Abstract
The incidence of type 1 diabetes has increased rapidly over recent decades, particularly in young children. We aimed to determine whether this rise was associated with changes in patterns of humoral islet autoimmunity at diagnosis. Autoantibodies to insulin (IAA), GAD (GADA), islet antigen-2 (IA-2A), and zinc transporter 8 (ZnT8A) were measured by radioimmunoassay in sera collected from children and young adults with newly diagnosed type 1 diabetes between 1985 and 2002. The influence of date of diagnosis on prevalence and level of autoantibodies was investigated by logistic regression with adjustment for age and HLA class II genetic risk. Prevalence of IA-2A and ZnT8A increased significantly over the period studied, and this was mirrored by raised levels of IA-2A, ZnT8A, and IA-2β autoantibodies (IA-2βA). IAA and GADA prevalence and levels did not change. Increases in IA-2A, ZnT8A, and IA-2βA at diagnosis during a period of rising incidence suggest that the process leading to type 1 diabetes is now characterized by a more intense humoral autoimmune response. Understanding how changes in environment or lifestyle alter the humoral autoimmune response to islet antigens should help explain why the incidence of type 1 diabetes is increasing and may suggest new strategies for preventing disease.
The incidence of type 1 diabetes is rising by 3–4% per year across Europe, North America, and Australia, with the greatest increase in children diagnosed at <5 years of age (1,2). These increases have been accompanied by a reduced frequency of the highest-risk HLA class II diabetes susceptibility genotype in patient populations and greater penetrance of lower-risk genotypes (3). The reason for the rise in incidence remains obscure but has been attributed to changes in environment and/or lifestyle (4). As type 1 diabetes results from autoimmune destruction of the pancreatic β-cells, increases in incidence could be associated with changes in the pattern of autoimmunity.
Autoantibodies to insulin (IAA), GAD (GADA), islet antigen-2 (IA-2A), and zinc transporter 8 (ZnT8A) are the most reliable markers of humoral autoimmune activity in diabetes and may offer key insights into changes in disease pathogenesis. As islet autoimmunity evolves, there is typically spreading of reactivity to different target autoantigens and epitopes (5). IAA are generally the first autoantibodies to be detected in infancy, followed by GADA (6), while IA-2A and ZnT8A tend to appear later and may mark a critical turning point in the process leading to β-cell destruction (5,7,8). Spreading of autoreactivity within antigens shows a distinct pattern; autoantibodies to epitopes in the juxtamembrane region of IA-2 (JMA) often arise early and develop independently of responses to protein tyrosine phosphatase epitopes of IA-2 (9), while antibodies to IA-2β (IA-2βA) may signal advanced autoimmunity and impending clinical onset of diabetes (10).
Despite the importance of autoantibodies in characterizing disease, changes in these markers at diagnosis have not been examined over the period of increasing type 1 diabetes incidence. We therefore investigated the prevalence and levels of autoantibodies at diagnosis, controlling for age and HLA-determined genetic risk, in a cohort of patients recruited to a U.K. population-based study of childhood diabetes during a time when type 1 diabetes incidence in the region rose by 2.2% per year (1,2).
RESEARCH DESIGN AND METHODS
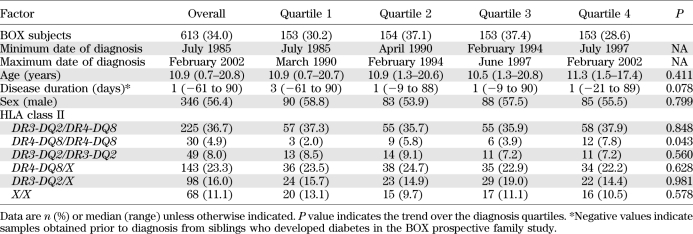
Newly diagnosed patients were recruited to the Bart’s-Oxford (BOX) study of childhood diabetes (11), a prospective, population-based study that has identified >95% of children and young people below age 21 years with type 1 diabetes diagnosed in the Oxford region, U.K., since 1985. Between 1985 and February 2002, 1,801 patients were referred to the study. Sera collected within 3 months of diagnosis (median 1 day [range −61 to 90]) were available from 613 of these patients (median age 11.0 years [0.7–20.9]) (12) (Table 1). GADA, IA-2A, and ZnT8A were tested in all sera. To avoid detecting antibodies to exogenous insulin, IAA testing was limited to 423 sera collected <2 weeks after diagnosis. IA-2A–positive sera were tested for IA-2βA and JMA. The BOX study was approved by local research ethics committees.
