Abstract
Objectives
Calcium independent group VIA phospholipase A2 (iPLA2β) is up-regulated in vascular smooth muscle cells in some diseases, but whether the up-regulated iPLA2β affects vascular morphology and blood pressure is unknown. The current study addresses this question by evaluating the basal- and angiotensin II infusion-induced vascular remodeling and hypertension in smooth muscle specific iPLA2β transgenic (iPLA2β -Tg) mice.
Method and Results
Blood pressure was monitored by radiotelemetry and vascular remodeling was assessed by morphologic analysis. We found that the angiotensin II-induced increase in diastolic pressure was significantly higher in iPLA2β-Tg than iPLA2β-Wt mice, whereas, the basal blood pressure was not significantly different. The media thickness and media∶lumen ratio of the mesenteric arteries were significantly increased in angiotensin II-infused iPLA2β-Tg mice. Analysis revealed no difference in vascular smooth muscle cell proliferation. In contrast, adenovirus-mediated iPLA2β overexpression in cultured vascular smooth muscle cells promoted angiotensin II-induced [3H]-leucine incorporation, indicating enhanced hypertrophy. Moreover, angiotensin II infusion-induced c-Jun phosphorylation in vascular smooth muscle cells overexpressing iPLA2β to higher levels, which was abolished by inhibition of 12/15 lipoxygenase. In addition, we found that angiotensin II up-regulated the endogenous iPLA2β protein in-vitro and in-vivo.
Conclusion
The present study reports that iPLA2β up-regulation exacerbates angiotensin II-induced vascular smooth muscle cell hypertrophy, vascular remodeling and hypertension via the 12/15 lipoxygenase and c-Jun pathways.
Introduction
Phospholipase A2 is a large family of enzymes that catalyze the hydrolysis of the sn-2 ester bond on phospholipids to produce free fatty acids (e.g., arachidonic acid) and lysophospholipids [1]. Calcium independent group VIA phospholipase A2 (iPLA2β), a member of the phospholipase A2 superfamily, is an intracellular protein that is catalytically active in the absence of calcium [1]. iPLA2β was initially implicated as a housekeeping enzyme in phospholipid remodeling; however, accumulating evidence indicates that it also plays fundamental roles in cellular signaling, causing cell activation, proliferation, migration or apoptosis [1]. Moreover, iPLA2β plays a significant role in a variety of diseases including neurodegenerative disorders [2], [3], cardiac ischemia induced arrhythmia [4], [5], [6] and tumor genesis and metastasis [7], [8]. However, whether iPLA2β is involved in hypertension remains unreported.
An estimated 29% of the United States adult (>18 years of age) population is hypertensive, exposing these individuals to an increased risk of mortality and cardiovascular events over their lifespan [9]. The mechanisms underlying primary hypertension remain incompletely understood. Evidence obtained from studies on cultured cells or isolated tissues demonstrates that iPLA2β is involved in the regulation of multiple vascular smooth muscle functions and thus may contribute to hypertension and associated vascular remodeling. We [10], [11] and others [12], [13], [14] have demonstrated that iPLA2β plays an important role in vascular smooth muscle contraction regulation. Inhibition of iPLA2β by the selective suicidal iPLA2 inhibitor BEL, anti-sense oligonucleotides or genetic deletion drastically inhibit arachidonic acid release induced by vasopressin [15], thrombin [16], and thapsigargin/A23187 [17]. Moreover, inhibition or genetic deletion of iPLA2β significantly reduce thrombin-induced DNA synthesis [16] and smooth muscle migration and proliferation, which was analyzed in isolated mesentery artery tissue in an explant assay [17], suggesting that iPLA2β is involved in smooth muscle migration and proliferation. Together, these data imply that vascular smooth muscle iPLA2β is pro-hypertensive. On the other hand, iPLA2β is also required for angiotensin II (Ang II) induced RGS2 transcription in vascular smooth muscle cells (VSMC) [18] and deleting iPLA2β promotes vascular constriction [14]. Since RGS2 is a negative regulator of Ang II-induced hypertension, these imply vascular smooth muscle iPLA2β can also be anti-hypertensive. Taken together, the precise role that vascular smooth muscle iPLA2β plays in hypertension and vascular remodeling in vivo remains to be determined.
The activity of iPLA2β is regulated at multiple levels including allosteric interaction with ATP [19] or calmodulin [20], covalent modification by acylation [21], and potentially by proteolysis and translocation [22]. Interestingly, recent evidence suggests that, in addition to post-translational regulation, the iPLA2β protein level is up-regulated under various pathological conditions; the mRNA and protein are up-regulated in Type 1 diabetic Akita mouse pancreatic cells [23], in astrocytes stimulated with pro-inflammatory lipopolysaccharide [24] and in C2C12 myotubes by ischemia [6]. In particular, we have found that iPLA2β is up-regulated in VSMC cultured in the presence of high glucose, and is up-regulated in the vasculature from streptozotocin-induced hyperglycemic rats or Type 2 diabetic db/db mice [11]. However, whether the up-regulated iPLA2β, among the numerous alterations present under these pathological conditions contributes to hypertension and vascular remodeling is unknown.
We have developed a smooth muscle specific iPLA2β transgenic mouse model to determine whether up-regulated iPLA2β modulates Ang II infusion-induced hypertension and vascular remodeling. Ang II is the major bioactive peptide of the renin-angiotensin-aldosterone system and its dysregulation is one of the major factors contributing to the pathogenesis of hypertension. Chronic subcutaneous infusion of Ang II induces hypertension and vascular remodeling and has been used extensively as a model to decipher the mechanisms underlying hypertension. Our in-vivo and in-vitro studies demonstrate that vascular smooth muscle iPLA2β up-regulation exacerbates Ang II-induced hypertension and vascular remodeling.
Methods
Ethics Statement
All animal work has been conducted according to relevant national and international guidelines. Animal protocols used in the study were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Kentucky, approved ID 00920M2005.
Animals
Smooth muscle specific overexpressing iPLA2β transgenic mice (iPLA2β-Tg) were generated, characterized, and backcrossed to C57/B6 for over 10 generations as described elsewhere (Liu et.al., manuscript submitted). The expression of iPLA2β is driven by a rabbit smooth muscle myosin heavy chain promoter. The 12/15 lipoxygenase knockout mice were purchased from The Jackson Laboratory.
Materials
Ang II was purchased from Sigma (St. Louis, MO). 17-Octadecynoic acid, MK886, Baicalein, and Luteolin were purchased from Cayman (Ann Arbor, MI). Nordihydroguaiaretic acid and Indomethacin were purchased from Biomol (Plymouth Meeting, PA). The primary antibodies to β-actin, phospho-c-Jun, phospho-p38 MAPK, total-p38 MAPK were purchased from Cell Signaling (Danvers, MA). The iPLA2β antibody was made in our lab, and the generation and characterization of the antibody was previously documented [18].
