Abstract
Previous observational studies using differing methodologies have yielded inconsistent results regarding the association between glycemic control and outcomes in diabetic patients receiving maintenance hemodialysis (MHD). We examined mortality predictability of A1C and random serum glucose over time in a contemporary cohort of 54,757 diabetic MHD patients (age 63 ± 13 years, 51% men, 30% African Americans, 19% Hispanics). Adjusted all-cause death hazard ratio (HR) for baseline A1C increments of 8.0–8.9, 9.0–9.9, and ≥10%, compared with 7.0–7.9% (reference), was 1.06 (95% CI 1.01–1.12), 1.05 (0.99–1.12), and 1.19 (1.12–1.28), respectively, and for time-averaged A1C was 1.11 (1.05–1.16), 1.36 (1.27–1.45), and 1.59 (1.46–1.72). A symmetric increase in mortality also occurred with time-averaged A1C levels in the low range (6.0–6.9%, HR 1.05 [95% CI 1.01–1.08]; 5.0–5.9%, 1.08 [1.04–1.11], and ≤5%, 1.35 [1.29–1.42]) compared with 7.0–7.9% in fully adjusted models. Adjusted all-cause death HR for time-averaged blood glucose 175–199, 200–249, 250–299, and ≥300 mg/dL, compared with 150–175 mg/dL (reference), was 1.03 (95% CI 0.99–1.07), 1.14 (1.10–1.19), 1.30 (1.23–1.37), and 1.66 (1.56–1.76), respectively. Hence, poor glycemic control (A1C ≥8% or serum glucose ≥200 mg/dL) appears to be associated with high all-cause and cardiovascular death in MHD patients. Very low glycemic levels are also associated with high mortality risk.
Diabetes is a potent cardiovascular risk factor in the general population as well as in people with end-stage renal disease (ESRD) undergoing maintenance dialysis treatment (1–5). Clinical trials have shown that tight glycemic control decreases the risk of developing retinopathy, nephropathy, and neuropathy in the general population (6,7). Furthermore, glycemic control—as measured by A1C—is a predictor of cardiovascular complications, including myocardial infarctions and hospitalizations for coronary artery disease (1,8). Some guidelines, such as those of the National Kidney Foundation Kidney Disease Outcomes Quality Initiative (KDOQI), have recommended that diabetic dialysis patients should follow the American Diabetes Association guidelines; however, there is no consistent evidence to support these recommendations for patients with ESRD (9–12). This lack of evidence is highlighted by the KDOQI recommendations, last updated in 2007, stating that “target A1C for people with diabetes should be <7%, irrespective of presence or absence CKD [chronic kidney disease]” (13).
There are several issues unique to the dialysis population that obligate a separate examination of glycemic control on outcomes in this cohort. Insulin and glucose homeostasis are affected by uremia, which may aggravate insulin resistance (14). Moreover, it may be difficult to accurately assess glycemic control in this population because of changes in erythrocyte survival in renal failure and the effects of erythropoiesis-stimulating agents on A1C levels (14,15).
Recently, three large randomized trials have indicated that intensive glucose lowering in patients with type 2 diabetes did not reduce the risks of cardiovascular disease, the most common source of ESRD mortality (16–19). Additionally, Williams and colleagues (20,21) reported a higher risk of death only in diabetic hemodialysis patients with A1C levels >11%. Shurraw et al. (22) found that higher casual glucose and A1C levels were not associated with mortality in maintenance hemodialysis (MHD) patients with or without diabetes. In contrast, we reported that after adjusting for potential confounders, higher A1C values were incrementally associated with higher death risks in patients on MHD (23). These large observational studies with differing methodologies and recruited patient populations reached somewhat contrasting conclusions regarding the association of A1C with survival in diabetic MHD patients. Hence, we undertook this study to further examine the predictive value of glycemic control on all-cause and cardiovascular mortality in a large, contemporary cohort of MHD patients. This extended cohort study also adds data on glucose levels, examines the effects of anemia and race, and provides new subset analyses.
RESEARCH DESIGN AND METHODS
We extracted, refined, and examined data from all individuals with ESRD who underwent MHD treatment from July 2001 through June 2006 in any 1 of the 580 outpatient dialysis facilities of DaVita Inc., a large dialysis organization in the U.S. (before its acquisition of units owned by Gambro). The study was approved by relevant institutional review committees. Patients were included who had been undergoing dialysis for at least 90 days, were being treated with MHD at the time of entry into the cohort, had a history of diabetes, and had at least one A1C measurement in the first quarter of entry into the cohort.
Clinical and demographic measures.
