Abstract
The pinewood nematode (PWN) Bursaphelenchus xylophilus, vectored primarily by the sawyer beetle, Monochamus alternatus, is an important invasive pest and causal agent of pine wilt disease of Chinese Masson pine, Pinus massoniana. Previous work demonstrated that the ratios and concentrations of α-pinene∶β-pinene differed between healthy trees and those trees containing blue-stain fungus (and M. alternatus pupae). However, the potential influence of the altered monoterpene ratios and concentrations on PWN and associated fungi remained unknown. Our current results show that low concentrations of the monoterpenes within petri dishes reduced PWN propagation, whereas the highest concentration of the monoterpenes increased PWN propagation. The propagation rate of PWN treated with the monoterpene ratio representative of blue-stain infected pine (α-pinene∶β-pinene = 1∶0.8, 137.6 mg/ml) was significantly higher than that (α-pinene∶β-pinene = 1∶0.1, 137.6 mg/ml) representative of healthy pines or those damaged by M. alternatus feeding, but without blue stain. Furthermore, inhibition of mycelial growth of associated fungi increased with the concentration of the monoterpenes α-pinene and β-pinene. Additionally, higher levels of β-pinene (α-pinene∶β-pinene = 1∶0.8) resulted in greater inhibition of the growth of the associated fungi Sporothrix sp.2 and Ophiostoma ips strains, but had no significant effects on the growth of Sporothrix sp.1, which is the best food resource for PWN. These results suggest that host monoterpenes generally reduce the reproduction of PWN. However, PWN utilizes high monoterpene concentrations and native blue-stain fungus Sporothrix sp.1 to improve its own propagation and overcome host resistance, which may provide clues to understanding the ecological mechanisms of PWN's successful invasion.
Introduction
Monoterpenes (a class of C10 terpenes that consist of two isoprene units) are volatile compounds that occur in conifers and other plants. Many monoterpenes can increase exponentially in response to fungal pathogen and insect herbivore attack [1]–[5]. A number of inducible terpenoids play important roles in mediating interactions between plants and other organisms [6]–[8]. For example, monoterpenes have complex functions in conifer-bark beetle-fungal interactions, which may thereby enhance the invasion success of red turpentine beetle (Dendroctonus valens LeConte) by the beetle-fungal complex [9]. Both α-pinene and volatiles from some bacterial associates are capable of stimulating the growth of the native American fungus Leptographum procerum, resulting in complex interactions between host volatiles, bacteria, and fungi that can affect D. valens and D. ponderosae fitness [10]. The monoterpene present in induced reaction tissue can be toxic to adult beetles (e.g. Ips pini) and their brood, and inhibitory to their associated fungi [2], [11]. Furthermore, monoterpenes function in plant defense. For example, monoterpenes synthesized by plants have antifungal and antibacterial properties [2], [12]–[14] and are toxic to other organisms [7], [15], [16].
The pinewood nematode, (PWN), Bursaphelenchus xylophilus (Steiner et Buhrer) Nickle, is the causal agent of pine wilt disease [17]–[18]. PWN, an invasive species native to North America, is primarily vectored by the sawyer beetle, Monochamus alternatus Hope (Coleoptera: Cerambycidae). In its introduced range in China and Japan, PWN is highly invasive and disruptive to Masson pine (Pinus massoniana Lamb.), which is one of the most widely planted conifers in Asia [18]–[20]. Maturation feeding and the pupal stage of M. alternatus are two important vector phases in the development of pine wilt disease, which is associated with PWN's phytophagous and mycophagous stages. PWN is initially phytophagous, entering healthy host trees via wounds caused during maturation feeding of M. alternatus and feeding on the epithelial cells of the resin ducts [18], [21]–[22]. During its mycophagous stage, PWN feeds upon fungi inhabiting the dead trees, then accumulate around M. alternatus pupal chambers, migrate onto the vector and are subsequently carried by emergent M. alternatus adults to new host trees [23]–[24]. The dying or dead tree is colonized by various wood-inhabiting fungi, including blue-stain fungi, which provide a further food source for PWN [25]–[26]. Blue-stain fungi cause blue-staining in trees and greatly affect the number of PWN carried by the vector beetles [27]–[28]. Extensive evidence has demonstrated the nematicidal activity of monoterpenoids against PWN [29]–[31]. Moreover, the specific ratio of monoterpenes has been shown to influence the PWN behavior [32]–[33]. Besides these effects on nematodes, monoterpenes have also been shown to inhibit the mycelial growth of blue-stain fungi [13], [34]–[36].
The monoterpenes α-pinene and β-pinene represent the main constituents of Masson pine, and their relative amounts and proportions change in relation to the progression of pine wilt disease [32], [37]–[38]. Previous studies of monoterpenes in Masson pine have revealed that ratios and concentrations of α-pinene∶β-pinene differed between healthy trees and those containing blue-stain fungus (and M. alternatus pupae) [32]. The extent to which the altered concentrations and ratios of monoterpenes influence PWN propagation and associated fungal growth during the two important phases of its vector beetle remains unknown. To investigate this question, we assessed the change of the monoterpenes α-pinene and β-pinene in Masson pine caused by feeding by M. alternatus adults. Then we simulated the specific monoterpene ratios and concentrations representative of healthy pines, blue-stain infected pupation chambers of M. alternatus, and pines fed on by M. alternatus adults, and examined their effects on propagation of PWN and the mycelial growth of three strains of blue-stain fungal associates. We also tested PWN propagation on these three strains of blue-stain fungi.
Results
Volatiles analysis
Analysis of the changes in the monoterpenes of Masson pine by GC revealed that M. alternatus feeding significantly increased concentration of α-pinene in pine branch by 1.21 times (F 1, 8 = 30.779; p = 0.041), it has little influence on β-pinene concentrations (F 1, 8 = 0.075, p = 0.623) (Table 1).
