Abstract
We investigated whether spinalized animals can produce inspiratory rhythm. We recorded spinal inspiratory phrenic (PNA) and cranial inspiratory hypoglossal (HNA) nerve activity in the perfused brainstem preparation of rat. Complete transverse transections were performed at 1.5 (pyramidal decussation) or 2 mm (first cervical spinal segment) caudal to obex. Excitatory drive was enhanced by either extracellular potassium, hypercapnia or by stimulating arterial chemoreceptors. Caudal transections immediately eliminated descending network drive for PNA, while the cranial inspiratory HNA remained unaffected. After transection, PNA bursting remained sporadic even during enhanced excitatory drive. This implies, cervical spinal circuits lack intrinsic rhythmogenic capacity. Rostral transections also abolished PNA immediately. However, HNA also progressively lost its amplitude and rhythm. Chemoreceptor activation only triggered tonic, non-rhythmic HNA. Thus the integrity of ponto-medullary circuitry was maintained. Our results suggest that an area overlapping the caudal nucleus retroambiguus provides critical ascending input to the ponto-medullary respiratory network for inspiratory rhythm generation.
Keywords: nucleus retroambiguus, respiratory rhythm generation, spinal cord
1. Introduction
It is assumed that, the generation of respiratory rhythm in mammals is determined primarily by two areas in the medulla, the pre-Bötzinger complex and the retrotrapezoid nucleus-parafacial respiratory group (RTN/pFRG) (for reviews see Feldman and Del Negro, 2006; Onimaru et al., 2009; Feldman, 2011). Nevertheless, whether the spinal cord contains respiratory rhythm generators or not has also been a contentious issue. In fact as early as the 1880 Langendorf showed that the spinal cord has the capacity to generate rhythmic respiratory activity in new-born puppies. Langendorff observed the occurrence of respiratory muscle contraction accompanied by changes in intra-thoracic pressure in animals following complete cervical transection. In 1905 Porter demonstrated that application of respiratory stimulants such as doxapram hydrochloride can assist the spontaneous recovery of respiratory rhythm following spinal transection (Porter, 1905). In a subsequent study Wertheimer (1905) argued that respiratory rhythm can be generated in spinalized animals even without such extraneous pharmacological stimulation. While it is possible that alteration in intrathoracic pressure could be linked to contractions of the trapezius and sterno-cleido-mastoideus muscles in these earlier studies, this issue was put to rest by Coglianese et al. (1977) who showed that the cyclic alteration in the intra-pleural pressure which occurred spontaneously after spinal cord transection, correlates with phrenic motor output. In a later study Aoki et al. (1980) found phrenic nerve bursts recorded from C1-spinalized cats in synchrony with the diaphragm EMG. Aoki et al. (1980) also identified respiratory-related neurons within the intermediate zone of the gray matter of the cervical spinal cord segments C1 and C2 and designated them as upper cervical inspiratory neurons.
Since extensive lesion of the medulla oblongata failed to arrest breathing (Speck and Feldman, 1982), it was thought that there could be respiratory rhythm generators in the spinal cord, that could generate breathing, when the bulbo-spinal drive to the phrenic motoneurons are severed (Sears et al., 1982; Sears, 1984, 1990; Dawkins et al., 1992, Lipski et al., 1993, Dubayle and Viala, 1996, Oku et al., 2008).
The start of 1990’s saw the advent of reduced in vitro preparations for use to study neuronal control of breathing and respiratory rhythm generation in particular. Smith et al. (1991) identify the pre-Bötzinger complex (pre-BötC), which has become accepted as a kernel for respiratory rhythm generation (see review by Feldman and del Negro, 2006). In addition the RTN/pFRG is either seen as conditional oscillator for expiratory activity (Feldman, 2011) or even as primary generator of inspiratory rhythmicity (Onimaru and Homma, 2003). Thus in recent years, with the pre-Bötzinger region and more recently the RTN/pFRG in the medulla occupying the helm of respiratory rhythm generation research, the focus on spinal mechanisms has dwindled enormously.
However the recent identification respiratory-related neurons in particular inspiratory and pre-inspiratory cells in the cervical spinal cord using imaging with voltage sensitive dye (Oku et al., 2008), raises a strong necessity to re-examine the role of spinal cord in respiratory rhythm generation.
Thus in this study, as a first step, we investigated the presence of the spinal rhythm generators using the in situ perfused brainstem preparation of juvenile rat (PBP). Our aim was to examine whether the spinal cord generates spontaneous respiratory motor activity independent of the ponto-medullary bulbo-spinal drive. For such investigation the PBP provides the advantage that changes in blood pressure or heart rate in response to acute spinal cord lesion (spinal shock) do not affect perfusion of the brainstem circuits as it may occur in vivo.