The creation of the cohort has previously been described (24–26). To minimize measurement variability, all repeated measures for each patient during any given calendar quarter, i.e., over a 13-week interval, were averaged and values were used in all models. Average values were obtained from up to 20 calendar quarters (q1–q20) for each laboratory and clinical measure for each patient over the 6-year cohort period. The first (baseline) studied quarter for each patient was the calendar quarter in which the patient’s vintage reached >90 days. The presence or absence of diabetes at baseline was obtained from DaVita Inc. data. Histories of tobacco smoking and preexisting comorbid conditions were obtained by linking the DaVita Inc. database to the Medical Evidence Form 2728 of the United States Renal Data System, and the latter were categorized into 10 comorbid conditions: ischemic heart disease, congestive heart failure, history of cardiac arrest, history of myocardial infarction, pericarditis, cardiac dysrhythmia, cerebrovascular events, peripheral vascular disease, chronic obstructive pulmonary disease, and cancer (27).
Patients were followed for outcomes through 30 June 2007. The recorded causes of death were obtained from the United States Renal Data System, and cardiovascular death was defined as death due to myocardial infarction, cardiac arrest, heart failure, cerebrovascular accident, and other cardiac causes.
Laboratory measures.
Blood samples were drawn using uniform techniques in all dialysis clinics and were transported to the DaVita Laboratory in Deland, Florida, within 24 h. All laboratory values, including A1C, were measured by automated and standardized methods. Most laboratory values were measured monthly. A1C was usually measured quarterly or semiannually. We divided patients into seven a priori categories based on A1C values: <5 and ≥10% and 1% increments in between, to examine the dose-response association between A1C categories and death risk. Additional analyses were performed after subdividing the population into two groups of A1C ≥7 and <7 and A1C ≥6 and <6%. We divided patients into eight a priori categories based upon randomly measured serum glucose values (<100, 100 to <125, 125 to <150, 150 to <175, 175 to <200, 200 to <250, 250 to <300, and ≥300 mg/dL) to examine the dose-response association between glucose categories and death risk. Finally, additional analyses were performed after dividing the population into two subgroups of glucose: ≥150 and <150 mg/dL.
Epidemiologic and statistical methods.
Survival analyses with Cox proportional hazards regression with repeated quarterly measures were used to examine whether glycemic control predicted survival for up to 6 years of follow-up. The primary analysis examined the associations between baseline A1C and glucose and all-cause mortality, with cardiovascular mortality serving as a secondary outcome measure. We also performed exploratory analyses in subgroups of patients based on age, sex, race, dialysis vintage, serum albumin category (≤3.8 or >3.8 g/dL), and anemia (serum hemoglobin ≤11 or >11 g/dL and serum ferritin ≤500 or >500 ng/mL). We also performed exploratory analyses according to race. To analyze the predictive value of time-averaged A1C and glucose and assess the association between different laboratory and clinical parameters and A1C levels, logistic regression analyses were performed. For each analysis, including subgroup analyses, three models were examined:
Unadjusted model that included mortality data, A1C/glucose categories, and entry calendar quarter (q1–q20).
Case-mix–adjusted model that included all of the above plus age, sex, race/ethnicity (African Americans and other self-categorized Blacks, Non-Hispanic Caucasians, Asians, Hispanics, and others), categories of dialysis vintage (<6 months, 6 months to 2 years, 2–5 years, and ≥5 years), primary insurance (Medicare, Medicaid, private, and others), marital status (married, single, divorced, widowed, and other or unknown), dialysis dose as indicated by Kt/V (single pool), and residual renal function during the entry quarter, i.e., urinary urea clearance.
Case-mix plus malnutrition-inflammation-complex syndrome (MICS)–adjusted model, which included all of the covariates in the case-mix model as well as 12 surrogates of nutritional status and inflammation, including BMI, total nitrogen appearance (also known as normalized protein catabolic rate [nPCR]), and 10 laboratory surrogates with known association with clinical outcomes in hemodialysis patients (28) including serum levels of albumin, total iron-binding capacity, ferritin, creatinine, phosphorus, calcium, bicarbonate, white blood cell count, lymphocyte percentage, and hemoglobin.
Missing covariate data were imputed by the multivariate regression imputation method as appropriate. For all analysis, two-sided P values are reported and results considered statistically significant if P < 0.05. All statistical analyses were carried out with SAS, version 9.1 (SAS Institute, Cary, NC).
RESULTS
Baseline data and correlations.
Over the 5-year period (July 2001–June 2006), 164,789 adult subjects received dialysis treatment in units owned by DaVita Inc. (Supplementary Fig. 1); of these, 141,762 patients were undergoing MHD at the time of entry into the cohort. The study cohort of 54,757 diabetic MHD patients (type 2 diabetes >96%) was identified after excluding individuals without diabetes (n = 61,519) and patients with diabetes without data on A1C (n = 25,486). Of the 54,757 eligible patients who formed the study cohort, 15,753 patients were prevalent in the first quarter (1 July 2001–30 September 2001) and 39,004 accumulated over the subsequent 19 quarters. The median follow-up time was 886 days.