Table 1. Changes in the monoterpenes of Masson pine material after Monochamus Alternatus feeding for 24 h.
| Treatment | Concentration (mg/ml) | |
| α-pinene (means ± SE) | β-pinene (means ± SE) | |
| Pine material before feeding | 0.0813±0.00261b | 0.0120±0.00661a |
| Pine material after feeding | 0.170±0.0332a | 0.0162±0.00598a |
Means in a column followed by different letter are significantly different at p<0.05, data were analyzed by a t-test for independent samples.
Concentration and ratio of α-pinene and β-pinene on the propagation of PWN
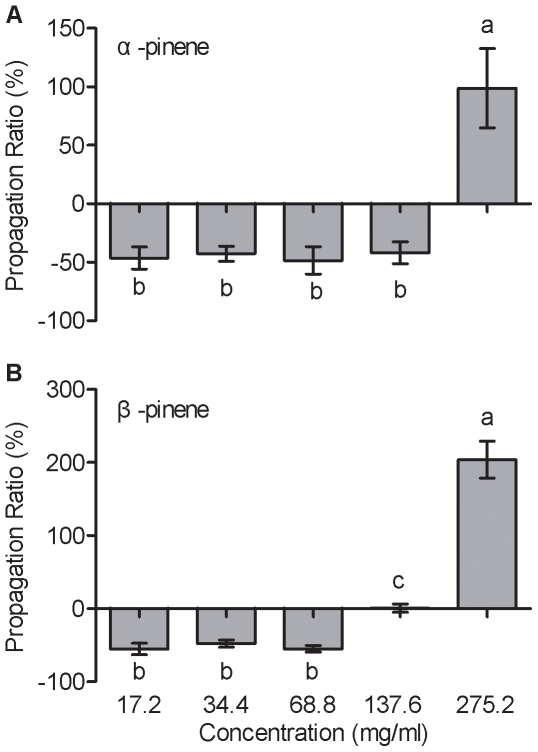
Propagation responses of PWN varied according to the concentrations of both α-pinene and β-pinene (Figure 1). Results indicate that there is no significant difference between low concentrations from 17.2 to 137.6 mg/ml (p>0.05), except for the concentration of β-pinene, 137.6 mg/ml (p<0.05; Figure 1B). At the highest concentration of 275.2 mg/ml, the propagation of PWN was significantly stimulated (α-pinene, Propagation Ratio (PR) = 98.80%; β-pinene, PR = 204.14%), and treatment with β-pinene had significantly higher propagation ratios than treatments with α-pinene (F 1, 14 = 0.815, p = 0.026). PWN propagation ratios were significantly higher at the highest monoterpene concentration compared to all other concentrations (Figure 1A, F 4, 39 = 13.686, p<0.05; Figure 1B, F 4, 39 = 80.763, p<0.05). In addition, the propagation rate of PWN treated with the resin ratio representative of blue-stain infected pine (α-pinene∶β-pinene = 1∶0.8, 137.6 mg/ml) was significantly higher (15.10%) than those treated with resin ratio associated with healthy pine/pine damaged by M. alternatus feeding (α-pinene∶β-pinene = 1∶0.1, 137.6 mg/ml) (F 1, 14 = 8.953, p = 0.01).
Figure 1. Propagation responses to different concentrations of α-pinene and β-pinene by pinewood nematode (PWN) using the cotton ball bioassay.
(A) α-pinene (17.2, 34.4, 68.8, 137.6, and 275.2 mg/ml), (B) β-pinene (17.2, 34.4, 68.8, 137.6, and 275.2 mg/ml). Each data bar represents the mean of eight independent replicates, and error bars represent standard errors of the mean. Different lowercase letters above or below bars indicate significant differences (Tukey's multiple comparison test; p<0.05).
Concentration and ratio of α-pinene and β-pinene on mycelial growth
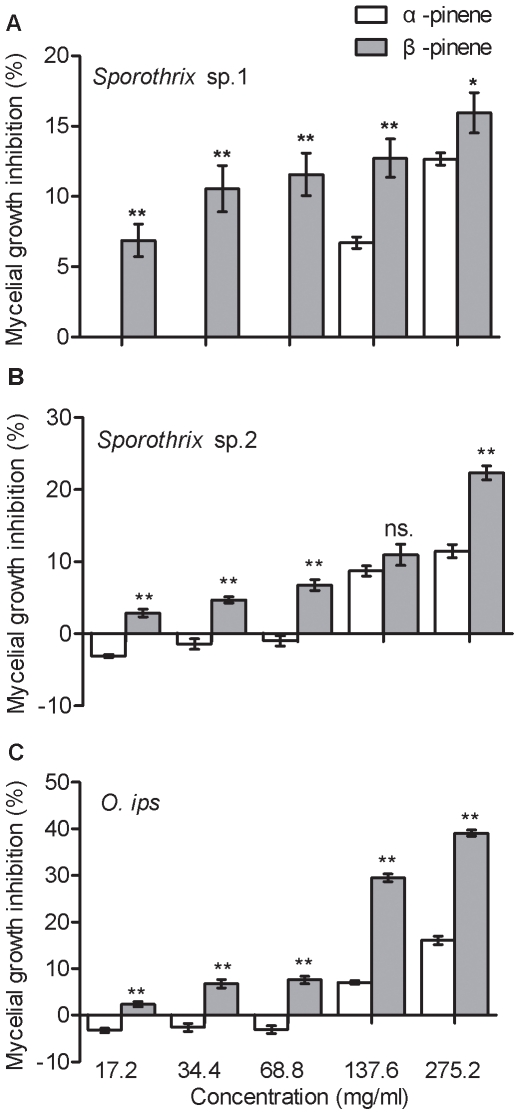
Results indicated that α-pinene and β-pinene inhibited mycelial growth of Sporothrix sp.1, Sporothrix sp.2, and Ophiostoma ips, and the effect was concentration-dependent (Figure 2). All concentrations of α-pinene alone showed weak activity, and there was a strong and significant correlation between the mycelial growth inhibition of fungal strains and the concentrations of α-pinene (y = 0.0532x-1.7975, r2 = 0.9561, p = 0.004 for Sporothrix sp.1; y = 0.0595x-3.4142, r2 = 0.8810, p = 0.018 for Sporothrix sp.2; y = 0.0800x-5.6840, r2 = 0.9571, p = 0.004 for O. ips). At the same concentrations, β-pinene exhibited stronger antifungal activities than α-pinene for each fungus (Figure 2) and the correlations between the inhibition of mycelial growth of fungal strains and the concentrations of β-pinene were also significant (y = 0.0288x+8.4650, r2 = 0.8376, p = 0.029 for Sporothrix sp.1; y = 0.0739x+1.6408, r2 = 0.9954, p<0.001 for Sporothrix sp.2; y = 0.148x+1.2992, r2 = 0.9170, p = 0.01 for O. ips).
