Abstract
Current therapies for type 1 diabetes (T1D) involve insulin replacement or transplantation of insulin-secreting tissue, both of which suffer from numerous limitations and complications. Here, we show that subcutaneous transplants of embryonic brown adipose tissue (BAT) can correct T1D in streptozotocin-treated mice (both immune competent and immune deficient) with severely impaired glucose tolerance and significant loss of adipose tissue. BAT transplants result in euglycemia, normalized glucose tolerance, reduced tissue inflammation, and reversal of clinical diabetes markers such as polyuria, polydipsia, and polyphagia. These effects are independent of insulin but correlate with recovery of the animals’ white adipose tissue. BAT transplants lead to significant increases in adiponectin and leptin, but with levels that are static and not responsive to glucose. Pharmacological blockade of the insulin receptor in BAT transplant mice leads to impaired glucose tolerance, similar to what is seen in nondiabetic animals, indicating that insulin receptor activity plays a role in the reversal of diabetes. One possible candidate for activating the insulin receptor is IGF-1, whose levels are also significantly elevated in BAT transplant mice. Thus, we propose that the combined action of multiple adipokines establishes a new equilibrium in the animal that allows for chronic glycemic control without insulin.
Type 1 diabetes (T1D) involves autoimmune-mediated destruction of β-cells, which leads to an absolute deficiency of insulin and loss of glycemic control. The ultimate cure for T1D would require permanent renormalization of blood glucose homeostasis. Although insulin is believed to be the major regulator of blood glucose levels, numerous other hormones are also involved (1). In particular, adipose tissue is a versatile endocrine organ secreting a variety of adipokines with both hyperglycemic and hypoglycemic properties (1–3). Two adipokines, adiponectin and leptin, have recently been reported to ameliorate diabetes phenotypes in the absence of exogenous insulin (4–7), indicating that specific adipokines may be able to compensate for a loss of insulin.
In addition to the destruction of pancreatic β-cells, T1D is associated with a loss of adipose tissue. Successful treatment of T1D results in spontaneous recovery of adipose tissue along with the restoration of normal glucose levels and dynamics (8–10). However, a two-way relationship between adipose tissue and blood glucose homeostasis has not yet been documented. Previous data following streptozotocin (STZ)-diabetic mice (a model of T1D) treated by embryonic pancreatic tissue transplants suggest that healthy adipose tissue contributes to the correction of T1D, even when insulin levels are low (11). In some immune-deficient nude mice (NCRNU; Taconic), we observed return to euglycemia and reversal of clinical signs of diabetes despite a lack of any measurable increase in plasma insulin. These animals exhibited a robust recovery of body weight and adipose tissue, as well as a simultaneous increase in plasma adiponectin and visfatin (11,12). Thus, we hypothesized that adipose tissue can compensate for a lack of insulin. Our previous subcutaneous transplants were performed using embryonic pancreatic buds with their surrounding tissue, resulting in remarkable replenishment of recipients’ subcutaneous white adipose tissue (WAT) (11,12). To delineate the contributions from different tissue types, we transplanted a panel of embryonic tissues subcutaneously into STZ-treated mice. Pancreatic bud transplants correct T1D as expected. Surprisingly, subcutaneous transplantation of embryonic brown adipose tissue (BAT) into immune-competent C57BL/6J T1D recipients results in marked improvement of glucose homeostasis and reversal of diabetes, accompanied by robust regeneration of the recipients’ subcutaneous WAT. None of the other transplanted tissue types produce similar results. The positive changes after the BAT transplant do not require exogenous insulin or immunosuppression, which indicates that healthy adipose tissue is sufficient to maintain glucose homeostasis in the absence of insulin.
RESEARCH DESIGN AND METHODS
Embryonic BAT was transplanted in the subcutaneous space of STZ-diabetic recipients. Recipient mice included C57BL/6J and NCRNU-M-M nude mice. Donor adipose tissue came from E16–E17 C57BL/6J embryos. Weight was recorded and basal-fed blood samples were collected at regular intervals from mice that received BAT transplants as well as normal nondiabetic control mice and untreated diabetic control mice. Intraperitoneal glucose tolerance tests (IPGTTs) were performed before BAT transplants and at monthly intervals after BAT transplants. Metabolic parameters such as blood glucose, insulin response to glucose and arginine, and plasma levels of adiponectin, leptin, glucagon, and IGF-1 were measured from transplant and control groups at regular intervals. Three months after BAT transplant, mice were subjected to an additional glucose tolerance test in the presence of S961, an inhibitor of insulin receptor. One insulin tolerance test was also performed between 2 and 3 months posttransplant. BAT transplant mice were killed at different time points after 3 months and tissues were collected. Pancreata were tested for insulin content by immunohistochemistry and radioimmunoassay, and adipose tissue at and around the transplant site was examined for uncoupling protein-1 (UCP-1), IGF-1, and signs of inflammation by immunohistochemistry.
Animals.