TABLE 1.
Subject characteristics subdivided by date of diagnosis
Autoantibody assays.
Autoantibodies to insulin, full-length GAD65, and intracytoplasmic (606–979) or juxtamembrane (609–631) regions of IA-2 were assayed by radioimmunoassay as previously described (12–14). IA-2βA and ZnT8A were assayed using similar protocols with plasmids encoding the protein tyrosine phosphatase domain of IA-2β (723–1015) or C-terminus of ZnT8 (268–369, 325R, or 325W) provided by Dr. Vito Lampasona. ZnT8A were determined by combining results from separate assays for autoantibodies recognizing either arginine (ZnT8RA) or tryptophan (ZnT8WA) at position 325 (15). IAA, GADA, IA-2βA, and ZnT8A results were expressed in arbitrary units and IA-2A in DK units/mL (14) derived from standard curves, with extrapolation of values above the top standard. JMA results were calculated as an index (12). Thresholds were set at the 97.5th percentile of 2,860 schoolchild sera for IAA, GADA, and IA-2A and of 523 schoolchild sera for ZnT8A. Thresholds for IA-2βA and JMA were set at the mean ± 3 SDs of 270 schoolchild sera.
Laboratory-defined sensitivity and specificity for assays in Diabetes Antibody Standardization Program workshops over the period of sample testing were as follows: IAA, 36 and 100% (2001); GADA, 84 and 92% (2001); IA-2A, 68 and 99% (2010); and ZnT8A, 60 and 100% (2010), respectively.
Interassay coefficients of varation of moderate/high and low positive samples, respectively, were 21 and 21% for IAA, 16 and 17% for GADA, 14 and 21% for IA-2A, 20 and 16% for ZnT8RA, 23 and 27% for ZnT8WA, 18 and 24% for JMA, and 14 and 19% for IA-2βA.
Genotyping.
HLA class II DRB1, DQA1, and DQB1 analysis was performed on blood and mouth swab DNA with sequence-specific primers as previously described (16). Haplotypes were established based on common patterns of linkage disequilibrium; those of interest were DRB1*03-DQA1*0501-DQB1*0201 (DR3-DQ2) and DRB1*04-DQA1*0301-DQB1*0302 (DR4-DQ8).
Statistical analysis.
Patients were divided into quartiles based on diagnosis date (Table 1). Genetic risk was analyzed as DR3-DQ2/DR4-DQ8, DR4-DQ8/DR4-DQ8, DR3-DQ2/ DR3-DQ2, DR4-DQ8/X, DR3-DQ2/X, and X/X (where X was not DR3-DQ2 or DR4-DQ8). Changes in autoantibody prevalence, sex, and genotype over the four time periods were assessed by χ2 test for trend. Changes in age and antibody level were examined using the Jonckheere-Terpstra test.
We used logistic regression models to adjust for factors previously shown to be associated with islet autoantibodies in the BOX study (sex, age at diagnosis, and DR-DQ genotype) (12,17). Models for number of autoantibodies were restricted to the 423 patients in whom IAA were tested. Statistical analyses were performed using the Statistical Package for Social Science 16 (SPSS, Chicago, IL).
RESULTS
Characteristics of the cohort.
The patients’ characteristics subdivided by date of diagnosis quartile are given in Table 1. The distributions of age, sex, date of diagnosis, and HLA genotypes in the 613 individuals studied were similar to those of the BOX study participants recruited during this period for whom serum was not available, apart from small differences in age at diagnosis and prevalence of DR4-DQ8 genotypes (Supplementary Table 1).