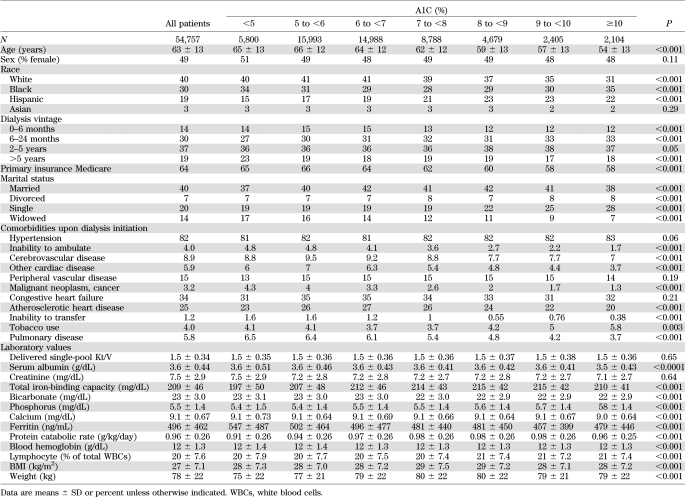
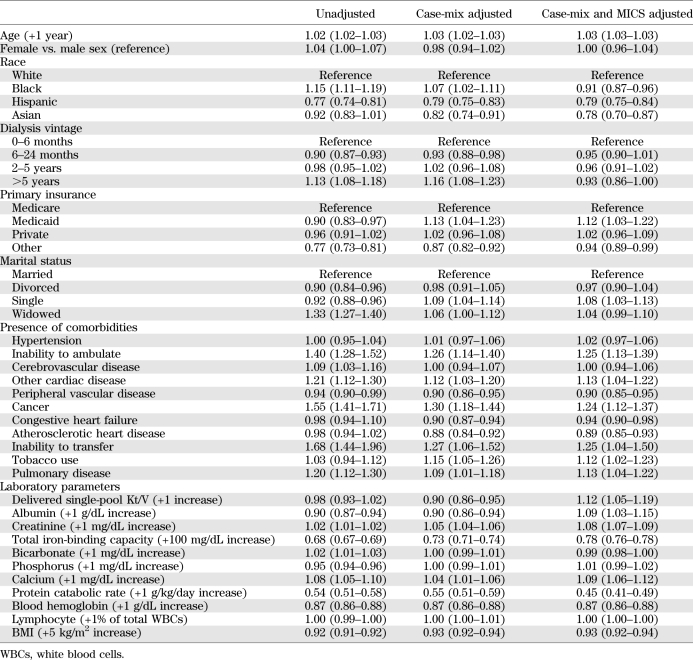
Table 1 shows baseline demographic, clinical, and laboratory characteristics of the studied MHD patients according to seven a priori categories based upon baseline A1C. Higher A1C levels were associated with younger age, fewer white and more Hispanic patients, and fewer Medicare patients.
TABLE 1.
Demographic, clinical, and laboratory values in 54,757 MHD patients and according to the categories of A1C
We found moderate but significant correlation between serum glucose and A1C level (r = 0.562) (Supplementary Fig. 2). In sensitivity analyses, we found relatively consistent correlations across different glucose categories and in different subgroups of patients (Supplementary Table 1). Of the 54,657 MHD patients with A1C data, 50,383 also had corresponding glucose data.
AlC and mortality.
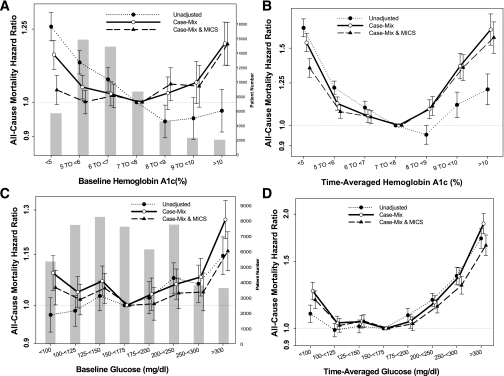
Figure 1A shows unadjusted and adjusted death hazard ratios (HRs) for groups based upon baseline A1C. Case-mix– and MICS-adjusted all-cause death HR for baseline A1C increments of 8.0–8.9, 9.0–9.9, and ≥10%, compared with 7.0–7.9% (reference), was 1.06 (95% CI 1.01–1.12), 1.05 (0.99–1.12), and 1.19 (1.12–1.28), respectively. However, a time-averaged A1C ≥8% was associated with a higher risk of all-cause mortality (Fig. 1B). In contrast with baseline analysis, an increased mortality risk was found in patients with low time-averaged A1C level. Case-mix– and MICS-adjusted all-cause death HR for time-averaged A1C increments of 6.0–6.9, 5.0–5.9, and ≤5%, compared with 7.0–7.9% (reference), was 1.05 (1.01–1.08), 1.08 (1.04–1.11), and 1.35 (1.29–1.42) (Fig. 1B).