Primary Cell Culture
Aortic VSMC were isolated from 12–13 wk old rats or mice and used at passages 5 to 10 as previously described [25].
Western Blot
After the indicated treatments, cells were collected, lysates were prepared, and the proteins were separated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to nitrocellulose membranes, which were then western blotted with the following antibodies: iPLA2β (1∶2500), β-actin (1∶2500), phospho-c-Jun (1∶1000), phospho-p38 MAPK (1∶2000), total-p38 MAPK (1∶2000), respectively. The proteins were quantified using the ECL Plus Western Blotting Detection System (GE Healthcare).
Blood Pressure Measurement
Eight pairs of 13-wk old male iPLA2β-Tg and iPLA2β-Wt (littermate) mice were anesthetized with isoflurane and implanted with telemetry probes (TA11PA-C10, Data Sciences International, St. Paul, MN) in the left carotid artery. After 7 to 10 days of recovery, basal blood pressure, heart rate, and locomotor activity data were collected continuously using the Dataquest A.R.T. system (Data Sciences International, St. Paul, MN) for 72 h. Osmotic mini-pumps (Alzet Model 2002) were then implanted, subcutaneously, to infuse Ang II (500 ng/kg/min, 14 days) or saline. During the infusion period, blood pressure data was collected continuously for 24 h every other day.
Morphometric Analysis of Vascular Remodeling
At the 14th day of Ang II or saline infusion, the mice were euthanized and perfused under physiological pressure with PBS and 4% paraformaldehyde. Thoracic aortas (2 mm from descending aorta) and secondary branches of the mesenteric arteries were isolated, cleaned, embedded, and cut into 5 µm sections. Sections were stained with HE (Hematoxylin-Eosin, Surgipath), or Elastin (Elastin Stain kit, Richard-Allan Scientific), or collagen (Masson Trichrome Stain, Richard-Allan Scientific). Images were captured and analyzed using an Olympus digital camera with Olympus MicroSuit-B3 Software. Elastin stained slides were used for morphometric analysis. The media thickness was determined by measuring the distance from the internal elastic lamina (IEL) to the external elastic lamina (EEL). For each slide, measurements from 4 points (12, 3, 6 and 9 o'clock positions) were averaged. The media area was determined by measuring the area between the IEL and EEL. The media∶lumen ratio was calculated based on the measured lumen area and media areas.
Immunohistochemistry
tlsb
OCT embedded 2nd order mesentery artery branches were sectioned and fixed for 30 min in paraformaldehyde. Sections were blocked with normal goat serum and incubated with anti-p-c-Jun (1∶100). Slides were then washed, incubated in fluorescently-labeled (1∶200, Alexa-Fluor 594, Invitrogen) secondary antibodies and counterstained with DAPI (300 nM).
iPLA2β Adenoviral Infection
The doxycycline-inducible iPLA2β-expressing adenoviral vectors were generated and purified as previously described [10]. Serum starved cells were infected for 12 h with Ad-iPLA2β (500 MOI) and Ad-Tet-on (2000 MOI). Doxycyclin (1 µg/ml) was added to the culture medium for 24 h to induce iPLA2β expression. The cells were then harvested for iPLA2β protein expression analysis or used for [3H]-leucine incorporation assay.
[3H]-Leucine Incorporation
After the indicated treatment, the VSMC were incubated with [3H]-leucine (0.25 µCi/well) for 24 h, washed, harvested and [3H]-leucine incorporation was quantified by liquid scintillation spectroscopy (Packard, Packard BioScience Company) as previously described [26].
Statistical Analysis
Each experiment was repeated a minimum of three times. Data were expressed as mean ± S.E. Statistical analysis was performed by using one- and/or two-way analysis of variance with repeated measurement for multiple groups. A post-hoc Bonferroni analysis was performed when appropriate (GraphPad Prism 4). Statistical significance was set at p<0.05.
Results
Smooth muscle specific iPLA2β overexpression exacerbates Ang II infusion-induced hypertension without affecting basal blood pressure
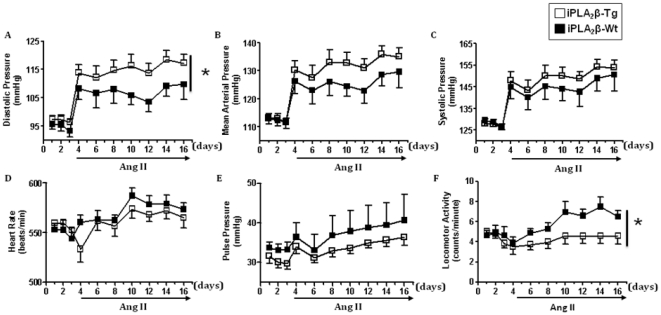
To examine the role of iPLA2β in basal blood pressure and in Ang II-induced hypertension, we utilized the smooth muscle specific iPLA2β-Tg mice that we previously developed (Liu et.al., manuscript submitted). No significant difference was detected in basal systolic (Fig. 1A); diastolic (Fig. 1B) or mean arterial blood pressure (Fig. 1C) between the iPLA2β-Tg and iPLA2β-Wt mice. Ang II infusion (500 ng/kg/min, 14 days) rapidly increased systolic, diastolic, and mean arterial pressure in both strains (Fig. 1A–C); saline infusion did not affect these parameters (Data not shown). Interestingly, diastolic pressure after Ang II infusion was significantly higher in iPLA2β-Tg mice (115±8.6 mmHg) than in the iPLA2β-Wt mice (107±10.3 mmHg) (Fig. 1B). Both the systolic and mean arterial pressures were higher in the iPLA2β-Tg mice after Ang II infusion, but the difference did not reach statistical significance. Additionally, no difference in the heart rate or pulse pressure was detected and the locomoter activity was lower in the iPLA2β-Tg mice than the iPLA2β-Wt mice (Fig. 1D–F).
Figure 1. Enhanced diastolic blood pressure increase in iPLA2β-Tg mice in response to Ang II infusion.
Blood pressure was measured in 13-wk old male, iPLA2β-Wt and iPLA2β-Tg mice by radiotelemetry. 24 h averages of systolic (A), diastolic (B), and mean arterial pressure (C), heart rate (D), pulse pressure (E), and locomotor activity (F) were collected prior to and during Ang II infusion (500 ng/kg/min, 14 days). n = 8; *: p<0.05 by two-way ANOVA with repeated measures.