Figure 2. Differences between the mycelial growth inhibition of blue-stain fungal strains (A Sporothrix sp.1, B Sporothrix sp.2, and C O. ips) on 2% malt extract agar in the presence of α-pinene and β-pinene at the same concentration.
Data were analyzed by a t-test for independent samples, for each blue-stain fungal strain in the presence of α-pinene and β-pinene at the same concentration. Each data bar represents the mean of eight independent replicates, and error bars represent standard errors of the mean (** p<0.01, * p<0.05 and ns. p>0.05).
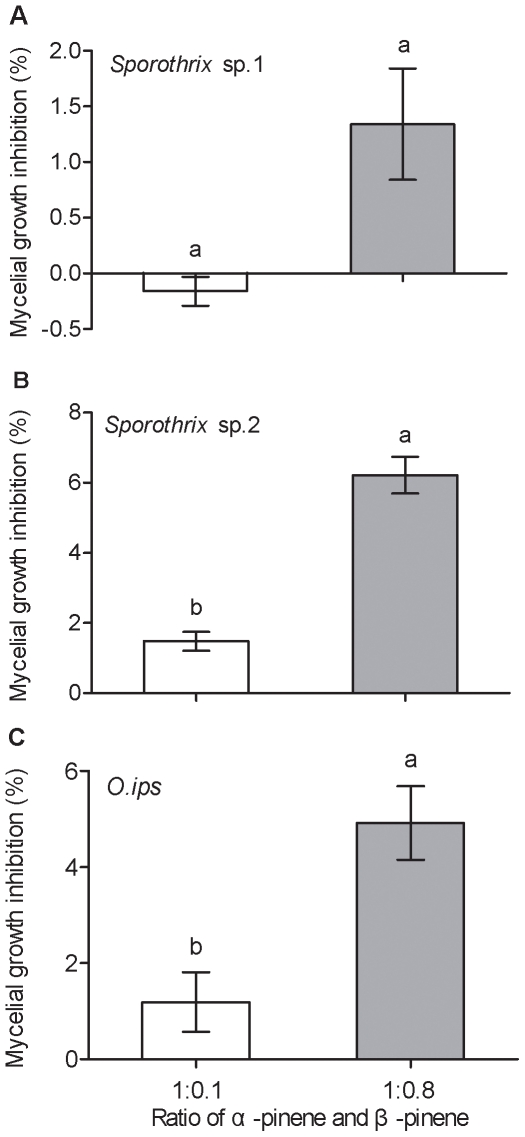
The resin ratio of α-pinene to β-pinene representative of blue-stain infected pine had a more significant negative effect than resin ratios associated with healthy pine/pine damaged by M. alternatus feeding on mycelial growth (Figure 3). Although the mycelial growth inhibition of Sporothrix sp.1 was less effective (F 1, 14 = 1.944, p = 0.315; Figure 3A), there were significant differences in the mycelial growth inhibition of Sporothrix sp.2 (F 1, 14 = 0.233, p = 0.009; Figure 3B) and O. ips (F 1, 14 = 0.438, p<0.001; Figure 3C) between the resin ratio of α-pinene∶β-pinene, 1∶0.1 and the resin ratio of α-pinene∶β-pinene, 1∶0.8 treatments, respectively.
Figure 3. Inhibition of mycelial growth of blue-stain fungus on 2% malt extract agar in the presence of different ratios of α-pinene and β-pinene at 137.6 mg/ml concentration.
(A) Sporothrix sp.1, (B) Sporothrix sp.2 and (C) O. ips. Data were analyzed by a t-test for independent samples for each blue-stain fungal strain. Significant differences within species are indicated by different lowercase letters (p<0.05). Values represent the means and standard errors of eight replicates for each treatment.
Propagation of nematodes on three blue-stain fungal strains
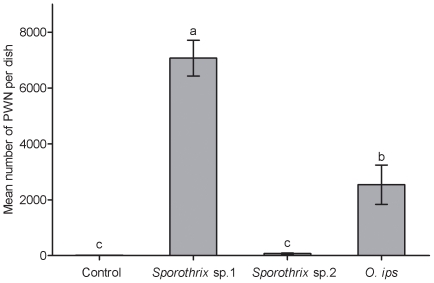
There were significant differences in the growth of PWN on Sporothrix sp.1, Sporothrix sp.2 and O. ips strains (F 3, 28 = 48.488, p<0.05; Figure 4). The mean number of nematodes was significantly higher on O. ips (mean ± SE, 2539.4±703.3) than on Sporothrix sp.2 (mean ± SE, 74.1±20.8) and controls (mean ± SE, 14.5±2.7), which was significantly less than that on Sporothrix sp.1 (mean ± SE, 7074.4±641.4, p<0.001). No significant statistical differences were found in the total numbers of nematodes between Sporothrix sp.2 and controls (p = 0.930).