FIG. 1.
HRs of all-cause mortality of the entire range of A1C in 54,757 MHD patients using standard Cox proportional hazards regression (A), a time-averaged model (B), and HRs of all-cause mortality of serum glucose in 50,383 diabetic MHD patients using standard Cox proportional hazards regression (C) and a time-averaged model (D). Case-mix model is adjusted for age, sex, race and ethnicity, categories of dialysis vintage, primary insurance, marital status, dialysis dose as indicated by Kt/V (single pool), and residual renal function during the entry quarter. MICS-adjusted model includes all of the case-mix covariates as well as BMI, nPCR, serum levels of albumin, total iron-binding capacity, ferritin, creatinine, phosphorus, calcium, bicarbonate, blood white blood cell count, lymphocyte percentage, and hemoglobin.
Hemoglobin level (≥11.0 or <11.0 g/dL) was identified as a nonsignificant modifier of the time-averaged A1C–mortality association (P value for interaction term, P = 0.67). In 43,806 or 80% of diabetic MHD patients, blood hemoglobin was ≥11.0 g/dL. Supplementary Fig. 3A and B shows the same analyses as shown in Fig. 1A for nonanemic (A) and anemic (B) MHD patients. Among nonanemic patients, time-averaged A1C levels of 8.0–8.9, 9.0–9.9, and ≥10% were associated with 9, 33, and 57% higher all-cause mortality, respectively (reference: A1C 7.0–7.9%; HR 1.09 [95% CI 1.03–1.15], 1.33 [1.23–1.43], and 1.57 [1.43–1.72]). However, only time-averaged A1C ≥9% was associated with a poor outcome in patients with hemoglobin <11.0 g/dL.
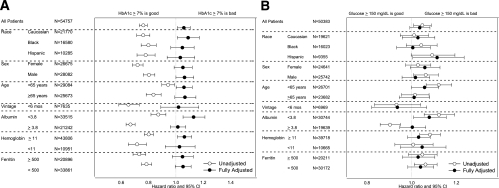
Race was identified as a significant modifier of the time-averaged A1C–mortality association (P value for interaction terms: black, P = 0.02; white, P = 0.09; Hispanic, P = 0.03). Supplementary Fig. 4A–C shows the same analyses as shown in Fig. 1A for white (A), black (B), and Hispanic (C) MHD patients. Among blacks and whites, time-averaged A1C ≥8.0% was associated with higher all-cause mortality. However, among high A1C values, only time-averaged A1C ≥10% was associated with a poor outcome in Hispanic patients. Subsequent subgroup analyses were performed to examine the HRs for all-cause mortality for patients with baseline A1C ≥7% among relevant demographic, clinical, and laboratory categories of MHD patients (Fig. 2A). All unadjusted analyses show that A1C >7% is protective against all-cause mortality. However, this diminished or reversed after adjustment for case-mix and MICS variables in all subgroups. In the entire MHD population, the HR for all-cause mortality in patients with baseline A1C ≥7% was 1.06 (95% CI 1.01–1.11) after adjustment for case-mix and MICS variables. A1C ≥7% was associated with higher mortality risk in white male patients, patients aged <65 years, patients with albumin <3.8 g/dL, and patients with hemoglobin ≥11.0 g/dL.
FIG. 2.
HRs of all-cause mortality for the dichotomized A1C >7% in different subgroups of 54,757 MHD patients (A) and HRs of all-cause mortality for the dichotomized glucose >150 mg/dL in different subgroups of 50,383 MHD patients (B). Fully adjusted model is controlled for age, sex, race and ethnicity, categories of dialysis vintage, primary insurance, marital status, dialysis dose as indicated by Kt/V (single pool), residual renal function during the entry quarter, BMI, nPCR, serum levels of albumin, total iron-binding capacity, ferritin, creatinine, phosphorus, calcium, bicarbonate, blood white blood cell count, lymphocyte percentage, and hemoglobin.
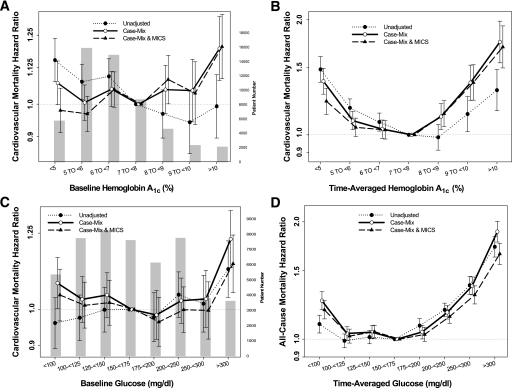
We repeated the analyses using cardiovascular death as the outcome. Figure 3A and B shows unadjusted and adjusted HRs according to the baseline and time-averaged A1C values. Similarly to all-cause mortality, increased cardiovascular mortality risk was associated with baseline A1C ≥10% and time-averaged A1C ≥8%.