Vascular remodeling induced by Ang II infusion is exacerbated in iPLA2-Tg mice
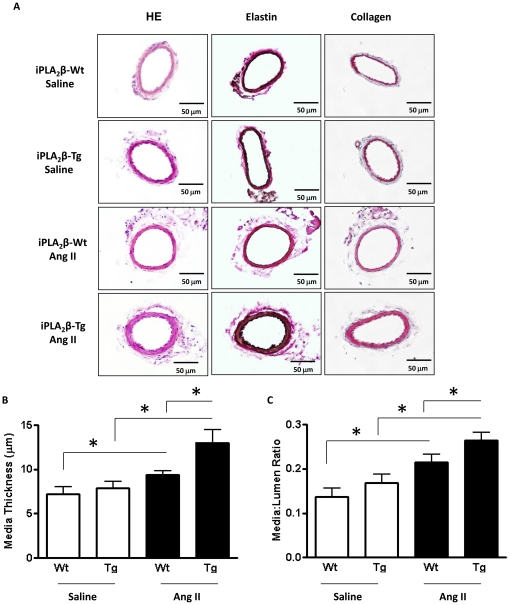
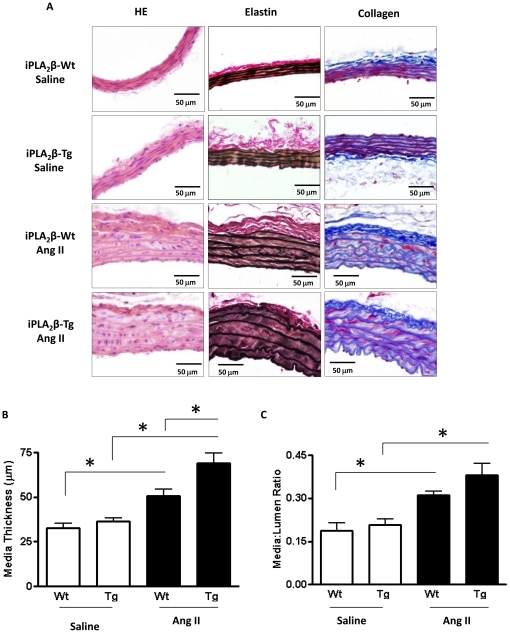
To investigate the mechanism underlying the exacerbation of Ang II infusion-induced blood pressure elevation in iPLA2β-Tg mice, we used morphometric analysis to determine whether iPLA2β overexpression affects Ang II-induced vascular remodeling. In the mesenteric arteries, iPLA2β overexpression alone did not significantly alter mesenteric artery collagen or elastin expression (Fig. 2A). However, iPLA2β overexpression significantly promoted the Ang II infusion-induced increase in media thickness (Fig. 2B) and media∶lumen ratio (Fig. 2C). In the thoracic aorta, iPLA2β overexpression also significantly promoted Ang II infusion-induced increases in media thickness (Fig. 3B) without altering the media∶lumen ratio (Fig. 3C) and the elastin or collagen expression (Fig. 3A).
Figure 2. Exacerbated mesenteric artery remodeling in iPLA2β-Tg mice in response to Ang II infusion.
Vascular remodeling was analyzed in embedded sections of the 2nd order branch of mesenteric arteries from the iPLA2β-Wt and iPLA2β-Tg mice infused with saline or Ang II (500 ng/kg/min, 14 days). Representative images of vessel sections stained with HE, elastin or collagen (A). The media thickness (B) and media∶lumen ratio (C) were quantified. n = 8, *: p<0.05, **: p<0.01, ***: p<0.001 by one-way ANOVA.
Figure 3. Exacerbated thoracic aorta remodeling in iPLA2β-Tg mice in response to Ang II infusion.
Vascular remodeling was analyzed in the thoracic aorta of the iPLA2β-Wt and iPLA2β-Tg infused with Ang II (500 ng/kg/min) or saline. Representative images of vessel sections stained with HE, elastin or collagen (A). The media thickness (B) and media∶lumen ratio (C) were quantified. n = 8, **: p<0.01, ***: p<0.001 by one way ANOVA.
iPLA2β overexpression enhances Ang II-induced vascular remodeling by promoting vascular smooth muscle hypertrophy via the 12/15 lipoxygenase pathway
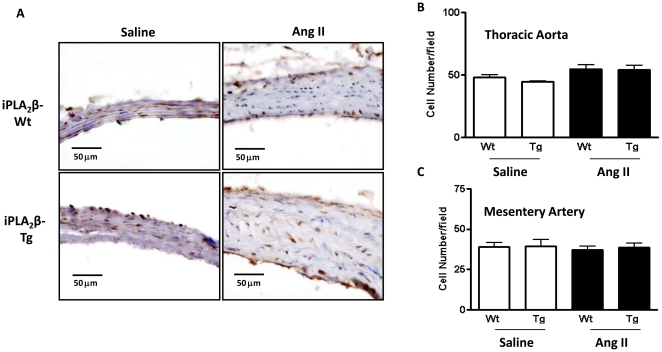
To elucidate the mechanism via which iPLA2β enhances Ang II-induced vascular remodeling, we investigated the effects of iPLA2β overexpression on VSMC proliferation and hypertrophy. The proliferation state was assessed by PCNA staining and cell counting. No significant difference was detected in PCNA staining [aorta (Fig. 4A) or mesentery artery (data not shown)] or cell number [aorta and mesentery artery (Fig. 4B,C)].
Figure 4. Lack of difference in cell proliferation between iPLA2β-Tg and iPLA2β-Wt mice by Ang II infusion.
Mice were infused with saline or Ang II (500 ng/kg/min, 14 days) and thoracic aortas were isolated and sectioned. (A) are representative images of the sections stained with anti-PCNA antibody. (B) and (C) are summaries of the cell number analysis using HE stained sections. n = 8.
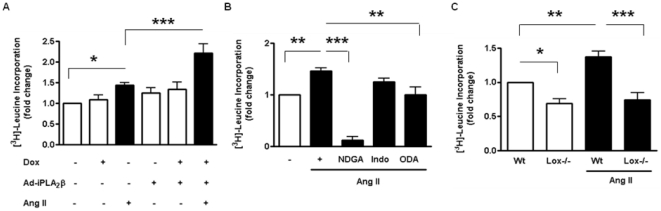
Next, we examined the effect of iPLA2β overexpression on Ang II-induced vascular smooth muscle hypertrophy in cultured VSMC. We found that exogenous overexpressed iPLA2β was gradually lost during the passage of VSMC isolated from the iPLA2β-Tg mice; therefore, we overexpressed iPLA2β by doxycycline inducible adenoviral vector mediated gene transfer as previously described [11], [18] and achieved an 11-fold increase in iPLA2β protein expression (data not shown). Ang II-induced [3H]-leucine incorporation was significantly enhanced by iPLA2β overexpression to 1.73-fold (Fig. 5A). As a control, neither doxycycline nor adenovirus treatment alone had an effect on the basal [3H]-leucine incorporation (Fig. 5A). Together, these results suggest that iPLA2β overexpression facilitates VSMC hypertrophy.
