Abstract
Flagella mediated motility in Pseudomonas fluorescens F113 is tightly regulated. We have previously shown that motility is repressed by the GacA/GacS system and by SadB through downregulation of the fleQ gene, encoding the master regulator of the synthesis of flagellar components, including the flagellin FliC. Here we show that both regulatory pathways converge in the regulation of transcription and possibly translation of the algU gene, which encodes a sigma factor. AlgU is required for multiple functions, including the expression of the amrZ gene which encodes a transcriptional repressor of fleQ. Gac regulation of algU occurs during exponential growth and is exerted through the RNA binding proteins RsmA and RsmE but not RsmI. RNA immunoprecipitation assays have shown that the RsmA protein binds to a polycistronic mRNA encoding algU, mucA, mucB and mucD, resulting in lower levels of algU. We propose a model for repression of the synthesis of the flagellar apparatus linking extracellular and intracellular signalling with the levels of AlgU and a new physiological role for the Gac system in the downregulation of flagella biosynthesis during exponential growth.
Introduction
The Gac system (GacA/GacS) conforms a conserved [1] global regulatory system that regulates the production of the majority of exoproducts and virulence factors in the pseudomonads, independently of their life-style [2]–[6]. In the opportunistic pathogen Pseudomonas aeruginosa, the Gac system positively regulates the production of the autoinducer N-butyryl-homoserine lactone and the formation of the virulence factors pyocyanin, cyanide, lipase [7] and elastase [8], being necessary for full virulence in animal and plant hosts [9]. The Gac system also regulates most of the virulence factors that have been identified in the insect pathogen P. entomophila [10]. In phytopathogenic pseudomonads, such as P. syringae, the Gac system has been implicated in lesion formation, production of protease and the phytotoxin syringomycin [11], swarming motility [12] and alginate production [13], acting as a master regulator [14]. In saprophytic pseudomonads such as P. fluorescens, P. putida, P. aureofaciens, and others, the Gac system has been shown to regulate the production of secondary metabolites such as the fungicide 2,4-diacetylphloroglucinol (DAPG), cyanide, pyoluteorin, phenazine, the phytohormone indole-3-acetic acid [15]–[18], extracellular enzymes and fluorescent siderophores [19], [20], and lipopeptides such as amphisin [21] and putisolvin [22]. Mutations in the Gac system often result in the loss of biocontrol ability [17], [23].
The Gac system acts as an activator, in the regulation of the production of most of these exoproducts. This system, in response to a yet unidentified signal produced during the transition to stationary phase [24], activates the transcription of several small regulatory RNAs termed rsmX, rsmY and rsmZ [25], [26]. Different Pseudomonas produce one, two or three of these sRNAs [15], [25], [27]. In turn, the small RNAs titrate RNA-binding proteins (RsmA, RsmE and in some strains RsmI) that in the absence of the small RNAs bind to the 5′ regions of target messenger RNAs repressing their translation [28], [29]. However, in a few cases, negative regulation by the Gac system has been observed. This is the case for rhamnolipids and lipase production, and swarming motility in P. aeruginosa PAO1 [30].
We have previously shown that swimming motility of Pseudomonas fluorescens F113 which is important for rhizosphere colonization and biocontrol ability is also under negative control by the Gac system, since mutants affected in either of the gac genes produce larger swimming haloes than the wild-type strain [31], [32].We have also shown that this downregulation occurs through the repression of the flagellar master regulatory gene fleQ, resulting in reduced production of proteins of the flagellar apparatus, including the flagellin FliC [33].
The sadB gene encodes a cytoplasmic signal transduction protein that was initially characterized as a protein implicated in surface attachment in the initial steps of biofilm formation [34] and in repressing swarming motility by rhamnolipid sensing [35]. This protein contains a modified HD(N)-GYP domain although no phosphodiesterase activity has been demonstrated [35]. SadB has also been implicated in downregulation of swimming motility in F113, and this regulation is also mediated by downregulation of fleQ [33]. The aim of this work was to investigate the mechanism of negative regulation of swimming motility by the Gac system and SadB, and how they converge in the repression of fleQ.
Results
The Gac system regulates motility through the Rsm pathway
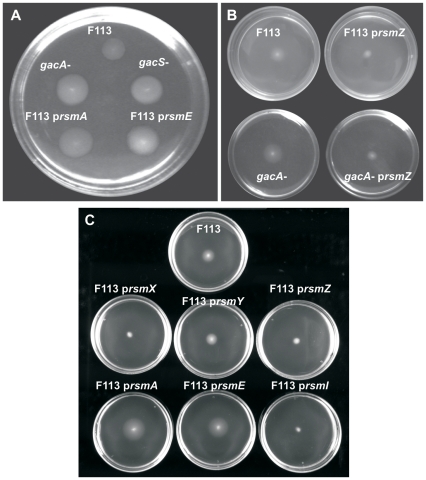
Sequence BLAST analysis of the genomic sequence of P. fluorescens F113 [36] showed that this bacterium possess genes encoding three Rsm proteins (rsmA,E and I) and three small RNAs (rsmX, Y and Z) homologous to their counterparts in other pseudomonads. Since Rsm proteins and rsm sRNAs have been shown to be in most cases redundant, we have chosen to analyze strains overexpresing rsmA, rsmE, rsmI and rsmX, Y and Z. To test whether negative regulation of motility occurred through the Rsm pathway, we hypothesized that in this case the overproduction of either of the Rsm proteins would mimic the phenotype of a gac mutant. In order to overexpress the P. fluorescens F113 rsmA, rsmE and rsmI genes, the amplified genes were cloned into vector pVLT31 (Table S1), under the control of the Ptac promoter and introduced into P. fluorescens F113 by triparental mating, to generate strains F113 prsmA, F113 prsmE and F113 prsmI. As shown in Fig. 1A and 1C, while the wild-type strain F113 showed normal motility, overexpression of either of the rsmA and E genes in F113 resulted in enhanced motility, a phenotype identical to the gacA and gacS mutants. However, overexpression of rsmI did not result in an increase in swimming motility, but in a slight decrease. Plasmid overexpression of the rsmZ and rsmX sRNA under the control of the same promoter (prsmZ and prsmX) resulted in a reduction of motility in the wild-type strain and suppressed the swimming phenotype of a gacA mutant (Fig. 1B–1C). Conversely, overexpression of rsmY did not have an effect on swimming motility. These results confirm that the Gac and Rsm systems act in the same pathway in repressing motility in P. fluorescens, although rsmI and Y do not participate in this regulation.
Figure 1. The Gac system regulates motility through the Rsm pathway.
(A) Analysis of the swimming motility of P. fluorescens F113 wild-type, F113 gacA mutant, F113 gacS mutant, F113 prsmA, and F113 prsmE. (B) Swimming motility of F113 wild-type strain and its isogenic gacA mutant harbouring the empty vector pVLT31 or pVLT31-rsmZ (prsmZ). (C) Swimming motility of F113 wild-type strain harbouring the empty vector pVLT31, pVLT31-rsmX (prsmX), pVLT31-rsmY (prsmY), pVLT31-rsmZ (prsmZ), pVLT31-rsmA (prsmA), pVLT31-rsmE (prsmE) or pVLT31-rsmI (prsmI).
