Abstract
The stress protein heat shock protein 60 (Hsp60) induces secretion of proinflammatory mediators from murine adipocytes. This study aimed to study Hsp60 as a mediator of adipose tissue inflammation and skeletal muscle cell (SkMC) insulin sensitivity and to quantify plasma Hsp60 concentrations in lean and obese individuals. Regulation of Hsp60 release and Hsp60-induced cytokine secretion and signaling was measured in human adipocytes and SkMCs. Adipocytes exhibited higher Hsp60 release than preadipocytes and SkMCs, which was further stimulated by cytokines and Toll-like receptor (TLR)-4 activation. Hsp60 activated extracellular signal–related kinase (ERK)-1/2, Jun NH2-terminal kinase (JNK), p38, nuclear factor (NF)-κB, and impaired insulin-stimulated Akt phosphorylation in adipocytes. Furthermore, Hsp60 stimulated adipocytes to secrete tumor necrosis factor-α, interleukin (IL)-6, and IL-8. In SkMCs, Hsp60 activated ERK1/2, JNK, and NF-κB and inhibits insulin signaling and insulin-stimulated glucose uptake. SkMCs released IL-6, IL-8, and monocyte chemoattractant protein-1 on Hsp60 stimulation. Plasma Hsp60 was higher in obese males than in lean males and correlated positively with BMI, blood pressure, leptin, and homeostasis model assessment–insulin resistance. In summary, Hsp60 is released by human adipocytes, increased in plasma of obese humans, and induces insulin resistance. This is accompanied by activation of proinflammatory signaling in human adipocytes and SkMCs. Thus, Hsp60 might be a factor underlying adipose tissue inflammation and obesity-associated metabolic disorders.
Obesity is frequently accompanied by metabolic disturbances such as insulin resistance and other components of the metabolic syndrome (1). Enlarged adipose tissue mass, especially in the visceral compartment, is one of the major risk factors for the development of type 2 diabetes (2). Adipocytes from obese subjects are characterized by altered metabolic and endocrine function with increased secretion of proinflammatory adipokines such as tumor necrosis factor (TNF)-α, interleukin (IL)-6, and resistin (3,4). However, until now, the physiological signals triggering the secretion of proinflammatory mediators from adipocytes remain largely unknown. The stress protein heat shock protein 60 (Hsp60) has been described as a potent inductor of proinflammatory mediators in innate immune cells such as macrophages and in adipocytes (5–8). Furthermore, elevated Hsp60 concentrations have been measured in the circulation of individuals with type 2 diabetes (9). Thus, Hsp60 could be a potential trigger of human adipocyte inflammation. Because insulin resistance is typical for obesity emerging early in the development of the metabolic syndrome and is highly associated with increased visceral adipose tissue mass, this study also aims at characterizing Hsp60 in the context of skeletal muscle insulin resistance. Here, we describe for the first time that Hsp60 is released from adipocytes and can therefore be identified as a novel adipokine, mediating paracrine proinflammatory effects on adipocytes as well as endocrine effects on other cell types such as skeletal muscle cell (SkMC). These findings are supported by our results that circulating Hsp60 levels are higher in obese individuals with and without type 2 diabetes than in lean individuals. The current study provides evidence that Hsp60 contributes to a negative crosstalk between adipose tissue and skeletal muscle.
RESEARCH DESIGN AND METHODS
Cell cultures.
Primary human preadipocytes were obtained from subcutaneous adipose tissue from lean or overweight females undergoing elective plastic surgery (BMI 28.1 ± 1.1 kg/m2, age 42.4 ± 2.8 years) and from PromoCell (Heidelberg, Germany) and were differentiated in vitro to adipocytes as described before (10). For isolation of mature adipocytes and the stromavascular fraction, the protocol was modified by decreasing the collagenase digestion period to 45 min. Mature adipocytes were collected by careful aspiration of the upper phase, while the lower phase was centrifuged at 1,100g to obtain the stromavascular fraction. All protocols were approved by the local ethics committee, and all participants gave written informed consent. Primary human SkMCs derived from healthy individuals (male: 16 and 21 years of age; female: 33 and 37 years of age) were obtained from PromoCell, cultivated, and differentiated as described before (10).
Antibodies and reagents.
Antibodies against phospho–extracellular signal–related kinase (ERK)-1/2 (Thr202/Tyr204), phospho-p38 (Thr180/Tyr182), phospho-SAPK/JNK (p46,Thr183/Tyr185), phospho–nuclear factor (NF)-κB (p65, Ser536), phospho-Akt (Ser473), phospho-GSK3α/β (Ser21/9), and β-actin (clone 13E5) were obtained from Cell Signaling Technology (Danvers, MA). Antitubulin antibodies were obtained from Calbiochem (Merck Biosciences, Schwalbach, Germany). Hsp60 antibodies (clone 24/HSP60) were purchased from BD Biosciences (San Diego, CA), and horseradish peroxidase–conjugated goat anti-rabbit and goat anti-mouse secondary antibodies were from Pierce Thermo Scientific (Bonn, Germany). Recombinant human Hsp60 was obtained from Loke Aps Diagnostics (Risskov, Denmark) or from StressGen Biotechnologies (Victoria, BC, Canada). Lipopolysaccharide (LPS) (Escherichia coli, 026:B6), lipoteichoic acid, ovalbumin (OVA), and porcine insulin were purchased from Sigma-Aldrich (Steinheim, Germany). Recombinant TNF-α, IL-1β, and interferon (IFN)-γ were obtained from Miltenyi Biotech (Bergisch Gladbach, Germany). The enzyme-linked immunosorbent assay (ELISA) kit for phospho–insulin receptor substrate (IRS)-1 (Ser307) was purchased from Cell Signaling Technology.
Hsp60 expression.
Primary human subcutaneous adipocytes and SkMCs obtained at different differentiation time points (day 0–14 and day 0–8, respectively) were lysed in a buffer containing 50 mmol/L HEPES, pH 7.4, 1% Triton, and protease inhibitors (Roche Diagnostics, Mannheim, Germany). Cell lysates were analyzed for Hsp60 expression by Western blot using the Lumi-Imager system (Roche Diagnostics).
Adipocyte release of Hsp60.