Figure 4. Population growth of pinewood nematode on three blue-stain fungi. Nematode numbers were counted ten days after inoculation in the fungal culture at an initial number of forty individuals per dish.
Values represent the means and standard errors of eight replicates for each treatment. Different lowercase letters above bars indicate significant differences (Tukey's multiple comparison test, p<0.05).
Discussion
Chemical analysis of Masson pine volatiles from pine material before and after M. alternatus feeding revealed a significantly higher concentration of α-pinene in the pine material after feeding compared to control twigs, but there were no significant differences in β-pinene concentrations (Table 1). This finding is similar to that reported in other studies of insect damage Masson pine [38]. The accumulation of high levels of monoterpenes by conifers in response to herbivore and pathogen attack is an important component of the host defense system, which is associated with terpene synthesis [7], [12]. In contrast, for the M. alternatus pupal phase, β-pinene levels in blue-stained pine samples were significantly higher than those of healthy samples, while α-pinene was lower in blue-stained pines than in healthy trees [32]. Our results suggest that the changes of the ratios and concentrations of with respect to these two vector phases influence the progression of pine wilt disease through the relations of the PWN and associated fungi, and may provide clues to understanding the invasion mechanism.
The growth of many species of nematodes such as Caenorhabditis elegans [39], Haemonchus contortus [40], and the PWN [30], can be inhibited by monoterpenes. Our experiments indicated that PWN propagation ratios were significantly increased by the highest monoterpene concentration treatment (Figure 1). In general, healthy P. taeda stands growing in North America are highly resistant to PWN and contain a higher concentration of volatile terpenoids than P. thunbergii [41]. Whereas the susceptible species (P. densiflora) was completely destroyed, highly resistant species (P. taeda) can be damaged by PWN [23]. So PWN may have ability to utilize high concentrations of volatile terpenoids to overcome hosts. During pupation of and maturation feeding by M. alternatus adults in Masson pine, both wood with blue-stain fungi and branches with feeding wounds exhibited increased monoterpene concentrations. This suggests the possibility that PWN may retain their niches and related ecological traits over time, to make use of elevated concentrations of terpenoids, and this is consistent with previous findings that demonstrated that the niche requirements in its native range matching habitat availability in the new environment should enhance invasion success [42]–[43]. In addition, the fact that the propagation rate of PWN treated with the resin ratio of α-pinene and β-pinene (1∶0.8) representative of blue-stain infected pine was significantly higher (15.10%) than those treated with resin (1∶0.1) associated with healthy pine/pine damaged by M. alternatus feeding suggests that tree defenses are likely to impose strong natural selection on nematode populations, indicating that individuals adapted to the altered ratio will have more offspring. Therefore, the altered concentrations and ratios of the monoterpenes α-pinene and β-pinene may directly increase PWN populations, which play an important in helping the PWN overcome its hosts' defenses and successful invasion [44]. Further experiments are needed to test the effects of the monoterpenes released from both P. taeda (a native host) and P. massoniana (a non-native host) on PWN to fully understand the propagation mechanisms of PWN.
We demonstrated that changes in the monoterpenes of Masson pine inhibit the mycelial growth of fungal strains at the concentrations observed during these two important vector phases of M. alternatus, which is similar to previous research [13], [34]–[36]. The strains Sporothrix sp.1, Sporothrix sp.2, and O. ips are also suitable for PWN propagation (Figure 4), which may indirectly reduce PWN potential colonization. The inhibition of fungal growth is positively correlated to concentrations of the monoterpenes α-pinene and β-pinene (Figure 2). Moreover, our results show that each fungus may respond differently to changing concentrations of each monoterpene, which is consistent with previous results [45]. At the same concentrations, β-pinene exhibited stronger antifungal activities than α-pinene for each fungus (Figure 2). However, β-pinene increases in the blue-stain infected samples associated with the pupal stage of M. alternatus [32], which may indirectly reduce the propagation of PWN since blue-stain fungi are one of main food sources for PWN. In general, volatiles emitted by plants are always a mixture of several compounds released in response to the invading pathogens [7]–[8]. However, the altered ratios of α-pinene and β-pinene did not inhibit the mycelial growth of the blue-stain fungus Sporothrix sp.1, but did inhibit growth of Sporothrix sp.2 and O. ips (Figure 3). The blue-stain fungus Sporothrix sp.1 grew fastest and generated the highest population growth of PWN, which provides a huge number of PWN to be vectored by M. alternatus to new host trees.
Why was the PWN so successful in its invasion of China and Japan? Previous studies have shown that a number of factors contribute to invasion success of exotic species, and several hypotheses have been proposed, including phytotoxins, cellulose, PWN and bacteria, and terpenoids [46]–[49]. PWN is an excellent model organism to study the complex native multitrophic species interactions in its introduced range, including susceptible native tree species (Masson pine), a native insect vector (M. alternatus), and native associated fungi. PWN has become a highly destructive and invasive forest pest. Our study focused on the potential influence of the altered monoterpenes during the two important vector phases on PWN and associated fungi. The results indicate that high concentrations of monoterpenes could increase the propagation of PWN and decrease growth of its associated fungi, which may provide clues to understanding the ecological mechanisms of PWN's successful invasion. The monoterpene concentrations we used were higher than those we found to occur in trees. As such, the exact role of naturally occurring monoterpenes in the PWN system remains worthy of further exploration.