Negative regulation of motility by the Gac system acts through downregulation of the fleQ gene transcription during exponential phase
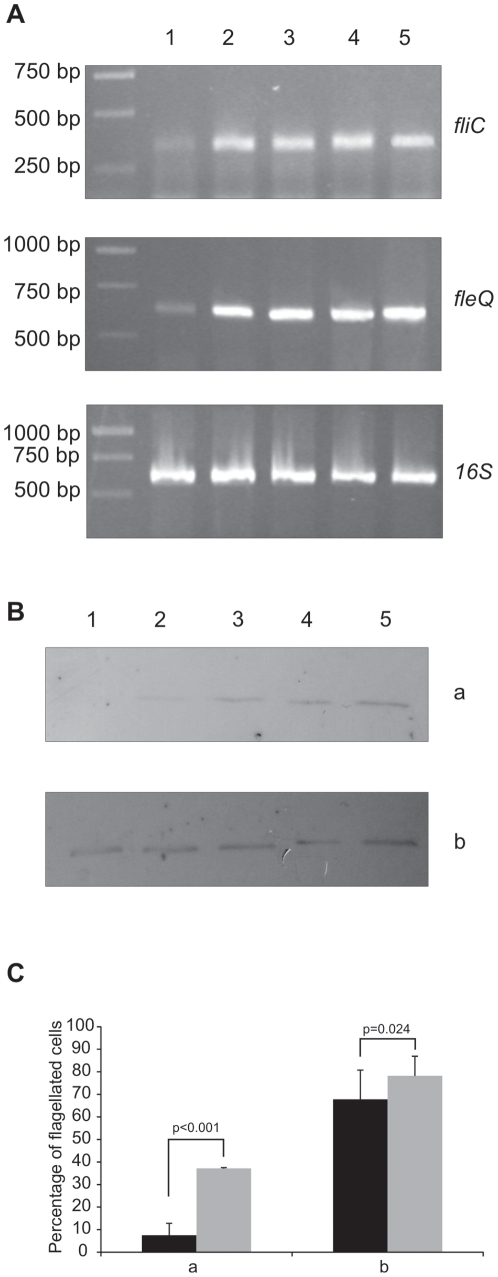
The fleQ gene encodes the major regulator of flagellar biosynthesis [37], [38] in pseudomonads. We have previously shown that hypermotile phenotypic variants of P. fluorescens F113 were characterized by overproduction of flagellin (FliC) and longer flagella [39]. Furthermore, we have shown that the GacAS pathway downregulates motility through repression of fleQ expression [33]. The expression of fleQ and fliC genes was also higher in the strains that overexpressed the rsmA and rsmE genes (Fig. 2A). These results clearly show that the negative regulation of motility by the Gac system acts through the Rsm pathway on the flagellar filament synthesis, by repressing the expression of the fleQ gene, resulting on a lower level of expression of genes encoding structural elements of the flagellum, including the fliC gene, which encodes flagellin.
Figure 2. Negative regulation of motility by the Gac system acts through downregulation of the fleQ gene transcription during exponential phase.
(A) RT-PCR expression analysis of fliC (primers fliCF-R), fleQ (primers fleQF-R), and 16S (primers 16SF-R) genes of F113 (1), gacA − (2), gacS − (3), F113 prsmA (4), and F113 prsmE (5). (B) Western blot analysis of external proteins from F113 (1), gacA − (2), gacS − (3), F113 prsmA (4), and F113 prsmE (5) during exponential phase (O.D.600 = 0.3) (a), and stationary phase (O.D.600 = 3.5) (b), reacted with an anti-flagellin antiserum. The observed band is approximately 35 KDa and corresponds to FliC. (C) Percentage of flagellated cells of F113 wild-type (black bar) or gacS − (grey bar) during exponential phase (O.D.600 = 0.3) (a), and stationary phase (O.D.600 = 3.5) (b). Statistical significance is shown.
Since the Gac system regulates secondary metabolism, especially at the transition from exponential to stationary growth, we hypothesized that the role of the Gac system on motility could be to downregulate flagellar synthesis during exponential growth. To test this hypothesis, total extracellular proteins from the wild-type strain, both gac mutants and the strains overexpressing the rsmA/E genes were precipitated from the growth medium during exponential phase (O.D.600 = 0.3) and late stationary phase (O.D.600 = 3.5). These proteins were probed with an anti-FliC (flagellin) antiserum [40]. As shown in Fig. 2B, during exponential phase the gac mutants and the strains overexpressing either of the rsm genes produced a higher amount of flagellin than the wild-type strain. However, during late stationary phase no differences in flagellin production were observed with the wild-type strain. Furthermore, transmission electron microscopy of negatively stained samples from the gac mutants and the wild-type strain showed that the percentage of flagellated cells were higher in the gacS mutant than in the wild-type strain during exponential growth (8% for wild-type strain, and 37% for gacS mutant) but not during stationary phase (68% for wild-type strain, and 78% for gacS mutant) (Fig. 2C). These results support the hypothesis of the role of the Gac system limiting flagella biosynthesis during exponential growth phase.
Gac-mediated downregulation of fleQ expression is independent of Vfr but dependent on AmrZ and AlgU
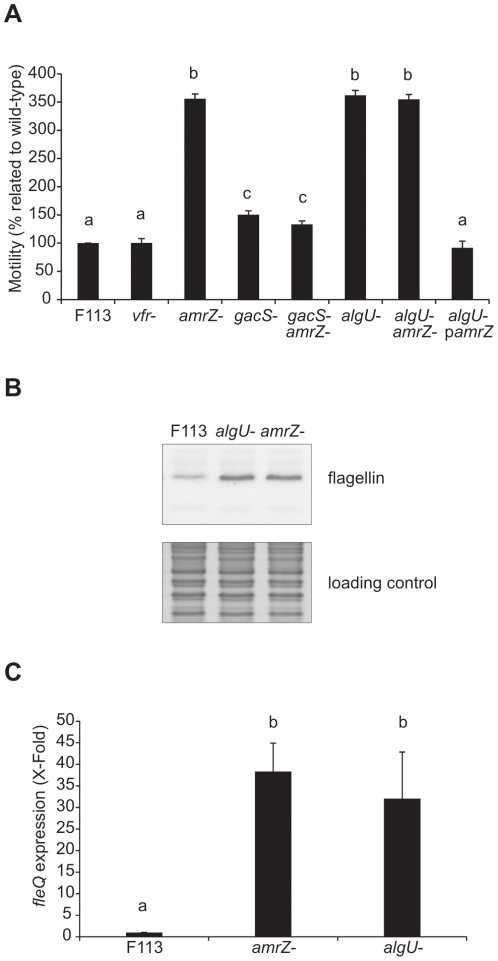
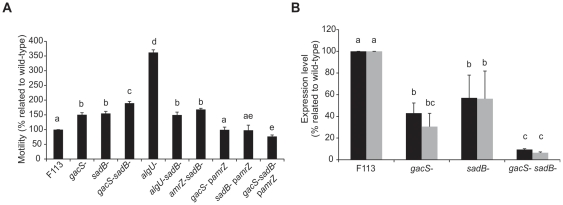
Gac regulation through the Rsm pathway takes place at the translational level since the RsmA and E proteins bind specific messenger RNAs blocking their translation [41], [42]. For negative regulation of motility, the RNA blocked should encode a repressor of fleQ transcription. Although several proteins such as MorA, FleN and AlgU have been shown to modulate fleQ expression in different pseudomonads [43]–[45], a direct role in repressing fleQ transcription by binding to the promoter region has been suggested for the global regulatory protein Vfr [46] and for AmrZ [47] in P. aeruginosa. Furthermore, Vfr has been implicated in the regulation of two Gac-controlled traits in P. aeruginosa: elastase and pyocyanin production [48]. Since the F113 fleQ promoter region contains a putative Vfr binding site, we decided to test whether Vfr was implicated in Gac-mediated fleQ downregulation. For this purpose, we used primers vfrF and vfrR (Table S2) to amplify an internal fragment of the vfr gene from F113 genomic DNA and cloned it into pVIK107 (Table S1). This construct was integrated into the F113 genome by homologous recombination and the resulting strain F113 vfr − was checked by PCR and Southern-blot, using the amplified vfr fragment as the probe. However, the vfr mutant did not show significant differences in motility compared to the wild-type strain (Fig. 3A), indicating that Vfr is not implicated in this pathway. In order to generate an amrZ mutant, an internal fragment of the gene was amplified with primers amrZF and amrZR (Table S2) and cloned into the pK19mobsacB vector (Table S1). The resulting plasmid was integrated into F113 genome by homologous recombination and the disruption of the gene was checked by PCR and Southern blot. As shown in Fig. 3A the amrZ mutant showed enhanced motility with respect to the wild-type strain and the gac mutants. Expression of fleQ and fliC was higher in the amrZ mutant than in the wild-type (Fig. 3B–3C). In order to test whether the Gac system and AmrZ were acting in the same pathway, a double mutant gacS-amrZ was constructed by disruption of the gacS gene in an amrZ mutant background (Table S1). The resulting double mutant had the same motility phenotype than the gacS mutant (Fig. 3A) showing genetic interaction, indicating that both genes participate in the same regulatory pathway. Since AlgU has also been implicated in regulation of motility in other pseudomonads, and it has been described as the sigma factor required for the expression of amrZ [47], we constructed an algU mutant by gene disruption by cloning an internal fragment of the algU gene into pK19mobsacB vector (Table S1). This plasmid was integrated into the F113 genome by homologous recombination and the disruption of algU was checked by PCR and Southern blot. The algU mutant showed a similar swimming phenotype than the amrZ mutant (Fig. 3A) and overexpressed fleQ and fliC (Fig. 3B–3C). A double mutant algU-amrZ showed the same phenotype than the independent mutants (Fig. 3A), indicating that both genes act in the same signalling pathway. Furthermore, ectopic expression of amrZ (pamrZ) in an algU mutant background, restored wild-type motility (Fig. 3A), showing that the phenotype of the algU mutant is caused by the lack of expression of amrZ.