Recipients were immune-deficient NCRNU-M-M nude mice (Taconic) and immune-competent C57BL/6J mice (Jackson Laboratories) rendered diabetic with STZ (125 mg/kg dissolved in ice-cold Na+ citrate buffer at pH 4.5, injected intraperitoneally, and repeated biweekly until diabetes induced). All recipients were 3–6 months of age at the time of transplants. Donor embryonic BAT was obtained from C57BL/6J embryos at gestational age E16.5–E17.5. Parents were purchased from Jackson Laboratories and maintained in the Vanderbilt animal care facility. Animals were fed standard laboratory chow and cared for according to the guidelines of the Vanderbilt Institutional Animal Care and Use Committee.
Isolation of donor tissue.
Pregnant females were anesthetized with ketamine/xylazine (110/10 mg/kg i.p.). A bilateral subcostal incision was made and extended by a midline transverse incision to expose the abdominal cavity. Uterine horns were exposed one at a time. Starting near the ovary, a longitudinal incision was made along the uterine horn. Embryos were removed and placed in sterile, ice-cold saline. The mouse was immediately killed by cervical dislocation. The embryos were rapidly dissected with Dumont forceps, and the embryonic BAT from the interscapular region was removed, placed in sterile, ice-cold saline, and transplanted into recipients as quickly as possible.
Transplantation.
Freshly isolated embryonic BAT was transplanted into diabetic NCRNU nude mice or C57BL/6J mice, underneath the skin of the dorsal body surface. Through a small (1–2 mm) incision, a subcutaneous pocket was made by blunt dissection. Donor tissue was introduced into the pocket with Dumont forceps and pushed in with blunt-ended microspatula. The incision was closed by gentle pressure with hemostats without sutures. Four to six lobules of embryonic BAT were introduced into each recipient. Surgeries were performed under general anesthesia with ketamine/xylazine (110/10 mg/kg i.p.), and postoperative analgesia was provided with buprenorphine 0.1 mg/kg/day subcutaneously as necessary.
Metabolic parameters.
STZ-treated mice with fasting blood glucose levels >300 mg/dL were selected as transplant candidates. Blood samples were collected from a tail snip under isolfurane/oxygen anesthesia for measurement of glucose, insulin, and other hormones. IPGTTs were performed a week before the transplant and then every month after the transplant until euthanasia for tissue collection. IPGTT involved blood collection from 6-h–fasted mice prior to (0 min) and 15, 30, 60, and 120 min after intraperitoneal injection of sterile glucose (Sigma) (2 g/kg body weight) under isofluorane/oxygen anesthesia. Basal nonfasting blood samples were also collected every 2 weeks when possible. Plasma samples were analyzed for insulin, adiponectin, leptin, glucagon, and IGF-1. At optimum euglycemia, usually ∼3 months after transplant, each mouse also underwent an insulin tolerance test and an IPGTT in the presence of S-961, an insulin receptor antagonist. During these tests, an initial glucose measurement was performed at −10 min immediately followed by intraperitoneal injection of S-961 100 nmol/kg, and the remaining blood collections proceeded as usual after injection of glucose at 0 min.
Postmortem tissue collection.
Mice were killed 2–6 months after transplantation, and adipose tissue and pancreas were harvested for histology and insulin measurements. The whole pancreas and the WAT from the subcutaneous space of the dorsal body surface were collected from BAT transplant groups as well as normal and diabetic control groups. Tissues were blotted to remove moisture, weighed, preserved in 4% paraformaldehyde, and washed in PBS for histological analysis.
Histology.
Histological sections of pancreata were analyzed by immunostaining for insulin and CD34. To verify the inflammatory status of adipose tissue, histological sections were immunostained with tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6). To monitor the progress of BAT transplant, adipose tissue sections encompassing the transplant site were harvested at 2, 3, and 6 months and immunostained for UCP-1 and IGF-1.
Pancreatic insulin content.
Freshly harvested pacreata were homogenized in acid-ethanol and placed on a shaker at 4°C for 48 h. Tissue extracts were centrifuged for 30 min at 4°C at 2,500 rpm, and supernatants were collected and analyzed for insulin with radioimmunoassay.
RESULTS
BAT transplants promote weight gain and euglycemia.
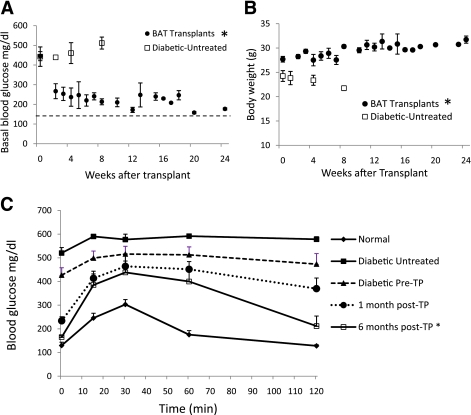
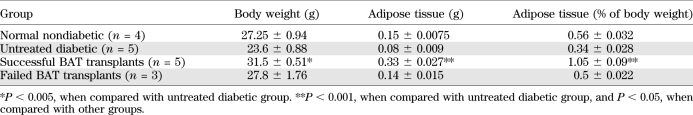
STZ-treated diabetic C57BL/6J mice receiving embryonic BAT transplants showed marked improvement of glucose homeostasis (Fig. 1). Successful BAT transplants were defined as those diabetic mice whose basal blood glucose levels decreased by at least 200 mg/dL within 8 weeks of receiving a BAT transplant. Figures 1–5 show the major results for immune-competent C57BL/6J mice, and the parallel findings in immune-deficient NCRNU nude mice (as well as additional data for C57BL/6J mice) are shown in Supplementary Figs. 1–7. Nine out of fourteen transplants were successful (Fig. 1A), resulting in euglycemia and reversal of clinical signs of diabetes. These mice exhibited gradual weight gain, as opposed to the weight loss seen in the untreated diabetic control groups (Fig. 1B). This weight gain was partially attributable to the replenishment and expansion of subcutaneous WAT. Animals in the BAT transplant group had increased subcutaneous WAT compared with all other groups, both in total and as percent of body weight (Table 1). Weight gain and blood glucose normalization were accompanied by reversal of clinical signs of diabetes such as polyuria, polydipsia, and polyphagia.
