Abstract
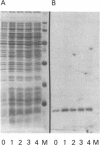
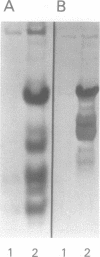
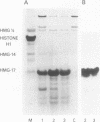
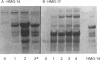
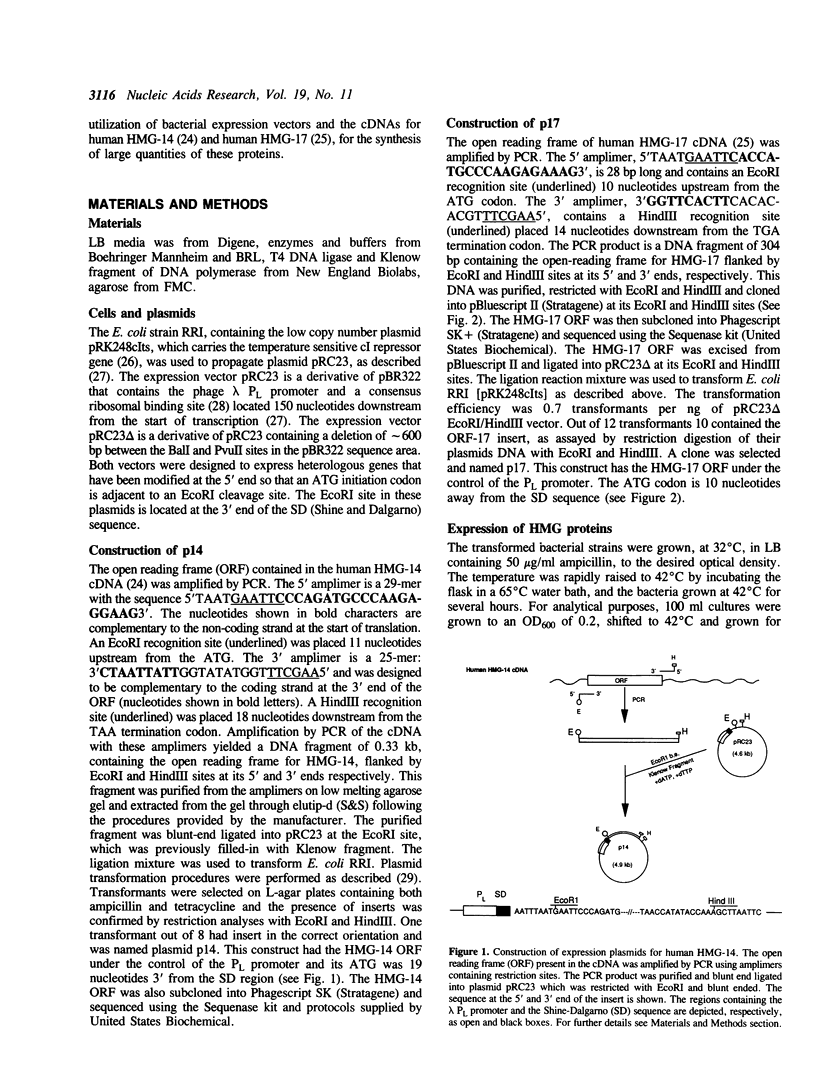
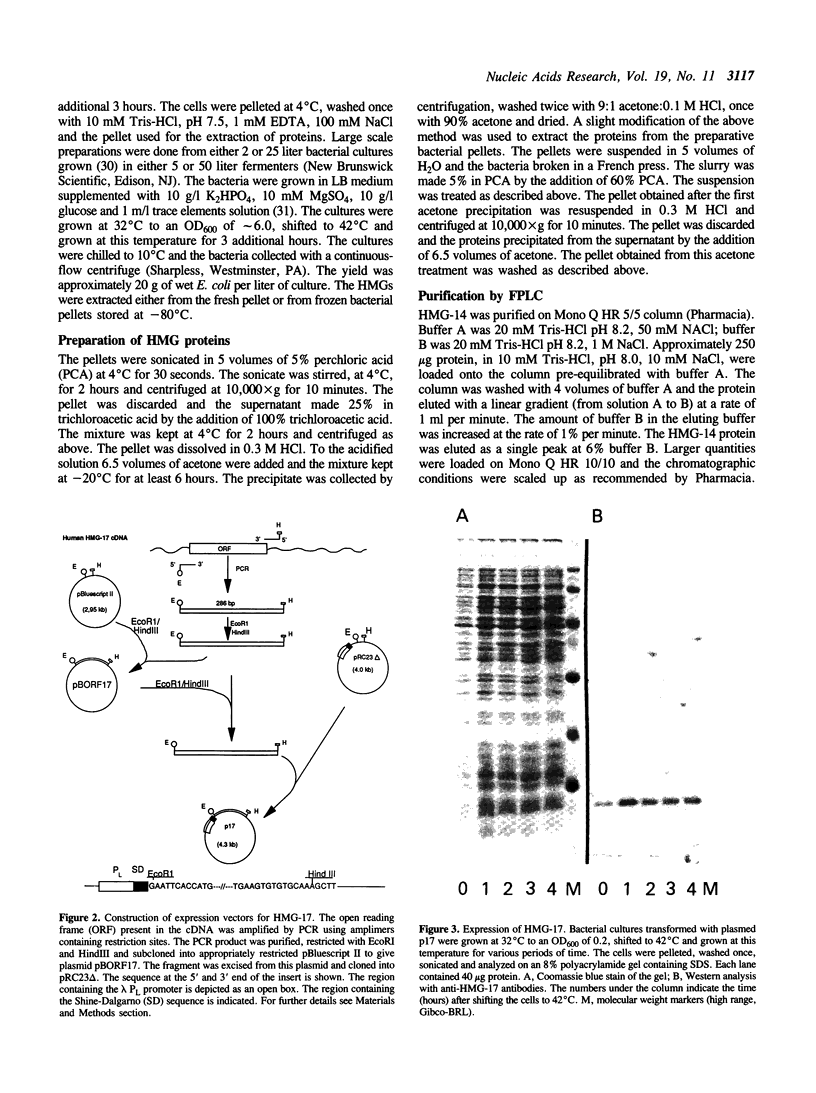
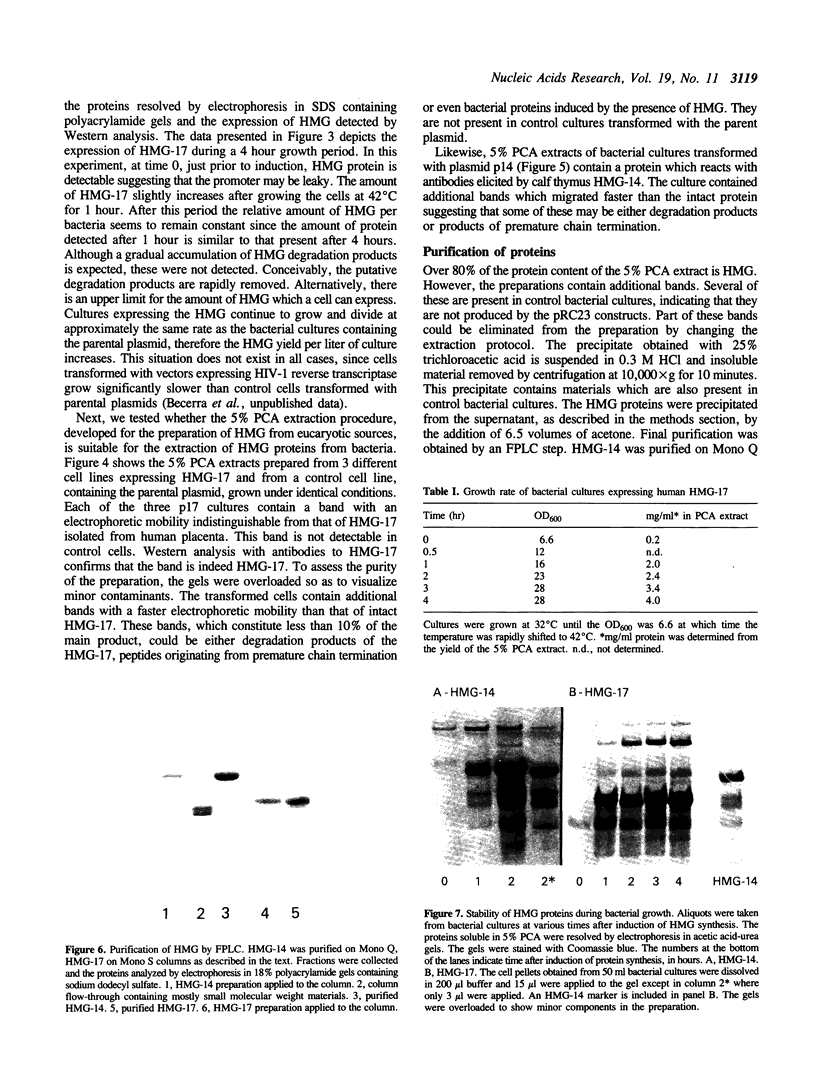
Vectors for expressing human chromosomal proteins HMG-14 and HMG-17 in bacterial cultures under the control of the temperature-inducible lambda PL promoter have been constructed. The open reading frames of the cDNAs have been amplified by the polymerase chain reaction (PCR), utilizing amplimers containing desired restriction sites, thereby facilitating precise location of the initiation codon downstream from a ribosomal binding site. Expression of the recombinant proteins does not significantly affect bacterial growth. The rate of synthesis of the recombinant proteins is maximal during the initial stages of induction and slows down appreciably with time. After an initial burst of protein synthesis, the level of the recombinant protein in the bacterial extracts remains constant at different times following induction. Methods for rapid extraction and purification of the recombinant proteins are described. The recombinant proteins are compared to the proteins isolated from eucaryotic cells by electrophoretic mobility, Western analysis and nucleosome core mobility-shift assays. The ability of the proteins to shift the mobility of the nucleosome cores, but not that of DNA, can be used as a functional assay for these HMG proteins. A source for large quantities of human chromosomal proteins HMG-14 and HMG-17 will facilitate studies on their structure, cellular function and mechanism of interaction with nucleosomes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albright S. C., Wiseman J. M., Lange R. A., Garrard W. T. Subunit structures of different electrophoretic forms of nucleosomes. J Biol Chem. 1980 Apr 25;255(8):3673–3684. [PubMed] [Google Scholar]
- Ausio J., Dong F., van Holde K. E. Use of selectively trypsinized nucleosome core particles to analyze the role of the histone "tails" in the stabilization of the nucleosome. J Mol Biol. 1989 Apr 5;206(3):451–463. doi: 10.1016/0022-2836(89)90493-2. [DOI] [PubMed] [Google Scholar]
