthus. Phytochemistry. 1990;29:251–256. [Google Scholar]
- Kiat TS, Pippen R, Yusof R, Ibrahim H, Khalid N, Rahman NA. Inhibitory activity of cyclohexenyl chalcone derivatives and flavonoids of Fingerroot, Boesenbergia rotunda (L.), towards dengue-2 virus ns3 protease. Bioorganic & Medicinal Chemistry Letters. 2006;16(12):3337–3340. doi: 10.1016/j.bmcl.2005.12.075. [DOI] [PubMed] [Google Scholar]
- Kuo YC, Yang LM, Lin LC. Isolation and inmunomodulatory effects of flavonoids from Syzygium samarangense. Planta Medica. 2004;70(1):1237–1239. doi: 10.1055/s-2004-835859. [DOI] [PubMed] [Google Scholar]
- Kuroyanagi M, Noro T, Fukushima S, Aiyama R, Ikuta A, Itokawa H, et al. Studies on the contituents of the seeds of Alpinia katsumadai hayata. Chemical & Pharmaceutical Bulletin. 1983;31(5):1544–1550. [Google Scholar]
- Lee JH, Jung HS, Giang PM, Jin X, Lee S, Son PT, et al. Blockade of nuclear factor-kb signaling pathway and anti-inflammatory activity of cardamomin, a chalcone analog from Alpinia conchigera. Journal of Pharmacology and Experimental Therapeutics. 2006;316(1):271–278. doi: 10.1124/jpet.105.092486. [DOI] [PubMed] [Google Scholar]
- Lespagnol A, Lespagnol C, Lesieur D, Cazin JC, Cazin M, Beerens H, et al. Analgesic, antiinflammatory and antimicrobial activities of chalcones and dihydrochalcones derived from salicylic acid. Chimica Therapeutica. 1972;7(5):365–369. [Google Scholar]
- Liu M, Li XQ, Weber C, Lee CY, Brown J, Liu RH. Antioxidant and antiproliferative activities of raspberries. Journal of Agricultural and Food Chemistry. 2002;50(10):2926–2930. doi: 10.1021/jf0111209. [DOI] [PubMed] [Google Scholar]
- Lu Y, Foo Y. Identification and quantification of major polyphenols in Apple pomace. Food Chemistry. 1997;59:187–194. [Google Scholar]
- Morton J. In: Fruits of Warm Climates. Morton Julia., editor. Winterville, NC: 1987. pp. 386–388. [Google Scholar]
- Nair AGR, Krishnan S, Ravikrishna C, Madhusudanan KP. New and rare flavonol glycosides from leaves of Syzygium samarangense. Fitoterapia. 1999;70(2):148–151. [Google Scholar]
- Nonaka G, Aiko Y, Aritake K, Nishioka I. Tannins and related compounds. CXIX. Samarangenins a and b, novel proanthocyanidins with doubly bonded structures, from Syzygium samarangens and S. aqueum. Chemical & Pharmaceutical Bulletin. 1992;40(10):2671–2673. [Google Scholar]
- Resurreccion-Magno MHC, Villasenor IM, Harada N, Monde K. Antihyperglycaemic flavonoids from Syzygium samarangense (Blume) merr. And perry. Phytotherapy Research. 2005;19(3):246–253. doi: 10.1002/ptr.1658. [DOI] [PubMed] [Google Scholar]
- Smith RC, Reeves JC, Dage RC, Schnettler RA. Antioxidant properties of 2-imidazolones and 2-imidazolthiones. Biochemical pharmacology. 1987;36(9):1457–1460. doi: 10.1016/0006-2952(87)90110-9. [DOI] [PubMed] [Google Scholar]
- Srivastava R, Shaw AK, Kulshreshtha DK. Triterpenoids and chalcone from Syzygium samarangense. Phytochemistry. 1995;38(3):687–689. [Google Scholar]
- Stark T, Bareuther S, Hofmann T. Sensory guided decomposition of roasted cocoa nibs (Theobroma cacao) and structure determination of taste active poliphenols. Journal of Agricultural and Food Chemistry. 2005;53(13):5407–5418. doi: 10.1021/jf050457y. [DOI] [PubMed] [Google Scholar]
- Svetaz L, Tapia A, Lopez SN, Furlan RLE, Petenatti E, Pioli R, et al. Antifungal chalcones and new caffeic acid esters from Zuccagnia punctata acting against soybean infecting fungi. Journal of Agricultural and Food Chemistry. 2004;52(11):3297–3300. doi: 10.1021/jf035213x. [DOI] [PubMed] [Google Scholar]
- Syahida A, Israf DA, Lajisa NH, Shaaria K, Mohamede H, Wahaba AA, et al. Cardamonin, inhibits pro-inflammatory mediators in activated raw 264.7 cells and whole blood. European Journal of Pharmacology. 2006;538(1-3):188–194. doi: 10.1016/j.ejphar.2006.03.070. [DOI] [PubMed] [Google Scholar]
- Tatsuzaki J, Bastow KF, Nakagawa-Goto K, Nakamura S, Itokawa H, Lee KH. Dehydrozingerone, chalcone, and isoeugenol analogues as in vitro anticancer agents. Journal of Natural Products. 2006;69(10):1445–1449. doi: 10.1021/np060252z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trakoontivakorn K, Nakahara H, Shinmoto M, Takenaka M, Onishi-Kameyama H, Ono M, et al. Structural analysis of a novel antimutagenic compound, 4-hydroxypanduratin a, and the antimutagenic activity of flavonoids in a Thai spice, fingerroot (Boesenbergia pandurata Schult.) against mutagenic heterocyclic amines. Journal of Agricultural and Food Chemistry. 2001;49(6):3046–3050. doi: 10.1021/jf010016o. [DOI] [PubMed] [Google Scholar]
- Wong KC, Lai FY. Volatile constituents from the fruits of four Syzygium species grown in Malaysia. Flavour and Fragrance Journal. 1996;11(1):61–66. [Google Scholar]
- Wu JH, Tung YT, Wang SY, Shyur LF, Kuo YH, Chang ST. Phenolic antioxidants from the heartwood of Acacia confusa. Journal of Agricultural and Food Chemistry. 2005;53(15):5917–5921. doi: 10.1021/jf050550m. [DOI] [PubMed] [Google Scholar]
- Yang H, Protiva P, Gil RR, Jiang B, Baggett S, Basile MJ, et al. Antioxidant and cytotoxic isoprenylated coumarins from Mammea americana. Planta Medica. 2005;71(9):852–860. doi: 10.1055/s-2005-871257. [DOI] [PubMed] [Google Scholar]
- Yildirim A, Mavi A, Kara A. Determination of antioxidant and antimicrobial activities of Rumex crispus L. Extracts. Journal of Agricultural and Food Chemistry. 2001;49(1):4083–4089. doi: 10.1021/jf0103572. [DOI] [PubMed] [Google Scholar]