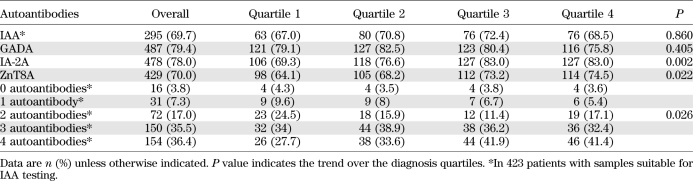
Overall, of 613 newly diagnosed patients, 487 (79%) were positive for GADA, 478 (78%) for IA-2A, and 429 (70%) for ZnT8A (Table 2). Of 423 individuals in whom IAA were measured, 407 (96%) were positive for at least one islet autoantibody, including 295 (70%) positive for IAA.
TABLE 2.
Autoantibody profile of patients subdivided by date of diagnosis
Autoantibody prevalence, level, and number vary with diagnosis quartile.
IA-2A prevalence increased between 1985 and 2002 (P = 0.002) (Table 2). In the first quartile, 69% of patients had IA-2A, compared with 83% in the last. This increased prevalence was accompanied by higher IA-2A levels (P = 0.025) (Fig. 1A).
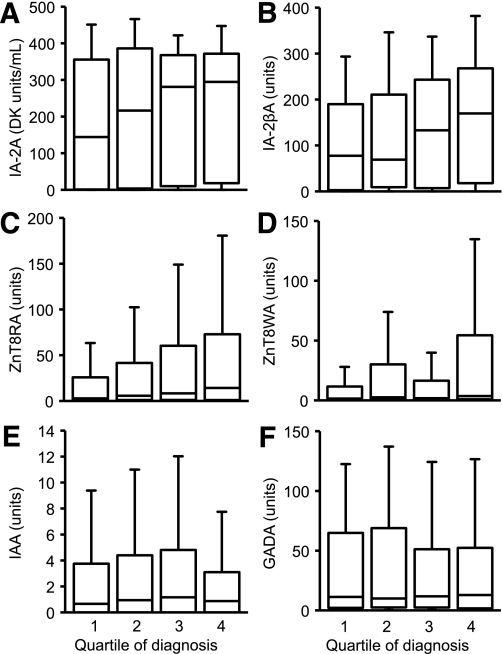
FIG. 1.
Plots showing IA-2A (A), IA-2βA (tested in IA-2A–positive patients) (B), ZnT8RA (C), ZnT8WA (D), IAA (E), and GADA (F) levels for four quartiles based on date of diagnosis. Median antibody levels are shown by the line in the box, the interquartile range is represented by the box, and the whiskers represent 1.5 times the interquartile range. The levels of IAA and GADA were stable, but levels of IA-2A, ZnT8RA, ZnT8WA, and IA-2βA increased significantly with diagnosis quartile.
Of the 478 IA-2A–positive patients, 228 (48%) had JMA and 408 (85%) had IA-2βA. There was no change in the prevalence or levels of JMA with diagnosis quartile. In contrast, the median level of IA-2βA increased twofold among IA-2A–positive patients over the period studied (P < 0.001, Fig. 1B). ZnT8A prevalence also increased from 64 to 75% over the study period (P = 0.022). This was paralleled by rises in levels of antibodies to both ZnT8 isoforms (325R and 325W) tested (P < 0.001 for both) (Figs. 1C and D).
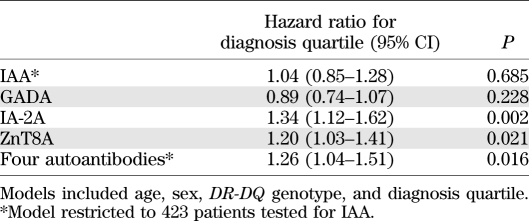
Logistic regression showed that after correction for age at diagnosis, sex, and DR-DQ genotype, diagnosis quartile was an independent determinant of prevalence of IA-2A (hazard ratio 1.34 [95% CI 1.12–1.62], P = 0.002) and ZnT8A (1.20 [1.03–1.41], P = 0.021) (Table 3). In contrast, the prevalence and levels of IAA and GADA were stable across the diagnosis quartiles even after adjustment for age, sex, and genotype (Fig. 1E and F).
