Abstract
Spinal tuberculosis is the most common cause of severe kyphosis in many parts of the world. Three percent of patients treated conservatively end up with a deformity greater than 60 degrees which can cause serious cosmetic, psychological, cardio-respiratory and neurological problems. Severe kyphotic deformities are usually the result of childhood spinal deformities and ‘Spine at risk’ radiological signs are helpful to identify children at risk of deformity. In children, a severe type of collapse, termed as ‘Buckling Collapse’ is also noted where the kyphosis is more than 120 degrees. Risk factors for buckling collapse include an age of less than seven years at the time of infection, thoracolumbar involvement, loss of more than two vertebral bodies and the presence of radiographic ‘Spine-at-risk’ signs. In correction of established deformity, posterior only surgery with a variety of osteotomies is now preferred. In patients with deformity of more than 90 degrees, an opening-closing wedge osteotomy must be done to prevent neurological deficit.
Introduction
Spinal tuberculosis is a common cause of a kyphotic deformity in most parts of the world. Although chemotherapy is highly effective in controlling tubercular infection, patients treated with chemotherapy alone have an average increase of 15° in deformity [1, 2] and 3% to 5% of the patients develop kyphosis greater than 60° [3–5]. A severe kyphosis can lead to immense cosmetic and psychological disturbance in growing children and can result in costo-pelvic impingement, secondary cardio-respiratory problems and late-onset paraplegia [5]. Correction of an established kyphosis is both difficult and hazardous with a high rate of complications, even in experienced hands [6]. It is essential that prevention of deformity be an integral part of any treatment schedule in spinal tuberculosis [5].
Natural history of progress of deformity
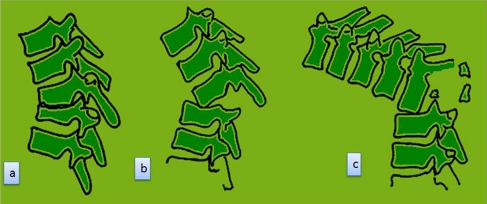
Tuberculosis preferentially affects the anterior elements of the vertebral column in more than 90% of patients. Although chemotherapy may inactivate the infection, vertebral collapse continues until the healthy vertebral bodies in the region of the kyphosis approximate anteriorly and consolidate. Depending on the extent of initial vertebral loss, three types of collapse and healing of the anterior column are noted [7] (Table 1), (Fig. 1). Adults have a lesser degree of deformity at presentation and also have a lesser increase in the deformity during Phase I for each vertebral body loss. Further the deformity remains virtually static after healing of the infection [8]. Children on the other hand, have a higher degree of deformity at presentation, a greater tendency for collapse during the active phase of the disease, and continued and variable progression even after the infection is cured and growth completed [7]. The thoracic lesions and thoracolumbar lesions have a significantly higher degree of deformity at presentation [8] and also show a greater degree of progression than lumbar or lumbosacral lesions.
Table 1.
Types of restabilisation in thoracolumbar spinal tuberculosis
| Type of restabilisation | Anterior column contact | Vertebral body loss | Facet joints | Final deformity |
|---|---|---|---|---|
| Type A | Wide | <0.75 | Intact | Spontaneous improvement. If increase <10° |
| Type B | Point contact | 0.75–1.5 | Subluxed or single level dislocation | Less than 60° |
| Type C | By 90° sagittal rotation of superior vertebrae | >1.5 | Dislocation of two or more | Can be more than 100° |
Fig. 1.