Materials and Methods
Source of Monochamus alternatus, nematodes and fungi
M. alternatus were harvested in March 2010 from naturally infested, dead Masson pine trees in Zhejiang Province. Bolts were placed in outdoor cages and emerging adults were collected daily in June 2010. M. alternatus pupal chambers, also collected from dead Masson pine trees in Zhejiang province in 2007, were placed in Baermann funnels to recover the nematodes. The nematodes were washed 3 times in M9 buffer (42.3 mM Na2HPO4, 22 mM KH2PO4, 85.6 mM NaCl, and 1 mM MgSO4, pH 7.0) [50], 3 times in sterile water, and were then cultured with the fungus Botrytis cinerea grown on 2% malt extract agar (MEA, 7 g Biolab malt extract, 7 g Biolab agar, and 350 ml deionised water, pH = 5.75). Prior to experiments, nematodes were rinsed from the culture dish lids with distilled water. Three fungal strains, Sporothrix sp.1 (CMW29982), Sporothrix sp.2 (CMW29978), and O. ips (CMW29981) were identified from pupal chambers of M. alternatus from Bursaphelenchus xylophilus–infested Masson pine in Zhejiang in 2007, China (our unpublished data). Sporothrix sp.1 and Sporothrix sp.2 are new, undescribed species in China. All cultures used in this study are maintained in the culture collection (CMW) of the Forestry and Agricultural Biotechnology Institute (FABI), University of Pretoria, Pretoria, South Africa.
Monochamus alternatus feeding and monoterpene analysis
Newly emerged M. alternatus were isolated, starved for 12 h, then reared by sleeving them with stainless steel mesh on the twigs of living Masson pine trees (five year old trees with height 180–200 cm and stem diameters 2.5–3.0 cm). Controls consisted of healthy Masson pine trees which did not have feeding by M. alternatus. After 24 h, approximately 500 mg of pine twigs with feeding wounds and healthy pine twigs were cut into ∼2 cm sections, and the entire branch sections were extracted with 4 ml hexane for 12 hr. Following extraction, the extracts were filtered through glass wool, and stored at −20°C. Just prior to GC (Agilent 7890A) analysis, extracts were filtered through 0.45 µm Teflon syringe filters.
The GC was fitted with a DB-WAX column (60 m length×0.25 mm i.d.×0.25 µm film, J&W Scientific, Folsom, CA, USA) and flame ionization detector (FID). The on-column injector temperature was 220°C and nitrogen (99.999%) was used as carrier gas (flow rate, 1 ml/min). The column temperature was programmed from an initial temperature of 50°C for 1 min, increased to 80°C at 3°C/min, increased to 120°C at 5°C/min, and then ramped to 230°C at 10°C/min (10 min hold time). Splitless injections of 1 µl were used for analyses, and mixtures of α-pinene and β-pinene were used as external standards. The identification of the chemical components of each sample was based on a comparison of their retention times with these standards. The concentrations of α-pinene and β-pinene were determined by extrapolation to external standard calibration curves of α-pinene and β-pinene (0.01, 0.025, 0.05, 0.25 and 0.5 mg/ml; r2 = 0.9999 for α-pinene and r2 = 0.9997 for β-pinene), respectively. All quantifications were calculated on calibrated GC-FID peak areas.
Monoterpene preparation
The monoterpenes α-pinene (Acros Organics, Purity 97%) and β-pinene (Alfa Aesar, Purity 99%) were chosen for this study because they represent the primary terpenoid constituents of Masson pine [32], and each was tested at five concentrations (17.2, 34.4, 68.8, 137.6, and 275.2 mg/ml) in hexane (Fisher chemicals, Purity 99%). The ratios of α-pinene and β-pinene used in experiments were 1∶0.1 (representative of healthy pine/pine damaged by M. alternatus feeding) and 1∶0.8 (representative of blue-stain infected pine) with the concentration of 137.6 mg/ml. These concentrations were chosen because both previous studies and our current study determined that these concentrations of α-pinene and β-pinene are representative of healthy pine samples, pine material after feeding, and blue-stain samples of P. massoniana, and thus provided a gradient of monoterpene concentration from which to test the propagative nematode's behavioral response [32]–[33], [37].
Concentration and ratio of α-pinene and β-pinene on propagation of PWN
A cotton ball bioassay was used to determine whether α-pinene and β-pinene exhibited propagation inhibition or stimulation toward PWN [30], [51]. Monoterpenes were prepared as described above, and the fungus B. cinerea was grown on 2% MEA. When the fungal colonies covered the petri dish (35 mm diameter), a mycelial disc (5 mm diameter) was taken out from the MEA medium center and placed in a suitable-sized sterile cotton ball (diameter approximately 5 mm). At this point, a 40 µl aqueous nematode suspension of eighty nematodes (juveniles∶adults = 40∶40) was injected. Subsequently, 50 µl of monoterpene solution were injected into the sterile cotton balls (eight replicates of each). Controls were injected with 50 µl of hexane. The dishes were then sealed with parafilm (Pechiney Plastic Packaging, Menasha, WI, USA) and incubated in the dark at 25°C for ten days. The living nematodes were separated from the culture by the Baermann funnel technique and counted [52].
Concentration and ratio of α-pinene and β-pinene on mycelial growth
To determine the effects of α-pinene and β-pinene on the growth of fungi, three fungal strains, i.e., Sporothrix sp.1, Sporothrix sp.2, and O. ips, were tested with a modified method of Hofstetter et al. (2005) [36]. A mycelial disc (5 mm diameter) was taken out from the periphery of an actively growing culture on 2% MEA for ten days and placed at the center of a 90×20 mm petri dish containing 20 ml of 2% MEA. For each treatment, 1 ml of test solution was added to a sterile filter paper (80 mm diameter) and the paper was placed onto the lid of a petri dish. Plates were rapidly sealed with parafilm and incubated at 25°C in darkness. Eight petri dish cultures for each treatment were used, including two controls (filter paper with 1 ml hexane and filter paper only). Radial growth was measured in four directions (0°, 90°, 180°, 270°) for each plate with the interval of 24 h for Sporothrix sp.1 and 48 hr for the other two until fungus reached the end of the petri dish. Tests were continued until one treatment of the fungus reached the end of the petri dish. Mean values from the final measurement of each dish were used for statistical analysis. Growth inhibition of treatment against control was calculated as a percentage, using the following formula: 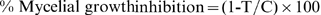 , where C is hyphal extension (mm, radius) of solvent controls and T is hyphal extension (mm, radius) of plates treated with monoterpenes [53].