Inspiratory motor output was recorded simultaneously from cervical spinal motoneurons in the phrenic nerve and medullary brainstem motoneurons in the hypoglossal nerve. Cervical transection disconnected the phrenic motor nucleus from ponto-medullary bulbo-spinal drive; whilst preserved ponto-medullary network activity was still monitored by hypoglossal nerve recording. This experimental setting allowed for the analysis of spinal cord rhythmogenic mechanism independent of brainstem activity.
2. Material and Methods
All experimental procedures were approved by the University of Leeds ethical review committee and conformed to the UK Animal (Home Office Scientific Procedures) Act 1986. Experiments were undertaken on the intra-arterially perfused brainstem preparation (PBP) of juvenile rats (Sprague-Dawley, postnatal day 21–24 of either sex, n = 15). Experimental procedures for the PBP were performed in full accordance with previously published studies (Paton, 1996; Dutschmann et al., 2007). In brief, rats were deeply anaesthetized with isoflurane (1-Chloro-2,2,2-trifluoroethyl-difluoromethylether, Abbott, Wiesbaden, Germany). Once respiration was depressed and the animal failed to respond to noxious pinch of tail or toe, it was transected below the diaphragm. Rats were decerebrated at the pre-collicular level and cerebellectomized. In a recording chamber the descending aorta was cannulated and perfused with Ringer’s (31°C) containing Ficoll (1.25%) using a peristaltic pump (Watson & Marlow, Rommerskirchen, Germany) at a flow rate of 28–32 ml min-1. The perfusate was filtered and passed through bubble traps (custom made). The perfusate leaking from the preparation was collected and re-circulated after its re-oxygenation. Two to 5 min after the start of perfusion, rhythmic contractions of the diaphragm resumed. In all preparations, respiratory-related movements were abolished by using vecuronium bromide (0.3 μg ml-1).
The perfusate contained (in mM): 125 NaCl, 24 NaHCO3, 2.5 CaCl2, 1.25 MgSO4, 4 KCL, 1.25 KH2PO4, 10 D-glucose and Ficoll (1.25 %; Sigma, Taufkirchen, Germany) to maintain colloid osmotic pressure. The osmolarity of the ACSF was 298±5 mosmol l-1 and on gassing with carbogen the pH was 7.35±0.05.
2.1 Recording of cardio-respiratory parameters
In all experiments the left phrenic and hypoglossal nerves were dissected to record inspiratory motor activity using glass suction electrodes. Nerve activity was amplified, filtered (8 Hz to 3 kHz), and integrated (time constant 100 ms). The heart was removed. MacLab and Chart software (ADInstruments, Australia) systems on Apple Macintosh were used for data recording, replay and analyses.
2.2 Transections
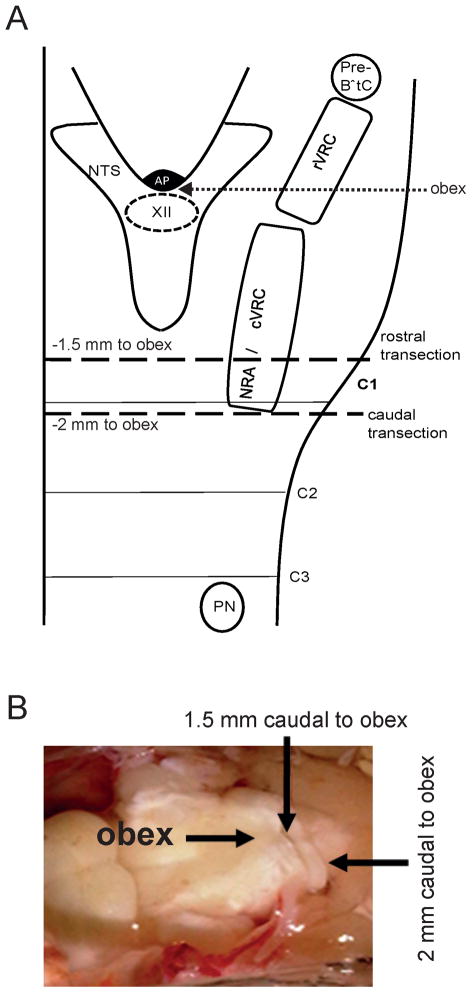
Transecting the spinal cord at the level of C1 removes all bulbospinal drive to the phrenic motor neurons. Transections were made via a single cut to minimize collateral damage and thus negate any tonic activity showing on recordings. Transections were performed at two locations, either at (n =10, −2 mm to obex) or rostral (n = 5, − 1.5 mm to obex) to cervical spinal cord segment 1 (see Figure 1 for anatomical co-ordinates). After 30 min extracellular potassium was raised to 8 mM (3 steps, 5 min interval, n = 6) or the perfusate is gassed with carbogen containing 12% CO2 (n = 4). In all experiments with transection 1.5 or 2 mm caudal to the obex sodium cyanide (NaCN) was bolus-injected into the perfusion circuit in order to elicit a chemoreceptor reflex by stimulating oxygen sensors in the carotid body.