To analyze the release of Hsp60 from human preadipocytes, mature adipocytes and SkMC 3.5 × 106 cells were seeded in 75-cm2 cell culture flasks for differentiation, and adipocyte conditioned medium (CM) was generated as described earlier (11). Adipocyte CM was collected and concentrated 200-fold by Amicon Ultra centrifugal filter units (Millipore, Schwalbach, Germany) before Western blot analysis. To induce Hsp60 release upon inflammatory stress or Toll-like receptor (TLR) activation, cells were treated with LPS (1 μg/mL); lipoteichoic acid (5 μg/mL); a cytokine mixture composed of TNF-α, IL-1β, and IFN-γ (1,000 units/mL for each cytokine); or each cytokine individually for 48 h. Concentrated cell culture supernatants were analyzed for Hsp60 concentrations by ELISA (Cusabio Biotech, Newark, DE).
Hsp60 binding and inhibition.
For Hsp60 binding studies, 0.5 × 106 human adipocytes or SkMCs were either directly incubated with DyLight649-labeled (Pierce, Rockford, IL) Hsp60 (Hsp60*, 45 min, 4°C) or preincubated with unlabeled Hsp60 or OVA as described before (12).
Assessment of insulin sensitivity of human SkMCs and adipocytes.
SkMCs were used for glucose uptake experiments at 4 days after start of differentiation. Uptake of 2-deoxyglucose was measured for 2 h after 30 min exposure to insulin (100 nmol/L) as described before (13). To analyze the effect of Hsp60 on Akt and GSK3α/β phosphorylation, human subcutaneous adipocytes and SkMCs were preincubated with medium or Hsp60 (0.5–20 μg/mL) for 24 h. Afterward, cells were stimulated with insulin (100 nmol/L) for 10 min. To investigate the Hsp60-induced activation of signal proteins, cells were stimulated for 0–60 min with medium, 2.5 nmol/L TNF-α (positive control), or Hsp60 (1–20 μg/mL). Subsequently, cells were washed with cold PBS, lysed (1–2 h, 4°C), sonified, and centrifuged (15 min, 10,000g, 4°C). For detection of activated signal proteins, appropriate antibodies were applied. Signals were visualized by the Lumi-Imager system.
Quantification of inflammatory mediators.
Human subcutaneous adipocytes or SkMCs (1 × 105 cells each) were seeded and differentiated in 48-well cell culture plates and exposed to control medium, 0.001–20 μg/mL recombinant human Hsp60 (StressGen Biotechnologies) or 1 μg/mL LPS. After 24 h, concentrations of TNF-α, monocyte chemoattractant protein (MCP)-1, regulated on activation normal T cell expressed and secreted (RANTES), macrophage inflammatory protein-1α (MIP-1α), IL-6, and IL-8 were measured in cell supernatants by multiplex beads assay (Luminex, Austin, TX).
Studies on Hsp60 in humans.
Hsp60 concentrations were determined by ELISA (Cusabio Biotech) in plasma obtained from 18 lean (BMI 23.4 ± 5.6 kg/m2, age 56 ± 14 years) and 23 obese (15 without and 8 with type 2 diabetes) men (BMI 44.5 ± 5.6 kg/m2, age 51 ± 14 years) in the fasted state before undergoing abdominal surgery at Ghent University Hospital. Anthropometric measurements were performed during preoperative examination. Subjects gave written informed consent to participate in this study, which was approved by the Ethical Review Board of the Ghent University Hospital and conducted according to most recent version of the Declaration of Helsinki. Adipose tissue lysates from paired subcutaneous and visceral fat of lean and obese individuals with or without type 2 diabetes were prepared as described before (14).
Statistical analysis.
Data were expressed as means ± SEM. Statistical analysis was performed using the Student t test or ANOVA. Correlations were performed by Pearson product-moment correlation. Statistical analyses were done with JMP (SAS Institute, Cary, NC) or Prism (GraphPad Software, San Diego, CA). Differences were considered statistically significant at P < 0.05.
RESULTS
Inflammatory stress induces release of Hsp60 by primary human subcutaneous adipocytes.
Human subcutaneous adipocytes express Hsp60 in comparable amounts in all differentiation stages (day 0–14) (Fig. 1A). Mature adipocytes freshly isolated from adipose tissue express significantly higher amounts of Hsp60 compared with the stromavascular fraction (Fig. 1B). To examine whether human subcutaneous adipocytes release Hsp60, a considerable amount of cell supernatant, termed “conditioned medium,” was harvested, concentrated 200-fold, and analyzed by Western blot, with adiponectin as a positive control (Fig. 1C). Hsp60 was already detected in 1 μL concentrated CM with increasing signal in 3 and 5 μL concentrated CM (Fig. 1C). Of note, Hsp60 concentration in CM was lower than adiponectin, since Hsp60 (in contrast to adiponectin) was only detectable by ELISA in concentrated CM. Adipocytes at day 14 of differentiation released about three times more Hsp60 than preadipocytes at day 0 (Fig. 1D). Moreover, human adipocytes were treated with proinflammatory cytokines (TNF-α, IL-1β, and IFN-γ, individually or as a mixture) to induce the release of Hsp60 under inflammatory conditions. The application of single cytokines evoked a significant Hsp60 secretion by IL-1β (3.9 ± 0.8-fold) and TNF-α (3.7 ± 0.3-fold), whereas the effect of IFN-γ was negligible (1.1 ± 0.3-fold) compared with medium control (Fig. 1E). Exposure of adipocytes to the cytokine mixture led to a 3.1 ± 0.9-fold secretion of Hsp60. The application of single cytokines to human preadipocytes revealed similar tendencies as for adipocytes (data not shown). Treatment of adipocytes with LPS as a TLR4 agonist stimulated Hsp60 release, whereas lipoteichoic acid as a TLR2 agonist had no effect on Hsp60 secretion (Fig. 1F).
FIG. 1.
Hsp60 expression and release by primary human subcutaneous adipocytes. A: The Hsp60 expression in primary human subcutaneous adipocytes was analyzed at different differentiation time points (day 0–14) with anti-Hsp60 and anti–β-actin antibodies (loading control) by Western blot analysis. Data represent means ± SEM (n = 3–4) and were normalized to β-actin and compared with day 0. B: Mature adipocytes and stromavascular fraction (SVF) were analyzed for Hsp60 expression. Data represent means ± SEM (n = 3) and were normalized to β-actin. *P < 0.05 vs. stromavascular fraction. C: Cell supernatants of primary human subcutaneous adipocytes were collected and 200-fold concentrated, and Hsp60 release was investigated by Western blot analysis. Adiponectin was used as a positive control. D: Concentrated supernatants from preadipocytes and adipocytes were analyzed for their Hsp60 content by ELISA. Data represent means ± SEM (n ≥ 4), *P < 0.05 vs. day 0. E and F: Cell supernatants of cytokine-treated (IFN-γ, IL-1β, TNF-α; individually and as a mixture; each 1,000 units/mL) and TLR agonist–treated (LPS 1 μg/mL, lipoteichoic acid [LTA] 5 μg/mL) adipocytes were analyzed by ELISA. Data represent means ± SEM (n = 3); *P < 0.05 vs. medium control.