FIG. 1.
Subcutaneous transplantation of embryonic BAT results in improved glucose homeostasis and weight gain. Basal nonfasting blood glucose (A) and body weight (B). Horizontal dashed line in A represents baseline glucose levels in nondiabetic mice of 146.13 ± 7.36 mg/dL. A: P < 0.05, between BAT transplant and untreated diabetic controls at each time point. B: P < 0.05, at 8 weeks posttransplant. C: Glucose tolerance tests on BAT transplant, normal, and diabetic control mice. *P < 0.05, when compared with untreated diabetic controls or diabetic pretransplant condition. Normal, pretransplant, and 1 month posttransplant (n = 9); 6 months posttransplant (n = 3); untreated diabetic controls (n = 5). (A high-quality color representation of this figure is available in the online issue.)
FIG. 5.
Inhibition of insulin receptor impairs glucose tolerance in euglycemic transplant mice. S981, a competitive antagonist of the insulin receptor, was administered immediately prior to glucose tolerance test. S961 resulted in impairment of glucose tolerance in both normal controls (n = 4) (A) and BAT transplant recipients euglycemic at 3 months (n = 5) (B). A: P < 0.05, when 60- and 120-min time points are compared with and without S961. B: P < 0.05, when 120-min time point is compared with and without S961.
TABLE 1.
Average body weight and content of subcutaneous adipose tissue collected from dorsal body surface at postmortem from representative subjects from each group
Prior to transplant, the diabetic mice exhibited basal blood glucose levels of 449 ± 22 mg/dL. Basal glucose levels decreased sharply and significantly within 2 weeks, dropping to 267 ± 38 mg/dL (Fig. 1A). These levels continued to fall until euthanasia at different end points between 3 and 6 months posttransplant. The BAT transplants resulted in improved glucose tolerance within 1 month of the transplant, and this tolerance became progressively closer to normal throughout the next 6 months (Fig. 1C and Supplementary Fig. 1A). This is in sharp contrast to untreated diabetic control mice, whose glucose tolerance progressively deteriorated with time (Supplementary Fig. 1B). There were no differences in plasma insulin levels measured in the BAT transplant mice and the STZ-treated diabetic control animals (Fig. 2). Insulin tolerance in successful BAT transplant mice at 2 or 3 months was not significantly different from nondiabetic control animals, and both were strikingly different than what was seen in STZ-treated diabetic mice (Supplementary Fig. 1B). In the six BAT transplant mice that were maintained over 3 months, there was a transient episode of hyperglycemia occurring between 3 and 5 months, with impaired glucose tolerance (Fig. 1A, Fig. 4A, and Supplementary Fig. 1A). However, all of the mice that were allowed to continue beyond 5 months (four out of the nine successful BAT transplants) reverted back to the lower baseline within 1 month of the hyperglycemic episode and recovered normal glucose tolerance similar to or better than their 3-month levels (Fig. 1A, Fig. 4A, and Supplementary Fig. 1A). These results were from immune-competent mice, and similar results were also seen after BAT transplants into STZ-treated nude mice (Supplementary Fig. 2). In nude mice, the BAT transplants performed better, achieving euglycemia and complete normalization of glucose tolerance by 2 months posttransplant.
FIG. 2.
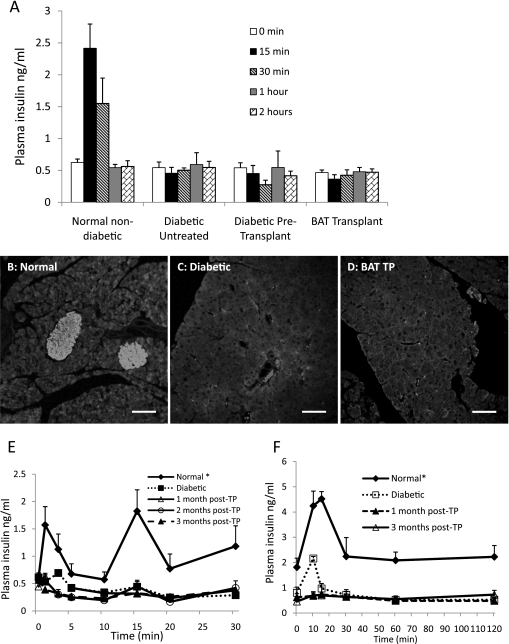
Glucose control is independent of insulin. A: Plasma insulin response to glucose challenge in 3 months posttransplants (n = 5), diabetic pretransplants (n = 9), untreated diabetic (n = 5), and normal nondiabetic controls (n = 7). P < 0.05, at 15 and 30 min between normal control and all other groups. Insulin immunofluorescence of pancreatic sections from 3 months post–BAT transplant mice (D) compared with those of normal (B) and untreated diabetic (C) controls. Scale bar, 50 μm. E: First-phase insulin release during IPGTT 1–3 months posttransplant, compared with normal and diabetic controls (n = 3). *P < 0.05, when compared with all other groups at 1- and 3-min points. F: Arginine-stimulated insulin release 1 and 3 months posttransplant, compared with normal and diabetic controls (n = 4). *P < 0.05, when compared with all other groups at each time point.