Fig. 1.
A. Schematic anatomical representation of medullary brainstem nuclei of the respiratory central pattern generator in relation to the position of the transections 1.5 and 2mm caudal to obex. pre-BötC, pre-Bötzinger complex; rVRG, rostral ventral respiratory group; cVRG, caudal ventral respiratory group; NRA, nucleus retroambiguus; NTS, nucleus of the solitary tract; AP, area postrema, XII, hypoglossal nucleus; PN; phrenic motoneurons. B. Photograph illustrating the anatomical location of the transections in a perfused brainstem preparation.
3. Results
3.1. Transection 2 mm caudal to obex
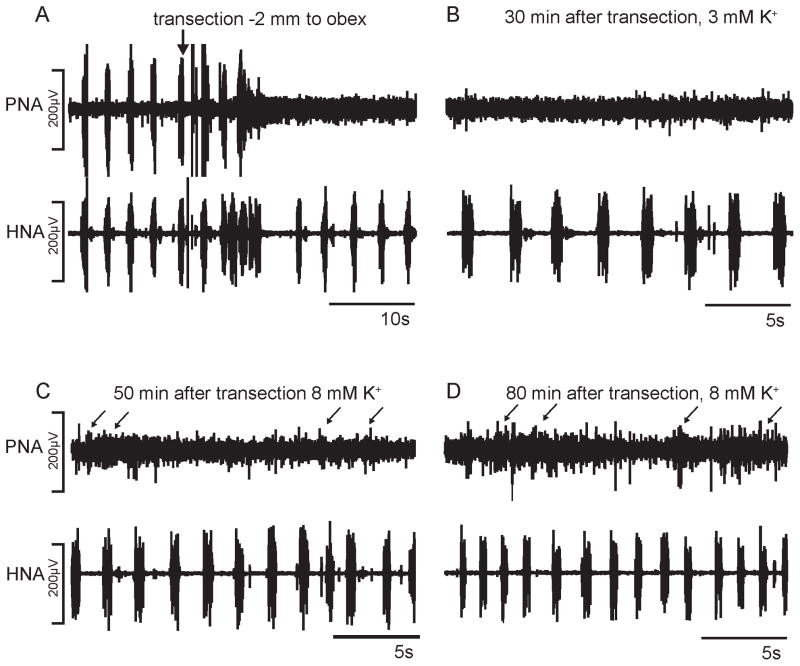
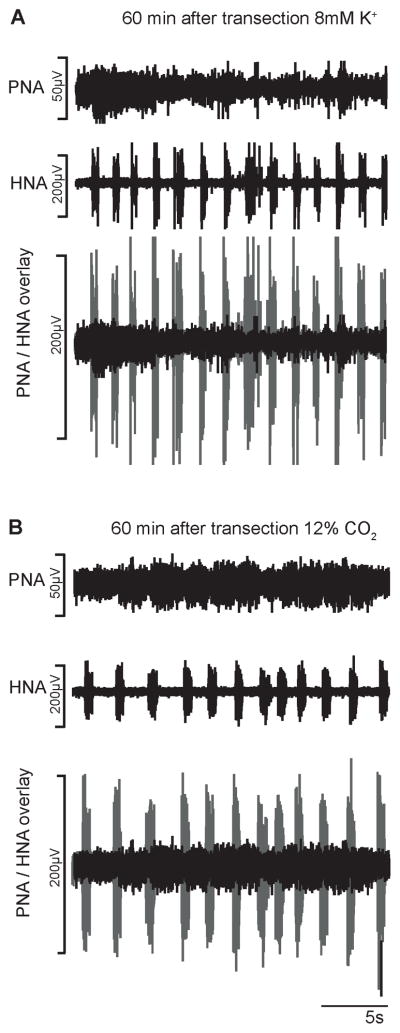
Figure 2 illustrates a typical representation of the phrenic and hypoglossal nerve activity (PNA, HNA) following complete transection 2 mm caudal to the obex. Acute transection (n = 8) abolished PNA instantly. However HNA was only transiently disturbed following transection and after a few seconds post-transection the hypoglossal nerve activity continued at a frequency and amplitude of pre-transection control. In two animals, low amplitude PNA bursts re-emerged in synchrony with HNA bursts after 2–5 min post-transection suggesting incomplete first transection (data not shown). In these two animals a second transection was performed which then abolished the phrenic nerve activity completely. Thirty minutes after complete C1 transection tonic PNA could be observed. Increasing the extracellular potassium concentration to 8mM (n = 6) after 45 – 60 min post-transection triggered a further increase in tonic PNA but also sporadic bursting, while HNA burst frequency increased by 50–60% (Figure 3). The majority of sporadic PNA bursts were short in duration (4–8% as compared to control). After 80–120 min post-transection PNA further increased and now showed sporadic bursting of longer duration (0.8 – 2.5 s) but PNA still never exhibited a stable rhythm. Increasing the CO2 in the perfusate (n = 4) had a similar effect to that of extracellular potassium manipulation, except that the short PNA bursting was less identifiable. The HNA bursting frequency increased by 20–25% and thus showed a physiological response to hypercapnia. Nevertheless, both experimental conditions triggered tonic PNA including sporadic bursting patterns (Figure 3). The sporadic PNA bursting pattern lasted 24 – 40 seconds