Hsp60 binds to primary human subcutaneous adipocytes.
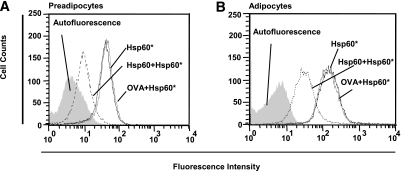
Applying fluorescent-labeled Hsp60 (Hsp60*) to human preadipocytes and mature adipocytes revealed specific binding of Hsp60* to both cell populations (Fig. 2). Specificity was proven through inhibition of Hsp60*-binding by preincubation with the unlabeled ligand (Hsp60) up to 85.0% for preadipocytes (Fig. 2A) and up to 81.4% for adipocytes (Fig. 2B), whereas incubation with OVA was without any effect.
FIG. 2.
Hsp60 binding to primary human subcutaneous adipocytes. A and B: Preadipocytes and mature adipocytes were incubated with 100 nmol/L Hsp60-DyLight649 (Hsp60*) in the absence or presence of 1 μmol/L unlabeled Hsp60 or OVA. Fluorescence intensities of the cells were plotted against cell counts and determined by fluorescence-activated cell sorter analysis.
Hsp60 affects insulin signaling in primary human subcutaneous adipocytes.
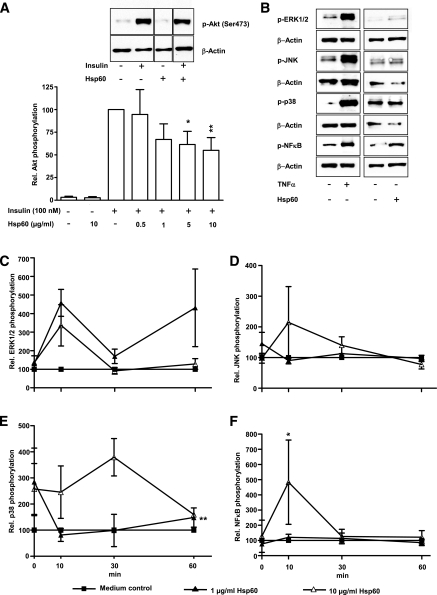
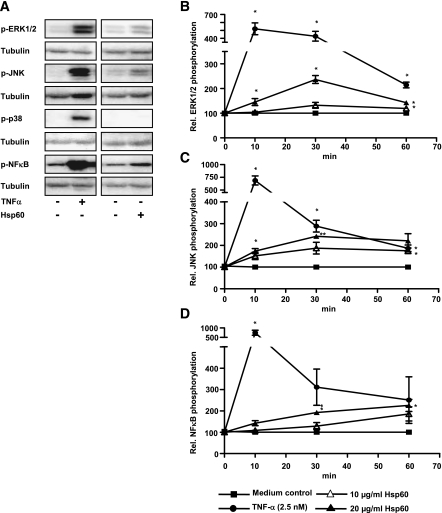
Human adipocytes revealed a dose-dependent significant decrease of insulin-stimulated Akt phosphorylation up to 55.0 ± 14.1% by 10 μg/mL Hsp60 (Fig. 3A). To elucidate the effect of Hsp60 on Akt phosphorylation, different mitogen-activated protein (MAP) kinases and the NF-κB pathway were investigated (Fig. 3B–F). Medium and TNF-α (2.5 nmol/L) were used as controls. A 4.6 ± 0.7-fold activation of the MAP kinase ERK1/2 occurred after a 10-min exposure to Hsp60 (1 μg/mL) (Fig. 3C). JNK activation reached its maximum after 10 min of incubation with 10 μg/mL Hsp60, since the phosphorylation increased 2.1 ± 1.2-fold over medium control (Fig. 3D). Activation of the MAP kinase p38 increased after 30 min (10 μg/mL Hsp60) up to 3.8 ± 0.7-fold (Fig. 3E), whereas the NF-κB pathway reached its activation peak (4.8 ± 2.8-fold) already after 10 min at the highest Hsp60 concentration (Fig. 3F).
FIG. 3.
Effect of Hsp60 on insulin signaling in primary human subcutaneous adipocytes. A: Human adipocytes were treated with medium or different Hsp60 concentrations (0.5–10 μg/mL) for 24 h. After stimulation with insulin (100 nmol/L, 10 min), total cell lysates were analyzed for Akt activation. The relative Akt phosphorylation after insulin stimulation was set at 100%. Lanes were excised from a single Western blot and displayed in the presented order. B: Representative Western blots of p-ERK1/2, p-JNK, p-p38, p-NFκB after stimulation with medium, Hsp60, or TNF-α. β-Actin was used for normalization. C–F: Human adipocytes were treated with medium, Hsp60 (1 and 10 μg/mL), or TNF-α (2.5 nmol/L) for 0–60 min. Total cell lysates were analyzed for activation of the MAP kinases ERK1/2, JNK, p38, and NF-κB. Data represent the means ± SEM of three independent experiments, were normalized to β-actin, and were compared with medium; *P < 0.05; **P < 0.01 vs. the corresponding insulin-stimulated control (A) and medium control (C–F), respectively. ■, Medium control; ▲, 1 μg/mL Hsp60; ∆, 10 μg/mL Hsp60.
Hsp60 induces the release of inflammatory mediators by primary human subcutaneous adipocytes.