Figure 5. iPLA2β overexpression promotes Ang II-induced [3H]-leucine incorporation in VSMC via the 12/15 lipoxygenase pathway.
[3H]-leucine incorporation was quantified by liquid scintillation spectroscopy in cultured iPLA2β-Wt VSMCs treated with Ang II (100 nM, 24 h) or saline after (A) Ad-iPLA2β (500 MOI) and Ad-tet-on (2000 MOI), and/or doxycycline (1 µg/ml); (B) saline or inhibitors of lipoxygenase (NDGA, 30 µM), or cyclooxygenase (Indomethacin, 50 µM) or P450 cytochrome C (ODA, 10 µM). (C) Lox−/− or Wt VSMCs stimulated with Ang II or saline. n = 3–4, *: p<0.05, **: p<0.01, ***: p<0.001 one-way ANOVA.
To dissect the mechanism downstream of iPLA2β that exacerbates Ang II-induced VSMC hypertrophy, we examined the role of the arachidonic acid metabolites from lipoxygenase, cyclooxygenase or P450 pathways on [3H]-leucine incorporation. The inhibition of lipoxygenase by NDGA nearly abolished both basal and Ang II-induced [3H]-leucine incorporation (Fig. 5B), whereas, inhibition of cyclooxygenase by Indomethacin had no effect. P450 inhibition by ODA significantly attenuated Ang II-stimulated [3H]-leucine incorporation (Fig. 5B). VSMC isolated from 12/15 lipoxygenase knockout (lox−/−) mice also showed a significant attenuation of Ang II stimulated [3H]-leucine incorporation (Fig. 5C), verifying the essential role of iPLA2β and the 12/15 lipoxygenase pathway in Ang II-induced VSMC hypertrophy.
iPLA2β overexpression promotes Ang II-induced c-Jun phosphorylation via the 12/15 lipoxygenase metabolites
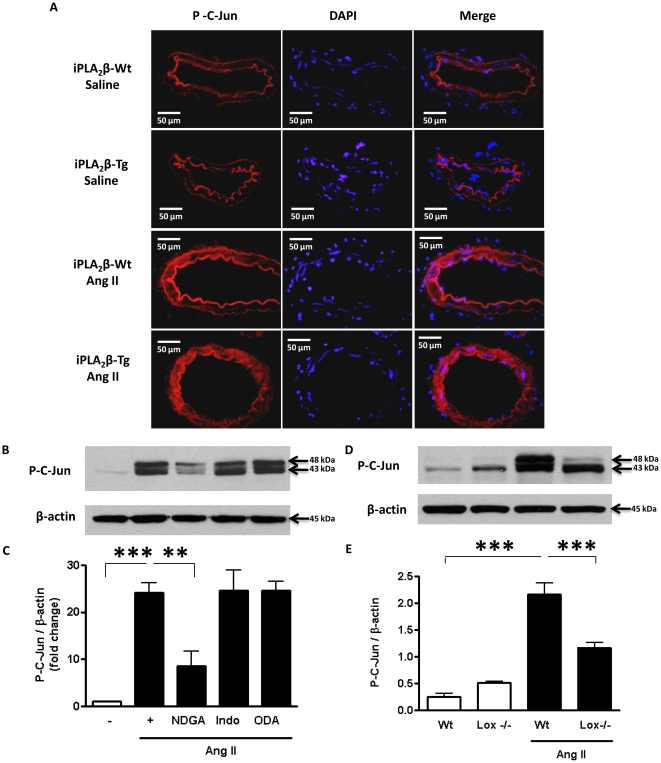
c-Jun phosphorylation has been demonstrated to be required for Ang II-induced VSMC hypertrophy. We therefore further investigated whether iPLA2β overexpression exacerbates Ang II-induced c-Jun phosphorylation by immunohistochemistry using mesentery artery sections from iPLA2β-Wt and iPLA2β-Tg mice. Ang II infusion enhanced the staining in both the iPLA2β-Wt and iPLA2β-Tg mouse sections (Fig. 6A). Moreover, the staining in the iPLA2β-Tg mice was much higher than that in the iPLA2β-Wt mice (Fig. 6A), indicating that iPLA2β overexpression promotes Ang II infusion-induced c-Jun phosphorylation.
Figure 6. iPLA2β overexpression promotes Ang II-induced c-Jun phosphorylation via the 12/15 lipoxygenase pathway.
(A) iPLA2β-Wt and iPLA2β-Tg mice were infused with Ang II (1000 ng/kg/min, 14 days) or saline and 2nd order branch of mesenteric arteries VSMC were isolated and embedded in OCT. Vessel sections were stained with anti-p-c-Jun antibody and DAPI. (B) and (D) are representative western blots, (C) and (E) are quantifications of the blots, of c-Jun phosphorylation in aortic VSMCs after the same treatments as in Fig. 3 B and C. n = 3–4, *:p<0.05, **: p<0.01, ***: p<0.001 one-way ANOVA.
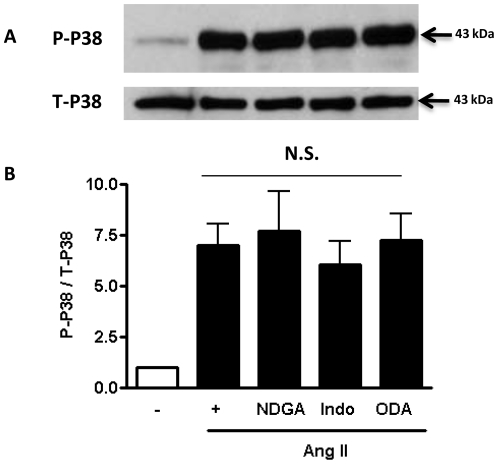
To further clarify the mechanism downstream of iPLA2β, we examined the role of the arachidonic acid metabolites in Ang II-induced c-Jun phosphorylation. The results demonstrate that inhibition of lipoxygenase by NDGA, but not inhibition of cyclooxygenase by indomethacin or P450 by ODA significantly attenuated Ang II-induced c-Jun phosphorylation (Fig. 6B, C). A significant attenuation of Ang II-stimulated c-Jun phosphorylation was also observed in VSMC isolated from 12/15 lipoxygenase knockout (lox−/−) mice (Fig. 6D, E), indicating a critical role of the 12/15 lipoxygenase pathway in Ang II-induced VSMC hypertrophy. Interestingly, the Ang II-induced p38 MAPK phosphorylation was not affected by any of the arachidonic acid metabolism enzyme inhibitors tested (Fig. 7A, B).