TABLE 3.
Influence of diagnosis quartile on autoantibody positivity by logistic regression
The number of antibodies detected increased across the quartiles (P = 0.026) (Table 2). Logistic regression showed that date of diagnosis quartile was an independent predictor for the presence of all four antibodies (P = 0.016).
DISCUSSION
We have shown that, during a period when the incidence of type 1 diabetes was rising in the Oxford region of the U.K. (1), the prevalence and levels of IA-2A and ZnT8A in patients at diagnosis increased. These increases were paralleled by a shift in autoantibody profile, with a higher proportion of the patients diagnosed more recently having all four antibodies and increased levels of IA-2βA, which are characteristic of more advanced islet autoimmunity (10).
A strength of this study is that the patients were recruited as part of the long-running BOX study of childhood diabetes, which is population based and has high levels of case ascertainment (1), allowing reliable monitoring of childhood type 1 diabetes incidence in the Oxford region since 1985. The size and detailed characterization of this cohort enabled us to investigate changes in autoantibodies while adjusting for age at diagnosis and HLA class II genotype, which could potentially drive changes in islet autoantibodies (6,12,15,17). There was an increase in the proportion of children in our region diagnosed at <5 years of age during this period (1). Although HLA risk genotype changed in the U.K. over half of a century (3), no further changes were observed among BOX study patients diagnosed over the shorter time span of this study.
We were only able to include a subset of the whole BOX study cohort in this analysis, but this subgroup is representative of all the young people with type 1 diabetes contemporaneously recruited to the BOX study. Inclusion in this substudy was determined by individual willingness to give a sample at the time of diagnosis. While this is a potential source of bias, there is no reason to think that this changed during the study period, and the proportion of BOX case subjects from whom samples were available for study was similar in each of the diagnosis quartiles (Table 1). In contrast to the BOX study as a whole (1), we did not see significant changes in age of diabetes onset between the diagnosis quartiles within our cohort, but this is likely explained by lower statistical power. Similarly, as IAA could only be measured in 69% of available samples to avoid detection of antibodies to exogenous insulin, the power to detect changes in prevalence and levels of IAA was also lower. Our findings for IAA cannot therefore be considered conclusive. To minimize effects of assay drift on our results, we used rigorous quality control and standardization procedures throughout, and most samples assayed for IA2A, ZnT8A, JMA, and IA-2βA were tested together over a few months.
What might have caused these changes in islet autoantibodies? We adjusted for age at diagnosis and HLA Class II genotype in our analysis; therefore, changes in these factors do not explain our findings, although we have not examined potential changes in the frequency of non-HLA genes (18). The BMI of U.K. children has increased during this period (19). Lifestyle changes could therefore play a role in stimulating autoantibody production through increased islet autoantigen expression (20,21), but there are currently no data directly supporting this association (22). Another possibility could be that recent patients were diagnosed earlier in disease pathogenesis, with greater β-cell mass remaining to stimulate autoantibody production, particularly of ZnT8A, which drop rapidly after diagnosis (23). Earlier diagnosis would, however, be expected to be reflected by less severe metabolic decompensation, and rates of ketoacidosis at diagnosis in the BOX region did not alter between 1986 and 2005 (24).
Our findings imply more intense and mature humoral autoimmunity in patients diagnosed more recently. The antibodies that increased over the study period, IA-2A and ZnT8A, usually appear after GADA and IAA (6). Furthermore, in the IA-2A–positive patients, levels of IA-2βA, which are associated with a more advanced phase of disease pathogenesis, rose in tandem with the increase in IA-2A, whereas JMA, which are often detected as part of the initial IA-2A response, appeared to be stable (9). IA-2A and ZnT8A were rarely found in the absence of IAA or GADA, which suggests that in recent cases IA-2 and ZnT8 are not being targeted more frequently as part of the primary autoimmune response but that disease progression is more commonly accompanied by inter- and intramolecular antigen spreading. This change to a more florid immunophenotype is consistent with the observation that progression from islet autoantibody positivity to diabetes was faster in young children born in 2004–2010 than in those born in 1989–2000, despite a similar frequency of islet autoantibodies (25). Understanding how changes in environment or lifestyle alter the humoral autoimmune response to islet antigens should help explain why the incidence of type 1 diabetes is increasing and may suggest new strategies for preventing disease.