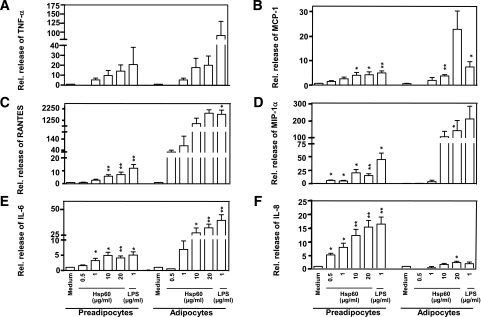
Human adipocytes were exposed to different Hsp60 concentrations (0.001–20 μg/mL) to identify stimulatory Hsp60 concentrations and to investigate dose-dependent Hsp60 effects. Hsp60 concentrations <0.5 μg/mL did not result in a measurable secretion of proinflammatory mediators (data not shown). Treatment with Hsp60 (>0.5 μg/mL) led to a dose-dependent significant secretion of TNF-α (up to 14.0 ± 6.3-fold), RANTES (up to 7.1 ± 2.0-fold), MIP-1α (up to 15.6 ± 3.5-fold), and IL-8 (up to 15.2 ± 2.4-fold) by preadipocytes compared with untreated cells (Fig. 4A–F). In mature adipocytes, Hsp60-stimulated secretion of TNF-α (up to 20.0 ± 9.1-fold), MCP-1 (up to 22.7 ± 7.3-fold), RANTES (up to 1,900.0 ± 302.0-fold), MIP-1α (up to 140.0 ± 59.1-fold), IL-6 (up to 32.0 ± 3.5-fold), and IL-8 (up to 2.4 ± 0.5-fold) and occurred in a concentration-dependent manner compared with unstimulated adipocytes (Fig. 4A–F).
FIG. 4.
Hsp60-induced release of inflammatory mediators from primary human subcutaneous adipocytes. Human preadipocytes and mature adipocytes remained untreated (medium control) or were exposed to increasing concentrations of Hsp60 (0.5–20 μg/mL) or LPS (1 μg/mL). After 24 h, TNF-α (A), MCP-1 (B), RANTES (C), MIP-1α (D), IL-6 (E), and IL-8 (F) concentrations were determined in cell culture supernatants by multiplex-beads assay. The data show means ± SEM from three independent experiments; *P < 0.05; **P < 0.01 vs. the corresponding medium control.
Hsp60 impairs insulin signaling and glucose uptake in primary human SkMCs.
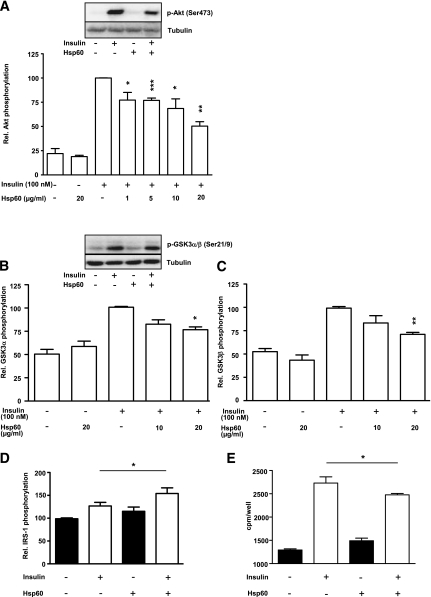
Hsp60-treated human SkMCs revealed a significant dose-dependent decrease in insulin-stimulated Akt phosphorylation from 77.3 ± 7.9% (for 1 μg/mL Hsp60) to 50.3 ± 4.6% (for 20 μg/mL Hsp60) compared with insulin control (Fig. 5A). Moreover, Hsp60 impaired insulin-stimulated phosphorylation of GSK3α and GSK3β (Fig. 5B and C). Our results demonstrate a significantly increased IRS-1 phosphorylation after application of insulin and Hsp60 (154.1 ± 12.4-fold) compared with insulin alone (126.8 ± 7.7-fold; Fig. 5D). Furthermore, Hsp60 exposure to human SkMCs significantly decreased insulin-stimulated glucose uptake (2,477.0 ± 29.3 cpm/well) compared with insulin-stimulated control (2,731.0 ± 134.2 cpm/well; Fig. 5E). Hsp60 treatment increases basal glucose uptake compared with control (1,491 cpm compared with 1,292 cpm). In previous studies, we also observed that SkMCs treated with adipocyte-CM display increased basal glucose uptake. Because both Hsp60 and adipocyte CM activate inflammatory and stress signaling pathways, one might suggest that activation of these pathways is responsible for this effect. To elucidate the role of Hsp60 in the activation of MAP kinases, Hsp60 and TNF-α were applied to SkMCs (Fig. 6A). Maximal activation of the MAP kinases ERK1/2 and JNK was reached after 30 min of incubation with 20 μg/mL Hsp60 (2.4 ± 0.3-fold increase of ERK1/2 and 2.4 ± 0.2-fold increase of JNK activation above medium control; Fig. 6A–C). The NF-κB pathway was activated in a more prolonged manner, with a maximal activation (2.3 ± 0.5-fold over medium control) after 60 min of exposure to 20 μg/mL Hsp60 (Fig. 6D). Activation of the MAP kinase p38 by Hsp60 could not be observed (data not shown).
FIG. 5.
Effect of Hsp60 on insulin signaling in human SkMCs. A–C: SkMCs were treated with medium or Hsp60 (1–20 μg/mL) for 24 h. After stimulation with insulin (100 nmol/L, 10 min), total cell lysates were analyzed for Akt (A) and GSK3α/β (B and C) activation. The relative Akt and GSK3α/β phosphorylation, respectively, after insulin stimulation was set at 100%. Data represent means ± SEM (n = 3–6), were normalized to tubulin, and were compared with the insulin-stimulated control; *P < 0.05; **P < 0.01; ***P < 0.001 vs. the corresponding insulin-stimulated control. D–E: Skeletal muscle cells were cultured for 24 h in the absence or presence of Hsp60 (20 μg/mL). IRS-1 phosphorylation (D) and glucose uptake (E) were assessed after acute stimulation with insulin, as outlined in research design and methods. Means ± SEM of three to four independent experiments are shown; *P < 0.05 vs. insulin-stimulated control.
FIG. 6.
Impact of Hsp60 on the activation of signaling pathways in human SkMCs. A–D: Human SkMCs were treated with medium, Hsp60 (10 and 20 μg/mL), or TNF-α (2.5 nmol/L) for 0–60 min. Representative Western blots of total cell lysates for activation of the MAP kinases ERK1/2 (B), JNK (C), and NF-κB (D) are depicted in A. Data represent means ± SEM of three independent experiments, were normalized to tubulin, and were compared with the unstimulated control; *P < 0.05; **P < 0.01 vs. the corresponding unstimulated medium control. ■, Medium control; ∆, 10 μg/mL Hsp60; ▲, 20 μg/mL Hsp60; ●, 2.5 nmol/L TNF-α.
