Abstract
Short interbirth interval has been associated with maternal complications and childhood autism and leukemia, possibly due to deficiencies in maternal micronutrients at conception or increased exposure to sibling infections. A possible association between interbirth interval and subsequent risk of childhood type 1 diabetes has not been investigated. A secondary analysis of 14 published observational studies of perinatal risk factors for type 1 diabetes was conducted. Risk estimates of diabetes by category of interbirth interval were calculated for each study. Random effects models were used to calculate pooled odds ratios (ORs) and investigate heterogeneity between studies. Overall, 2,787 children with type 1 diabetes were included. There was a reduction in the risk of childhood type 1 diabetes in children born to mothers after interbirth intervals <3 years compared with longer interbirth intervals (OR 0.82 [95% CI 0.72–0.93]). Adjustments for various potential confounders little altered this estimate. In conclusion, there was evidence of a 20% reduction in the risk of childhood diabetes in children born to mothers after interbirth intervals <3 years.
Childhood type 1 diabetes is caused by the autoimmune destruction of the pancreatic β-cells. The marked increases in incidence in recent decades (1) suggest a role for environmental exposures. Researchers have been particularly interested in environmental exposures in early life, and associations, although weak in magnitude, have been observed with caesarean section delivery (2), maternal age (3), and birth weight (4).
It has long been recognized that short interbirth interval (the time since the immediately preceding birth) is associated with increased risk of adverse pregnancy outcomes such as preterm birth and low birth weight (5). Recently, studies have shown associations between short interbirth interval and an increased risk of diseases in the offspring including childhood autism (6) and schizophrenia (7) and a reduced risk of childhood leukemia (8). The mechanism behind these findings is unknown, but researchers have suggested that short interbirth intervals may not allow complete restoration of maternal micronutrients at the time of conception (7,9), may lead to increased maternal stress (7), and may increase exposure to childhood infections from immediately older siblings (7). These mechanisms are of potential relevance to childhood type 1 diabetes because associations with type 1 diabetes have been observed with maternal micronutrient levels during pregnancy (such as vitamin D [10]), stressful life events during pregnancy (11), and day care attendance (a surrogate for infections in early life) (12). The aim of this study was to conduct the first investigation into the association between interbirth interval and childhood diabetes risk.
RESEARCH DESIGN AND METHODS
The authors of 29 studies who previously contributed to a meta-analysis of the association between birth order and type 1 diabetes (13) were contacted and invited to participate in this study if they could calculate interbirth interval for their study participants (usually from the date of birth or ages of other siblings). Authors of 14 of these studies (14–22) had recorded the dates of birth of older siblings and provided raw datasets or calculated estimates of the association between interbirth interval and diabetes before and after adjustments for potential confounders (if available). Interbirth interval was calculated as time since last live birth and was categorized based upon predefined categories used in a study of autism (6) (<21, 21–32, 33–44, and ≥45 months) and in a study of leukemia (8) (firstborns, <36 months, and ≥36 months).
Statistical analysis.
Odds ratios (ORs) and SEs were calculated for the association between each category of interbirth interval and type 1 diabetes for each study. Unconditional and conditional logistic regression was used to calculate the ORs and SEs for unmatched and matched case-control studies, respectively. In one cohort study with varying length of participant follow-up, Cox regression analysis was used to estimate hazard ratios and their SEs as a measure of association (which are approximate ORs for rare diseases such as type 1 diabetes [23]). A year of birth term was added to Cox regression analysis models to adjust the hazard ratios for any differences in year of birth between case and control subjects resulting from this study design. Combinations of other potential confounders were added as covariates in the regression models for each study before random-effects models were used to calculate pooled ORs (24). Tests for heterogeneity were conducted, and the I2 statistic was calculated (25). A subgroup analysis was conducted by age at diabetes diagnosis, and pooled estimates were compared by age at onset using standard tests for heterogeneity (26). All statistical analyses were performed using Stata 9.0 (Stata, College Station, TX).