, where C is hyphal extension (mm, radius) of solvent controls and T is hyphal extension (mm, radius) of plates treated with monoterpenes [53].
Propagation of nematodes on three blue-stain fungal strains
Population growth tests of nematodes were conducted with three blue-stain fungal strains (Sporothrix sp.1, Sporothrix sp.2, O. ips) grown on 2% MEA (2% MEA medium without fungus as control). When the fungal colonies covered the whole petri dish (35 mm diameter), eighty nematodes (juveniles∶adults = 40∶40) suspended in 40 µl of sterile water were inoculated in the middle of each dish (8 replicates). 10 days after the dishes were incubated at 25°C in darkness, nematodes were destructively sampled and separately extracted by the Baermann funnel technique [52].
Statistical Analyses
Data were analyzed with the statistical software SPSS 17.0 for Windows (SPSS Inc., Chicago, IL, USA). We used a t-test for independent samples to compare changes in Masson pine monoterpenes between infested (M. alternatus) pines and healthy pines, and also compared the mycelial growth inhibition of each blue-stain fungus treated with different ratios and concentrations of α-pinene and β-pinene. The relationship between the relative mycelial growth rate of each blue-stain fungus and the concentration of α-pinene or β-pinene was determined by linear regression [54]. The Propagation Ratio (PR) was determined using the formula: 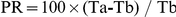 where Ta is the number of nematodes after chemical treatment and Tb is the number of nematodes after hexane treatment. Propagation ratio, mycelial growth inhibition and nematode population growth were analyzed with ANOVA and means were separated with Tukey's multiple comparison test.
where Ta is the number of nematodes after chemical treatment and Tb is the number of nematodes after hexane treatment. Propagation ratio, mycelial growth inhibition and nematode population growth were analyzed with ANOVA and means were separated with Tukey's multiple comparison test.
Ethics statement
All necessary permits were obtained for the described field studies from the Forest Pest Control and Quarantine Bureau of Zhejiang Province. These field studies do not involve endangered or protected species.
Acknowledgments
We sincerely thank Drs Zhudong Liu (Institute of Zoology, Chinese Academy of Sciences) and Li Li (Gui Zhou Normal University) for their comments, and Dr. Jacob Wickham, NSF Internationnal Postdoc Fellow at Institute of Chemistry, Chinese Academy of Sciences, for his critical review on the earlier versions of the manuscript. We greatly appreciate the constructive comments by three anonymous reviewers that helped improve the manuscript. We also thank Ping Jiang (Forest Pest Control and Quarantine Bureau of Zhejiang Province) for his assistance with field work.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by National Natural Science Foundation of China (30730074 and 30970369), National Basic Science Research Program (2009CB119200 and 2012CB114100) and Chinese Academy of Science Key Knowledge Innovation Program (KSCX2-EW-J-2). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Raffa KF. Tallamy DW, Raupp MJ, editors. Induced reactions in conifer–bark beetle systems. In Phytochemical Induction by Herbivores. 1991. pp. 245–276. John Wiley and Sons Inc., New York.
- 2.Raffa KF, Smalley EB. Interactions of pre-attack and induced monoterpene concentrations in host conifer defense against bark beetle-fungal complexes. Oecologia. 1995;102:285–295. doi: 10.1007/BF00329795. [DOI] [PubMed] [Google Scholar]
- 3.Klepzig KD, Kruger EL, Smalley EB, Raffa KF. Effects of biotic and abiotic stress on induced accumulation of terpenes and phenolics in red pines inoculated with bark beetle-vectored fungus. J Chem Ecol. 1995;21:601–626. doi: 10.1007/BF02033704. [DOI] [PubMed] [Google Scholar]
- 4.Tomlin ES, Antonejevic E, Alfaro RI, Borden JH. Changes in volatile terpene and diterpene resin acid composition of resistant and susceptible white spruce leaders exposed to simulated white pine weevil damage. Tree Physiol. 2000;20:1087–1095. doi: 10.1093/treephys/20.16.1087. [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez-Saona CR, Rodriguez-Saona LE, Frost CJ. Herbivore-induced volatiles in the perennial shrub, Vaccinium corymbosum, and their role in inter- branch signaling. J Chem Ecol. 2009;35:163–175. doi: 10.1007/s10886-008-9579-z. [DOI] [PubMed] [Google Scholar]