Figure 3. Gac-mediated downregulation of fleQ expression is independent of Vfr but dependent on AmrZ and AlgU.
(A) Analysis of the swimming motility of F113 wild-type, mutants in the Gac-AlgU cascade, and complemented amrZ (pamrZ). Different letters indicate significant statistical difference (p<0.05). At 18 h, F113 wild-type strain swimming halo diameter is 11±0.55 (mm). (B) Western blot analysis of extracellular proteins from F113 wild-type strain and its isogenic mutants amrZ − and algU −, reacted with an anti-flagellin antiserum. Loading control corresponds to a Coomassie-stained gel portion. (C) qRT-PCR expression analysis of the fleQ gene (primers qfleQF-R) in F113 wild-type, amrZ − and algU −. 16S gene expression (primers 16SF-R) was used for normalization. To control for DNA contamination, PCR of RNA was performed using the same primer pairs. Different letters indicate significant statistical differences (p<0.05).
RsmA binds the algUmucABD polycistronic mRNA
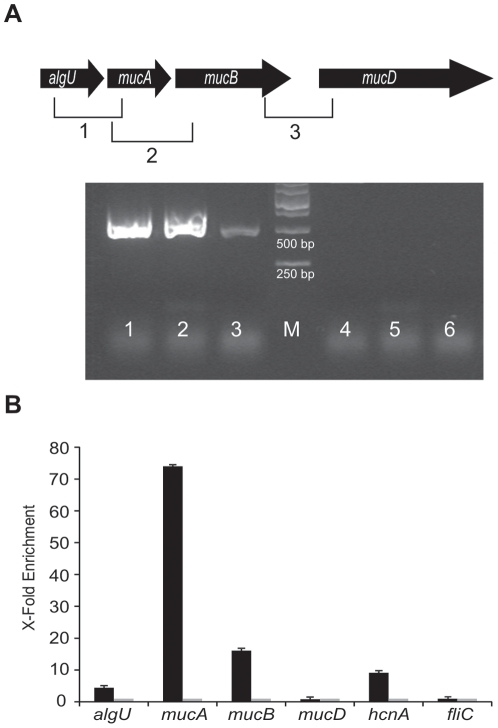
In P fluorescens F113 the algU gene is followed by three genes encoding the antisigma factors MucA and B and the protease MucD. In order to test whether these four genes form an operon, an RT-PCR experiment was performed by designing primers for the co-amplification of adjacent genes. As shown in Fig. 4A, amplicons of the expected size were obtained for algU-mucA, mucA-mucB and mucB-mucD indicating that the three genes are encoded in a polycistronic mRNA. In order to test the hypothesis of RsmA binding to this mRNA, an RNA immunoprecipitation assay was performed. For this assay a C-Terminus HA-tagged RsmA protein was generated by PCR and cloned into expression vector pVLT31 (Table S1). This RsmA-HA protein was functional, since overexpression of the construct mimics the phenotype of the overexpression of the rsmA gene (not shown). RNA immunoprecipitation showed that the RsmA protein binds to the algUmucABD mRNA (Fig. 4B). Furthermore, binding of RsmA to this RNA was stronger than binding to the hcnA mRNA, that has been previously shown to be post-transcriptionally regulated by binding of RsmA/RsmE to its 5′ region [28], [29]. Binding of RsmA seems to be located in the region upstream of mucA, since the higher amount of immunoprecipitated RNA is located in this region.
Figure 4. RsmA binds the algUmucABD polycistronic mRNA.
(A) RT-PCR of adjacent genes in the polycistronic mRNA algU-mucA-mucB-mucD. PCR of cDNA using the primer pairs qalgUF-qmucAR (lane 1), qmucAF-qmucBR (lane 2) and qmucBF2-qmucDR (lane 3) or PCR of RNA using the same primer pairs qalgUF-mucAR (lane 4), qmucAF-qmucBR (lane 5) and qmucBF2-qmucDR (lane 6), M marker. (B) RNA-IP assay of F113 wild-type strain harbouring the pVLT31-rsmAHA plasmid. qRT-PCR of HA-immunoprecipitated RNA (black bar) or IgG-immunoprecipitated RNA (mock, grey bar) using the primer pairs qalgUF-R (algU), qmucAF-R (mucA), qmucBF-R (mucB), qmucDF-R (mucD), qhcnAF-R (hcnA) and qfliCF-R (fliC). The fliC gene was used for normalization.
SadB and GacAS regulate algU expression
We have previously shown that not only GacAS but also SadB downregulates motility through FleQ [33]. In order to test whether fleQ regulation by SadB was through the AlgU-AmrZ pathway, double mutants algU-sadB and amrZ-sadB were constructed. As shown in Fig. 5A, both double mutants presented the same phenotype than the sadB mutant, indicating that the three genes act in the same pathway. We have previously shown [33] that a sadB-gacS mutant presents an additive swimming phenotype, indicating that the Gac and SadB pathways converge on the regulation of algU. We also analyzed the expression of the algU and amrZ in the gacS, sadB and gacS-sadB mutant backgrounds. Expression of both genes was clearly reduced in the individual mutants and very low in the double mutant (Fig. 5B), confirming the cooperative regulation of algU by the Gac and SadB pathways. Furthermore, ectopic expression of the amrZ gene (pamrZ) in the gacS, sadB and gacS-sadB mutants complemented the swimming phenotype of the mutants (Fig. 5A).
Figure 5. SadB and GacAS regulate algU expression.
(A) Analysis of the swimming motility of F113 wild-type, mutants in the Gac-SadB-AlgU cascade, and amrZ complementation (pamrZ). Different letters indicate significant statistical differences (p<0.05). At 18 h, F113 wild-type strain swimming halo diameter is 11±0.55 (mm). (B) qRT-PCR expression analysis of algU (black) and amrZ (grey) genes (primers qalgUF-R and qamrZF-R, respectively) in F113 wild-type, gacS −, sadB −, and double mutant gacS-sadB. 16S gene expression (primers 16SF-R) was used for normalization. Different letters indicate significant statistical differences (p<0.05).