but were always characterized by low amplitudes and thus never matched activity still generated by the intact ponto-medullary respiratory network (see overlay of HNA and PNA in Figure 3). Post-lesion NaCN injections (n = 5) triggered consistently a chemoreceptor response comprising of high frequency 5 – 8 HNA bursts in all aimals (data not shown). In n = 3 experiments NaCN (multiple injections, 4–5/animal) evoked predominantly tonic PNA but could evoke weak rhythmic activity. In the remaining two experiments PNA however did not respond to NaCN injection.
Fig. 2.
Phrenic and hypoglossal nerve recordings (PNA, HNA) of transection experiment 2 mm caudal to obex. A. Acute response to transection. B. – D. PNA and HNA at various time points after the transection.
Fig. 3.
Phrenic and hypoglossal nerve recordings (PNA, HNA) 1h after transection. A. Identifiable but sporadic low amplitude PNA bursts after increase of extracellular K+. B. Sporadic PNA bursts during hypercapnia (12% CO2). Comparison of eupneic HNA amplitude and spinal cord generated PNA is illustrated in overlays of PNA (black) and HNA (gray).
3.2 Transection 1.5 mm caudal to obex
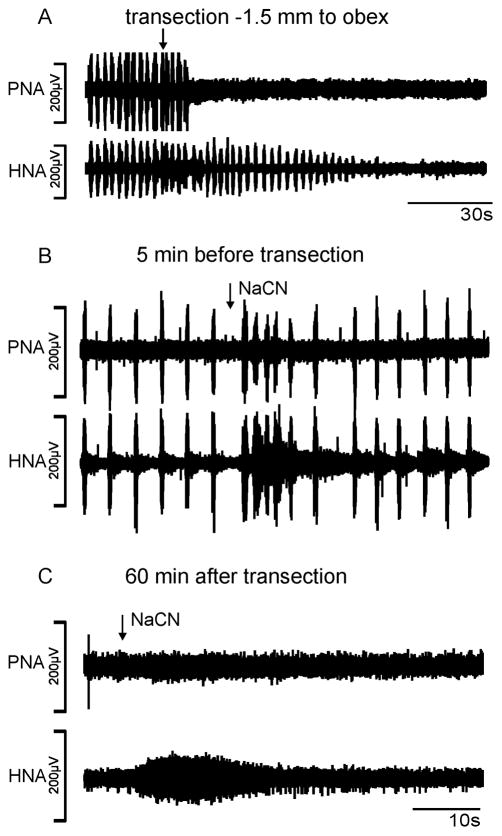
Figure 4 illustrates a typical representation of the phrenic and hypoglossal nerve activity following complete transection 1.5 mm caudal to the obex. The transection (n = 5) caused instant cessation of the phrenic nerve. The hypoglossal nerve (n = 4) following initial irritation, progressively decreased its amplitude 3 – 5 min post-transection and eventually, completely losing rhythmic function. In one animal however, the hypoglossal nerve discharge persisted although with greatly reduced amplitude (6% of control) and frequency (25% of control). In all cases even a hypercapnic stimulation (12% CO2) could not restore stable rhythm either in the hypoglossal or the phrenic nerve. Moreover arterial bolus injections (n = 5) of NaCN at various post-transection periods (e.g. 20, 60 and 90 min post-transection) capable of triggering a chemoreceptor response transient tachypnea during control (see Figure 4) also failed to initiate hypoglossal nerve rhythmicity. In all animals (n = 5) a single tonic burst in the hypoglossal nerve for 8–12 s (Figure 4). PNA showed a modest increase in tonic activity in two animals, while in the other 3 animals NaCN injections had no effect.
Fig. 4.