FIG. 3.
HRs of cardiovascular mortality of the entire range of A1C in 54,757 MHD patients using standard Cox proportional hazards regression (A), a time-averaged model (B), and HRs of cardiovascular mortality of serum glucose in 50,383 diabetic MHD patients using standard Cox proportional hazards regression (C) and a time-averaged model (D). Case-mix model is adjusted for age, sex, race and ethnicity, categories of dialysis vintage, primary insurance, marital status, dialysis dose as indicated by Kt/V (single pool), and residual renal function during the entry quarter. MICS-adjusted model includes all of the case-mix covariates as well as BMI, nPCR, serum levels of albumin, total iron-binding capacity, ferritin, creatinine, phosphorus, calcium, bicarbonate, blood white blood cell count, lymphocyte percentage, and hemoglobin.
Glucose and mortality.
Figure 1C shows unadjusted and adjusted death HRs for groups based upon baseline glucose. Case-mix– and MICS-adjusted all-cause death HR for baseline glucose increments of 200–249, 250–299, and ≥300 mg/dL, compared with 150–175 mg/dL (reference), was 1.03 (95% CI 0.99–1.08), 1.04 (0.99–1.09), and 1.16 (1.10–1.22), respectively. However, a time-averaged glucose ≥200 mg/dL was associated with a higher risk of all-cause mortality (Fig. 1D).
Hemoglobin level (≥11.0 or <11.0 g/dL) was not identified as a significant modifier of the baseline glucose–mortality association. Supplementary Fig. 5A and B shows the same analyses as shown in Supplementary Fig. 3 for nonanemic (A) and anemic (B) MHD patients. Among anemic and nonanemic patients, a baseline glucose ≥200 mg/dL was associated with higher all-cause mortality (reference: glucose 150–175 mg/dL). Supplementary Fig. 6A–C shows the same analyses as shown in Supplementary Fig. 4 for white (A), black (B), and Hispanic (C) MHD patients.
Subsequent subgroup analyses were performed to examine the HRs for all-cause mortality for patients with baseline glucose ≥150 mg/dL among relevant demographic, clinical, and laboratory categories of MHD patients including race, sex, age, vintage, and selected laboratory measures (Fig. 2B). In the entire MHD population, the HR for all-cause mortality in patients with baseline glucose ≥150 mg/dL was 1.04 (95% CI 0.99–1.08) after adjustment for case-mix and MICS variables. A baseline glucose ≥150 mg/dL was associated with higher mortality risk in Hispanic patients and patients with albumin <3.8 g/dL.
We repeated the analyses using cardiovascular death as the outcome. Figure 3C and D shows unadjusted and adjusted HRs according to the baseline and time-averaged glucose values. Similarly to all-cause mortality, cardiovascular mortality risk was associated with a baseline glucose ≥300 mg/dL and a time-averaged glucose ≥200 mg/dL.
Correlates of low A1C.
To examine the likelihood of unusually low A1C in diabetic HD patients, we performed a multivariate logistic regression analysis comparing the odds of low (<6%) A1C to the nonlow A1C group (≥6%) (Table 2). In our case-mix–adjusted model, each gram per deciliter increase in serum albumin (odds ratio 0.90 [0.86–0.94]) and blood hemoglobin level (0.87 [0.86–0.88]), each gram per kilogram per day increase of nPCR (0.55 [0.51–0.59]), and each kilogram per meters squared increase in BMI level (0.93 [0.92–0.94]) translated into a 10, 13, 45, and 7% lower risk of A1C level <6%, respectively.
TABLE 2.
Multivariate logistic regression models showing clinical parameters and their odds ratios (95% CI) for low (<6%) A1C compared with the nonlow A1C group (≥6%) as reference
DISCUSSION
In this large-scale and contemporary cohort of 54,757 diabetic MHD patients, we report that a time-averaged A1C ≥8% or time-averaged serum glucose ≥200 mg/dL appears to be associated with higher all-cause and cardiovascular mortality. This association was particularly robust in diabetic MHD patients with hemoglobin levels ≥11 g/dL. Subgroup analyses showed that the baseline A1C threshold for higher all-cause mortality was higher in Caucasians, men, and patients with albumin level <3.8 g/dL (A1C ≥7%). We also report that the likelihood of having low baseline A1C (<6%) was associated with lower values for BMI, albumin, creatinine, and nPCR levels, indicating a link between A1C level and malnutrition and inflammation burden. These findings may have important clinical implications, especially since they imply that moderate hyperglycemia may not be a risk factor for death for this population.