RESULTS
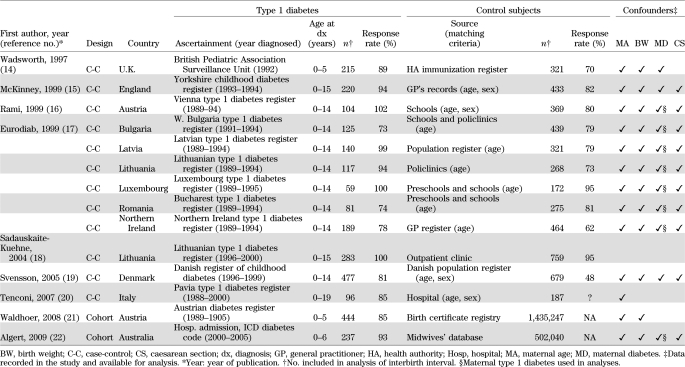
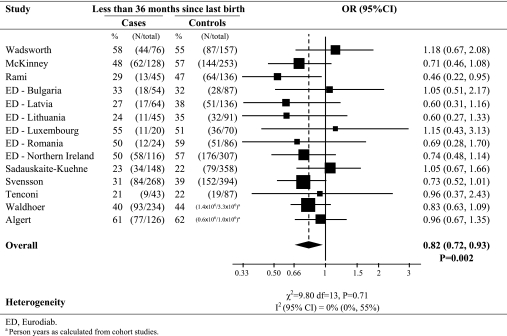
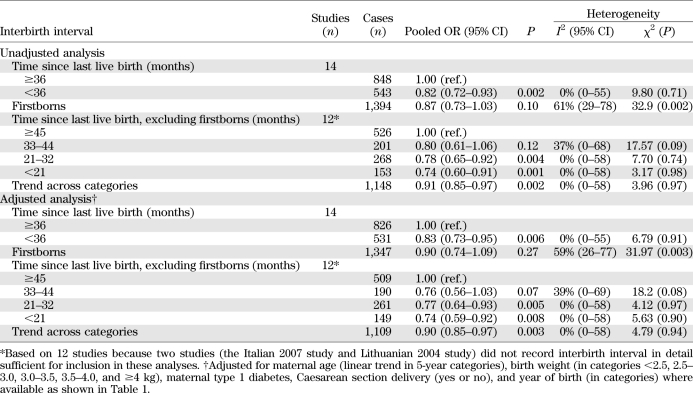
The characteristics of the 14 contributing studies are shown in Table 1. The associations between interbirth interval and type 1 diabetes (with 2,787 cases of type 1 diabetes) are shown in Fig. 1 and Table 2. Overall, children born to mothers with a short time since last birth (<3 years) had a significant 18% reduction in their subsequent risk of developing type 1 diabetes (OR 0.82 [95% CI 0.72–0.93]; P = 0.002) compared with children born to mothers with a long time since last birth (≥3 years). There was little evidence of heterogeneity between study centers in this association (I2 = 0%; heterogeneity P = 0.71). In contrast, there was little evidence of a difference in subsequent risk of type 1 diabetes in firstborns compared with children born with a long time since last pregnancy (OR 0.87; P = 0.10), although there was marked heterogeneity in this association between centers (I2 = 61%; heterogeneity P = 0.002).
TABLE 1.
Characteristics of studies contributing data to the pooled analysis of interbirth interval and type 1 diabetes, ordered by publication date
FIG. 1.
Pooled analysis of risk of type 1 diabetes in children born after a shorter interbirth interval (<36 months since previous birth) compared with a longer interbirth interval (≥36 months since previous birth), excluding firstborns.
TABLE 2.
Pooled analysis of association between interbirth interval and type 1 diabetes before and after adjustments for confounders
Table 2 also shows evidence of a dose-response relationship with larger reductions in diabetes risk with shorter interbirth intervals (test for trend P = 0.002). Compared with the longest time since previous birth (over 45 months), the risk of type 1 diabetes was reduced by 20% (OR 0.80) in children with immediately preceding birth between 33 an 44 months, by 22% (OR 0.78) in children with immediately preceding birth between 21 and 32 months, and by 26% (OR 0.74) in children with immediately preceding birth <21 months. There was little evidence of heterogeneity in these associations across studies.
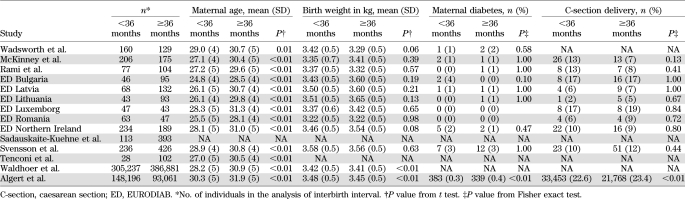
Table 3 shows maternal and child characteristics by interbirth interval. In the majority of studies, there was little evidence of a difference in birth weight, caesarean section delivery, or maternal diabetes, but maternal age was slightly lower by, on average, 3 years after interbirth interval <3 years compared with >3 years. Table 2 shows the findings for interbirth interval after adjustment for these potential confounders. In general, the associations between type 1 diabetes and interbirth interval were little altered after adjustment for maternal age, caesarean section delivery, maternal type 1 diabetes, birth weight, and gestational age in studies in which these variables were available (Table 1). Additionally, in 10 studies with data, adjustment for breast-feeding (at 1 month or similar) little altered the reduction in diabetes risk in children born to mothers with a short time (<3 years) since last birth (adjusted OR 0.75 [95% CI 0.63–0.90]).