ACKNOWLEDGMENTS
A.E.L. was funded by a PhD studentship provided by the Medical Research Council, London, U.K. The BOX study was supported by the Medical Research Council, Diabetes UK, and the Wellcome Trust.
No potential conflicts of interest relevant to this article were reported.
A.E.L. and A.J.K.W. researched data, contributed to the discussion, and wrote the manuscript. K.M.G. researched data, contributed to the discussion, and reviewed and edited the manuscript. S.R. researched data and reviewed and edited the manuscript. P.J.B. researched data, contributed to the discussion, wrote the manuscript, and coordinated the BOX study. A.J.K.W. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in abstract form at the 47th Annual Meeting of the European Association for the Study of Diabetes, Lisbon, Portugal, 12–16 September 2011.
The authors thank Rachel Aitken at the University of Bristol for her technical help and Vito Lampasona, San Raffaele Scientific Institute, Milan, Italy, for providing the ZnT8 and IA-2β plasmids. They are also grateful to the diabetes team and families in the Oxford region for participating in the BOX study.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db11-0962/-/DC1.
REFERENCES
- 1.Gardner SG, Bingley PJ, Sawtell PA, Weeks S, Gale EA; The Bart’s-Oxford Study Group Rising incidence of insulin dependent diabetes in children aged under 5 years in the Oxford region: time trend analysis. BMJ 1997;315:713–717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patterson CC, Dahlquist GG, Gyürüs E, Green A, Soltész G; EURODIAB Study Group Incidence trends for childhood type 1 diabetes in Europe during 1989-2003 and predicted new cases 2005-20: a multicentre prospective registration study. Lancet 2009;373:2027–2033 [DOI] [PubMed] [Google Scholar]
- 3.Gillespie KM, Bain SC, Barnett AH, et al. The rising incidence of childhood type 1 diabetes and reduced contribution of high-risk HLA haplotypes. Lancet 2004;364:1699–1700 [DOI] [PubMed] [Google Scholar]
- 4.Gale EA. Spring harvest? Reflections on the rise of type 1 diabetes. Diabetologia 2005;48:2445–2450 [DOI] [PubMed] [Google Scholar]
- 5.Yu L, Rewers M, Gianani R, et al. Antiislet autoantibodies usually develop sequentially rather than simultaneously. J Clin Endocrinol Metab 1996;81:4264–4267 [DOI] [PubMed] [Google Scholar]
- 6.Ziegler AG, Nepom GT. Prediction and pathogenesis in type 1 diabetes. Immunity 2010;32:468–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wenzlau JM, Juhl K, Yu L, et al. The cation efflux transporter ZnT8 (Slc30A8) is a major autoantigen in human type 1 diabetes. Proc Natl Acad Sci USA 2007;104:17040–17045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Decochez K, De Leeuw IH, Keymeulen B, et al. ; Belgian Diabetes Registry IA-2 autoantibodies predict impending type I diabetes in siblings of patients. Diabetologia 2002;45:1658–1666 [DOI] [PubMed] [Google Scholar]
- 9.Naserke HE, Ziegler A-G, Lampasona V, Bonifacio E. Early development and spreading of autoantibodies to epitopes of IA-2 and their association with progression to type 1 diabetes. J Immunol 1998;161:6963–6969 [PubMed] [Google Scholar]
- 10.Achenbach P, Bonifacio E, Williams AJ, Ziegler AG, Gale EA, Bingley PJ; ENDIT Group Autoantibodies to IA-2beta improve diabetes risk assessment in high-risk relatives. Diabetologia 2008;51:488–492 [DOI] [PubMed] [Google Scholar]