The literature on the relationship between glycemic control and survival in CKD population is somewhat limited. However, a study using data from patients treated in units owned by the Fresenius Group was unable to demonstrate any association between A1C and 1-year survival in 24,875 hemodialysis patients (11). These findings contrast with those of several other observational studies: Wu et al. (29) studied 137 hemodialysis patients with type 2 diabetes and reported that the cumulative survival was lower in the group with poor glycemic control. Similarly, we have previously shown that higher A1C is associated with increased death risk in patients treated with hemodialysis in time-dependent analyses (23). Recently, a study published this year (30) that examined the time-dependent association between A1C levels and mortality and cardiovascular events in diabetic dialysis patients reported a significantly increased all-cause mortality among patients reporting A1C levels <6% (31,32). Additionally, Williams et al. (21) reported a higher risk for death only in type 2 diabetic hemodialysis patients with A1C levels >11% when using baseline and time-dependent models. Moreover, we found in a contemporary peritoneal dialysis population that only poor glycemic control (A1C ≥8% and/or glucose ≥300 mg/dL) appeared to be associated incrementally with lower survival in peritoneal dialysis patients (33). These studies provide additional evidence that very poor glycemic control is associated with higher mortality in dialysis patients. However, peritoneal dialysis patients have a different glycemic burden than MHD patients, including glucose load from the peritoneal dialysate.
There are several possible mechanisms that might explain the relationship between glycemic control and survival of MHD patients. Poor glycemic control might result directly in macrovascular complications, possibly secondary to the generation of advanced glycation end products (AGEs), and, hence, shorten survival of these patients. However, higher AGE levels in 312 hemodialysis patients were found, paradoxically, to be associated with better survival (34). The determination of whether the benefit of high serum AGEs in these types of observational studies is an epiphenomenon or reflects a better nutritional status requires further study. Furthermore, comorbid conditions might make glycemic control unsatisfactory, and the higher risk for death may be secondary to the comorbid conditions rather than the poor glycemic control itself. An interventional study of the impact of glycemic control is needed to confirm the reported findings.
In this observational study, we found that compared with patients with A1C 7.0–7.9% (reference), patients with time-averaged A1C increments of 6.0–6.9, 5.0–5.9, and ≤5% had 5, 8, and 35% higher all-cause mortality risk, respectively. A similar association was found in different observational trials in dialysis populations (21,23,33). Moreover, in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial, a prospective interventional study in 10,251 patients with diabetes and without renal failure investigated whether A1C <6%, to be attained by intensive glucose control, reduces cardiovascular events and mortality. Surprisingly, they found an increase in all-cause mortality in the intensive therapy group compared with the standard therapy group (16). There are at least two potential mechanisms that might explain the relationship between low A1C level and survival of MHD patients. It is possible that intensive diabetes control increases the risk for hypoglycemic episodes, which with increasing frequency increases the risk of dying in the long-term follow-up period. Another potential explanation is that low A1C level is a surrogate marker of protein-energy wasting, which is a well-know predictor of mortality in MHD patients (35). This was supported by our observations. In our logistic regression model, the markers of protein-energy wasting such as albumin, creatinine, and BMI indicated a correlation with having a low A1C level. After adjusting the MICS covariables, we found that this association was abolished or sometimes inversed, indicating that MICS is in the causal pathway.
The information on comorbidity in our study was limited to that obtained from Medical Evidence Form 2728, a form through which comorbid conditions are significantly underreported (36). Moreover, we did not have any data available on the medications, if any, to treat diabetes or their doses, and we did not study patient adherence with therapy. Furthermore, the required dose of these medications can be confounded by the residual renal function and its deterioration over time (37). Another potential limitation is the use of nonfasting (random) blood draw for A1C and glucose as well as a lack of explicit laboratory markers of inflammation such as C-reactive protein. However, we used data on serum albumin, ferritin, total iron-binding capacity, blood white blood cell count, lymphocyte percentage, and hemoglobin, which have significant associations with inflammation in dialysis patients (28). Moreover, it is known that A1C significantly underestimates glycemic control in hemodialysis patients (38). Finally, the use of time-averaged measures in this analysis allowed us to reduce variability observed over time and to examine overall trends in the association between glycemic control and mortality; however, these methods may mask significant increases or decreases in laboratory parameters important to survival.
In conclusion, poor glycemic control (A1C ≥8% or serum glucose ≥200 mg/dL) appears to be associated with decreased survival in the general population of diabetic MHD patients. Our study suggests that moderate hyperglycemia increases the risk for all-cause or cardiovascular mortality of diabetic MHD patients, especially in certain subgroups (Caucasians, men, and those with serum albumin ≤3.8 g/dL). Moreover, the presence of protein-energy wasting contributes to the higher risk of low (<6%) A1C level. Admittedly, mortality is only one measure of the deleterious impact of poor glycemic control. Other potential benefits of glycemic control, including slowing the rate of progression of microvascular disease and rate of loss of residual renal function, are possible and were not studied herein. Clinical trials are needed to better define the target A1C levels in different subgroups of diabetic MHD patients.