FIG. 4.
Long-term glucose control shows a correlation with adipokines rather than insulin. Fasting blood glucose (A), glucose-stimulated insulin (B), adiponectin (C), and leptin (D) levels over time from BAT transplant mice are shown. Although insulin levels in BAT transplant mice remain consistently subnormal, adiponectin and leptin levels show fluctuations that mirror the changes in blood glucose. A–C: Normal (n = 5), BAT transplant, and diabetic (n = 7). D: Normal (n = 5), BAT transplant (n = 7), and diabetic (n = 6). A: P < 0.0005, when diabetic and pretransplant (0 weeks) groups are compared with all other groups. B: P < 0.005, between normal control and all other groups. C: P < 0.001, between BAT transplants and pretransplant (0 weeks) except for 16 weeks; P < 0.001, between BAT transplants and untreated diabetic controls at 8, 12, and 24 weeks. D: P < 0.05, between BAT transplants and normal, diabetic, and pretransplant controls at 4, 8, and 20 weeks; P < 0.005, at 24 weeks posttransplant. Plasma glucagon (E) and IGF-1 (F) levels from 5- and 6-month BAT transplants compared with normal and diabetic controls. BAT transplants are associated with a decrease of glucagon and increase of IGF-1 (n = 4). E: P < 0.0005, between BAT transplants and diabetic controls; P < 0.05, between BAT transplants and normal controls. F: P < 0.05, between BAT transplants and diabetic or normal controls.
BAT transplants act independent of insulin.
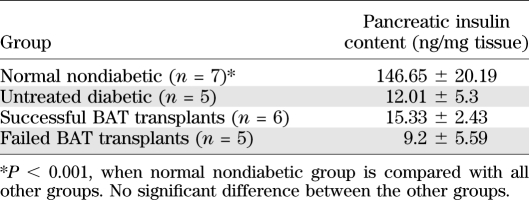
Plasma insulin levels in BAT transplant animals remained statistically identical to those from untreated diabetic mice. Unlike nondiabetic control mice, BAT transplant mice showed no insulin increase in response to a glucose challenge, a response that was comparable to the diabetic pretransplant situation, as well as untreated diabetic controls (Fig. 2A). The first phase of insulin release was missing in the transplant recipients (Fig. 2E), nor was it observed as the mice reach euglycemia after transplants. Arginine, which elicits a robust insulin response above and beyond glucose stimulated insulin secretion from normal mice, also failed to stimulate an insulin response from transplant mice (Fig. 2F). To determine any possible contribution from endogenous insulin, we examined pancreatic morphology by histology and insulin immunofluorescence. Histological analysis did not reveal any intact islets or any cellular insulin immunostaining in the pancreata from BAT transplant animals (Fig. 2D). Islet morphology and cellular insulin staining was lacking in both diabetic control (Fig. 2C) and BAT transplant animals, in contrast to normal control animals (Fig. 2B). Pancreatic insulin content in the BAT transplant mice did not differ significantly from diabetic mice, both of which were >10-fold lower than normal nondiabetic control animals (Table 2).
TABLE 2.
Average pancreatic insulin content at postmortem from each group
Euglycemia negatively correlates with adipose tissue inflammation.
STZ-treated animals exhibited significant adipose tissue inflammation (13,14) (Fig. 3B). This inflammation was characterized by elevation of adipokines, such as IL-6 and TNF-α. In the BAT transplant animals, there was recovery and expansion of subcutaneous WAT, which contributes to the observed weight gain. Immunofluorescence staining of WAT from the recipient’s own fat bed (i.e., not taken from the transplant site) showed low levels of the proinflammatory cytokines IL-6 and TNF-α (Fig. 3C), with adipocyte diameters of 22.68 ± 1.52 μm. Both these parameters were similar to those seen in nondiabetic control animals (Fig. 3A), where adipocyte diameters were 22.74 ± 1.89 μm. In contrast, adipose tissue from untreated diabetic control animals (Fig. 3B), exhibited increased IL-6 and TNF-α levels, increased adipocyte diameters of 35.88 ± 3.57 μm, and infiltration of macrophages resulting in thickening of cell membranes, all of which is consistent with a severe inflammatory phenotype. Similar inflammatory phenotypes were seen in unsuccessful BAT transplants (i.e., transplant recipients that never achieved euglycemia) (Supplementary Fig. 3) and successful BAT transplants that reverted to diabetes after 4 months (Fig. 3D), although these groups exhibited even larger adipocytes of 78.53 ± 5.24 μm. Thus, inflammation appears to be directly related to loss of ability of adipose tissue to maintain glucose homeostasis.