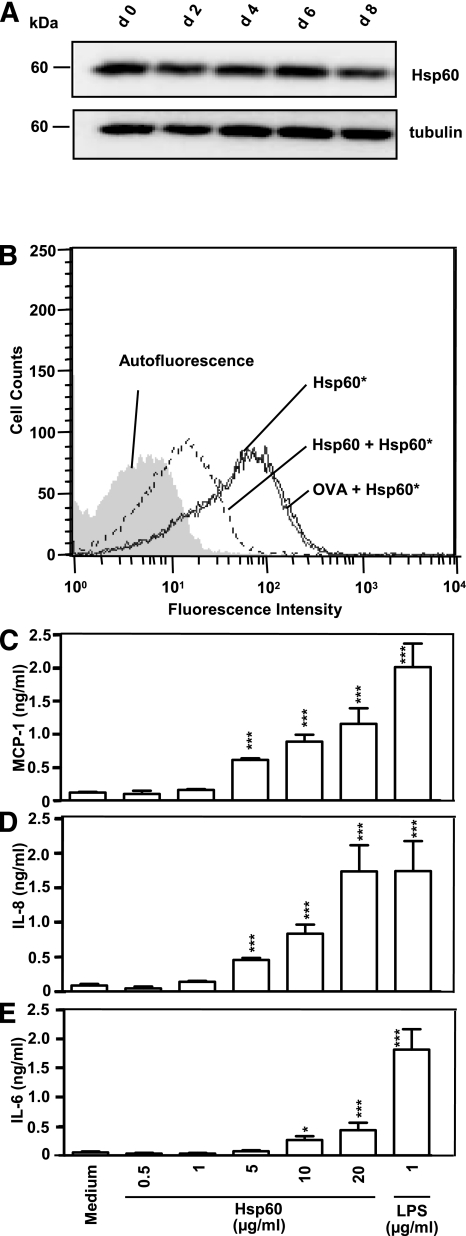
Hsp60 is expressed, but not released, from human SkMCs.
Immunoblot analysis of unstimulated SkMC lysates revealed a consistent expression of Hsp60 in all analyzed maturation states (day 0–8; Fig. 7A). Hsp60 expression in SkMCs is donor dependent and slightly, but not significantly, lower than in adipocytes (data not shown). To investigate if human SkMCs themselves serve as a source for extracellular Hsp60, cell supernatants of unstimulated SkMCs were generated, concentrated to the same extent as the CM from adipocytes, and analyzed by ELISA. Hsp60 release by human SkMCs was not detectable.
FIG. 7.
Hsp60 binding capacity and Hsp60 reactivity to human SkMCs. A: Hsp60 expression in SkMCs was investigated at different differentiation time points (day 0–8) with antibodies directed against Hsp60 and tubulin (loading control) by Western blot analysis. B: Human SkMCs were incubated with 100 nmol/L Hsp60-DyLight649 (Hsp60*) in the absence or presence of 1 μmol/L unlabeled Hsp60 or OVA. Fluorescence intensities of the cells were plotted against cell counts and determined by fluorescence-activated cell sorter analysis. C–E: SkMCs were treated with medium, increasing Hsp60 concentrations (0.5–20 μg/mL) or LPS (1 μg/mL). After 24 h, MCP-1 (C), IL-8 (D), and IL-6 (E) concentrations were measured in cell culture supernatants by multiplex-beads assay. The data show means ± SEM from three independent determinations; *P < 0.05; ***P < 0.001 vs. the corresponding medium control.
Hsp60 binds to human SkMCs and induces the release of cytokines.
Hsp60* was applied to human myoblasts and found to bind specifically to these cells (Fig. 7B). The Hsp60* binding signal was drastically reduced (81.6% inhibition) only in the presence of unlabeled Hsp60, but not with OVA as the control (Fig. 7B). Human SkMCs were exposed to different Hsp60 concentrations (0.001–20 μg/mL), and measurable cytokine levels resulted from Hsp60 concentrations ≥0.5 μg/mL. Hsp60 (20 μg/mL) induced a significant release of MCP-1 (1.2 ± 0.2 ng/mL) compared with medium control (0.1 ± 0.0 ng/mL), IL-8 (1.7 ± 0.4 ng/mL) compared with medium control (0.1 ± 0.0 ng/mL), and IL-6 (0.4 ± 0.1 ng/mL) compared with medium control (0.1 ± 0.0 ng/mL) from human SkMCs (Fig. 7C–E).
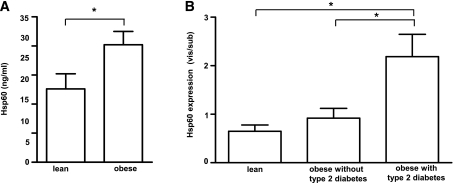
Hsp60 levels are elevated in plasma of obese individuals.
Plasma Hsp60 levels were higher in obese (20.3 ± 11.0 ng/mL Hsp60) than in lean (12.6 ± 11.0 ng/mL Hsp60) men (Fig. 8A; for clinical characterization of patients, see Supplementary Table 1).Within the obese group, Hsp60 levels did not differ between males with (n = 13) or without (n = 8) type 2 diabetes (data not shown). Nevertheless, plasma Hsp60 related weakly but positively with BMI (P = 0.04; r = 0.34), diastolic (P = 0.04; r = 0.35) and systolic (P = 0.03; r = 0.35) blood pressure, plasma leptin (P = 0.04; r = 0.34), and homeostasis model assessment–insulin resistance (HOMA-IR) (P = 0.05; r = 0.35) and inversely with quantitative insulin sensitivity check index (QUICKI) (P = 0.04; r = 0.38). Hsp60 expression in visceral relative to subcutaneous adipose tissue was greater in obese patients with type 2 diabetes (n = 8) than in obese (n = 7) or lean (n = 9) patients without type 2 diabetes (Fig. 8B).
FIG. 8.
Hsp60 plasma levels in lean and obese men and correlations to clinical parameters. A: Plasma levels from 18 lean and 23 obese men grouped into patients with type 2 diabetes (n = 8) and without type 2 diabetes (n = 13) were analyzed for circulating Hsp60 by ELISA. Data show means ± SEM. *P < 0.05 vs. lean subjects. B: Hsp60 expression was measured in tissue lysates of paired subcutaneous and visceral adipose tissue from lean patients (n = 9), obese patients without type 2 diabetes (n = 7), and obese patients with type 2 diabetes (n = 8). The data show means ± SEM of expression in visceral adipose tissue compared with subcutaneous adipose tissue in each individual (vis/sub). *P < 0.05 vs. the respective group.