Phrenic and hypoglossal nerve recordings (PNA, HNA) of transection experiment 1.5 mm caudal to obex. A. Acute response to transection. B. NaCN evoked chemoreflex tachypnea prior to transection (same time scale as C). C. Absence of rhythmic PNA and HNA during NaCN evoked chemoreceptor reflex after transection.
4. Discussion
Our results show that some spontaneous phrenic nerve bursting independent of bulbo-spinal drive can occur in high cervical spinalized animals and are in part in agreement with observations made in previous studies (Aoki et al., 1980; Coglianese et al., 1977; Dawkins et al., 1992; Dubayle and Viala, 1996; Lipski et al., 1993; Oku et al., 2008; Sears et al., 1982; Sears, 1984, 1990). A unique aspect of our study is that we used hypoglossal nerve activity (HNA) and phrenic nerve activity (PNA) as indicators for generation of inspiratory rhythm. Examining HNA confirms that the entire ponto-medullary respiratory central pattern generator activity is maintained despite rhythmic PNA was instantly abolished after transection 2 mm caudal to obex. During post-lesion recording low amplitude PNA bursting (see Figure 3) emerged but remained sporadic even under increased excitatory drive induced by hypercapnia, high extracellular potassium or arterial chemoreceptor stimulation. Under the condition of high K+ more sustained phrenic activity was characterized only by very short bursts. The mild excitatory effect of hypercapnia on spinal PNA observed in the present study contradicts findings by Jodkowski and Lipski (1986) showing that hypercapnia had an inhibitory effect on respiratory motoneurons. The present results indicate that the observed sporadic rhythm may have been generated by spinal cord interneurons rather than motoneurons. Under this assumption one can speculate that a more robust rhythm generated by interneurons is not expressed because in parallel the motoneurons show decreased excitability.
Considering the low amplitude or very variable duration of PNA compared to normal eupneic drive preserved in the hypoglossal nerve the present study does not indicate that spinal cord generated PNA activity is sufficient to effect diaphragmatic contraction thus causing inspiratory airflow.
The serendipitous finding of the present study is that complete transections 1.5 mm caudal to obex not only abolished PNA but also triggered a progressive fading of HNA. Rhythmic HNA could be not recovered even with carotid chemoreceptor stimulation. Therefore our results suggest that the most caudal aspects of the medulla oblongata, overlapping with the nucleus retroambiguus (NRA), provide a critical synaptic input to the ponto-medullary pattern generator which is required for the generation of eupneic inspiratory rhythm.
4.1. Location of cervical spinal respiratory group
Coglianese et al. (1977) and Aoki et al. (1980) both hypothesized the existence of cervical spinal respiratory group within the ventral horn of C1–C2. Coglianese et al. (1977) performed complete spinal transections at the level of C1 on 8 anesthetized dogs. In two dogs spontaneous rhythmic respiratory activity returned in within 12 hours post-transection and in the remaining 6 animals spontaneous breathing returned only after administration of doxapram [l-ethyl- 4-(2-morpholinoethyl)-3,3-diphenyl-2-pyrrolidinone HCl hydrate]. While Doxapram is a known respiratory stimulant activating carotid body chemoreceptors, it is also thought to have a weak excitatory effect on the spinal cord (Coglianese et al., 1977). From these results Coglianese et al (1977) concluded that a respiratory rhythm generation mechanism exists within the spinal cord. Subsequent evidence came from investigations conducted on spinalized cats (Aoki et al., 1980). In this study 13 out of 18 cats spinalized at the level of C1 showed spontaneous respiratory activity within 2 hours post-transection. Without additional drug administration, the amplitude of diaphragm electromyogram was approximately 40% that of control, the inspiratory phase shortened, however rhythmicity of the discharge was maintained.
The spinal respiratory pattern generator hypothesis was supported by several studies showing the existence of inspiratory and pre-inspiratory neurons at level of the upper cervical spinal cord (Douse et al., 1992; Lipski and Duffin, 1986; Yuan et al., 2000). Using optical imaging, Oku et al (2008) recently identified that the cervical respiratory group extends across the ventral region from the spino-medullary junction to the C2 level. Because this study reported that the bulk of respiratory neurons identified were at the spino-medullary junction, with only sparse number of units in caudal C1 and C2 segments, we performed rostral transections to preserve the caudal aspects of the medulla oblongata. The rostral transection caused immediate cessation of the phrenic nerve while the hypoglossal nerve, an accepted indicator of inspiratory rhythm in situ or in vitro displayed a progressive loss of burst amplitude post-transection. This finding cannot be explained if seen via the concept of pre-BötC or retrotrapezoid/parafacial area generated eupneic rhythm. Transection at 1.5 mm caudal to the obex should leave the rostral medullary projections to the hypoglossal motor nuclei intact. Even stimulation of arterial chemoreceptors (with NaCN) could not trigger any rhythmic hypoglossal nerve function post-transection 1.5 mm caudal to the obex. Nevertheless NaCN evoked tonic hypoglossal nerve activity demonstrating that sensory synaptic information was processed by the chemoreceptor reflex-mediating circuits. This would involve the sensory relay in the caudal nucleus tractus solitarius and its efferent targets within the entire medullary respiratory central pattern generators and as well as the consequent motor output. Thus, our study elucidates for the first time that a group of neurons caudal to obex might be essential for the generation of eupnea, thus constituting the caudal respiratory rhythmogenic region.