- Bernard H. U., Helinski D. R. Use of the lambda phage promoter PL to promote gene expression in hybrid plasmid cloning vehicles. Methods Enzymol. 1979;68:482–492. doi: 10.1016/0076-6879(79)68037-0. [DOI] [PubMed] [Google Scholar]
- Brotherton T. W., Reneker J., Ginder G. D. Binding of HMG 17 to mononucleosomes of the avian beta-globin gene cluster in erythroid and non-erythroid cells. Nucleic Acids Res. 1990 Apr 25;18(8):2011–2016. doi: 10.1093/nar/18.8.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustin M., Hopkins R. B., Isenberg I. Immunological relatedness of high mobility group chromosomal proteins from calf thymus. J Biol Chem. 1978 Mar 10;253(5):1694–1699. [PubMed] [Google Scholar]
- Bustin M., Lehn D. A., Landsman D. Structural features of the HMG chromosomal proteins and their genes. Biochim Biophys Acta. 1990 Jul 30;1049(3):231–243. doi: 10.1016/0167-4781(90)90092-g. [DOI] [PubMed] [Google Scholar]
- Bustin M. Preparation and application of immunological probes for nucleosomes. Methods Enzymol. 1989;170:214–251. doi: 10.1016/0076-6879(89)70049-5. [DOI] [PubMed] [Google Scholar]
- Cook G. R., Minch M., Schroth G. P., Bradbury E. M. Analysis of the binding of high mobility group protein 17 to the nucleosome core particle by 1H NMR spectroscopy. J Biol Chem. 1989 Jan 25;264(3):1799–1803. [PubMed] [Google Scholar]
- Crowl R., Seamans C., Lomedico P., McAndrew S. Versatile expression vectors for high-level synthesis of cloned gene products in Escherichia coli. Gene. 1985;38(1-3):31–38. doi: 10.1016/0378-1119(85)90200-8. [DOI] [PubMed] [Google Scholar]
- Dorbic T., Wittig B. Chromatin from transcribed genes contains HMG17 only downstream from the starting point of transcription. EMBO J. 1987 Aug;6(8):2393–2399. doi: 10.1002/j.1460-2075.1987.tb02517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorbic T., Wittig B. Isolation of oligonucleosomes from active chromatin using HMG17-specific monoclonal antibodies. Nucleic Acids Res. 1986 Apr 25;14(8):3363–3376. doi: 10.1093/nar/14.8.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druckmann S., Mendelson E., Landsman D., Bustin M. Immunofractionation of DNA sequences associated with HMG-17 in chromatin. Exp Cell Res. 1986 Oct;166(2):486–496. doi: 10.1016/0014-4827(86)90493-3. [DOI] [PubMed] [Google Scholar]