DISCUSSION
The current study demonstrates that Hsp60 is a novel adipokine that could contribute to inflammatory processes in an autocrine manner within adipose tissue and to the development of peripheral insulin resistance in an endocrine fashion. The observation that Hsp60 is not only expressed but also released clearly indicates that Hsp60 is an intracellular chaperone and also a secretion product. Other heat shock proteins can also be secreted from viable cells such as cardiomyocytes, glial cells, and peripheral blood mononuclear cells (15,16). To date, the origin of circulating Hsp60 remains elusive, but adipose tissue might be considered as one of the sources. It has been reported that circulating levels of Hsp60 were elevated in subjects with inflammatory diseases such as arthritis, atherosclerosis, or type 2 diabetes (9,17,18). Here, we demonstrate that obese patients with or without diabetes also have higher plasma Hsp60 concentrations, suggesting that adipose tissue is a possible source of circulating Hsp60. Analysis of Hsp60 levels in concentrated cell culture supernatants confirmed adipocytes as a putative origin of circulating Hsp60, since Hsp60 was mainly released from untreated human adipocytes, in a lesser extent from preadipocytes, but not from human SkMCs. The latter could be explained by Hsp60 concentrations below the detection limit, even in highly concentrated CM from SkMCs. However, further investigations are needed to clarify Hsp60 sources in vivo. Furthermore, we simulated inflammation by applying a cytokine mixture to human adipocytes, to induce the release of Hsp60. To elucidate the individual contribution of these cytokines to these processes, cells were also exposed to each cytokine individually. Hsp60 secretion was observed after the application of TNF-α and IL-1β in comparable amounts to that obtained after incubation with the cytokine mixture, but not after IFN-γ treatment. These results indicate that macrophages/monocytes and adipocytes themselves might be an inducer of Hsp60 release by adipocytes, since they have been described as sources of TNF-α and IL-1β (7,19). Besides IL-1β and TNF-α, other stimuli for the regulation of Hsp60 expression might be considered. Increased Hsp60 expression was reported for primary human astrocytes in response to cytokines as diverse as IL-1β and TNF-α but also IL-4, IL-6, and IL-10 (20).
In general, the amounts of Hsp60 released by adipocytes are relatively low and not measurable in unconcentrated CM by ELISA, a problem that is often encountered in unconcentrated cell culture supernatants. In fact, various known adipokines that are found in high concentrations in the circulation are relatively low in adipocyte CM (21). Nevertheless, it can be hypothesized that extracellular levels of Hsp60 mediating auto- and paracrine effects might be significantly higher. Furthermore, the fact that circulating Hsp60 levels are higher in obese subjects than in lean control subjects and that Hsp60 concentrations are correlated with circulating leptin further supports the assumption that adipocytes are a source for circulating Hsp60 in vivo.
Hsp60-binding studies revealed that Hsp60 binds specifically and in a dose-dependent manner to adipocytes, representing the typical characteristics of a ligand-receptor interaction. These findings are consistent with results obtained from the murine adipocyte cell line 3T3-L1 (12). Attempts to characterize the Hsp60 receptor structure(s) on innate immune cells identified TLR2, TLR4, and CD14 as components responsible for the proinflammatory effects of Hsp60 (22–25). Activation of TLR4 but not TLR2, on the other hand, induces the release of Hsp60 from adipocytes. The identity of the Hsp60 receptor complex on human adipocytes, however, remains elusive.
Because Hsp60 can be released from human subcutaneous adipocytes and moreover binds specifically to human adipocytes, autocrine effects of Hsp60 can be assumed. We obtained Hsp60-mediated activation of the MAP kinases ERK1/2, JNK, and p38 and the transcription factor NF-κB in human adipocytes. The activation of similar MAP kinase patterns has been described for other adipokines, such as chemerin and MCP-1 (26), suggesting that different adipokines are capable of inducing similar proinflammatory responses. Moreover, Hsp60 induced secretion of proinflammatory mediators. All these Hsp60-mediated effects may indicate that Hsp60 could be involved in adipose tissue inflammation by both inducing acute proinflammatory signaling and an enhanced release of proinflammatory mediators. The resulting elevated adipokine levels, e.g., for TNF-α, IL-6, or MCP-1, are well described to be obesity-related and may indirectly induce the development of insulin resistance in human adipocytes and other insulin-sensitive tissues (1,27). The first evidence that Hsp60 might contribute to these processes is given, since Hsp60 provoked a significant decrease of the insulin-stimulated Akt and GSK3α/β phosphorylation in human adipocytes (Akt) and SkMCs (Akt, GSK3α/β), thereby triggering the development of an insulin resistance. These findings were confirmed by our results demonstrating significant effects of Hsp60 on IRS-1 phosphorylation as well as Hsp60-mediated impairment of glucose uptake in human SkMCs, indicating Hsp60 as a putative mediator in the development of insulin resistance. Therefore, Hsp60 might have direct effects on insulin signaling and also may indirectly induce insulin resistance by increasing proinflammatory adipokines, known to interfere with insulin signaling.
There are several putative mechanisms by which Hsp60 and cytokine concentrations could reach pathophysiological levels in obese and/or type 2 diabetic patients. Heat shock proteins are involved in the activation of innate immune cells and in the resulting macrophage-infiltration of adipose tissue by the release of chemokines such as MCP-1 (5,6,28). Macrophage infiltration positively correlated with increased adipocyte size and body mass in human subcutaneous tissue, leading to elevated cytokine levels, which finally may contribute to the development of insulin resistance in adipocytes (29,30). Therefore, besides adipose tissue macrophages, adipocytes themselves must be considered as important players in the development of obesity-related insulin resistance (31). However, other mechanisms such as obesity-related hypoxia might contribute to inflammatory processes in adipose tissue. Previously, it was depicted that hypoxia is associated with an increased Hsp60 expression in human vessels (32) and that Hsp60 was translocated to the plasma membrane in the heart (33). Because hypoxia has been reported to occur in obese individuals (34), one might speculate that hypoxic conditions could contribute to elevated Hsp60 expression levels, possibly leading to an increased release of Hsp60 observed in obese individuals.