Figure 7. Lack of effects of inhibiting arachidonic acid metabolism on Ang II induced p38 MAPK activation.
VSMC were isolated from the aortas of iPLA2β-Wt mice and incubated with inhibitors of lipoxygenase (NDGA, 30 µM), cyclooxygenase (Indomethacin, 50 µM) or P450 cytochrome C (ODA, 10 µM) and stimulated with Ang II (100 nM) or saline for 1 h. Then the total- or phosphorylated p38 MAPK was determined by Western Blot. n = 3–4.
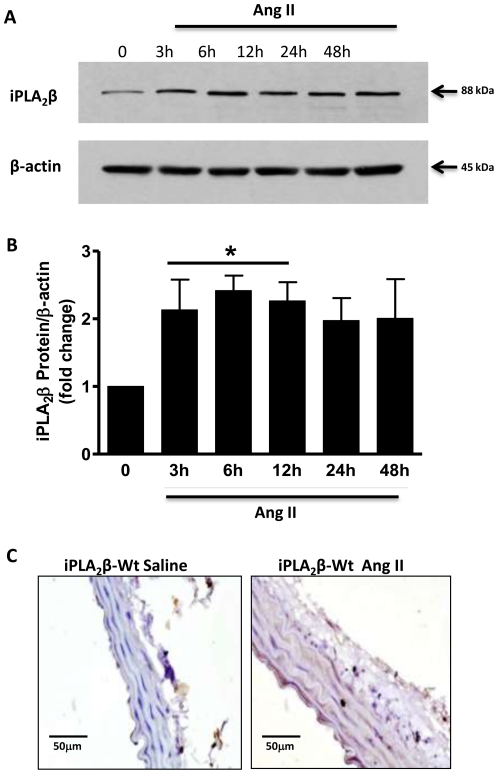
Ang II increases endogenous iPLA2β protein expression in VSMC
To further explore a possible role of endogenous iPLA2β in Ang II's effects, we determined whether iPLA2β protein is up-regulated by Ang II in-vitro and in-vivo. In cultured iPLA2β-Wt VSMC, Ang II treatment significantly increased iPLA2β protein expression within the time frame from 3 h to 48 h (Fig. 8A–B). In-vivo, Ang II infusion enhanced iPLA2β protein expression in the thoracic aorta as indicated by immunohistochemical analysis (Fig. 8C, note brown color accumulation in the media layer).
Figure 8. Ang II enhances iPLA2β protein expression in cultured VSMC and in-vivo.
24 h serum-deprived rat aortic VSMC were stimulated with Ang II (100 nM) for various time intervals as indicated. iPLA2β protein abundance was determined by Western Blot (A and B). Representative immunohistochemical images of iPLA2β protein staining in C57/B6 thoracic aorta from iPLA2β-Wt and iPLA2β-Tg mice infused with saline or Ang II (500 ng/kg/min, 14 days) (C). n = 4–5; *: p<0.05 by one-way ANOVA analysis.
Discussion
In the present study, we investigate the role of up-regulated smooth muscle iPLA2β in Ang II-induced VSMC hypertrophy, vascular remodeling and hypertension. Several novel findings are reported here. First, using a smooth muscle specific iPLA2β-Tg mouse model, we find that overexpression of iPLA2β in smooth muscle exacerbates Ang II infusion-induced blood pressure increase and vessel remodeling. Second, we demonstrate that the up-regulated iPLA2β acts via the 12/15 lipoxygenase pathway to induce c-Jun phosphorylation and to promote Ang II-induced VSMC hypertrophy. Third, Ang II up-regulates iPLA2β protein in cultured VSMCs and in-vivo during Ang II-infusion.
Metabolites of free arachidonic acid produced by the three major pathways, cyclooxygenase [27], [28], lipoxygenase [29], [30] and cytochrome P450 [31] have been demonstrated to play a role in hypertension. Different classes of phospholipase A2 may be responsible for the release of arachidonic acid, which is rate-limiting. Among these phospholipases, the cytosolic calcium-dependent cPLA2 has been shown to play an important role in the development of hypertension induced by chronic NO inhibition [32]. However, to the best of our knowledge, whether the cytosolic calcium-independent phospholipase A2, iPLA2β, participates in hypertension has not been reported. The current study for the first time demonstrates that iPLA2β overexpression in smooth muscle does not alter basal blood pressure, but exacerbates Ang II infusion induced hypertension and vascular remodeling. The lack of effect on basal blood pressure by iPLA2β overexpression is likely due to the fact that under resting conditions iPLA2β is presumably bound to calmodulin thus catalytically inactive [13]. Upon Ang II stimulation, iPLA2β is dissociated from calmodulin and activated therefore exacerbating Ang II infusion-induced blood pressure increase. Several lines of evidence suggest that the enhanced Ang II infusion-induced blood pressure increase is specifically caused by iPLA2β overexpression in vascular smooth muscle. First, neither the locomotor activity nor heart rate is increased in the iPLA2β-Tg mice (Fig. 1D, F), suggesting that locomotor activity and heart rate are not responsible for the exaggerated increase in blood pressure in response to Ang II-infusion. Second, among the diastolic, systolic and mean arterial pressure, the diastolic pressure exhibited the most enhancement by iPLA2β overexpression (Fig. 1). It is established that enhanced diastolic pressure is associated with an increase in peripheral resistance, whereas, enhanced systolic pressure is reliant on an increase in large conduit vessel stiffness and stroke volume [33]. The selective diastolic pressure enhancement observed in iPLA2β-Tg mice, in response to Ang II infusion, suggests that it is the increase in resistance of the vasculature that accounts for the enhanced blood pressure response. Third, morphometric analysis of the mesentery arteries reveals that the media thickness and media∶lumen ratio are significantly increased in the iPLA2β-Tg. This enhanced vessel remodeling in small arteries is expected to increase peripheral resistance and thus contributes to the enhanced diastolic blood pressure observed in the iPLA2β-Tg mice. Finally, the systolic blood pressure was similar in the iPLA2β-Tg and control mice. This is consistent with the observation that there was no significant difference in the aorta elastin and collagen content, which implies that the stiffness of the conduit vessels is similar in the iPLA2β-Tg and iPLA2β-Wt mice.