- 11.Bingley PJ, Gale EAM. Incidence of insulin dependent diabetes in England: a study in the Oxford region, 1985-6. BMJ 1989;298:558–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams AJ, Aitken RJ, Chandler MA, Gillespie KM, Lampasona V, Bingley PJ. Autoantibodies to islet antigen-2 are associated with HLA-DRB1*07 and DRB1*09 haplotypes as well as DRB1*04 at onset of type 1 diabetes: the possible role of HLA-DQA in autoimmunity to IA-2. Diabetologia 2008;51:1444–1448 [DOI] [PubMed] [Google Scholar]
- 13.Bingley PJ, Bonifacio E, Williams AJ, Genovese S, Bottazzo GF, Gale EA. Prediction of IDDM in the general population: strategies based on combinations of autoantibody markers. Diabetes 1997;46:1701–1710 [DOI] [PubMed] [Google Scholar]
- 14.Bonifacio E, Yu L, Williams AK, et al. Harmonization of glutamic acid decarboxylase and islet antigen-2 autoantibody assays for national institute of diabetes and digestive and kidney diseases consortia. J Clin Endocrinol Metab 2010;95:3360–3367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wenzlau JM, Liu Y, Yu L, et al. A common nonsynonymous single nucleotide polymorphism in the SLC30A8 gene determines ZnT8 autoantibody specificity in type 1 diabetes. Diabetes 2008;57:2693–2697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lambert AP, Gillespie KM, Thomson G, et al. Absolute risk of childhood-onset type 1 diabetes defined by human leukocyte antigen class II genotype: a population-based study in the United Kingdom. J Clin Endocrinol Metab 2004;89:4037–4043 [DOI] [PubMed] [Google Scholar]
- 17.Williams AJ, Norcross AJ, Dix RJ, Gillespie KM, Gale EA, Bingley PJ. The prevalence of insulin autoantibodies at the onset of Type 1 diabetes is higher in males than females during adolescence. Diabetologia 2003;46:1354–1356 [DOI] [PubMed] [Google Scholar]
- 18.Lipponen K, Gombos Z, Kiviniemi M, et al. Effect of HLA class I and class II alleles on progression from autoantibody positivity to overt type 1 diabetes in children with risk-associated class II genotypes. Diabetes 2010;59:3253–3256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lobstein TJ, James WP, Cole TJ. Increasing levels of excess weight among children in England. Int J Obes Relat Metab Disord 2003;27:1136–1138 [DOI] [PubMed] [Google Scholar]
- 20.Buschard K, Brogren CH, Röpke C, Rygaard J. Antigen expression of the pancreatic beta-cells is dependent on their functional state, as shown by a specific, BB rat monoclonal autoantibody IC2. APMIS 1988;96:342–346 [DOI] [PubMed] [Google Scholar]
- 21.Wilkin TJ. The accelerator hypothesis: weight gain as the missing link between type I and type II diabetes. Diabetologia 2001;44:914–922 [DOI] [PubMed] [Google Scholar]
- 22.Winkler C, Marienfeld S, Zwilling M, Bonifacio E, Ziegler AG. Is islet autoimmunity related to insulin sensitivity or body weight in children of parents with type 1 diabetes? Diabetologia 2009;52:2072–2078 [DOI] [PubMed] [Google Scholar]
- 23.Wenzlau JM, Walter M, Gardner TJ, et al. Kinetics of the post-onset decline in zinc transporter 8 autoantibodies in type 1 diabetic human subjects. J Clin Endocrinol Metab 2010;95:4712–4719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ali K, Wilson IV, Edge JA, Bingley PJ. Diabetic ketoacidosis at diagnosis has not declined in children over the last 20 years: data from the Bart’s-Oxford Study (Abstract). Diabet Med 2009;26 (Suppl. 1):3419125758 [Google Scholar]
- 25.Ziegler AG, Pflueger M, Winkler C, et al. Accelerated progression from islet autoimmunity to diabetes is causing the escalating incidence of type 1 diabetes in young children. J Autoimmun 2011;37:3–7 [DOI] [PMC free article] [PubMed] [Google Scholar]