TABLE 3.
Maternal and child characteristics for children born after shorter interbirth interval (<36 months since previous birth) compared with longer interbirth interval (≥36 months since previous birth) by study, excluding firstborns
Analysis was also conducted by age at diagnosis. The association between type 1 diabetes risk and time since last birth (<3 vs. ≥3 years) appeared slightly stronger in children >5 years old at diagnosis (in 11 studies with available data, OR 0.74 [95% CI 0.61–0.89]; P = 0.002) than in children <5 years old at diagnosis (in 13 studies with available data, 0.96 [0.76–1.21]; P = 0.74), but the interaction test was not significant (interaction test P = 0.09).
Additional sensitivity analyses were conducted. The risk of diabetes in children born to mothers with a short time since last birth (<3 years) compared with a long time since last birth (≥3 years) was similar to the overall association when restricted to second-born children only (in 12 studies, OR 0.70 [95% CI 0.57–0.86]) and when also adjusted for birth order as well as maternal age (in 12 studies, 0.76 [0.65–0.88]). This association was also similar when participants were excluded if they had an older sibling with diabetes (in nine studies with available data, 0.71 [0.57–0.89]) and when stillbirths were included in the calculation of interbirth interval (in eight studies with available data, 0.73 [0.59–0.90]).
DISCUSSION
This study has identified a reduction in type 1 diabetes risk of ~20% in children born to mothers who gave birth in the previous 3 years. This reduction was consistent across the 14 study centers. This is, to our knowledge, the first study to investigate interbirth interval and type 1 diabetes.
The main strength of this study is that it contains data from 14 centers including 2,787 cases of type 1 diabetes with consistent categorization of interbirth interval (using previously specified categorizations from studies of leukemia [8] and autism [6]). A further strength was the use of population-based diabetes registers to identify cases (in 12 of the 14 studies) and the selection of control subjects from largely population-based sources. The study has various weaknesses. As with all observational studies, it is not possible to rule out residual confounding: that children born to mothers after shorter interbirth intervals also have other characteristics that could decrease their risk of type 1 diabetes. In our analysis, we were able to adjust consistently for maternal age, caesarean section, birth weight, maternal diabetes, birth order, and breast-feeding, but it is not possible to rule out the effect of other unknown confounders. One such candidate is miscarriage and abortion history, and it is possible that mothers with longer interbirth intervals may have been more likely to have had miscarriages or abortions; however, to our knowledge there is no evidence that miscarriage history affects childhood-onset diabetes risk and the reports of an association between abortion and childhood diabetes risk have been inconsistent (27–29).
Bias could have occurred if parents delayed pregnancy after the diagnosis of a child with type 1 diabetes because their next child, who would have an increased risk of type 1 diabetes, would tend to be born after a longer interbirth interval. However, it seems unlikely that this bias would have much influence because the incidence of diabetes is low in early life. Furthermore, in a subset of nine studies, children whose older siblings had diabetes could be removed and the main finding was similar. The main analysis was conducted on interbirth interval calculated after the removal of stillbirths (where possible), but an additional analysis was conducted including stillbirths and results were little altered. Half-siblings were excluded from the analysis in nine studies, as it was often unclear whether they had the same natural mother or whether the half-sibling was present in the house when the study participant was an infant. This may introduce some measurement error, but it would be expected that such error would dilute real associations rather than create spurious ones. The included studies were identified if they had contributed to a previous systematic review of birth order (13), instead of taken from literature searches, because to our knowledge data on interbirth interval and type 1 diabetes have not been published.
The cause of any reduction in the risk of childhood type 1 diabetes in children born after shorter interbirth intervals is unknown. Previous studies (9) showing increased risks of low birth weight after short interbirth intervals have suggested that incomplete restoration of maternal micronutrients, particularly folate, at conception is responsible. However, as our study observed a reduced risk of type 1 diabetes after short interbirth intervals, this seems unlikely to be involved. Another potential mechanism behind the association is maternal stress. A Danish study previously demonstrated that children born after short interbirth interval were more likely to be unplanned (30), potentially increasing maternal stress. However, this also seems like an unlikely explanation, as previous studies have shown increased risks of type 1 diabetes with stressful life events during pregnancy (11), particularly bereavements and family stress (31).