Phospholipase A2 and arachidonic acid metabolites are involved in hypertension caused by various mechanisms; however, it is interesting to note that blood pressure under basal physiological conditions was not affected by iPLA2β smooth muscle overexpression (present study), or by deleting cPLA2 [34] or 12/15 lipoxygenase [30]. This suggests that the arachidonic acid metabolites do not alter basal blood pressure homeostasis, but play a significant role in pathological hypertension induced by Ang II infusion (present study), renal artery occlusion, or blockade of NO [30], [35]. These observations raise the possibility that the phospholipase A2/arachidonic acid pathway is a therapeutic target for selective lowering of blood pressure under pathological conditions without disturbing normal physiological blood pressure.
The exacerbation of vascular remodeling in iPLA2β-Tg mice can be a consequence in response to increased blood pressure or an enhancement of Ang II's direct effect on vasculature. Our results suggest that increasing iPLA2β enhances Ang II signaling in the vasculature. In cultured VSMC, we found that iPLA2β overexpression enhanced Ang II-induced [3H]-leucine incorporation, indicating promotion of Ang II's direct effects on VSMC account for, at least in part, the exacerbation of vascular remodeling. Increased VSMC proliferation and/or hypertrophy may underlie the enhanced vascular remodeling. Our results demonstrate selectively enhanced hypertrophy in the absence of detectable alterations of proliferation in both mesenteric arteries and descending aortas in iPLA2β-Tg mice. This further suggests that iPLA2β overexpression amplifies Ang II mediated hypertrophic pathways and is consistent with the observations of others who found that Ang II selectively induces hypertrophy in cultured VSMCs [36] and in-vivo in the descending aorta [37].
Among the elaborate signaling network activated by Ang II, the lipoxygenase [38], c-Jun NH2-terminal kinase (JNK) [39] and p38 MAPK [40] pathways are known to be essential mediators of Ang II induced VSMC hypertrophy. This study demonstrates that iPLA2β overexpression potentiates Ang II-induced VSMC hypertrophy via activation of the lipoxygenase pathway since [3H]-leucine incorporation was inhibited by NDGA (Fig. 5B) or by genetic deletion of 12/15 lipoxygenase (Fig. 5C). Interestingly, only the JNK activation, as indicated by increased c-Jun phosphorylation (Fig. 6B–E), but not p38 MAPK (Fig. 7A,B) is affected by inhibition of the 12/15 lipoxygenase pathway. This is consistent with the report that p38 MAPK is upstream of and required for iPLA2β activation induced by thrombin [16].
In addition to iPLA2, cPLA2 has been shown to be activated by Ang II [41], [42] and is required for Ang II-induced arachidonic acid release [43] and hypertrophy [44] in VSMC. The relationship between the cPLA2 and iPLA2 in the regulation of arachidonic acid release and cell hypertrophy remain to be established. It is conceivable that iPLA2 and cPLA2 act in a sequential manner. iPLA2 has been demonstrated to play a critical role in store-operated calcium influx [45] and that is crucial for cPLA2α activation [35]. Indeed, iPLA2 has been shown to mediate the early phase whereas cPLA2 mediates the late phase of arachidonic acid release in response to thapsigargin and ionophore A23187 in VSMCs [17].
Emerging evidence suggests that iPLA2β protein level is increased under disease conditions including ischemia [6], Type I diabetes mellitus in pancreatic β-cells [23] and vascular tissues [11], in addition to being regulated by complex post-translational modifications. Whereas, thrombin activation of iPLA2 is not associated with iPLA2 protein level alterations [16], the current study demonstrates that Ang II activation of iPLA2β is associated with up-regulation of iPLA2β protein in cultured VSMC and in-vivo in the vascular tissue (Fig. 8A–C). This is the first evidence that a G-protein-coupled-receptor agonist can up-regulate iPLA2β protein, suggesting that the regulation of iPLA2β protein is agonist specific. However, whether iPLA2β protein up-regulation is required for Ang II infusion induced hypertension requires further investigation using iPLA2β knockout mice.
In summary, the present study is the first to demonstrate a novel role of up-regulated vascular smooth muscle iPLA2β in Ang II-induced hypertension, vascular remodeling and VSMC hypertrophy. This effect of iPLA2β is mediated via the lipoxygenase pathway, which induces JNK activation. Ang II stimulation up-regulates iPLA2β protein expression and these results suggest that up-regulated iPLA2β has significant effects on the vasculature.
Acknowledgments
We are grateful to Ming Zhang for her technical assistance and to Wendy Katz at the COBRE research core facility (NIH-COBRE, 1P20RR021954) for embedding and sectioning vascular samples.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by National Institute of Health (NIH) grants HL-082791 to M.C.G. and HL-088389 to Z.G. L.E.C. was supported by an American Heart Association (AHA) Fellowship-09PRE2300152 and a National Institute of Health (NIH) T32-HL072743. The funders had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
References
- 1.Murakami M, Taketomi Y, Miki Y, Sato H, Hirabayashi T, et al. Recent progress in phospholipase A research: from cells to animals to humans. Progress in lipid research. 2011;50:152–192. doi: 10.1016/j.plipres.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 2.Khateeb S, Flusser H, Ofir R, Shelef I, Narkis G, et al. PLA2G6 mutation underlies infantile neuroaxonal dystrophy. Am J Hum Genet. 2006;79:942–948. doi: 10.1086/508572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morgan NV, Westaway SK, Morton JE, Gregory A, Gissen P, et al. PLA2G6, encoding a phospholipase A2, is mutated in neurodegenerative disorders with high brain iron. Nat Genet. 2006;38:752–754. doi: 10.1038/ng1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ford DA, Hazen SL, Saffitz JE, Gross RW. The rapid and reversible activation of a calcium-independent plasmalogen-selective phospholipase A2 during myocardial ischemia. J Clin Invest. 1991;88:331–335. doi: 10.1172/JCI115296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mancuso DJ, Abendschein DR, Jenkins CM, Han X, Saffitz JE, et al. Cardiac ischemia activates calcium-independent phospholipase A2beta, precipitating ventricular tachyarrhythmias in transgenic mice: rescue of the lethal electrophysiologic phenotype by mechanism-based inhibition. The Journal of biological chemistry. 2003;278:22231–22236. doi: 10.1074/jbc.C300033200. [DOI] [PubMed] [Google Scholar]