4.2. Does the nucleus retroambiguus contain spinal respiratory rhythm generators?
This caudal rhythmogenic region could involve neurons of the nucleus retroambiguus (NRA). The NRA was originally considered by Merrill (1970 Merrill (1974) as an important component of spinal respiratory pattern generators, owing to different types of inspiratory and expiratory neurons found in the region. The inspiratory and expiratory cells seem to be topographically organized, with the rostral NRA predominantly containing inspiratory cells, while the caudal NRA, expiratory cells. Anatomical tract-tracing studies have shown that NRA receives dense afferent inputs from all medullary nuclei and particular from those that form the respiratory central pattern generators. (Gerrits and Holstege, 1996). The NRA’s descending spinal projections include; to the motor-neurons of the diaphragm, intercostal and abdominal muscles (Merrill, 1970; Holstege, 1991). Ascending innervation of the NRA supplies laryngeal motor-neurons (Holstege, 1991), various respiratory areas within the medulla (including presumably the central respiratory pattern generators) and the pontine Kölliker-Fuse nucleus (Holstege, 1989). Chemical activation of the NRA depending upon the site of stimulation generates either a laryngeal or abdominal breathing pattern (Subramanian and Holstege, 2009). In addition, in particular the caudal NRA has been proposed to be involved in maintenance of abdominal tone and pelvic pressure, constituting the functional pathway for expulsive behaviors such as vomiting, retching, coughing and emesis (Miller and Yates, 1993; Nonaka and Miller, 1991, Subramanian and Holstege, 2009). With respect to respiratory rhythm, chemical activation of the NRA alters both inspiratory and expiratory durations (Bongianni et al., 1994; Subramanian and Holstege, 2009). Our data indicate that ascending synaptic information from the NRA to the ponto-medullary respiratory central pattern generator could be critical for the generation of eupnea. Thus the precise neuronal interactions within the NRA and its ascending effect on the respiratory central pattern generator, requires further investigation.
4.3. Reasons for a weak phrenic rhythm following C1 spinal cord transection
The reasons for unstable or weak rhythmic PNA after C1 post-transection could be associated with the experimental procedures. Here we employed the decerebrated perfused brainstem preparation. Although the respiratory motor pattern generated in situ is comparable to in vivo (Paton and St-John, 2000; Dutschmann and Paton, 2002), the low temperature of the preparation (31°C) could have suppressed the emergence of robust spinal cord respiratory rhythm, because the associated low metabolic rate could have blocked or delayed the onset of rhythmic activity. Moreover, in all our experiments a muscle relaxant was applied. It is known that rhythmicity and amplitude of cervical spinal cord generated phrenic nerve activity is disrupted with administration of a muscle relaxant (Aoki et al., 1980). Also as in the case of spinal locomotor activity (Orlovsky and Shik, 1976, Rossignol et al., 1998) pharmacological agents such as NMDA, DOPA, 5HT and clonidine may also be required to trigger spinal respiratory activity (Aoki et al, 1980; Coglianese et al., 1977). However upon such pharmacological stimulation, any induced respiratory activity could be an entrained derivative of the rhythmic spinal locomotor output as previously demonstrated in the DOPA treatment of spinalized rabbits (Viala and Buser 1969; Viala et al., 1979). In our experiments, we have not undertaken such pharmacological activation. Also we have removed the thoracic and lumbar spinal cord in its entirety. This means the sporadic phrenic bursting is not a derivative of lumbar spinal locomotor activity and was generated by the cervical spinal cord the proposed region of the spinal cord rhythm generator.
Highlights.
We tested whether cervical spinal or caudal medullary areas can generate rhythm.
We recorded inspiratory activity from hypoglossal and phrenic nerves (HNA, PNA).
Spinal respiratory rhythm was absent without descending network drive.
Caudal medullary transection ceased inspiratory rhythm in PNA and HNA.
The caudal nucleus retroambiguus is required for inspiratory rhythm generation.