Several studies indicate that circulating Hsp60 levels are increased not only in patients with type 2 diabetes but also in patients with coronary heart disease (35). In vitro studies further underline that Hsp60 has endocrine effects on cardiomyocytes. Hsp60 was found to be increased early in heart failure accompanied by increased release from cardiomyocytes, where it induces apoptosis via TLR4 (36,37). Furthermore, Hsp60 induces proliferation of vascular smooth muscle cells (38), which might also contribute to cardiovascular disease (39). In the current study, we revealed a positive association between elevated Hsp60 concentrations and blood pressure, which might contribute to the development of cardiovascular diseases. Our study is the first demonstrating that Hsp60 might also be a relevant mediator for skeletal muscle and adipose tissue insulin resistance, thereby contributing to the development of type 2 diabetes. In an acute way, Hsp60 activates proinflammatory signaling cascades in primary human SkMCs similarly to MCP-1 and chemerin (10,26). Prolonged incubation with Hsp60 induces insulin resistance at the level of Akt and GSK3α/β in these cells at both physiological and pathophysiological concentrations. These findings could be verified through Hsp60-mediated impaired glucose uptake in SkMCs and a positive association between Hsp60 concentration and HOMA-IR, as well as a negative correlation with QUICKI. Most interestingly, Hsp60 treatment of SkMCs results in a marked secretion of myokines such as MCP-1, which is associated with skeletal muscle inflammation (40). Increased release of MCP-1 in Hsp60-treated SkMCs is in line with a reported increase in MCP-1 secretion in these cells after stimulation with adipocyte CM (41). Because Hsp60 is released by adipocytes, Hsp60 content in adipocyte CM might at least partly explain its effect on MCP-1 release.
In summary, inflammatory stress induces the release of Hsp60 by human adipocytes, and Hsp60 exerts autocrine/paracrine effects on adipocytes characterized by an increased release of proinflammatory adipokines, increased inflammatory signaling, and insulin resistance. Furthermore, the current study reveals that Hsp60 has endocrine effects on SkMCs, inducing insulin resistance. Our clinical data reveal positive associations of circulating Hsp60 concentrations with BMI, leptin, HOMA-IR, and blood pressure. Therefore, there is rising evidence that circulating Hsp60 levels are increased in obesity, leading to the conception that Hsp60 might represent a novel adipokine involved in adipose tissue inflammation, thereby contributing to the development of insulin resistance in human adipocytes and SkMCs.
ACKNOWLEDGMENTS
This work was supported by the Bundesministerium für Gesundheit and by the Ministerium für Innovation, Wissenschaft, Forschung und Technologie des Landes Nordrhein-Westfalen, in part by a grant from the Bundesministerium für Bildung und Forschung (BMBF) to the German Center for Diabetes Research (DZD e.V.), European Union COST (European Cooperation in Science and Technology), the Commission of the European Communities (Collaborative Project ADAPT [Adipokines as Drug Targets to Combat Adverse Effects of Excess Adipose Tissue]), and the Deutsche Forschungsgemeinschaft (DFG).
No potential conflicts of interest relevant to this article were reported.
T.M. and H.S. researched data and wrote the manuscript. P.Z., A.G., J.K., and S.F. researched data. D.M.O., P.P., and J.R. performed clinical research. T.M., H.S., and C.H. designed and initiated the experimental procedures. M.R., J.E., and C.H. contributed to discussion and reviewed the manuscript. C.H. is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank Professor Jutta Liebau, Department of Plastic Surgery, Florence-Nightingale-Hospital Düsseldorf, and Dr. Christoph Andree, Department of Plastic Surgery, Sana-Hospital Düsseldorf-Gerresheim, for support in obtaining adipose tissue samples. The technical assistance of Jutta Brüggemann, Angelika Horrighs, Andrea Cramer, and Manuela Elsen is gratefully acknowledged.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db10-1574/-/DC1.
REFERENCES
- 1.Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest 2005;115:1111–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bloomgarden ZT. Obesity and diabetes. Diabetes Care 2000;23:1584–1590 [DOI] [PubMed] [Google Scholar]
- 3.Mohamed-Ali V, Pinkney JH, Coppack SW. Adipose tissue as an endocrine and paracrine organ. Int J Obes Relat Metab Disord 1998;22:1145–1158 [DOI] [PubMed] [Google Scholar]
- 4.Park HS, Park JY, Yu R. Relationship of obesity and visceral adiposity with serum concentrations of CRP, TNF-alpha and IL-6. Diabetes Res Clin Pract 2005;69:29–35 [DOI] [PubMed] [Google Scholar]
- 5.Gülden E, Märker T, Kriebel J, Kolb-Bachofen V, Burkart V, Habich C. Heat shock protein 60: evidence for receptor-mediated induction of proinflammatory mediators during adipocyte differentiation. FEBS Lett 2009;583:2877–2881 [DOI] [PubMed] [Google Scholar]
- 6.Gülden E, Mollérus S, Brüggemann J, Burkart V, Habich C. Heat shock protein 60 induces inflammatory mediators in mouse adipocytes. FEBS Lett 2008;582:2731–2736 [DOI] [PubMed] [Google Scholar]
- 7.Habich C, Baumgart K, Kolb H, Burkart V. The receptor for heat shock protein 60 on macrophages is saturable, specific, and distinct from receptors for other heat shock proteins. J Immunol 2002;168:569–576 [DOI] [PubMed] [Google Scholar]
- 8.Habich C, Burkart V. Heat shock protein 60: regulatory role on innate immune cells. Cell Mol Life Sci 2007;64:742–751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dasu MR, Devaraj S, Park S, Jialal I. Increased Toll-like receptor (TLR) activation and TLR ligands in recently diagnosed type 2 diabetic subjects. Diabetes Care 2010;33:861–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sell H, Laurencikiene J, Taube A, et al. Chemerin is a novel adipocyte-derived factor inducing insulin resistance in primary human skeletal muscle cells. Diabetes 2009;58:2731–2740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dietze-Schroeder D, Sell H, Uhlig M, Koenen M, Eckel J. Autocrine action of adiponectin on human fat cells prevents the release of insulin resistance-inducing factors. Diabetes 2005;54:2003–2011 [DOI] [PubMed] [Google Scholar]