Discussion
Although for most traits the Gac system acts as a positive regulator, for some traits such as swarming motility and rhamnolipids and lipase production it may function as a negative regulator [30]. This negative role of the Gac system is especially clear for swimming motility in P. fluorescens F113, since mutations in the gacA or gacS genes results in increased motility [31], [32]. The relevance of this trait and of the Gac system for rhizosphere colonization is highlighted by the fact that phenotypic variants arising during rhizosphere colonization harbour mutations in the gac genes, being more motile than the wild-type strain. Furthermore, several of these variants, selected because of their increased motility, were more competitive for rhizosphere colonization than the wild-type strain [32]. The finding that the FliC and FliD proteins are among the most highly overproduced proteins in gac mutants, in P. aeruginosa [27] and P. fluorescens [3], suggests that negative regulation of motility by the Gac system may be a general feature in pseudomonads.
Activation through the Gac system occurs post-transcriptionally. Briefly, an unidentified signal stimulates autophosphorylation of the GacS sensor [24]. The phosphate group is then transferred to the response regulator GacA by a phospho-relay mechanism, activating directly or indirectly the transcription of genes encoding small RNAs, termed rsmX, Y and Z [49]. These riboregulators bind to RNA-binding proteins such as RsmA, E and I, which have the ability to bind specific mRNAs blocking their translation [28], [50]. In such system, an active Gac system results in the Rsm proteins bound to the small regulatory RNAs and therefore the target mRNAs are translated. Conversely, in the absence of a functional Gac system (for instance strains harbouring a gac mutation), the Rsm proteins would be bound to their target mRNAs that would not be translated [51]. This model easily explains positive regulation, since translation of the target mRNAs is required for the production of the trait. Here we show that the Rsm pathway (excluding rsmI and Y) is also used for negative regulation of motility in P. fluorescens, since overexpression of either of the rsmA or E genes mimics the phenotypes of the gac mutants. Our results also show that for repression of swimming motility, the RsmA and RsmE proteins are functionally equivalent (Fig. 1A). This functional equivalence has also been shown for other positively regulated traits such as exoprotease, hydrogen cyanide, and 2,4-diacetylphloroglucinol in P. fluorescens CHAO [42]. However, it is not true for all Rsm-controlled traits. We have shown here that neither rsmI nor rsmY participate in negative regulation of motility in strain F113. It has also been shown that in P. aeruginosa, the BfiSR two-component system regulates biofilm formation through rsmZ but not through rsmY [52]. It is interesting to note that several pseudomonads, such as P. aeruginosa, produce a single Rsm protein [53] whereas other as it is the case for P. fluorescens F113, produce more than two Rsm-like proteins [42].
We have previously shown that hypermotile phenotypic variants of strain F113 isolated from the rhizosphere harboured gac mutations, produced higher amounts of the FliC protein and possessed longer flagella than the wild-type strain [39]. Since the major activator of flagellar synthesis is the FleQ protein [38], we decided to test whether the Gac system acted through the fleQ gene to regulate swimming motility. Our results clearly show that the Gac system dramatically influences the level of transcription of the fleQ and fliC genes and that this influence is enforced through the RsmA and RsmE proteins (Fig. 2A). These results are consistent with those reported in P. aeruginosa that showed that in a gacA and rsmYZ mutants, FliC and FliD (the flagellar cap protein) had increased expression (between 7.5 and 10.2-fold) when compared to wild-type strain, being the most overproduced proteins in both mutants [27].
Since Gac regulation of motility occurs through the Rsm pathway, a direct effect on the transcription of activators such as fleQ can be discarded. Two alternative ways are possible. The RsmA and E proteins could bind to the mRNA of the transcriptional activators stabilizing them or the Rsm proteins would bind to the mRNAs encoding transcriptional repressors of the activator genes. The former possibility has been shown to occur with the RsmA homologue CsrA in Escherichia coli [54]. In this bacterium, CsrA binds the mRNA of the flhDC genes, which encode the master operon regulating flagellar biosynthesis. The second possibility, i.e. the RsmA and E binding of a mRNAs encoding a transcriptional repressor of fleQ could explain the observed phenotype of gac mutants. We have tested two putative repressors, Vfr and AmrZ [46], [47]. Our results discard the implication of Vfr in Gac regulation of swimming motility (Fig. 3A). Conversely, we have shown that Gac-mediated regulation occurs through the AmrZ repressor. This repressor has been shown to mediate the transition from motile P. aeruginosa cells to the non-motile (aflagellated) mucoid phenotype [47]. In P. aeruginosa, this transition occurs during chronic infections and the expression of amrZ requires the AlgU sigma factor. Similarly, in P. fluorescens F113 we have shown here a similar regulatory cascade. A functional algU gene is required for the expression of amrZ and repression of fleQ, resulting in reduced flagellar production.
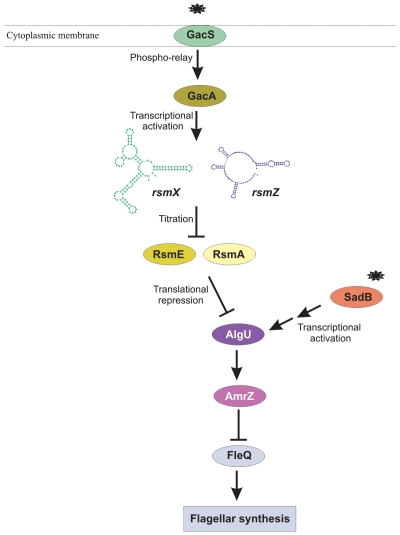
We have also extended this regulatory network by showing that the expression of algU and possibly its translation, is co-ordinately regulated by the Gac system and the sadB gene. This has allowed us to propose a model for the environmental regulation of motility through repression of the synthesis of components of the flagellar apparatus (Fig. 6). According to this model, a yet unidentified environmental signal is perceived by the GacS protein that autophosphorilates and phosphorilates the GacA protein [49]. Phosphorilated GacA is responsible for the expression of small regulatory RNAs, able to titrate the Rsm proteins [5], [26]. The Rsm proteins are also able to bind a polycistronic mRNA, encoding the algU, mucA, mucB and mucD genes (Fig. 4) in competition with the rsmX/Z sRNAs, resulting in a decrease in the transcription/translation of the algU gene. The algU gene is also transcriptionally regulated by SadB in response to a cytoplasmatic signal, possibly c-diGMP [55]. AlgU is the sigma factor required for amrZ expression and AmrZ downregulates fleQ expression, resulting in a lower production of flagellar components, including the flagellin FliC. This model links signal perception by a membrane receptor (GacS) and a cytoplasmic receptor (SadB) with the production of the components of the flagellar apparatus activated by FleQ and identifies AlgU as an important node for the environmental regulation of motility. In this sense, the kinB gene which requires AlgU for expression [56]–[58], is also implicated in motility regulation in P. fluorescens F113, since a kinB mutant shows hypermotility [33]. It has been also shown that in P. syringae, AlgW a periplasmic protease that controls the levels of AlgU, is a key negative regulator of flagellin abundance [59].
Figure 6. Hypothetical model for the environmental regulation of flagellar synthesis in P. fluorescens F113 through the Gac-SadB cascades.
Arrows indicate positive control and perpendicular lines negative control.
Gac-mediated positive regulation typically occurs in the transition from exponential to stationary phase. In this sense, the Gac system has been defined as a global activator of secondary metabolism in stationary phase. Furthermore, a relation between the Gac system and RpoS, the stationary phase sigma factor, has been described [14], [60], [61]. Here, we present evidence showing that in P. fluorescens the Gac system is also active during exponential phase, being able to repress flagellar synthesis at this stage. This role is also supported by the finding of gacS expression being maximal at mid-exponential phase [62]. Therefore, we propose a second physiological role for the Gac system: the repression or downregulation of specific traits like flagella synthesis during exponential phase.