FIG. 3.
BAT transplant failure is associated with inflammatory changes in adipose tissue. Histological sections of replenished WAT in euglycemic mouse 3 months posttransplant (C) is compared with normal (A) and diabetic (B) controls, and a transplant recipient that reverted to diabetes at 4 months (D). Sections are immunostained for TNF-α (green) and IL-6 (red). All tissues contain some green autofluorsecence, and TNF-α expression is indicated by bright green spots. TNF-α immunofluorescence appears near the right border in the normal section (A), toward the bottom in the transplant (TP) (C), and throughout the section in B and D, colocalizing with IL-6 and appearing yellow-orange. In addition to the presence of larger amounts of inflammatory markers, the failed adipose tissue shows other signs of inflammation, such as enlargement of adipocytes and infiltration of macrophages resulting in thickening of cell membranes. Sections shown are representative of four BAT transplants, two untreated diabetic controls, and two normal animals analyzed. Scale bar, 50 μm. (A high-quality digital representation of this figure is available in the online issue.)
The eventual fate of the transplanted BAT was monitored by histological examination of the transplant site at different time points. UCP-1 was observed by immunostaining at 2 months posttransplant, but not at 3 months or beyond (Supplementary Fig. 6). The UCP-1 staining was localized to areas smaller than the original embryonic BAT transplant, and the level of UCP-1 staining decreased over time. Although UCP-1 staining declined over time, BAT appeared to expand into the WAT, as seen in the tissue morphology at 3 and 6 months (Supplementary Fig. 6C and D and Supplementary Fig. 7E).
Noninsulin regulators of glucose homeostasis.
To determine the molecular mechanisms underlying the insulin-independent glucose control, we measured the blood plasma levels of two candidate adipokines, adiponectin and leptin (Fig. 4). Several studies from different sources indicate the ability of adiponectin to improve cell growth and increase insulin sensitivity in peripheral tissues, particularly adipocytes (15–19). In the BAT transplant animals, plasma adiponectin levels showed considerable correlation with glucose homeostasis: low levels pretransplant, with gradually increasing levels as glucose homeostasis improved (Fig. 4C). The adiponectin levels directly mirrored the plasma glucose levels (Fig. 4A). Basal and glucose-stimulated levels of adiponectin were not significantly different, and there was no change of adiponectin levels in response to a glucose challenge (Supplementary Fig. 4). Mirroring the behavior of plasma glucose levels, plasma adiponectin levels also exhibited a marked decrease at 4 months, corresponding with the transient hyperglycemic spike, and then returned to high levels, corresponding with the recovery of euglycemia.
Plasma leptin levels showed a similar time course after BAT transplant, and also exhibited a dip and recovery at ∼4 months posttransplant (Fig. 4D). As is seen for adiponectin, there is also a distinct correlation between improved glucose homeostasis and increased leptin levels. Leptin is reported to affect glucose homeostasis by suppressing glucagon and decreasing lipogenesis (6,7). Thus, we examined glucagon levels in the BAT transplant recipients compared with other groups (Fig. 4E). Plasma glucagon levels increased in the STZ-treated animals and decreased significantly 3 months posttransplant. This decrease in glucagon was accompanied by an elevation of plasma IGF-1 levels (Fig. 4F), which is similar to what is observed after exogenous leptin treatment (6). Notably, the glucagon levels in BAT transplant recipients were significantly lower than those in the nondiabetic control group, and IGF-1 levels were significantly higher. Thus, suppression of glucagon may be a key mechanism of glucose homeostasis that becomes even more important in the absence of insulin, as has also been reported in recent studies (20,21). In addition, IGF-1 may also compensate for insulin function, as evidenced by the experiments with insulin receptor inhibition described in the next section. Upon histological examination of adipose tissue sections from the transplant site at 2, 3, and 6 months, immunostaining for IGF-1 was consistently observed in the BAT, whereas IGF-1 staining in the expanded WAT varied from mild to none (Supplementary Fig. 7).
Role of the insulin receptor.
In the BAT transplant model, diabetes is corrected without an increase in endogenous insulin or addition of exogenous insulin. However, the adipose tissue appears to be normal, and activation of the insulin receptor is believed to be critical for adipose tissue health and survival (22). To explore the possible interactions between the elevated adipokines and the insulin receptor, we monitored glucose tolerance in the presence of S961, a competitive inhibitor of the insulin receptor (23). In nondiabetic control mice, S961 caused an impairment of glucose tolerance (Fig. 5A). In the presence of S961, blood glucose levels increased after the addition of a glucose bolus, but did not show a significant return to basal levels even after 2 hours. In animals with successful BAT transplants, S961 caused a similar impairment of glucose tolerance (Fig. 5B), indicating that the insulin receptor plays a role in the insulin-independent regulation of glucose homeostasis. STZ-treated nude mice also exhibited similar glucose tolerance test results in the presence of S961 (Supplementary Fig. 5).