- 12.Märker T, Kriebel J, Wohlrab U, Habich C. Heat shock protein 60 and adipocytes: characterization of a ligand-receptor interaction. Biochem Biophys Res Commun 2010;391:1634–1640 [DOI] [PubMed] [Google Scholar]
- 13.Uhlig M, Passlack W, Eckel J. Functional role of Rab11 in GLUT4 trafficking in cardiomyocytes. Mol Cell Endocrinol 2005;235:1–9 [DOI] [PubMed] [Google Scholar]
- 14.Dutour A, Achard V, Sell H, et al. Secretory type II phospholipase A2 is produced and secreted by epicardial adipose tissue and overexpressed in patients with coronary artery disease. J Clin Endocrinol Metab 2010;95:963–967 [DOI] [PubMed] [Google Scholar]
- 15.Gupta S, Knowlton AA. HSP60 trafficking in adult cardiac myocytes: role of the exosomal pathway. Am J Physiol Heart Circ Physiol 2007;292:H3052–H3056 [DOI] [PubMed] [Google Scholar]
- 16.Ireland HE, Leoni F, Altaie O, et al. Measuring the secretion of heat shock proteins from cells. Methods 2007;43:176–183 [DOI] [PubMed] [Google Scholar]
- 17.Pockley AG, Bulmer J, Hanks BM, Wright BH. Identification of human heat shock protein 60 (Hsp60) and anti-Hsp60 antibodies in the peripheral circulation of normal individuals. Cell Stress Chaperones 1999;4:29–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu CT, Ou LS, Yeh KW, Lee WI, Huang JL. Serum heat shock protein 60 can predict remission of flare-up in juvenile idiopathic arthritis. Clin Rheumatol 2011:30;959–965 [DOI] [PubMed] [Google Scholar]
- 19.Dasu MR, Jialal I. Free fatty acids in the presence of high glucose amplify monocyte inflammation via Toll-like receptors. Am J Physiol Endocrinol Metab 2011;300:E145–E154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bajramović JJ, Bsibsi M, Geutskens SB, et al. Differential expression of stress proteins in human adult astrocytes in response to cytokines. J Neuroimmunol 2000;106:14–22 [DOI] [PubMed] [Google Scholar]
- 21.Famulla S, Lamers D, Hartwig S, et al. Pigment epithelium-derived factor is one of the most abundant proteins secreted by human adipocytes and induces insulin resistance and inflammatory signaling in muscle and fat cells. Int J Obes 2011;35:762–772 [DOI] [PubMed] [Google Scholar]
- 22.Habich C, Kempe K, van der Zee R, Burkart V, Kolb H. Different heat shock protein 60 species share pro-inflammatory activity but not binding sites on macrophages. FEBS Lett 2003;533:105–109 [DOI] [PubMed] [Google Scholar]
- 23.Kol A, Lichtman AH, Finberg RW, Libby P, Kurt-Jones EA. Cutting edge: heat shock protein (HSP) 60 activates the innate immune response: CD14 is an essential receptor for HSP60 activation of mononuclear cells. J Immunol 2000;164:13–17 [DOI] [PubMed] [Google Scholar]
- 24.Ohashi K, Burkart V, Flohé S, Kolb H. Cutting edge: heat shock protein 60 is a putative endogenous ligand of the Toll-like receptor-4 complex. J Immunol 2000;164:558–561 [DOI] [PubMed] [Google Scholar]
- 25.Vabulas RM, Ahmad-Nejad P, da Costa C, et al. Endocytosed HSP60s use Toll-like receptor 2 (TLR2) and TLR4 to activate the Toll/interleukin-1 receptor signaling pathway in innate immune cells. J Biol Chem 2001;276:31332–31339 [DOI] [PubMed] [Google Scholar]
- 26.Sell H, Dietze-Schroeder D, Kaiser U, Eckel J. Monocyte chemotactic protein-1 is a potential player in the negative cross-talk between adipose tissue and skeletal muscle. Endocrinology 2006;147:2458–2467 [DOI] [PubMed] [Google Scholar]
- 27.Hirosumi J, Tuncman G, Chang L, et al. A central role for JNK in obesity and insulin resistance. Nature 2002;420:333–336 [DOI] [PubMed] [Google Scholar]
- 28.Tsan MF, Gao B. Heat shock proteins and immune system. J Leukoc Biol 2009;85:905–910 [DOI] [PubMed] [Google Scholar]
- 29.Grimble RF. Inflammatory status and insulin resistance. Curr Opin Clin Nutr Metab Care 2002;5:551–559 [DOI] [PubMed] [Google Scholar]
- 30.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 2003;112:1796–1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu H, Barnes GT, Yang Q, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 2003;112:1821–1830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hammerer-Lercher A, Mair J, Bonatti J, Watzka SB, Puschendorf B, Dirnhofer S. Hypoxia induces heat shock protein expression in human coronary artery bypass grafts. Cardiovasc Res 2001;50:115–124 [DOI] [PubMed] [Google Scholar]
- 33.Gupta S, Knowlton AA. HSP60, Bax, apoptosis and the heart. J Cell Mol Med 2005;9:51–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pasarica M, Sereda OR, Redman LM, et al. Reduced adipose tissue oxygenation in human obesity: evidence for rarefaction, macrophage chemotaxis, and inflammation without an angiogenic response. Diabetes 2009;58:718–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang X, He M, Cheng L, et al. Elevated heat shock protein 60 levels are associated with higher risk of coronary heart disease in Chinese. Circulation 2008;118:2687–2693 [DOI] [PubMed] [Google Scholar]
- 36.Kim SC, Stice JP, Chen L, et al. Extracellular heat shock protein 60, cardiac myocytes, and apoptosis. Circ Res 2009;105:1186–1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin L, Kim SC, Wang Y, et al. HSP60 in heart failure: abnormal distribution and role in cardiac myocyte apoptosis. Am J Physiol Heart Circ Physiol 2007;293:H2238–H2247 [DOI] [PubMed] [Google Scholar]
- 38.de Graaf R, Kloppenburg G, Kitslaar PJ, Bruggeman CA, Stassen F. Human heat shock protein 60 stimulates vascular smooth muscle cell proliferation through Toll-like receptors 2 and 4. Microbes Infect 2006;8:1859–1865 [DOI] [PubMed] [Google Scholar]
- 39.Ellins E, Shamaei-Tousi A, Steptoe A, et al. The relationship between carotid stiffness and circulating levels of heat shock protein 60 in middle-aged men and women. J Hypertens 2008;26:2389–2392 [DOI] [PubMed] [Google Scholar]
- 40.De Rossi M, Bernasconi P, Baggi F, de Waal Malefyt R, Mantegazza R. Cytokines and chemokines are both expressed by human myoblasts: possible relevance for the immune pathogenesis of muscle inflammation. Int Immunol 2000;12:1329–1335 [DOI] [PubMed] [Google Scholar]
- 41.Sell H, Eckardt K, Taube A, et al. Skeletal muscle insulin resistance induced by adipocyte-conditioned medium: underlying mechanisms and reversibility. Am J Physiol Endocrinol Metab 2008;294:E1070–E1077 [DOI] [PubMed] [Google Scholar]