ACKNOWLEDGMENTS
This study was partly supported by a research grant from the National Institutes of Health (DK077341) to K.K.-Z. K.K.-Z. also receives funding from the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (R01 DK078106) and a philanthropic grant from Mr. Harold Simmons. M.Z.M. received grants from the National Developmental Agency (KTIA-OTKA-EU 7KP-HUMAN-MB08-A-81231) from the Research and Technological Innovation Fund and was also supported by the Hungarian Kidney Foundation.
This study was also supported by a research grant from DaVita Inc. (to K.K.-Z.). No other potential conflicts of interest relevant to this article were reported.
J.R., M.Z.M., C.P.K., and M.W. contributed to analysis and interpretation of data and to writing the manuscript. A.S. contributed to writing the manuscript. A.R.N. contributed to analysis and interpretation of data. K.K.-Z. designed, organized, and coordinated the study; managed data entry; contributed to data analysis and interpretation of data; and wrote the manuscript. K.K.-Z. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in abstract form at the 44th Annual Meeting of the American Society of Nephrology, Philadelphia, Pennsylvania, 8–13 November 2011.
The authors thank DaVita Clinical Research for providing the clinical data, analysis, and review for this research project.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db11-1015/-/DC1.
REFERENCES
- 1.Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med 2003;348:383–393 [DOI] [PubMed] [Google Scholar]
- 2.United States Renal Data System. Excerpts from the USRDS 2005 Annual Data Report: Atlas of End-Stage Renal Disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. Am J Kid Dis 2006;47(Suppl. 1):1–286 [Google Scholar]
- 3.Friedman EA. Renal syndromes in diabetes. Endocrinol Metab Clin North Am 1996;25:293–324 [DOI] [PubMed] [Google Scholar]
- 4.Abbott KC, Bakris GL. Treatment of the diabetic patient: focus on cardiovascular and renal risk reduction. Prog Brain Res 2002;139:289–298 [DOI] [PubMed] [Google Scholar]
- 5.Kimmel PL, Varela MP, Peterson RA, et al. Interdialytic weight gain and survival in hemodialysis patients: effects of duration of ESRD and diabetes mellitus. Kidney Int 2000;57:1141–1151 [DOI] [PubMed] [Google Scholar]
- 6.Warram JH, Manson JE, Krolewski AS. Glycosylated hemoglobin and the risk of retinopathy in insulin-dependent diabetes mellitus. N Engl J Med 1995;332:1305–1306 [DOI] [PubMed] [Google Scholar]
- 7.The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 8.Chaturvedi N, Fuller JH; EURODIAB IDDM Complications Study Group Glycosylated hemoglobin and the risk of microalbuminuria in insulin-dependent diabetes mellitus. N Engl J Med 1995;333:940–941 [DOI] [PubMed] [Google Scholar]
- 9.American Diabetes Association Standards of medical care in diabetes—2010. Diabetes Care 2010;33(Suppl. 1):S11–S61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.K/DOQI clinical practice guidelines for cardiovascular disease in dialysis patients Am J Kidney Dis 2005;45(Suppl. 3):S46–S48 [PubMed] [Google Scholar]
- 11.Williams ME, Lacson E, Jr, Teng M, Ofsthun N, Lazarus JM. Hemodialyzed type I and type II diabetic patients in the US: characteristics, glycemic control, and survival. Kidney Int 2006;70:1503–1509 [DOI] [PubMed] [Google Scholar]
- 12.Feldt-Rasmussen B. Is there a need to optimize glycemic control in hemodialyzed diabetic patients? Kidney Int 2006;70:1392–1394 [DOI] [PubMed] [Google Scholar]
- 13.KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Diabetes and Chronic Kidney Disease Am J Kidney Dis 2007;49:S12–S154 [DOI] [PubMed] [Google Scholar]
- 14.Rubenstein AH, Mako ME, Horwitz DL. Insulin and the kidney. Nephron 1975;15:306–326 [DOI] [PubMed] [Google Scholar]
- 15.Ly J, Marticorena R, Donnelly S. Red blood cell survival in chronic renal failure. Am J Kidney Dis 2004;44:715–719 [PubMed] [Google Scholar]
- 16.Gerstein HC, Miller ME, Byington RP, et al. ; Action to Control Cardiovascular Risk in Diabetes Study Group Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358:2545–2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patel A, MacMahon S, Chalmers J, et al. ; ADVANCE Collaborative Group Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008;358:2560–2572 [DOI] [PubMed] [Google Scholar]
- 18.Duckworth W, Abraira C, Moritz T, et al. ; VADT Investigators Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009;360:129–139 [DOI] [PubMed] [Google Scholar]