DISCUSSION
Here, we have shown that regeneration of healthy adipose tissue can reverse clinical diabetes and re-establish glucose homeostasis with no detectable contribution from insulin (Figs. 1 and 2). The correction of diabetes phenotypes by BAT transplants in STZ-treated mice can persist for >6 months, without immunosuppression or hormone supplements (Fig. 1). Our data suggest that these BAT transplants achieve chronic regulation of glucose through a steady-state elevation of alternative hormones, such as adiponectin, leptin, and IGF-1 (Fig. 4). This regulation correlates with the maintenance of healthy and noninflamed adipose tissue, as demonstrated by the inflammatory changes in adipose tissue associated with the diabetic state (Fig. 3). Although insulin is not required, activation of the insulin receptor still appears to play a physiological role because its blockade leads to impaired glucose tolerance in the transplant recipients (Fig. 5).
The lack of insulin response is confirmed by consistently low plasma insulin levels in the transplant recipients (Fig. 1A) and a lack of insulin content (Table 2) in the pancreas postmortem. Previous studies have reported recovery of β-cell mass and function in STZ-treated mice (24,25). However, plasma insulin levels and insulin response to a glucose or arginine challenge remained consistently low, comparable to untreated diabetic controls and in stark contrast to normal controls (Fig. 2A, E, and F). Further, histological examination from successful BAT transplant recipients showed no insulin immunoreactivity in their pancreata (Fig. 2D). Thus, recovery of islet mass and function does not play a significant role in the glucose homeostasis of BAT transplant animals.
Although insulin is not required, activation of the insulin receptor does appear to be involved (Fig. 5). This involvement is in contrast to the proposed mechanisms underlying leptin treatment that focus solely on its role in the inhibition of glucagon action (7). Molecules that activate the insulin receptor may include IGF-1, whose levels are significantly elevated in BAT transplant mice compared with both normal and diabetic groups (Fig. 3F). We also find that IGF-1 is expressed in the transplanted BAT (Supplementary Fig. 7). Several studies have reported the beneficial effects of IGF-1 in improving glucose metabolism and glucose tolerance (26–30). Administration of recombinant IGF-1 improves glucose and lipid metabolism in a range of conditions, including type 2 diabetes, obesity, and T1D (26–29), whereas low IGF-1 levels are associated with impaired glucose tolerance (30). Our histological studies show consistent presence of IGF-1 in the transplanted BAT, with a lesser degree of immunostaining in the expanded WAT. Although part of the IGF-1 may arise from the transplant site, it is likely that elevated IGF-1 levels in plasma are generated from elsewhere, possibly stimulated by increased leptin produced from new WAT. Recent studies show the ability of leptin to stimulate the expression of IGF-1 in various tissues (31–33).
A central role for adipose tissue hormones in glucose homeostasis is increasingly supported in the literature. Certain adipokines, such as resistin, retinol binding protein, and TNF-α, have long been recognized for their ability to exacerbate hyperglycemia (34–37). However, several more recent studies show that individual adipokines can correct diabetes independent of insulin (4–7). In particular, leptin has been shown to restore euglycemia in STZ-treated animals, putatively though its role in reducing hyperglucagonemia (6,7,20,21). Adiponectin gene therapy has also been shown to ameliorate both type 1 and type 2 diabetes (4,5), an effect that is proposed to act through increased insulin receptor sensitivity (15,16). Previous studies further show that adiponectin and leptin each bind to specific receptors while also influencing the actions of other hormones through indirect mechanisms (2,38). Our data show that fluctuations of plasma adiponectin and leptin levels correlate directly with glucose homeostasis in the absence of insulin, along with elevated levels of IGF-1 and reduced levels of plasma glucagon. Taken together, these data suggest a model where glucose homeostasis is regulated by the combination of adipokine activation of insulin receptor signaling (or an equivalent downstream pathway), possible replacement of insulin function with IGF-1, and leptin-mediated reduction of hyperglucagonemia. Another possibility is that adiponectin improved the overall health of peripheral tissues and increased sensitivity to very low levels of insulin that normally would not have been sufficient to maintain euglycemia. Indeed, because approximately one-third of the transplants failed (i.e., the replenished adipose tissue failed to reverse diabetes and showed inflammatory changes postmortem), it is possible there is still some requirement for subdetectable levels of insulin. If such a requirement exists, it might be present only at the outset of the BAT transplant. In any case, such a need may be satisfied clinically with the residual insulin levels seen in recently diagnosed T1D patients. Alternatively, this need could be met by provision of subphysiological levels of insulin (which would not produce the side effects associated with traditional insulin therapy) or by other methods, such as administration of hormone-sensitive lipase levels sufficient to maintain adipose tissue.