Methods
Bacterial strains, plasmids, and growth conditions
Bacterial strains, plasmids and primers used in this study are shown in Table S1 and S2. All the Pseudomonas fluorescens strains used here are derivatives of the biocontrol strain F113 [63]. All the PCR fragments obtained in this study were initially cloned in the pGEM®-T Easy vector according to manufacturer's instructions (Promega). Mutants were obtained by single homologous recombination of amplified internal fragment from the gene using primer pairs vfrF-vfrR, amrZF-amrZR and algUF-algUR (Table S2) and cloned into the suicide vector pVIK107 [64], pK19mobsadB [65] or pG18mob2 [66]. Mutants were checked by Southern blotting and by PCR. Overexpression of rsmA, rsmE, rsmI, rsmX, rsmY, rsmZ and amrZ genes was achieved by cloning them under the control of the IPTG-inducible promoter present in the pVLT31 plasmid [67], for this purpose primers rsmAextF-rsmAextR, rsmEextF-rsmEextR, rsmIextF-rsmIextR, rsmXextF-rsmXextR, rsmYextF-rsmYextR, rsmZextF-rsmZextR and amrZextF-amrZextR were used (Table S2). Hemagglutinin peptide (HA) was fused in-frame to RsmA protein at the C-terminal by PCR using primers rsmAextF and HArsmAR (Table S2), and the PCR product was ligated to pVLT31 vector. Plasmids were mobilized into P. fluorescens by triparental mating, using pRK600 as the helper plasmid [68]. P. fluorescens strains were grown in SA medium [69] overnight at 28°C; solid growth medium contained 1.5% (w/v) purified agar. Escherichia coli strains were grown overnight in Luria-Bertani (LB) medium [70] at 37°C. The following antibiotics were used, when required, at the indicated concentrations: rifampicin (Rif), 100 µg/mL; ampicillin (Amp), 100 µg/mL; tetracycline (Tet), 10 µg/mL for E. coli or 70 µg/mL for P. fluorescens; kanamycin (Km), 25 µg/mL for E. coli or 50 µg/mL for P. fluorescens; and gentamicin (Gm), 10 µg/mL for E. coli or 4 µg/mL for P. fluorescens.
Transmission electron microscopy
Formvar-coated grids were placed on the top of a drop of bacterial culture either at 0.3 or 3.5 O.D.600 for 30 s to allow bacterial adhesion. Liquid was eliminated with filter paper and grids were stained for 1 min with a 1% solution of potassium phosphotungstate and washed 3 times for 1 min with a drop of water. Grids were air-dried and observed in a Jeol JEM1010 microscope.
DNA techniques
Standard methods [71] were used for DNA extraction, gene cloning, plasmid preparation and agarose gel electrophoresis. Southern blots were performed with a non-radioactive detection kit (DIG Luminescent Detection Kit for Nucleic Acids), and a chemiluminescence method was used to detect hybridization signals according to the instructions of the manufacturer (Roche Boehringer Mannheim). PCR reactions were performed using the Tth enzyme (Biotools) under standard conditions. DNA sequencing was done by chain-termination method using DyeDeoxy terminator cycle sequencing kit as described by the manufacturer (Applied Biosystems).
The sequences of the P. fluorescens F113 rsmA, rsmE, rsmI, rsmX, rsmY, gacS, sadB, vfr, amrZ, algU-mucA-mucB has been deposited in GenBank under accession numbers: rsmA EU165536, rsmE EU165537, rsmI JN382566, rsmX JN382569, rsmY JN382570, gacS JN382567, sadB JN382568, vfr JN382563, amrZ JN382562, algU-mucA-mucB JN382565. The complete genomic sequence of Pseudomonas fluorescens F113 has been deposited in GenBank under accession number CP003150
Swimming assays
SA medium plates containing 0.3% purified agar were used to test swimming abilities. The cells from exponentially growing cultures were inoculated into the plates using a toothpick. Swimming haloes were measured after 18 h of inoculation. Every assay was performed three times with three replicates each time.
Protein extraction and Western blots
Proteins were extracted from 200 mL exponential (O.D.600 = 0.3) and stationary (O.D.600 = 3.5) phase grown cultures. In order to detach the flagella, the cultures were agitated by vortexing for 2 min and then centrifuged for 20 min at 12,000 r.p.m and extracellular proteins were extracted from the supernatant by precipitation for 2 h at 4°C with 10% (w/v) trichloroacetic acid, followed by two washes with chilled acetone, and were finally resuspended in Laemmli buffer [72]. Proteins were resolved by 12% SDS-PAGE and stained with Coomassie blue. The same electrophoretic conditions were used for Western blotting. Acrylamide gels were transferred onto nitrocellulose membranes for 1 h under standard conditions. The membranes were incubated with a 1∶10,000 dilution of an anti-flagellin antiserum [40] for 16 h at 4°C and then with a peroxidase-tagged secondary antibody (anti-rabbit immunoglobulin) for 1 h at room temperature. The enhanced chemiluminescence (ECL) method and Hyperfilm ECL (Amersham Biosciences) were used for development.
Gene expression analysis
Total RNA was extracted using Trizol® according to manufacturer's specifications (Invitrogen) from P. fluorescens strains grown at 0.8 O.D.600 in LB medium. Genomic DNA remains were removed by RQ1 RNase-Free DNase treatment (Promega) for 30 minutes at 37°C. After that, RNA was purified using Trizol®. The concentration of RNA was spectrophotometrically determined in a Nanodrop® and integrity was verified in denaturing agarose gels. All RNA samples were stored at −80°C.
RT-PCRs were carried out using Illustra Ready-To-Go™ RT-PCR Beads kit from Amersham GE Healthcare. qRT-PCRs were performed in two steps: a first step of cDNA synthesis using the SuperScript®III First-Strand Synthesis System from Invitrogen and a second step of qPCR using the Power SYBR®Green PCR Master Mix from Applied Biosystems. In both cases, gene expression was measured into different backgrounds and normalized by using 16S RNA as internal control. Every assay was performed three times with three replicates each time.
RNA immunoprecipitation
RNA immunoprecipitation (RNA-IP) was performed using the same procedure as that described by Lin et al. [73] with some modifications. Briefly, 3 h post-induction with 1 mM IPTG, F113 strain harbouring the pVLT31-rsmAHA plasmid (Table S1) was fixed with 1% formaldehyde for 10 min at room temperature. Cross-linking was quenched by adding glycine to a final concentration of 120 mM, and then cells were sedimented by centrifugation at 5,000 rpm for 15 min at 4°C and washed twice with ice-cold Phosphate-buffered saline (PBS). The cells were lysed in a non-ionic sonication buffer (50 mM Tris-HCl pH 8, 150 mM NaCl, 5 mM EDTA, 1% Triton X-100, 0.5% NP-40, 1 mM DTT) containing protease inhibitor cocktail (Roche) and RNaseOUT™ (Invitrogen) and sonicated in a Bioruptor™ UCD-200 TM (conditions: power H, 30 sec ON-30 sec OFF, 10 min). Debris was removed by centrifugation, and lysate was divided and immunoprecipitated with 5 µg of either anti-HA antibody (12CA5, Roche) or appropriate control IgG (sc-2025, Santa Cruz Biotechnology) and 30 µL of Dynabeads® protein G (Invitrogen). After washing with sonication buffer four times and with TE twice at 4°C, samples were treated with RQ1 RNase-Free DNase (Promega) for 30 min at 37°C. Reverse transcription (RT) was carried out directly on magnetic bead-bound complexes with random hexanucleotide primers using SuperScript®III First-Strand Synthesis System (Invitrogen) according to the manufacturer's protocol. The cDNAs from pulled down fractions were quantified by qPCR as above using the primer pairs shown in Table S2. Every assay was performed three times with three replicates each time.