- 19.Skyler JS, Bergenstal R, Bonow RO, et al. ; American Diabetes Association American College of Cardiology Foundation American Heart Association Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA Diabetes Trials: a position statement of the American Diabetes Association and a Scientific Statement of the American College of Cardiology Foundation and the American Heart Association. J Am Coll Cardiol 2009;53:298–304 [DOI] [PubMed] [Google Scholar]
- 20.Ix JH. Hemoglobin A1C in hemodialysis patients: should one size fit all? Clin J Am Soc Nephrol 2010;5:1539–1541 [DOI] [PubMed] [Google Scholar]
- 21.Williams ME, Lacson E, Jr, Wang W, Lazarus JM, Hakim R. Glycemic control and extended hemodialysis survival in patients with diabetes mellitus: comparative results of traditional and time-dependent Cox model analyses. Clin J Am Soc Nephrol 2010;5:1595–1601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shurraw S, Majumdar SR, Thadhani R, Wiebe N, Tonelli M; Alberta Kidney Disease Network Glycemic control and the risk of death in 1,484 patients receiving maintenance hemodialysis. Am J Kidney Dis 2010;55:875–884 [DOI] [PubMed] [Google Scholar]
- 23.Kalantar-Zadeh K, Kopple JD, Regidor DL, et al. A1C and survival in maintenance hemodialysis patients. Diabetes Care 2007;30:1049–1055 [DOI] [PubMed] [Google Scholar]
- 24.Molnar MZ, Streja E, Kovesdy CP, et al. High platelet count as a link between renal cachexia and cardiovascular mortality in end-stage renal disease patients. Am J Clin Nutr 2011;94:945–954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ricks J, Molnar MZ, Kovesdy CP, et al. Racial and ethnic differences in the association of body mass index and survival in maintenance hemodialysis patients. Am J Kidney Dis 2011;58:574–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Streja E, Kovesdy CP, Molnar MZ, et al. Role of nutritional status and inflammation in higher survival of African American and Hispanic hemodialysis patients. Am J Kidney Dis 2011;57:883–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rattanasompattikul M, Feroze U, Molnar MZ, et al. Charlson comorbidity score is a strong predictor of mortality in hemodialysis patients. Int Urol Nephrol. 30 November 2011 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kilpatrick RD, McAllister CJ, Kovesdy CP, Derose SF, Kopple JD, Kalantar-Zadeh K. Association between serum lipids and survival in hemodialysis patients and impact of race. J Am Soc Nephrol 2007;18:293–303 [DOI] [PubMed] [Google Scholar]
- 29.Wu MS, Yu CC, Yang CW, et al. Poor pre-dialysis glycaemic control is a predictor of mortality in type II diabetic patients on maintenance haemodialysis. Nephrol Dial Transplant 1997;12:2105–2110 [DOI] [PubMed] [Google Scholar]
- 30.Sturm G, Lamina C, Zitt E, et al. Association of HbA1C values with mortality and cardiovascular events in diabetic dialysis patients. The INVOR study and review of the literature. PLoS ONE 2011;6:e20093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kovesdy CP, Park JC, Kalantar-Zadeh K. Glycemic control and burnt-out diabetes in ESRD. Semin Dial 2010;23:148–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kalantar-Zadeh K, Derose SF, Nicholas S, Benner D, Sharma K, Kovesdy CP. Burnt-out diabetes: impact of chronic kidney disease progression on the natural course of diabetes mellitus. J Ren Nutr 2009;19:33–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duong U, Mehrotra R, Molnar MZ, et al. Glycemic control and survival in peritoneal dialysis patients with diabetes mellitus. Clin J Am Soc Nephrol 2011;6:1041–1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwedler SB, Metzger T, Schinzel R, Wanner C. Advanced glycation end products and mortality in hemodialysis patients. Kidney Int 2002;62:301–310 [DOI] [PubMed] [Google Scholar]
- 35.Rambod M, Bross R, Zitterkoph J, et al. Association of Malnutrition-Inflammation Score with quality of life and mortality in hemodialysis patients: a 5-year prospective cohort study. Am J Kidney Dis 2009;53:298–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Longenecker JC, Coresh J, Klag MJ, et al. Validation of comorbid conditions on the end-stage renal disease medical evidence report: the CHOICE study. Choices for Healthy Outcomes in Caring for ESRD. J Am Soc Nephrol 2000;11:520–529 [DOI] [PubMed] [Google Scholar]
- 37.McMurray SD, Johnson G, Davis S, McDougall K. Diabetes education and care management significantly improve patient outcomes in the dialysis unit. Am J Kidney Dis 2002;40:566–575 [DOI] [PubMed] [Google Scholar]
- 38.Freedman BI, Shenoy RN, Planer JA, et al. Comparison of glycated albumin and hemoglobin A1C concentrations in diabetic subjects on peritoneal and hemodialysis. Perit Dial Int 2010;30:72–79 [DOI] [PubMed] [Google Scholar]