Although the possibility of insulin-independent glucose regulation by adipokines has been established in previous studies, development of therapies based on these effects would still be hampered by limitations of compound delivery. Such therapies would likely require persistent injections or infusion pumps that necessitate careful dosage calculations to avoid deleterious effects. Successful treatments could eventually rely on gene therapy, but this approach is still unproven for clinical use. Our data demonstrate the ability of whole adipose tissue to compensate for loss of the insulin-secreting β-cells, providing a simpler therapeutic approach. Transplantation of embryonic BAT at appropriate gestational ages stimulates regeneration of the recipients’ subcutaneous WAT, which in turn secretes a combination of adipokines that bring about euglycemia. This approach does not require any further intervention and thus could constitute a legitimate cure. Therapies based on this approach would provide a safe and convenient method that uses the known effects of adipokines for T1D treatment without the complications associated with regular delivery of exogenous compounds.
ACKNOWLEDGMENTS
This work was supported by development funds from the Vanderbilt University School of Medicine. Some experiments were facilitated by core laboratories supported by the Vanderbilt Diabetes Center (DK-20593), Digestive Diseases Center (DK-58404), and Vanderbilt-Ingram Cancer Center (CA-68485).
No potential conflicts of interest relevant to this article were reported.
S.C.G. designed and carried out experiments, analyzed data, and cowrote the manuscript. D.W.P. designed experiments, analyzed data, and cowrote the manuscript. S.C.G. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank R.K.P. Benninger (Vanderbilt University) and A. Ustione (Vanderbilt University) for advice on quantitative imaging, A.C. Powers (Vanderbilt University) for advice on experimental design and comments on the manuscript, R.D. Cone (Vanderbilt University) and G. Bell (University of Chicago, Chicago, IL) for comments on the manuscript, and W.S. Head (Vanderbilt University) for laboratory management.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db11-0510/-/DC1.
REFERENCES
- 1.Casey A. Hormonal control of metabolism: regulation of plasma glucose. Surgery 2003;21:128a–128d [Google Scholar]
- 2.Wozniak SE, Gee LL, Wachtel MS, Frezza EE. Adipose tissue: the new endocrine organ? A review article. Dig Dis Sci 2009;54:1847–1856 [DOI] [PubMed] [Google Scholar]
- 3.Ahima RS. Adipose tissue as an endocrine organ. Obesity (Silver Spring) 2006;14(Suppl. 5):242S–249S [DOI] [PubMed] [Google Scholar]
- 4.Hu X, She M, Hou H, et al. Adiponectin decreases plasma glucose and improves insulin sensitivity in diabetic Swine. Acta Biochim Biophys Sin (Shanghai) 2007;39:131–136 [DOI] [PubMed] [Google Scholar]
- 5.Fukushima M, Hattori Y, Tsukada H, et al. Adiponectin gene therapy of streptozotocin-induced diabetic mice using hydrodynamic injection. J Gene Med 2007;9:976–985 [DOI] [PubMed] [Google Scholar]
- 6.Yu X, Park BH, Wang MY, Wang ZV, Unger RH. Making insulin-deficient type 1 diabetic rodents thrive without insulin. Proc Natl Acad Sci USA 2008;105:14070–14075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang MY, Chen L, Clark GO, et al. Leptin therapy in insulin-deficient type I diabetes. Proc Natl Acad Sci USA 2010;107:4813–4819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conway B, Miller RG, Costacou T, et al. Temporal patterns in overweight and obesity in type 1 diabetes. Diabet Med 2010;27:398–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conway B, Miller RG, Costacou T, et al. Adiposity and mortality in type 1 diabetes. Int J Obes (Lond) 2009;33:796–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nathan DM, Zinman B, Cleary PA, et al. ; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Research Group Modern-day clinical course of type 1 diabetes mellitus after 30 years’ duration: the diabetes control and complications trial/epidemiology of diabetes interventions and complications and Pittsburgh epidemiology of diabetes complications experience (1983-2005). Arch Intern Med 2009;169:1307–1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gunawardana SC, Benninger RK, Piston DW. Subcutaneous transplantation of embryonic pancreas for correction of type 1 diabetes. Am J Physiol Endocrinol Metab 2009;296:E323–E332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gunawardana SC, Benninger RK, Piston DW. Blood glucose regulation through adipose tissue hormones following subcutaneous transplantation of pancreas. Abstract presented at the Keystone Symposia, 20–25 January 2009, at Fairmont Banff Springs, Banff, Alberta, Canada [Google Scholar]
- 13.Lin Y, Berg AH, Iyengar P, et al. The hyperglycemia-induced inflammatory response in adipocytes: the role of reactive oxygen species. J Biol Chem 2005;280:4617–4626 [DOI] [PubMed] [Google Scholar]