- 6.Poulsen KA, Pedersen SF, Kolko M, Lambert IH. Induction of group VIA phospholipase A2 activity during in vitro ischemia in C2C12 myotubes is associated with changes in the level of its splice variants. American journal of physiology Cell physiology. 2007;293:C1605–1615. doi: 10.1152/ajpcell.00012.2007. [DOI] [PubMed] [Google Scholar]
- 7.Li H, Zhao Z, Wei G, Yan L, Wang D, et al. Group VIA phospholipase A2 in both host and tumor cells is involved in ovarian cancer development. FASEB J. 2010;24:4103–4116. doi: 10.1096/fj.10-161356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McHowat J, Gullickson G, Hoover RG, Sharma J, Turk J, et al. Platelet-activating factor and metastasis: calcium-independent phospholipase A2beta deficiency protects against breast cancer metastasis to the lung. Am J Physiol Cell Physiol. 2011;300:C825–832. doi: 10.1152/ajpcell.00502.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, et al. Heart disease and stroke statistics–2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:480–486. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- 10.Guo Z, Su W, Ma Z, Smith GM, Gong MC. Ca2+-independent Phospholipase A2 Is Required for Agonist-induced Ca2+ Sensitization of Contraction in Vascular Smooth Muscle. J Biol Chem. 2003;278:1856–1863. doi: 10.1074/jbc.M211075200. [DOI] [PubMed] [Google Scholar]
- 11.Xie Z, Gong MC, Su W, Xie D, Turk J, et al. Role of calcium-independent phospholipase A2beta in high glucose-induced activation of RhoA, Rho kinase, and CPI-17 in cultured vascular smooth muscle cells and vascular smooth muscle hypercontractility in diabetic animals. The Journal of biological chemistry. 2010;285:8628–8638. doi: 10.1074/jbc.M109.057711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boittin FX, Gribi F, Serir K, Beny JL. Ca2+-independent PLA2 controls endothelial store-operated Ca2+ entry and vascular tone in intact aorta. Am J Physiol Heart Circ Physiol. 2008;295:H2466–2474. doi: 10.1152/ajpheart.00639.2008. [DOI] [PubMed] [Google Scholar]
- 13.Park KM, Trucillo M, Serban N, Cohen RA, Bolotina VM. Role of iPLA2 and store-operated channels in agonist-induced Ca2+ influx and constriction in cerebral, mesenteric, and carotid arteries. Am J Physiol Heart Circ Physiol. 2008;294:H1183–1187. doi: 10.1152/ajpheart.01148.2007. [DOI] [PubMed] [Google Scholar]
- 14.Dietrich HH, Abendschein DR, Moon SH, Nayeb-Hashemi N, Mancuso DJ, et al. Genetic ablation of calcium-independent phospholipase A(2)beta causes hypercontractility and markedly attenuates endothelium-dependent relaxation to acetylcholine. Am J Physiol Heart Circ Physiol. 2010;298:H2208–2220. doi: 10.1152/ajpheart.00839.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolf MJ, Wang J, Turk J, Gross RW. Depletion of intracellular calcium stores activates smooth muscle cell calcium-independent phospholipase A2. A novel mechanism underlying arachidonic acid mobilization. J Biol Chem. 1997;272:1522–1526. doi: 10.1074/jbc.272.3.1522. [DOI] [PubMed] [Google Scholar]
- 16.Yellaturu CR, Rao GN. A requirement for calcium-independent phospholipase A2 in thrombin-induced arachidonic acid release and growth in vascular smooth muscle cells. J Biol Chem. 2003;278:43831–43837. doi: 10.1074/jbc.M301472200. [DOI] [PubMed] [Google Scholar]
- 17.Moon SH, Jenkins CM, Mancuso DJ, Turk J, Gross RW. Smooth muscle cell arachidonic acid release, migration, and proliferation are markedly attenuated in mice null for calcium-independent phospholipase A2beta. J Biol Chem. 2008;283:33975–33987. doi: 10.1074/jbc.M805817200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xie Z, Gong MC, Su W, Turk J, Guo Z. Group VIA phospholipase A2 (iPLA2beta) participates in angiotensin II-induced transcriptional up-regulation of regulator of g-protein signaling-2 in vascular smooth muscle cells. The Journal of biological chemistry. 2007;282:25278–25289. doi: 10.1074/jbc.M611206200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hazen SL, Gross RW. ATP-dependent regulation of rabbit myocardial cytosolic calcium-independent phospholipase A2. J Biol Chem. 1991;266:14526–14534. [PubMed] [Google Scholar]
- 20.Jenkins CM, Wolf MJ, Mancuso DJ, Gross RW. Identification of the calmodulin-binding domain of recombinant calcium-independent phospholipase A2beta. implications for structure and function. J Biol Chem. 2001;276:7129–7135. doi: 10.1074/jbc.M010439200. [DOI] [PubMed] [Google Scholar]
- 21.Jenkins CM, Yan W, Mancuso DJ, Gross RW. Highly selective hydrolysis of fatty acyl-CoAs by calcium-independent phospholipase A2beta. Enzyme autoacylation and acyl-CoA-mediated reversal of calmodulin inhibition of phospholipase A2 activity. J Biol Chem. 2006;281:15615–15624. doi: 10.1074/jbc.M511623200. [DOI] [PubMed] [Google Scholar]
- 22.Zhao X, Wang D, Zhao Z, Xiao Y, Sengupta S, et al. Caspase-3-dependent activation of calcium-independent phospholipase A2 enhances cell migration in non-apoptotic ovarian cancer cells. J Biol Chem. 2006;281:29357–29368. doi: 10.1074/jbc.M513105200. [DOI] [PubMed] [Google Scholar]
- 23.Lei X, Zhang S, Barbour SE, Bohrer A, Ford EL, et al. Spontaneous development of endoplasmic reticulum stress that can lead to diabetes mellitus is associated with higher calcium-independent phospholipase A2 expression: a role for regulation by SREBP-1. The Journal of biological chemistry. 2010;285:6693–6705. doi: 10.1074/jbc.M109.084293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Strokin M, Sergeeva M, Reiser G. Proinflammatory treatment of astrocytes with lipopolysaccharide results in augmented Ca2+ signaling through increased expression of via phospholipase A2 (iPLA2). Am J Physiol Cell Physiol. 2011;300:C542–549. doi: 10.1152/ajpcell.00428.2010. [DOI] [PubMed] [Google Scholar]