- 6.Byun-Mckay A, Godard KA, Toudefallah M, Maritin DM, Alfaro R, et al. Wound-induced terpene synthase gene expression in Sitka spruce that exhibit resistance or susceptibility to attack by the white pine weevil. Plant Physiol. 2006;140:1009–1021. doi: 10.1104/pp.105.071803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keeling CI, Bohlmann J. Genes, enzymes and chemicals of terpenoid diversity in the constitutive and induced defence of conifers against insects and pathogens. New Phytol. 2006;170:657–675. doi: 10.1111/j.1469-8137.2006.01716.x. [DOI] [PubMed] [Google Scholar]
- 8.Mumm R, Hilker M. Direct and indirect chemical defence of pine against folivorous insects. Trends Plant Sci. 2006;11:351–358. doi: 10.1016/j.tplants.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 9.Lu M, Wingfield MJ, Gillette NE, Mori SR, Sun JH. Complex interactions among host pines and fungi vectored by an invasive bark beetle. New Phytol. 2010;187:859–866. doi: 10.1111/j.1469-8137.2010.03316.x. [DOI] [PubMed] [Google Scholar]
- 10.Adams AS, Currie CR, Cardoza YJ, Klepzig KD, Raffa KF. Effects of bacteria and tree chemistry on the growth and reproduction of bark beetle fungal symbionts. Can J For Res. 2009;39:1133–1147. [Google Scholar]
- 11.Erbilgin N, Powell JS, Raffa KF. Effect of varying monoterpene concentrations on the response of Ips pini (Coleoptera: Scolytidae) to its aggregation pheromone: implications for pest management and ecology of bark beetles. Agric For Entomol. 2003;5:269–274. [Google Scholar]
- 12.Lewisohn E, Gijzen M, Croteau R. Defense mechanisms of conifers. Differences in constitutive and wound-induced monoterepene biosynthesis among species. Plant Physiol. 1991;96:44–49. doi: 10.1104/pp.96.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klepzig KD, Smalley EB, Raffa KF. Combined chemical defenses against an insect-fungal complex. J Chem Ecol. 1996;22:1367–1388. doi: 10.1007/BF02027719. [DOI] [PubMed] [Google Scholar]
- 14.Ludley KE, Robinson CH, Jickells S, Chamberlain PM, Whitaker J. Differential response of ectomycorrhizal and saprotrophic fungal mycelium from coniferous forest soils to selected monoterpenes. Soil Biol Biochem. 2008;40:669–678. [Google Scholar]
- 15.Gershenzon J, Dudareva N. The function of terpene natural products in the natural world. Nat Chem Biol. 2007;3:408–414. doi: 10.1038/nchembio.2007.5. [DOI] [PubMed] [Google Scholar]
- 16.Smith RH. The fumigant toxicity of three pine resins to Dendroctonus brevicomis and D. Jeffreyi. J Econ Entomol. 1961;54:365–369. [Google Scholar]
- 17.Togashi K, Shigesada N. Spread of the pinewood nematode vectored by the Japanese pine sawyer: modelling and analytical approaches. Popul Ecol. 2006;48:271–283. [Google Scholar]
- 18.Jones JT, Moens M, Mota M, Li H, Kikuchi T. Bursaphelenchus xylophilus: opportunities in comparative genomics and molecular host-parasite interactions. Mol Plant Pathol. 2008;9:357–368. doi: 10.1111/j.1364-3703.2007.00461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fu LG, Li N, Mills RR. Pinaceae in flora of China. Tome 4. In: Wu ZY, Raven PH, editors. Cycadaceae through Fagaceae. Beijing: Science Press & St Louis: Missouri Botanical Garden Press; 1999. 453 [Google Scholar]
- 20.Richardson DM. Ecology and Biogeography of Pinus. 1998. Cambridge University Press, Cambridge, UK.
- 21.Mamiya Y, Enda N. Transmission of Bursaphelenchus lignicolus (Nematoda: Aphelenchoididae) by Monochamus alternatus (Coleoptera: Cerambycidae). Nematologica. 1972;18:159–166. [Google Scholar]
- 22.Aikawa T, Togashi K. An effect of pine volatiles on departure of Bursaphelenchus xylophilus (Nematoda: Aphelenchoididae) by Monochamus alternatus (Coleoptera : Cerambycidae). Appl Entomol Zool. 1998;33:231–237. [Google Scholar]
- 23.Mamiya Y. Pathology of the pine wilt disease caused by Bursaphelenchus xylophilus. Annu Rev Phytopathol. 1983;21:201–220. doi: 10.1146/annurev.py.21.090183.001221. [DOI] [PubMed] [Google Scholar]
- 24.Togashi K. Factors affecting the number of Bursaphelenchus xylophilus (Nematoda: Aphelenchoididae) carried by newly emerged adults of Monochamus alternatus (Coleoptera: Cerambycidae). Appl Entomol Zool. 1989;24:379–386. [Google Scholar]
- 25.Maehara N, Futai K. Population changes of the pinewood nematode, Bursaphelenchus xylophilus (Nematoda: Aphelenchoididae), on fungi growing in pine-branch segments. Appl Entomol Zool. 2000;35:413–417. [Google Scholar]
- 26.Oh WS, Jeong PY, Joo HJ, Lee JE, Moon YS, et al. Identification and characterization of a dual-acting antinematodal agent against the pinewood nematode, Bursaphelenchus xylophilus. PLoS One. 2009;4:e7593. doi: 10.1371/journal.pone.0007593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seifert KA. Wingfield MJ, Seifert KA, Webber JF, editors. Sapstain of commercial lumber by species of Ophiostoma and Ceratocystis. Ceratocystis and Ophiostoma: taxonomy, ecology, and pathogenicity. 1993. pp. 141–151. APS, St. Paul.