- 14.Yessoufou A, Moutairou K, Khan NA. A model of insulin resistance in mice, born to diabetic pregnancy, is associated with alterations of transcription-related genes in pancreas and epididymal adipose tissue. J Obes 2011;pii:654967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luo N, Liu J, Chung BH, et al. Macrophage adiponectin expression improves insulin sensitivity and protects against inflammation and atherosclerosis. Diabetes 2010;59:791–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fu Y, Luo N, Klein RL, Garvey WT. Adiponectin promotes adipocyte differentiation, insulin sensitivity, and lipid accumulation. J Lipid Res 2005;46:1369–1379 [DOI] [PubMed] [Google Scholar]
- 17.Li S, Shin HJ, Ding EL, van Dam RM. Adiponectin levels and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA 2009;302:179–188 [DOI] [PubMed] [Google Scholar]
- 18.Snehalatha C, Mukesh B, Simon M, Viswanathan V, Haffner SM, Ramachandran A. Plasma adiponectin is an independent predictor of type 2 diabetes in Asian Indians. Diabetes Care 2003;26:3226–3229 [DOI] [PubMed] [Google Scholar]
- 19.Kaas A, Pfleger C, Hansen L, et al. ; Hvidøre Study Group on Childhood Diabetes Association of adiponectin, interleukin (IL)-1ra, inducible protein 10, IL-6 and number of islet autoantibodies with progression patterns of type 1 diabetes the first year after diagnosis. Clin Exp Immunol 2010;161:444–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee Y, Wang MY, Du XQ, Charron MJ, Unger RH. Glucagon receptor knockout prevents insulin-deficient type 1 diabetes in mice. Diabetes 2011;60:391–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edgerton DS, Cherrington AD. Glucagon as a critical factor in the pathology of diabetes. Diabetes 2011;60:377–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laviola L, Perrini S, Cignarelli A, Giorgino F. Insulin signalling in human adipose tissue. Arch Physiol Biochem 2006;112:82–88 [DOI] [PubMed] [Google Scholar]
- 23.Schäffer L, Brand CL, Hansen BF, et al. A novel high-affinity peptide antagonist to the insulin receptor. Biochem Biophys Res Commun 2008;376:380–383 [DOI] [PubMed] [Google Scholar]
- 24.Grossman EJ, Lee DD, Tao J. Glycemic control promotes pancreatic beta-cell regeneration in streptozotocin-induced diabetic mice. PLoS One 2010;5:e8749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yin D, Tao J, Lee DD, et al. Recovery of islet beta-cell function in streptozotocin- induced diabetic mice: an indirect role for the spleen. Diabetes 2006;55:3256–3263 [DOI] [PubMed] [Google Scholar]
- 26.LeRoith D, Yakar S. Mechanisms of disease: metabolic effects of growth hormone and insulin-like growth factor 1. Nat Clin Pract Endocrinol Metab 2007;3:302–310 [DOI] [PubMed] [Google Scholar]
- 27.Zenobi PD, Jaeggi-Groisman SE, Riesen WF, Røder ME, Froesch ER. Insulin-like growth factor-I improves glucose and lipid metabolism in type 2 diabetes mellitus. J Clin Invest 1992;90:2234–2241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rao MN, Mulligan K, Tai V, et al. Effects of insulin-like growth factor (IGF)-I/IGF-binding protein-3 treatment on glucose metabolism and fat distribution in human immunodeficiency virus-infected patients with abdominal obesity and insulin resistance. J Clin Endocrinol Metab 2010;95:4361–4366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zenobi PD, Glatz Y, Keller A, et al. Beneficial metabolic effects of insulin-like growth factor I in patients with severe insulin-resistant diabetes type A. Eur J Endocrinol 1994;131:251–257 [DOI] [PubMed] [Google Scholar]
- 30.Dunger D, Yuen K, Ong K. Insulin-like growth factor I and impaired glucose tolerance. Horm Res 2004;62(Suppl. 1):101–107 [DOI] [PubMed] [Google Scholar]
- 31.Dumond H, Presle N, Terlain B, et al. Evidence for a key role of leptin in osteoarthritis. Arthritis Rheum 2003;48:3118–3129 [DOI] [PubMed] [Google Scholar]
- 32.Soliman AT, ElZalabany MM, Salama M, Ansari BM. Serum leptin concentrations during severe protein-energy malnutrition: correlation with growth parameters and endocrine function. Metabolism 2000;49:819–825 [DOI] [PubMed] [Google Scholar]
- 33.Hamrick MW, Ferrari SL. Leptin and the sympathetic connection of fat to bone. Osteoporos Int 2008;19:905–912 [DOI] [PubMed] [Google Scholar]
- 34.Tamori Y, Sakaue H, Kasuga M. RBP4, an unexpected adipokine. Nat Med 2006;12:30–31; discussion 31 [DOI] [PubMed] [Google Scholar]
- 35.Ondrak KS, Hackney AC. Body composition differences in normal weight, obese-overweight and anorexic adolescents: role of adipocytokines. Med Sport Sci 2010;55:32–42 [DOI] [PubMed] [Google Scholar]
- 36.Choi SH, Kwak SH, Youn BS, et al. High plasma retinol binding protein-4 and low plasma adiponectin concentrations are associated with severity of glucose intolerance in women with previous gestational diabetes mellitus. J Clin Endocrinol Metab 2008;93:3142–3148 [DOI] [PubMed] [Google Scholar]
- 37.Schreyer SA, Chua SC, Jr, LeBoeuf RC, LeBoeuf RC. Obesity and diabetes in TNF-alpha receptor- deficient mice. J Clin Invest 1998;102:402–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev 2005;26:439–451 [DOI] [PubMed] [Google Scholar]