- Einck L., Bustin M. Inhibition of transcription in somatic cells by microinjection of antibodies to chromosomal proteins. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6735–6739. doi: 10.1073/pnas.80.22.6735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fass R., Clem T. R., Shiloach J. Use of a novel air separation system in a fed-batch fermentative culture of Escherichia coli. Appl Environ Microbiol. 1989 May;55(5):1305–1307. doi: 10.1128/aem.55.5.1305-1307.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziano V., Ramakrishnan V. Interaction of HMG14 with chromatin. J Mol Biol. 1990 Aug 20;214(4):897–910. doi: 10.1016/0022-2836(90)90344-L. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Johnson P. F., McKnight S. L. Eukaryotic transcriptional regulatory proteins. Annu Rev Biochem. 1989;58:799–839. doi: 10.1146/annurev.bi.58.070189.004055. [DOI] [PubMed] [Google Scholar]
- Landsman D., Soares N., Gonzalez F. J., Bustin M. Chromosomal protein HMG-17. Complete human cDNA sequence and evidence for a multigene family. J Biol Chem. 1986 Jun 5;261(16):7479–7484. [PubMed] [Google Scholar]
- Landsman D., Srikantha T., Westermann R., Bustin M. Chromosomal protein HMG-14. Complete human cDNA sequence and evidence for a multigene family. J Biol Chem. 1986 Dec 5;261(34):16082–16086. [PubMed] [Google Scholar]
- Mardian J. K., Paton A. E., Bunick G. J., Olins D. E. Nucleosome cores have two specific binding sites for nonhistone chromosomal proteins HMG 14 and HMG 17. Science. 1980 Sep 26;209(4464):1534–1536. doi: 10.1126/science.7433974. [DOI] [PubMed] [Google Scholar]
- Pash J., Popescu N., Matocha M., Rapoport S., Bustin M. Chromosomal protein HMG-14 gene maps to the Down syndrome region of human chromosome 21 and is overexpressed in mouse trisomy 16. Proc Natl Acad Sci U S A. 1990 May;87(10):3836–3840. doi: 10.1073/pnas.87.10.3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton A. E., Wilkinson-Singley E., Olins D. E. Nonhistone nuclear high mobility group proteins 14 and 17 stabilize nucleosome core particles. J Biol Chem. 1983 Nov 10;258(21):13221–13229. [PubMed] [Google Scholar]