- 28.Maehara N, Tsuda K, Yamasaki M, Shirakikawa S, Futai K. Effect of blue-stain fungi on the number of Bursaphelenchus xylophilus (Nematoda: Aphelenchoididae) carried by Monochamus alternatus (Coleoptera: Cerambycidae). Nematology. 2005;7:161–167. [Google Scholar]
- 29.Park IK, Park JY, Kim KH, Choi KS, Choi IH, et al. Nematicidal activity of plant essential oils and components from garlic (Allium sativum) and cinnamon (Cinnamomum verum) oils against the pine wood nematode (Bursaphelenchus xylophilus). Nematology. 2005;7:767–774. [Google Scholar]
- 30.Kong JO, Park IK, Choi KS, Shin SC, Ahn YJ. Nematicidal and propagation activities of thyme red and white oil compounds toward Bursaphelenchus xylophilus (Nematoda: Parasitaphelenchidae). J Nematol. 2007;39:237–242. [PMC free article] [PubMed] [Google Scholar]
- 31.Choi IH, Kim JH, Shin SC, Park IK. Nematicidal activity of monoterpenoids against the pine wood nematode (Bursaphelenchus xylophilus). Russ J Nematol. 2007;15:35–40. [Google Scholar]
- 32.Zhao LL, Wei W, Kang L, Sun JH. Chemotaxis of the pinewood nematode, Bursaphelenchus xylophilus, to volatiles associated with host pine, Pinus massoniana, and its vector Monochamus alternatus. J Chem Ecol. 2007;33:1207–1216. doi: 10.1007/s10886-007-9289-y. [DOI] [PubMed] [Google Scholar]
- 33.Zhao LL, Jiang P, Humble LM, Sun JH. Within-tree distribution and attractant sampling of propagative pinewood nematode, Bursaphelenchus xylophilus: An early diagnosis approach. For Ecol Manag. 2009;258:1932–1937. [Google Scholar]
- 34.Bridges J. Effects of terpenoid compounds on growth of symbiotic fungi associated with the southern pine beetle. Phytopathology. 1987;77:83–85. [Google Scholar]
- 35.De Groot RC. Growth of wood-inhabiting fungi in saturated atmospheres of monoterpenoids. Mycologia. 1972;64:863–870. [PubMed] [Google Scholar]
- 36.Hofstetter R, Mahfouz J, Klepzig KD, Ayres MP. Effects of tree phytochemistry on the interactions among endophloedic fungi associated with the southern pine beetle. J Chem Ecol. 2005;31:539–560. doi: 10.1007/s10886-005-2035-4. [DOI] [PubMed] [Google Scholar]
- 37.Fan JT, Sun JH, Shi J. Attraction of the Japanese pine sawyer, Monochamus alternatus, to volatiles from stressed host in China. Ann For Sci. 2007;64:67–71. [Google Scholar]
- 38.Su JW, Zeng JP, Qin XW, Ge F. Effect of needle damage on the release rate of Masson pine (Pinus massoniana) volatiles. J Plant Res. 2009;122:193–200. doi: 10.1007/s10265-008-0203-7. [DOI] [PubMed] [Google Scholar]
- 39.Lei J, Leser M, Enan E. Nematicidal activity of two monoterpenoids and SER-2 tyramine receptor of Caenorhabditis elegans. Biochem Pharmacol. 2010;79:1062–1071. doi: 10.1016/j.bcp.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 40.Camurca-Vasconcelos ALF, Bevilaqua CML, Morais SM, Maciel MV, Costa CTC, et al. Anthelmintic activity of Croton zehntneri and Lippia sidoides essential oils. Vet Parasitol. 2007;148:288–294. doi: 10.1016/j.vetpar.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 41.Kuroda K. Mechanism of cavitation development in the pine wilt disease. Eur J For Pathol. 1991;21:82–89. [Google Scholar]
- 42.Korsu K, Huusko A, Muotka T. Invasion of north European streams by brook trout: hostile takeover or pre-adapted habitat niche segregation? Biol Invasions. 2010;12:1363–1375. [Google Scholar]
- 43.Wiens JJ, Ackerly DD, Allen AP, Anacher BL, Buckley LB, et al. Niche conservatism as an emerging principle in ecology and conservation biology. Ecol Lett. 2010;13:1310–1324. doi: 10.1111/j.1461-0248.2010.01515.x. [DOI] [PubMed] [Google Scholar]
- 44.Cheng XY, Xie PZ, Cheng FX, Xu RM, Xie BY. Competitive displacement of the native species Bursaphelenchus mucronatus by an alien species Bursaphelenchus xylophilus (Nematoda: Aphelenchida: Aphelenchoidi- dae): a case of successful invasion. Bio Invasions. 2009;11:205–213. [Google Scholar]
- 45.Paine TD, Hanlon CC. Influence of oleoresin constituents from Pinus ponderosa and Pinus jeffreyi on growth of mycangial fungi from Dendroctonus ponderosae and Dendroctonus jeffreyi. J Chem Ecol. 1994;20:2551–2563. doi: 10.1007/BF02036191. [DOI] [PubMed] [Google Scholar]
- 46.Olesen JM, Eskildsen LI, Venkatasamy S. Invasion of pollination networks on oceanic islands: importance of invader complexes and endemic super generalists. Divers Distrib. 2002;8:181–192. [Google Scholar]
- 47.Richardson DM, Allsopp N, D'Antonio CM, Milton SJ, Rejmánek M. Plant invasions–the role of mutualisms. Biol Rev. 2000;75:65–93. doi: 10.1017/s0006323199005435. [DOI] [PubMed] [Google Scholar]
- 48.Torchin ME, Lafferty KD, Dobson AP, Mckenzie VJ, Kuris AM. Introduced species and their missing parasites. Nature. 2003;421:628–630. doi: 10.1038/nature01346. [DOI] [PubMed] [Google Scholar]
- 49.Wang Z, Wang CY, Fang ZM, Zhang DL, Liu L, et al. Advances in research of pathogenic mechanism of pine wilt disease. Afr J Microbiol Res. 2010;4:437–442. [Google Scholar]
- 50.Kikuchi TJ, Jones T, Aikawa T, Kosaka H, Ogura N. A family of glycosyl hydrolase family 45 cellulases from the pine wood nematode Bursaphelenchus xylophilus. FEBS Lett. 2004;572:201–205. doi: 10.1016/j.febslet.2004.07.039. [DOI] [PubMed] [Google Scholar]
- 51.Kawazu K, Nishii Y, Ishiii K, Tada M. A convenient screening method for nematicidal activity. Agric Biol Chem. 1980;44:631–635. [Google Scholar]
- 52.Van Bezooijen J. 2006. Methods and techniques for nematology, Wageningen.
- 53.Blodett JT, Stanosz GR. Differential inhibition of Sphaeropsis sapinea morphotypes by a phenolic compound and several monoterpenes of red pine. Phytopathology. 1997;87:606–609. doi: 10.1094/PHYTO.1997.87.6.606. [DOI] [PubMed] [Google Scholar]
- 54.Kerlinger FN, Pedhazur EJ. Multiple regression in behavioral research. New York: Holt, Rinehart, and Winston; 1973. pp. 282–284. [Google Scholar]