Statistical methods
SPSS program was used for all statistical analyses. The data in Figs. 3A, 3C, 5A and 5B were compared using one way analysis of variance (ANOVA) followed by Bonferroni's multiple comparison test (set at 0.05) and in Fig. 2C using Student's t-test for independent samples (p<0.05).
Supporting Information
Strains and Plasmids used.
(PDF)
Primers used.
(PDF)
Acknowledgments
We are grateful to Ine Mulders and Ben Lugtenberg for the anti-flagellin antiserum and to the Unidad de Microscopía Electronica de Transmission (SIDI-UAM) for assistance with the transmission electron microscopy. We are also grateful to Dr María Sánchez-Contreras for critical reading of the manuscript.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was funded by the Spanish Ministry of Science and Education grant BIO2009-08596, and by the Research Program MICROAMBIENTE-CM from Comunidad de Madrid. AN is the recipient of an FPI fellowship from the Spanish Ministry of Science and Education. EB is the recipient of a predoctoral contract from Comunidad de Madrid. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.de Souza JT, Mazzola M, Raaijmakers JM. Conservation of the response regulator gene gacA in Pseudomonas species. Environ Microbiol. 2003;5:1328–1340. doi: 10.1111/j.1462-2920.2003.00438.x. [DOI] [PubMed] [Google Scholar]
- 2.Heeb S, Haas D. Regulatory roles of the GacS/GacA two-component system in plant-associated and other Gram-negative bacteria. Mol Plant Microbe Interact. 2001;14:1351–1363. doi: 10.1094/MPMI.2001.14.12.1351. [DOI] [PubMed] [Google Scholar]
- 3.Hassan KA, Johnson A, Shaffer BT, Ren Q, Kidarsa TA, et al. Inactivation of the GacA response regulator in Pseudomonas fluorescens Pf-5 has far-reaching transcriptomic consequences. Environ Microbiol. 2010;12:899–915. doi: 10.1111/j.1462-2920.2009.02134.x. [DOI] [PubMed] [Google Scholar]
- 4.Haas D, Défago G. Biological control of soil-borne pathogens by fluorescent pseudomonads. Nature Reviews Microbiology. 2005;3:307–319. doi: 10.1038/nrmicro1129. [DOI] [PubMed] [Google Scholar]
- 5.Lapouge K, Schubert M, Allain FH, Haas D. Gac/Rsm signal transduction pathway of gamma-proteobacteria: from RNA recognition to regulation of social behaviour. Mol Microbiol. 2008;67:241–253. doi: 10.1111/j.1365-2958.2007.06042.x. [DOI] [PubMed] [Google Scholar]
- 6.Sonnleitner E, Haas D. Small RNAs as regulators of primary and secondary metabolism in Pseudomonas species. Appl Microbiol Biotechnol. 2011;91:63–79. doi: 10.1007/s00253-011-3332-1. [DOI] [PubMed] [Google Scholar]
- 7.Reimmann C, Beyeler M, Latifi A, Winteler H, Foglino M, et al. The global activator GacA of Pseudomonas aeruginosa PAO positively controls the production of the autoinducer N-butyryl-homoserine lactone and the formation of the virulence factors pyocyanin, cyanide, and lipase. Mol Microbiol. 1997;24:309–319. doi: 10.1046/j.1365-2958.1997.3291701.x. [DOI] [PubMed] [Google Scholar]
- 8.Burrowes E, Abbas A, O'Neill A, Adams C, O'Gara F. Characterisation of the regulatory RNA rsmB from Pseudomonas aeruginosa PAO1. Res Microbiol. 2005;156:7–16. doi: 10.1016/j.resmic.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 9.Rahme LG, Stevens EJ, Wolfort SF, Shao J, Tompkins RG, et al. Common virulence factors for bacterial pathogenicity in plants and animals. Science. 1995;268:1899–1902. doi: 10.1126/science.7604262. [DOI] [PubMed] [Google Scholar]
- 10.Vodovar N, Vallenet D, Cruveiller S, Rouy Z, Barbe V, et al. Complete genome sequence of the entomopathogenic and metabolically versatile soil bacterium Pseudomonas entomophila. Nat Biotechnol. 2006;24:673–679. doi: 10.1038/nbt1212. [DOI] [PubMed] [Google Scholar]
- 11.Rich JJ, Kinscherf TG, Kitten T, Willis DK. Genetic evidence that the gacA gene encodes the cognate response regulator for the lemA sensor in Pseudomonas syringae. J Bacteriol. 1994;176:7468–7475. doi: 10.1128/jb.176.24.7468-7475.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kinscherf TG, Willis DK. Swarming by Pseudomonas syringae B728a requires gacS (lemA) and gacA but not the acyl-homoserine lactone biosynthetic gene ahlI. J Bacteriol. 1999;181:4133–4136. doi: 10.1128/jb.181.13.4133-4136.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Willis DK, Holmstadt JJ, Kinscherf TG. Genetic evidence that loss of virulence associated with gacS or gacA mutations in Pseudomonas syringae B728a does not result from effects on alginate production. Appl Environ Microbiol. 2001;67:1400–1403. doi: 10.1128/AEM.67.3.1400-1403.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chatterjee A, Cui YY, Yang HL, Collmer A, Alfano JR, et al. GacA, the response regulator of a two-component system, acts as a master regulator in Pseudomonas syringae pv. tomato DC3000 by controlling regulatory RNA, transcriptional activators, and alternate sigma factors. Mol Plant Microbe Interact. 2003;16:1106–1117. doi: 10.1094/MPMI.2003.16.12.1106. [DOI] [PubMed] [Google Scholar]
- 15.Aarons S, Abbas A, Adams C, Fenton A, O'Gara F. A regulatory RNA (PrrB RNA) modulates expression of secondary metabolite genes in Pseudomonas fluorescens F113. J Bacteriol. 2000;182:3913–3919. doi: 10.1128/jb.182.14.3913-3919.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kang BR, Yang KY, Cho BH, Han TH, Kim IS, et al. Production of indole-3-acetic acid in the plant-beneficial strain Pseudomonas chlororaphis O6 is negatively regulated by the global sensor kinase GacS. Curr Microbiol. 2006;52:473–476. doi: 10.1007/s00284-005-0427-x. [DOI] [PubMed] [Google Scholar]
- 17.Laville J, Voisard C, Keel C, Maurhofer M, Defago G, et al. Global control in Pseudomonas fluorescens mediating antibiotic synthesis and suppression of black root-rot of tobacco. Proc Natl Acad Sci U S A. 1992;89:1562–1566. doi: 10.1073/pnas.89.5.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang ZG, Pierson LS. A second quorum-sensing system regulates cell surface properties but not phenazine antibiotic production in Pseudomonas aureofaciens. Appl Environ Microbiol. 2001;67:4305–4315. doi: 10.1128/AEM.67.9.4305-4315.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liao CH, McCallus DE, Wells JM, Tzean SS, Kang GY. The repB gene required for production of extracellular enzymes and fluorescent siderophores in Pseudomonas viridiflava is an analog of the gacA gene of Pseudomonas syringae. Can J Microbiol. 1996;42:177–182. doi: 10.1139/m96-026. [DOI] [PubMed] [Google Scholar]
- 20.Sacherer P, Defago G, Haas D. Extracellular protease and phospholipase-C are controlled by the global regulatory gene gacA in the biocontrol strain Pseudomonas fluorescens CHA0. FEMS Microbiol Lett. 1994;116:155–160. doi: 10.1111/j.1574-6968.1994.tb06694.x. [DOI] [PubMed] [Google Scholar]