Acknowledgments
The study was supported by a start up grant of Leeds University and and NHLBI (Cluster Grant R33 HL087377, M. Dutschmann).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aoki M, Mori S, Kawahara K, Watanabe H, Ebata N. Generation of spontaneous respiratory rhythm in high spinal cats. Brain Res. 1980;202:51–63. [PubMed] [Google Scholar]
- Bongianni F, Corda M, Fontana GA, Pantaleo T. Chemical activation of caudal medullary expiratory neurones alters the pattern of breathing in the cat. J Physiol. 1994;474:497–507. doi: 10.1113/jphysiol.1994.sp020040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coglianese CJ, Peiss CN, Wurster RD. Rhythmic phrenic nerve activity and respiratory activity in spinal dogs. Respir Physiol. 1977;29:247–254. doi: 10.1016/0034-5687(77)90001-9. [DOI] [PubMed] [Google Scholar]
- Dawkins MA, Foreman RD, Farber JP. Short latency excitation of upper cervical respiratory neurons by vagal stimulation in the rat. Brain Res. 1992;594:319–322. doi: 10.1016/0006-8993(92)91143-3. [DOI] [PubMed] [Google Scholar]
- Douse MA, Duffin J, Brooks D, Fedorko L. Role of upper cervical inspiratory neurons studied by cross-correlation in the cat. Exp Brain Res. 1992;90:153–62. doi: 10.1007/BF00229267. [DOI] [PubMed] [Google Scholar]
- Dubayle D, Viala D. Localization of the spinal respiratory rhythm generator by an in vitro electrophysiological approach. Neuroreport. 1996;7:1175–80. doi: 10.1097/00001756-199604260-00016. [DOI] [PubMed] [Google Scholar]
- Dutschmann M, Paton JF. Inhibitory synaptic mechanisms regulating upper airway patency. Respir Physiol Neurobiol. 2002;131:57–63. doi: 10.1016/s1569-9048(02)00037-x. [DOI] [PubMed] [Google Scholar]
- Dutschmann M, Kron M, Mörschel M, Gestreau C. Activation of Orexin B receptors in the pontine Kölliker-Fuse nucleus modulates pre-inspiratory hypoglossal motor activity in rat. Respir Physiol Neurobiol. 2007;159:232–235. doi: 10.1016/j.resp.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Feldman JL, Del Negro CA. Looking for inspiration: new perspectives on respiratory rhythm. Nat Rev Neurosci. 2006;7:232–242. doi: 10.1038/nrn1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman JL. Chapter 14--looking forward to breathing. Prog Brain Res. 2011;188:213–218. doi: 10.1016/B978-0-444-53825-3.00019-X. [DOI] [PubMed] [Google Scholar]
- Gerrits PO, Holstege G. Pontine and medullary projections to the nucleus retroambiguus: A wheat germ agglutinin horseradish peroxidase and autoradiographic tracing study in the cat. J Comp Neurol. 1996;373:173–185. doi: 10.1002/(SICI)1096-9861(19960916)373:2<173::AID-CNE2>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Holstege G. Anatomical study of the final common pathway for vocalization in the cat. J Comp Neurol. 1989;284:242–252. doi: 10.1002/cne.902840208. [DOI] [PubMed] [Google Scholar]
- Holstege G. Descending motor pathways and the spinal motor system. Limbic and non-limbic components. Prog Brain Res. 1991;87:307–421. doi: 10.1016/s0079-6123(08)63057-5. [DOI] [PubMed] [Google Scholar]
- Langendorff O. Studien über die Innervation der Atembewegungen. Arch Physiol. 1880;3:518–549. [Google Scholar]
- Jodkowski JS, Lipski J. Decreased excitability of respiratory motoneurons during hypercapnia in the acute spinal cat. Brain Res. 1986;386:296–304. doi: 10.1016/0006-8993(86)90166-6. [DOI] [PubMed] [Google Scholar]
- Lipski J, Duffin J, Kruszewska B, ZHANG X. Upper cervical inspiratory neurons in the rat: an electrophysiological and morphological study. Exp Brain Res. 1993;95:477–87. doi: 10.1007/BF00227141. [DOI] [PubMed] [Google Scholar]
- Lipski J, Duffin J. An electrophysiological investigation of propriospinal inspiratory neurons in the upper cervical cord of the cat. Exp Brain Res. 1986;61:625–37. doi: 10.1007/BF00237589. [DOI] [PubMed] [Google Scholar]
- Merill EG. Lateral respiratory neurones of medulla - their associations with nucleus ambiguus, nucleus retroambiguus, spinal accessory nucleus and spinal cord. Brain Res. 1970;24:11–28. doi: 10.1016/0006-8993(70)90271-4. [DOI] [PubMed] [Google Scholar]