- 25.Pang H, Guo Z, Xie Z, Su W, Gong MC. Divergent kinase signaling mediates agonist-induced phosphorylation of phosphatase inhibitory proteins PHI-1 and CPI-17 in vascular smooth muscle cells. Am J Physiol Cell Physiol. 2006;290:C892–899. doi: 10.1152/ajpcell.00378.2005. [DOI] [PubMed] [Google Scholar]
- 26.Yaghini FA, Li F, Malik KU. Expression and mechanism of spleen tyrosine kinase activation by angiotensin II and its implication in protein synthesis in rat vascular smooth muscle cells. J Biol Chem. 2007;282:16878–16890. doi: 10.1074/jbc.M610494200. [DOI] [PubMed] [Google Scholar]
- 27.Qi Z, Hao CM, Langenbach RI, Breyer RM, Redha R, et al. Opposite effects of cyclooxygenase-1 and -2 activity on the pressor response to angiotensin II. J Clin Invest. 2002;110:61–69. doi: 10.1172/JCI14752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fujino T, Nakagawa N, Yuhki K, Hara A, Yamada T, et al. Decreased susceptibility to renovascular hypertension in mice lacking the prostaglandin I2 receptor IP. J Clin Invest. 2004;114:805–812. doi: 10.1172/JCI21382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nozawa K, Tuck ML, Golub M, Eggena P, Nadler JL, et al. Inhibition of lipoxygenase pathway reduces blood pressure in renovascular hypertensive rats. Am J Physiol. 1990;259:H1774–1780. doi: 10.1152/ajpheart.1990.259.6.H1774. [DOI] [PubMed] [Google Scholar]
- 30.Anning PB, Coles B, Bermudez-Fajardo A, Martin PE, Levison BS, et al. Elevated endothelial nitric oxide bioactivity and resistance to angiotensin-dependent hypertension in 12/15-lipoxygenase knockout mice. The American journal of pathology. 2005;166:653–662. doi: 10.1016/S0002-9440(10)62287-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jennings BL, Sahan-Firat S, Estes AM, Das K, Farjana N, et al. Cytochrome P450 1B1 contributes to angiotensin II-induced hypertension and associated pathophysiology. Hypertension. 2010;56:667–674. doi: 10.1161/HYPERTENSIONAHA.110.154518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tanaka K, Yamamoto Y, Ogino K, Tsujimoto S, Saito M, et al. Cytosolic phospholipase A2alpha contributes to blood pressure increases and endothelial dysfunction under chronic NO inhibition. Arterioscler Thromb Vasc Biol. 2011;31:1133–1138. doi: 10.1161/ATVBAHA.110.218370. [DOI] [PubMed] [Google Scholar]
- 33.McEniery CM, Yasmin, Wallace S, Maki-Petaja K, McDonnell B, et al. Increased stroke volume and aortic stiffness contribute to isolated systolic hypertension in young adults. Hypertension. 2005;46:221–226. doi: 10.1161/01.HYP.0000165310.84801.e0. [DOI] [PubMed] [Google Scholar]
- 34.Ichinose F, Ullrich R, Sapirstein A, Jones RC, Bonventre JV, et al. Cytosolic phospholipase A(2) in hypoxic pulmonary vasoconstriction. J Clin Invest. 2002;109:1493–1500. doi: 10.1172/JCI14294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chang WC, Parekh AB. Close functional coupling between Ca2+ release-activated Ca2+ channels, arachidonic acid release, and leukotriene C4 secretion. The Journal of biological chemistry. 2004;279:29994–29999. doi: 10.1074/jbc.M403969200. [DOI] [PubMed] [Google Scholar]
- 36.Geisterfer AA, Peach MJ, Owens GK. Angiotensin II induces hypertrophy, not hyperplasia, of cultured rat aortic smooth muscle cells. Circ Res. 1988;62:749–756. doi: 10.1161/01.res.62.4.749. [DOI] [PubMed] [Google Scholar]
- 37.Owens AP, 3rd, Subramanian V, Moorleghen JJ, Guo Z, McNamara CA, et al. Angiotensin II induces a region-specific hyperplasia of the ascending aorta through regulation of inhibitor of differentiation 3. Circ Res. 2010;106:611–619. doi: 10.1161/CIRCRESAHA.109.212837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Natarajan R, Gonzales N, Lanting L, Nadler J. Role of the lipoxygenase pathway in angiotensin II-induced vascular smooth muscle cell hypertrophy. Hypertension. 1994;23:I142–147. doi: 10.1161/01.hyp.23.1_suppl.i142. [DOI] [PubMed] [Google Scholar]
- 39.Viedt C, Soto U, Krieger-Brauer HI, Fei J, Elsing C, et al. Differential activation of mitogen-activated protein kinases in smooth muscle cells by angiotensin II: involvement of p22phox and reactive oxygen species. Arterioscler Thromb Vasc Biol. 2000;20:940–948. doi: 10.1161/01.atv.20.4.940. [DOI] [PubMed] [Google Scholar]
- 40.Ushio-Fukai M, Alexander RW, Akers M, Griendling KK. p38 Mitogen-activated protein kinase is a critical component of the redox-sensitive signaling pathways activated by angiotensin II. Role in vascular smooth muscle cell hypertrophy. J Biol Chem. 1998;273:15022–15029. doi: 10.1074/jbc.273.24.15022. [DOI] [PubMed] [Google Scholar]
- 41.Freeman EJ, Ruehr ML, Dorman RV. ANG II-induced translocation of cytosolic PLA2 to the nucleus in vascular smooth muscle cells. Am J Physiol. 1998;274:C282–288. doi: 10.1152/ajpcell.1998.274.1.C282. [DOI] [PubMed] [Google Scholar]
- 42.Rao GN, Lassegue B, Alexander RW, Griendling KK. Angiotensin II stimulates phosphorylation of high-molecular-mass cytosolic phospholipase A2 in vascular smooth-muscle cells. The Biochemical journal. 1994;299(Pt 1):197–201. doi: 10.1042/bj2990197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li F, Malik KU. Angiotensin II-induced Akt activation is mediated by metabolites of arachidonic acid generated by CaMKII-stimulated Ca2(+)-dependent phospholipase A2. Am J Physiol Heart Circ Physiol. 2005;288:H2306–2316. doi: 10.1152/ajpheart.00571.2004. [DOI] [PubMed] [Google Scholar]
- 44.Zafari AM, Ushio-Fukai M, Minieri CA, Akers M, Lassegue B, et al. Arachidonic acid metabolites mediate angiotensin II-induced NADH/NADPH oxidase activity and hypertrophy in vascular smooth muscle cells. Antioxid Redox Signal. 1999;1:167–179. doi: 10.1089/ars.1999.1.2-167. [DOI] [PubMed] [Google Scholar]
- 45.Smani T, Zakharov SI, Csutora P, Leno E, Trepakova ES, et al. A novel mechanism for the store-operated calcium influx pathway. Nat Cell Biol. 2004;6:113–120. doi: 10.1038/ncb1089. [DOI] [PubMed] [Google Scholar]