- Sandeen G., Wood W. I., Felsenfeld G. The interaction of high mobility proteins HMG14 and 17 with nucleosomes. Nucleic Acids Res. 1980 Sep 11;8(17):3757–3778. doi: 10.1093/nar/8.17.3757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasi R., Hüvös P. E., Fasman G. D. A conformational study of the binding of a high mobility group protein with chromatin. J Biol Chem. 1982 Oct 10;257(19):11448–11454. [PubMed] [Google Scholar]
- Schröter H., Bode J. The binding sites for large and small high-mobility-group (HMG) proteins. Studies on HMG-nucleosome interactions in vitro. Eur J Biochem. 1982 Oct;127(2):429–436. doi: 10.1111/j.1432-1033.1982.tb06890.x. [DOI] [PubMed] [Google Scholar]
- Shick V. V., Belyavsky A. V., Mirzabekov A. D. Primary organization of nucleosomes. Interaction of non-histone high mobility group proteins 14 and 17 with nucleosomes, as revealed by DNA-protein crosslinking and immunoaffinity isolation. J Mol Biol. 1985 Sep 20;185(2):329–339. doi: 10.1016/0022-2836(85)90407-3. [DOI] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein A., Townsend T. HMG 14/17 binding affinities and DNAase I sensitivities of nucleoprotein particles. Nucleic Acids Res. 1983 Oct 11;11(19):6803–6819. doi: 10.1093/nar/11.19.6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow P. S., Varshavsky A. Affinity of HMG17 for a mononucleosome is not influenced by the presence of ubiquitin-H2A semihistone but strongly depends on DNA fragment size. Nucleic Acids Res. 1983 Jan 25;11(2):387–401. doi: 10.1093/nar/11.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uberbacher E. C., Mardian J. K., Rossi R. M., Olins D. E., Bunick G. J. Neutron scattering studies and modeling of high mobility group 14 core nucleosome complex. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5258–5262. doi: 10.1073/pnas.79.17.5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisbrod S. Active chromatin. Nature. 1982 May 27;297(5864):289–295. doi: 10.1038/297289a0. [DOI] [PubMed] [Google Scholar]
- Weisbrod S., Weintraub H. Isolation of actively transcribed nucleosomes using immobilized HMG 14 and 17 and an analysis of alpha-globin chromatin. Cell. 1981 Feb;23(2):391–400. doi: 10.1016/0092-8674(81)90134-3. [DOI] [PubMed] [Google Scholar]
- Westermann R., Grossbach U. Localization of nuclear proteins related to high mobility group protein 14 (HMG 14) in polytene chromosomes. Chromosoma. 1984;90(5):355–365. doi: 10.1007/BF00294162. [DOI] [PubMed] [Google Scholar]