- Merill EG. Finding a respiratory function for the medullary respiratory neurons. In: Bellairs R, Gray EG, editors. Essays on the Nervous System. Clarendon Oxford; 1974. pp. 451–486. [Google Scholar]
- Miller AD, Yates BJ. Evaluation of role of upper cervical inspiratory neurons in respiration, emesis and cough. Brain Res. 1993;606:143–147. doi: 10.1016/0006-8993(93)91582-d. [DOI] [PubMed] [Google Scholar]
- Nonaka S, Miller AD. Behavior of upper cervical inspiratory propriospinal neurons during fictive vomiting. J Neurophysiol. 1991;65:1492–500. doi: 10.1152/jn.1991.65.6.1492. [DOI] [PubMed] [Google Scholar]
- Oku Y, Okabe A, Hayakawa T, Okada Y. Respiratory neuron group in the high cervical spinal cord discovered by optical imaging. Neuroreport. 2008;19:1739–43. doi: 10.1097/WNR.0b013e328318edb5. [DOI] [PubMed] [Google Scholar]
- Orlovsky M, Shik L. Control of locomotion: a neurophysiological analysis of the cat locomotor system. In: Guyton AC, editor. International Review of Physiology, Neurophysiology II. Vol. 10. University Park Press; Baltimore: 1976. pp. 281–317. [Google Scholar]
- Onimaru H, Ikeda K, Kawakami K. Phox2b, RTN/pFRG neurons and espiratory rhythmogenesis. Respir Physiol Neurobiol. 2009;168:13–18. doi: 10.1016/j.resp.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Paton JFR. A working heart-brainstem preparation of the mouse. J Neurosci Meth. 1996;65:63–68. doi: 10.1016/0165-0270(95)00147-6. [DOI] [PubMed] [Google Scholar]
- Porter WT. The Path of the Respiratory Impulse from the Bulb to the Phrenic Nuclei. J Physiol. 1905;17:455–485. doi: 10.1113/jphysiol.1895.sp000553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol S, Chau C, Burstein E, Giroux N, Bouyer L, Barbeau H, Reader TA. Pharmacological activation and modulation of the central pattern generator for locomotion in the cat. Ann N Y Acad Sci. 1998;860:346–59. doi: 10.1111/j.1749-6632.1998.tb09061.x. [DOI] [PubMed] [Google Scholar]
- Sears TA, Berger AJ, Phillipson EA. Reciprocal tonic activation of inspiratory and expiratory motoneurones by chemical drives. Nature. 1982:728–30. doi: 10.1038/299728a0. [DOI] [PubMed] [Google Scholar]
- Sears TA. Spinal integration and rhythm generation in breathing. Bull Eur Physiopathol Respir. 1984;20:399–401. [PubMed] [Google Scholar]
- Sears TA. Central rhythm generation and spinal integration. Chest. 1990;97:45S–51S. doi: 10.1378/chest.97.3_supplement.45s-a. [DOI] [PubMed] [Google Scholar]
- Speck DF, Feldman JL. The effects of microstimulation and microlesions in the ventral and dorsal respiratory groups in medulla of cat. J Neurosci. 1982;2:744–757. doi: 10.1523/JNEUROSCI.02-06-00744.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-John WM, Paton JF. Characterizations of eupnea, apneusis and gasping in aperfused rat preparation. Respir Physiol. 2000;123:201–213. doi: 10.1016/s0034-5687(00)00177-8. [DOI] [PubMed] [Google Scholar]
- Subramanian HH, Holstege G. The nucleus retroambiguus control of respiration. J Neurosci. 2009;29(12):3824–3832. doi: 10.1523/JNEUROSCI.0607-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viala D, Buser P. The effects of DOPA and 5-HTP on rhythmic efferent discharges in hind limb nerves in the rabbit. Brain Res. 1969;12:437–443. doi: 10.1016/0006-8993(69)90011-0. [DOI] [PubMed] [Google Scholar]
- Viala D, Vidal C, Freton E. Coordinated rhythmic bursting in respiratory and locomotor muscle nerves in the spinal rabbit. Neurosci Lett. 1979;11:155–159. doi: 10.1016/0304-3940(79)90119-8. [DOI] [PubMed] [Google Scholar]
- Wertheimer E. Sur les modifications de la respiration produites par les injections intraveineuses de soude chez les animaux a moelle cervicale sectionée. CR Soc Biol. 1905:668–669. [Google Scholar]
- Yuan Y, Chandler MJ, Foreman RD, Farber JP. Effects of abdominal or cardiopulmonary sympathetic afferents on upper cervical inspiratory neurons. Am J Physiol Regul Integr Comp Physiol. 2000;278:R1289–95. doi: 10.1152/ajpregu.2000.278.5.R1289. [DOI] [PubMed] [Google Scholar]