- 21.Koch B, Nielsen TH, Sorensen D, Andersen JB, Christophersen C, et al. Lipopeptide production in Pseudomonas sp. strain DSS73 is regulated by components of sugar beet seed exudate via the Gac two-component regulatory system. Appl Environ Microbiol. 2002;68:4509–4516. doi: 10.1128/AEM.68.9.4509-4516.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dubern JF, Lagendijk EL, Lugtenberg BJJ, Bloemberg GV. The heat shock genes dnaK, dnaJ, and grpE are involved in regulation of putisolvin biosynthesis in Pseudomonas putida PCL1445. J Bacteriol. 2005;187:5967–5976. doi: 10.1128/JB.187.17.5967-5976.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barahona E, Navazo A, Martinez-Granero F, Zea-Bonilla T, Perez-Jimenez RM, et al. A Pseudomonas fluorescens F113 mutant with enhanced competitive colonization ability shows improved biocontrol activity against fungal root pathogens. Appl Environ Microbiol. 2011;77:5412–5419. doi: 10.1128/AEM.00320-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zuber S, Carruthers F, Keel C, Mattart A, Blumer C, et al. GacS sensor domains pertinent to the regulation of exoproduct formation and to the biocontrol potential of Pseudomonas fluorescens CHA0. Mol Plant Microbe Interact. 2003;16:634–644. doi: 10.1094/MPMI.2003.16.7.634. [DOI] [PubMed] [Google Scholar]
- 25.Kay E, Dubuis C, Haas D. Three small RNAs jointly ensure secondary metabolism and biocontrol in Pseudomonas fluorescens CHA0. Proc Natl Acad Sci U S A. 2005;102:17136–17141. doi: 10.1073/pnas.0505673102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Valverde C, Heeb S, Keel C, Haas D. RsmY, a small regulatory RNA, is required in concert with RsmZ for GacA-dependent expression of biocontrol traits in Pseudomonas fluorescens CHA0. Mol Microbiol. 2003;50:1361–1379. doi: 10.1046/j.1365-2958.2003.03774.x. [DOI] [PubMed] [Google Scholar]
- 27.Kay E, Humair B, Dénervaud V, Riedel K, Spahr S, et al. Two GacA-dependent small RNAs modulate the quorum-sensing response in Pseudomonas aeruginosa. J Bacteriol. 2006;188:6026–6033. doi: 10.1128/JB.00409-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lapouge K, Sineva E, Lindell M, Starke K, Baker CS, et al. Mechanism of hcnA mRNA recognition in the Gac/Rsm signal transduction pathway of Pseudomonas fluorescens. Mol Microbiol. 2007;66:341–356. doi: 10.1111/j.1365-2958.2007.05909.x. [DOI] [PubMed] [Google Scholar]
- 29.Schubert M, Lapouge K, Duss O, Oberstrass FC, Jelesarov I, et al. Molecular basis of messenger RNA recognition by the specific bacterial repressing clamp RsmA/CsrA. Nature Structural & Molecular Biology. 2007;14:807–813. doi: 10.1038/nsmb1285. [DOI] [PubMed] [Google Scholar]
- 30.Heurlier K, Williams F, Heeb S, Dormond C, Pessi G, et al. Positive control of swarming, rhamnolipid synthesis, and lipase production by the posttranscriptional RsmA/rsmZ system in Pseudomonas aeruginosa PAO1. J Bacteriol. 2004;186:2936–2945. doi: 10.1128/JB.186.10.2936-2945.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martínez-Granero F, Capdevila S, Sánchez-Contreras M, Martín M, Rivilla R. Two site-specific recombinases are implicated in phenotypic variation and competitive rhizosphere colonization in Pseudomonas fluorescens. Microbiology. 2005;151:975–983. doi: 10.1099/mic.0.27583-0. [DOI] [PubMed] [Google Scholar]
- 32.Martínez-Granero F, Rivilla R, Martin M. Rhizosphere selection of highly motile phenotypic variants of Pseudomonas fluorescens with enhanced competitive colonization ability. Appl Environ Microbiol. 2006;72:3429–3434. doi: 10.1128/AEM.72.5.3429-3434.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caiazza NC, O'Toole GA. SadB is required for the transition from reversible to irreversible attachment during biofilm formation by Pseudomonas aeruginosa PA14. J Bacteriol. 2004;186:4476–4485. doi: 10.1128/JB.186.14.4476-4485.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Caiazza NC, Shanks RM, O'Toole GA. Rhamnolipids modulate swarming motility patterns of Pseudomonas aeruginosa. J Bacteriol. 2005;187:7351–7361. doi: 10.1128/JB.187.21.7351-7361.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Redondo-Nieto M, Barret M, Morrisey J, Germaine K, Martínez-Granero F, et al. Genome sequence of the biocontrol strain Pseudomonas fluorescens F113. J Bacteriol. doi: 10.1128/JB.06601-11. In press. doi: 10.1128/JB.06601-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Capdevila S, Martínez-Granero FM, Sánchez-Contreras M, Rivilla R, Martín M. Analysis of Pseudomonas fluorescens F113 genes implicated in flagellar filament synthesis and their role in competitive root colonization. Microbiology. 2004;150:3889–3897. doi: 10.1099/mic.0.27362-0. [DOI] [PubMed] [Google Scholar]
- 38.Dasgupta N, Wolfgang MC, Goodman AL, Arora SK, Jyot J, et al. A four-tiered transcriptional regulatory circuit controls flagellar biogenesis in Pseudomonas aeruginosa. Mol Microbiol. 2003;50:809–824. doi: 10.1046/j.1365-2958.2003.03740.x. [DOI] [PubMed] [Google Scholar]
- 39.Sánchez-Contreras M, Martín M, Villacieros M, O'Gara F, Bonilla I, et al. Phenotypic selection and phase variation occur during alfalfa root colonization by Pseudomonas fluorescens F113. J Bacteriol. 2002;184:1587–1596. doi: 10.1128/JB.184.6.1587-1596.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Weger LA, van der vlugt CIM, Wijfjes AHM, Bakker PAHM, Schippers B, et al. Flagella of a plant-growth-stimulating Pseudomonas fluorescens strain are required for colonization of potato roots. J Bacteriol. 1987;169:2769–2773. doi: 10.1128/jb.169.6.2769-2773.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blumer C, Heeb S, Pessi G, Haas D. Global GacA-steered control of cyanide and exoprotease production in Pseudomonas fluorescens involves specific ribosome binding sites. Proc Natl Acad Sci U S A. 1999;96:14073–14078. doi: 10.1073/pnas.96.24.14073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reimmann C, Valverde C, Kay E, Haas D. Posttranscriptional repression of GacS/GacA-controlled genes by the RNA-binding protein RsmE acting together with RsmA in biocontrol strain Pseudomonas fluorescens CHA0. J Bacteriol. 2005;187:276–285. doi: 10.1128/JB.187.1.276-285.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Choy WK, Zhou L, Syn CKC, Zhang LH, Swarup S. MorA defines a new class of regulators affecting flagellar development and biofilm formation in diverse Pseudomonas species. J Bacteriol. 2004;186:7221–7228. doi: 10.1128/JB.186.21.7221-7228.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dasgupta N, Ramphal R. Interaction of the antiactivator FleN with the transcriptional activator FleQ regulates flagellar number in Pseudomonas aeruginosa. J Bacteriol. 2001;183:6636–6644. doi: 10.1128/JB.183.22.6636-6644.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tart AH, Wolfgang MC, Wozniak DJ. The Alternative Sigma Factor AlgT Represses Pseudomonas aeruginosa Flagellum Biosynthesis by Inhibiting Expression of fleQ. J Bacteriol. 2005;187:7955–7962. doi: 10.1128/JB.187.23.7955-7962.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dasgupta N, Ferrell EP, Kanack KJ, West SEH, Ramphal R. fleQ, the gene encoding the major flagellar regulator of Pseudomonas aeruginosa, is s70 dependent and is downregulated by Vfr, a homolog of Escherichia coli cyclic AMP receptor protein. J Bacteriol. 2002;184:5240–5250. doi: 10.1128/JB.184.19.5240-5250.