Previous studies have shown that children who are second or higher in birth order have a reduced risk of type 1 diabetes (13). Authors have speculated that second or later birth order children may have increased exposure to sibling infections and that this may be protective through the hygiene hypothesis (which suggests that the immune system requires stimulation by infections and other immune challenges in early life to achieve a mature and balanced repertoire of responses [32]). It is possible that exposure to sibling infection may be greater in children born after short interbirth intervals, as their immediately older sibling will be of similar age. In our analysis, there were indications that the association between interbirth interval and childhood diabetes may be stronger for children diagnosed at older ages, perhaps because early-onset diabetes may have a stronger genetic component (33).
In conclusion, short interbirth interval is associated with a 20% reduction in type 1 diabetes risk. The magnitude of the association makes it difficult to rule out residual confounding. Confirmation of this finding in independent studies is necessary.
ACKNOWLEDGMENTS
No potential conflicts of interest relevant to this article were reported.
C.R.C. performed statistical analysis, wrote the manuscript, and reviewed and edited the manuscript. J.S., T.W., J.L., V.S.-K., C.L.R., R.C.P., E.J.K.W., G.B., B.U., E.S., G.D., C.I.-T., C.E.d.B, G.S., and C.C.P. researched data and reviewed and edited the manuscript. C.R.C. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank G. Dahlquist, MD, PhD (Umea University, Umea, Sweden), a coordinator of the EURODIAB Substudy 2.
REFERENCES
- 1.Patterson CC, Dahlquist GG, Gyürüs E, Green A, Soltész G; EURODIAB Study Group Incidence trends for childhood type 1 diabetes in Europe during 1989-2003 and predicted new cases 2005-20: a multicentre prospective registration study. Lancet 2009;373:2027–2033 [DOI] [PubMed] [Google Scholar]
- 2.Cardwell CR, Stene LC, Joner G, et al. Caesarean section is associated with an increased risk of childhood-onset type 1 diabetes mellitus: a meta-analysis of observational studies. Diabetologia 2008;51:726–735 [DOI] [PubMed] [Google Scholar]
- 3.Cardwell CR, Stene LC, Joner G, et al. Maternal age at birth and childhood type 1 diabetes: a pooled analysis of 30 observational studies. Diabetes 2010;59:486–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cardwell CR, Stene LC, Joner G, et al. Birthweight and the risk of childhood-onset type 1 diabetes: a meta-analysis of observational studies using individual patient data. Diabetologia 2010;53:641–651 [DOI] [PubMed] [Google Scholar]
- 5.Conde-Agudelo A, Rosas-Bermúdez A, Kafury-Goeta AC. Birth spacing and risk of adverse perinatal outcomes: a meta-analysis. JAMA 2006;295:1809–1823 [DOI] [PubMed] [Google Scholar]
- 6.Cheslack-Postava K, Liu K, Bearman PS. Closely spaced pregnancies are associated with increased odds of autism in California sibling births. Pediatrics 2011;127:246–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smits L, Pedersen C, Mortensen P, van Os J. Association between short birth intervals and schizophrenia in the offspring. Schizophr Res 2004;70:49–56 [DOI] [PubMed] [Google Scholar]
- 8.Johnson KJ, Soler JT, Puumala SE, Ross JA, Spector LG. Parental and infant characteristics and childhood leukemia in Minnesota. BMC Pediatr 2008;8:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smits LJ, Essed GG. Short interpregnancy intervals and unfavourable pregnancy outcome: role of folate depletion. Lancet 2001;358:2074–2077 [DOI] [PubMed] [Google Scholar]
- 10.Stene LC, Ulriksen J, Magnus P, Joner G. Use of cod liver oil during pregnancy associated with lower risk of type I diabetes in the offspring. Diabetologia 2000;43:1093–1098 [DOI] [PubMed] [Google Scholar]
- 11.Virk J, Li J, Vestergaard M, Obel C, Lu M, Olsen J. Early life disease programming during the preconception and prenatal period: making the link between stressful life events and type-1 diabetes. PLoS ONE 2010;5:e11523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaila B, Taback SP. The effect of day care exposure on the risk of developing type 1 diabetes: a meta-analysis of case-control studies. Diabetes Care 2001;24:1353–1358 [DOI] [PubMed] [Google Scholar]