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tart AH, Blanks MJ, Wozniak DJ. The AlgT-dependent transcriptional regulator AmrZ (AlgZ) inhibits flagellum biosynthesis in mucoid, nonmotile Pseudomonas aeruginosa cystic fibrosis isolates. J Bacteriol. 2006;188:6483–6489. doi: 10.1128/JB.00636-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Beatson SA, Whitchurch CB, Sargent JL, Levesque RC, Mattick JS. Differential regulation of twitching motility and elastase production by Vfr in Pseudomonas aeruginosa. J Bacteriol. 2002;184:3605–3613. doi: 10.1128/JB.184.13.3605-3613.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goodman AL, Merighi M, Hyodo M, Ventre I, Filloux A, et al. Direct interaction between sensor kinase proteins mediates acute and chronic disease phenotypes in a bacterial pathogen. Genes Dev. 2009;23:249–259. doi: 10.1101/gad.1739009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Valverde C, Lindell M, Wagner EGH, Haas D. A repeated GGA motif is critical for the activity and stability of the riboregulator rsmY of Pseudomonas fluorescens. J Biol Chem. 2004;279:25066–25074. doi: 10.1074/jbc.M401870200. [DOI] [PubMed] [Google Scholar]
- 51.Brencic A, Lory S. Determination of the regulon and identification of novel mRNA targets of Pseudomonas aeruginosa RsmA. Mol Microbiol. 2009;72:612–632. doi: 10.1111/j.1365-2958.2009.06670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Petrova OE, Sauer K. The novel two-component regulatory system BfiSR regulates biofilm development by controlling the small RNA rsmZ through CafA. J Bacteriol. 2010;192:5275–5288. doi: 10.1128/JB.00387-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pessi G, Williams F, Hindle Z, Heurlier K, Holden MTG, et al. The global posttranscriptional regulator RsmA modulates production of virulence determinants and N-acylhomoserine lactones in Pseudomonas aeruginosa. J Bacteriol. 2001;183:6676–6683. doi: 10.1128/JB.183.22.6676-6683.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wei BDL, Brun-Zinkernagel AM, Simecka JW, Pruss BM, Babitzke P, et al. Positive regulation of motility and flhDC expression by the RNA-binding protein CsrA of Escherichia coli. Mol Microbiol. 2001;40:245–256. doi: 10.1046/j.1365-2958.2001.02380.x. [DOI] [PubMed] [Google Scholar]
- 55.Kuchma SL, Brothers KM, Merritt JH, Liberati NT, Ausubel FM, et al. BifA, a cyclic-di-GMP phosphodiesterase, inversely regulates biofilm formation and swarming motility by Pseudomonas aeruginosa PA14. J Bacteriol. 2007;189:8165–8178. doi: 10.1128/JB.00586-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Damron FH, Qiu D, Yu HD. The Pseudomonas aeruginosa sensor kinase KinB negatively controls alginate production through AlgW-dependent MucA proteolysis. J Bacteriol. 2009;191:2285–2295. doi: 10.1128/JB.01490-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wozniak DJ, Ohman DE. Transcriptional analysis of the Pseudomonas aeruginosa genes algR, algB, and algD reveals a hierarchy of alginate gene expression which is modulated by algT. J Bacteriol. 1994;176:6007–6014. doi: 10.1128/jb.176.19.6007-6014.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ma S, Wozniak DJ, Ohman DE. Identification of the histidine protein kinase KinB in Pseudomonas aeruginosa and its phosphorylation of the alginate regulator AlgB. J Biol Chem. 1997;272:17952–17960. doi: 10.1074/jbc.272.29.17952. [DOI] [PubMed] [Google Scholar]
- 59.Schreiber KJ, Desveaux D. AlgW regulates multiple Pseudomonas syringae virulence strategies. Mol Microbiol. 2011;80:364–377. doi: 10.1111/j.1365-2958.2011.07571.x. [DOI] [PubMed] [Google Scholar]
- 60.Heeb S, Valverde C, Gigot-Bonnefoy C, Haas D. Role of the stress sigma factor RpoS in GacA/RsmA-controlled secondary metabolism and resistance to oxidative stress in Pseudomonas fluorescens CHA0. FEMS Microbiol Lett. 2005;243:251–258. doi: 10.1016/j.femsle.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 61.Whistler CA, Corbell NA, Sarniguet A, Ream W, Loper JE. The two-component regulators GacS and GacA influence accumulation of the stationary-phase sigma factor ss and the stress response in Pseudomonas fluorescens Pf-5. J Bacteriol. 1998;180:6635–6641. doi: 10.1128/jb.180.24.6635-6641.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dubern JF, Bloemberg GV. Influence of environmental conditions on putisolvins I and II production in Pseudomonas putida strain PCL1445. FEMS Microbiol Lett. 2006;263:169–175. doi: 10.1111/j.1574-6968.2006.00406.x. [DOI] [PubMed] [Google Scholar]
- 63.Shanahan P, O'Sullivan DJ, Simpson P, Glennon JD, O'Gara F. Isolation of 2,4-diacetylphloroglucinol from a fluorescent pseudomonad and investigation of physiological parameters influencing its production. Appl Environ Microbiol. 1992;58:353–358. doi: 10.1128/aem.58.1.353-358.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kalogeraki VS, Winans SC. Suicide plasmids containing promoterless reporter genes can simultaneously disrupt and create fusions to target genes of diverse bacteria. Gene. 1997;188:69–75. doi: 10.1016/s0378-1119(96)00778-0. [DOI] [PubMed] [Google Scholar]
- 65.Schäfer A, Tauch A, Jager W, Kalinowski J, Thierbach G, et al. Small mobilizable multipurpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: Selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene. 1994;145:69–73. doi: 10.1016/0378-1119(94)90324-7. [DOI] [PubMed] [Google Scholar]
- 66.Kirchner O, Tauch A. Tools for genetic engineering in the amino acid-producing bacterium Corynebacterium glutamicum. J Biotechnol. 2003;104:287–299. doi: 10.1016/s0168-1656(03)00148-2. [DOI] [PubMed] [Google Scholar]
- 67.de Lorenzo V, Eltis L, Kessler B, Timmis KN. Analysis of Pseudomonas gene products using lacIq/Ptrp-lac plasmids and transposons that confer conditional phenotypes. Gene. 1993;123:17–24. doi: 10.1016/0378-1119(93)90533-9. [DOI] [PubMed] [Google Scholar]
- 68.Finan TM, Kunkel B, De Vos GF, Signer ER. Second symbiotic megaplasmid in Rhizobium meliloti carrying exopolysaccharide and thiamine synthesis genes. J Bacteriol. 1986;167:66–72. doi: 10.1128/jb.167.1.66-72.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Scher FM, Baker R. Effects of Pseudomonas putida and a synthetic iron chelator on induction of soil suppressiveness to Fusarium wilt pathogens. Phytopathology. 1982;72:1567–1573. [Google Scholar]
- 70.Bertani G. Studies on Lysogenesis. 1. The Mode of Phage Liberation by Lysogenic Escherichia coli. J Bacteriol. 1951;62:293–300. doi: 10.1128/jb.62.3.293-300.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sambrook J, Fritsch EF, Maniatics T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.; 1989. [Google Scholar]
- 72.Laemmli UK. Cleavage of structural proteins during assembly of head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 73.Lin C, Yang L, Yang JJ, Huang Y, Liu ZR. ATPase/helicase activities of p68 RNA helicase are required for pre-mRNA splicing but not for assembly of the spliceosome. Mol Cell Biol. 2005;25:7484–7493. doi: 10.1128/MCB.25.17.7484-7493.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Strains and Plasmids used.
(PDF)
Primers used.
(PDF)