- 13.Cardwell CR, Stene LC, Joner G, et al. Birth order and childhood type 1 diabetes risk: a pooled analysis of 31 observational studies. Int J Epidemiol 2011;40:363–374 [DOI] [PubMed] [Google Scholar]
- 14.Wadsworth EJK, Shield JPH, Hunt LP, Baum JD. A case-control study of environmental factors associated with diabetes in the under 5s. Diabet Med 1997;14:390–396 [DOI] [PubMed] [Google Scholar]
- 15.McKinney PA, Parslow R, Gurney KA, Law GR, Bodansky HJ, Williams R. Perinatal and neonatal determinants of childhood type 1 diabetes. A case-control study in Yorkshire, U.K. Diabetes Care 1999;22:928–932 [DOI] [PubMed] [Google Scholar]
- 16.Rami B, Schneider U, Imhof A, Waldhör T, Schober E. Risk factors for type I diabetes mellitus in children in Austria. Eur J Pediatr 1999;158:362–366 [DOI] [PubMed] [Google Scholar]
- 17.Dahlquist GG, Patterson C, Soltesz G. Perinatal risk factors for childhood type 1 diabetes in Europe. The EURODIAB Substudy 2 Study Group. Diabetes Care 1999;22:1698–1702 [DOI] [PubMed] [Google Scholar]
- 18.Sadauskaite-Kuehne V, Ludvigsson J, Padaiga Z, Jasinskiene E, Samuelsson U. Longer breastfeeding is an independent protective factor against development of type 1 diabetes mellitus in childhood. Diabetes Metab Res Rev 2004;20:150–157 [DOI] [PubMed] [Google Scholar]
- 19.Svensson J, Carstensen B, Mortensen HB, Borch-Johnsen K; Danish Study Group of Childhood Diabetes Early childhood risk factors associated with type 1 diabetes—is gender important? Eur J Epidemiol 2005;20:429–434 [DOI] [PubMed] [Google Scholar]
- 20.Tenconi MT, Devoti G, Comelli M, et al. ; Pavia T1DM Registry Group Major childhood infectious diseases and other determinants associated with type 1 diabetes: a case-control study. Acta Diabetol 2007;44:14–19 [DOI] [PubMed] [Google Scholar]
- 21.Waldhoer T, Rami B, Schober E; Austrian Diabetes Incidence Study Group Perinatal risk factors for early childhood onset type 1 diabetes in Austria - a population-based study (1989-2005). Pediatr Diabetes 2008;9:178–181 [DOI] [PubMed] [Google Scholar]
- 22.Algert CS, McElduff A, Morris JM, Roberts CL. Perinatal risk factors for early onset of Type 1 diabetes in a 2000-2005 birth cohort. Diabet Med 2009;26:1193–1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kirkwood BR, Sterne JAC. Essential Medical Statistics. Oxford, Blackwell Science Ltd, 2003, p. 160–162 [Google Scholar]
- 24.Stukel TA, Demidenko E, Dykes J, Karagas MR. Two-stage methods for the analysis of pooled data. Stat Med 2001;20:2115–2130 [DOI] [PubMed] [Google Scholar]
- 25.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Altman DG, Bland JM. Interaction revisited: the difference between two estimates. BMJ 2003;326:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bache I, Bock T, Vølund A, Buschard K. Previous maternal abortion, longer gestation, and younger maternal age decrease the risk of type 1 diabetes among male offspring. Diabetes Care 1999;22:1063–1065 [DOI] [PubMed] [Google Scholar]
- 28.Robertson L, Harrild K. Maternal and neonatal risk factors for childhood type 1 diabetes: a matched case-control study. BMC Public Health 2010;10:281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tai TY, Wang CY, Lin LL, Lee LT, Tsai ST, Chen CJ. A case-control study on risk factors for Type 1 diabetes in Taipei City. Diabetes Res Clin Pract 1998;42:197–203 [DOI] [PubMed] [Google Scholar]
- 30.Kaharuza FM, Sabroe S, Basso O. Choice and chance: determinants of short interpregnancy intervals in Denmark. Acta Obstet Gynecol Scand 2001;80:532–538 [PubMed] [Google Scholar]
- 31.Robinson N, Lloyd CE, Fuller JH, Yateman NA. Psychosocial factors and the onset of type 1 diabetes. Diabet Med 1989;6:53–58 [DOI] [PubMed] [Google Scholar]
- 32.Gale EA. A missing link in the hygiene hypothesis? Diabetologia 2002;45:588–594 [DOI] [PubMed] [Google Scholar]
- 33.Harjutsalo V, Podar T, Tuomilehto J. Cumulative incidence of type 1 diabetes in 10,168 siblings of Finnish young-onset type 1 diabetic patients. Diabetes 2005;54:563–569 [DOI] [PubMed] [Google Scholar]