Pattern of healing: following destruction of the anterior column, restabilisation occurs by one of the three methods. (a) In patients with partially destroyed vertebrae, restabilisation occured with a wide contact area. There was no dislocation of the facet joints. (b) In patients with dislocation of a single facet joint, restabilisation occurred by point contact. (c) In patients with loss of two or three vertebrae in the thoracolumbar region, the facets dislocated at multiple levels. The superior segment rotated by 90° so the anterior surface of the superior vertebra rested on the superior surface of the inferior vertebra. The superior segment became horizontal
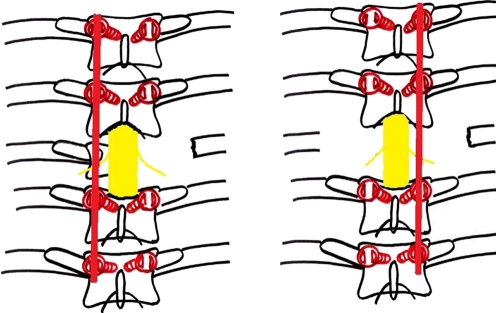
Buckling collapse
In children with severe destruction of the spine, a peculiar pattern of collapse is seen which has been termed as “Buckling Collapse” [9]. These children usually have complete destruction of more than two vertebral bodies. During collapse, dislocation of facet joints occurs at multiple levels and this leads to a kyphosis of more than 120°. The anterior surface of many vertebral segments above and below the deformity approximate to stabilise the vertebral column and the entire spine is converted into two large compensatory curves. Many vertebral segments thus became horizontally oriented with stress shielding of their growth plates. This causes longitudinal overgrowth of these vertebral segments (Fig. 2) and leads to stretching of the spinal cord at the apex of the kyphosis with a potential for secondary, late onset paraplegia [7, 8]. Risk factors for buckling collapse include an age of less than seven years at the time of the disease, thoracolumbar involvement, loss of more than two vertebral bodies, and presence of radiographic spine-at-risk signs [9].
Fig. 2.
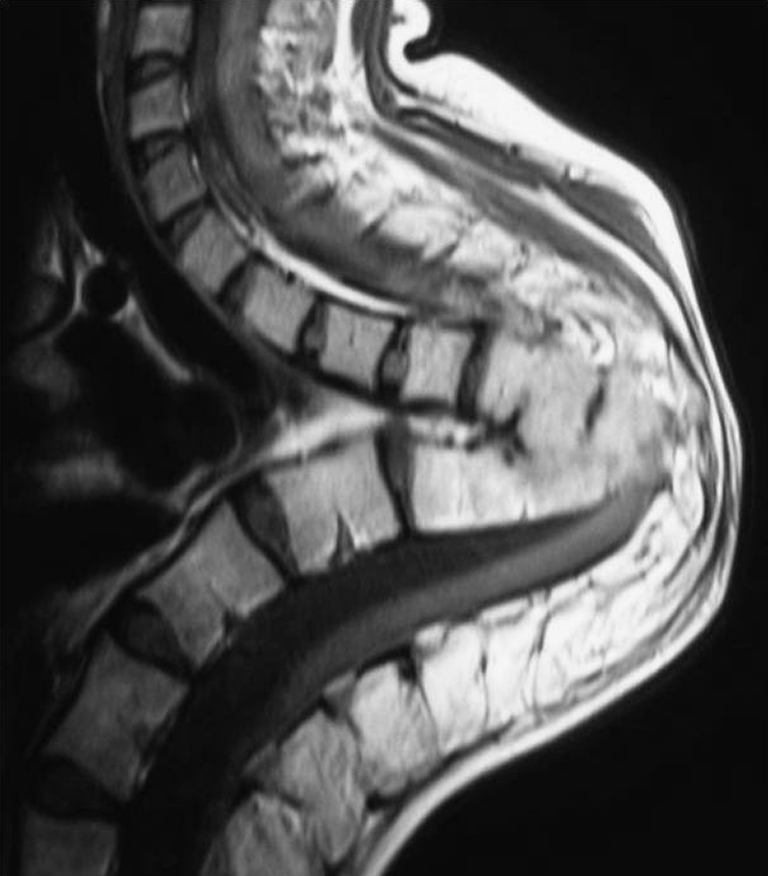
An example of buckling collapse, the cephalad arm of the kyphosis is completely horizontal. Longitudinal overgrowth of multiple segments, a cause of late onset paraplegia, is seen
Surgery for spinal tuberculosis
The indications of surgery in spinal tuberculosis include:
Progressive neuro-deficit
Persisting pain due to instability
Severe deformity
Identifying the subset of patients at risk for progression of deformity is essential as timely surgical intervention can prevent the development of severe deformity. Risk factors for severe progression are as follows: [2, 7–9].
An age below ten years and loss of one or one and a half vertebral bodies.
A pre-treatment kyphosis angle of greater than 30 degrees, especially in children.
Cervical thoracic and thoracolumbar junctional lesions.
Presence of ‘spine at risk’ radiological signs.
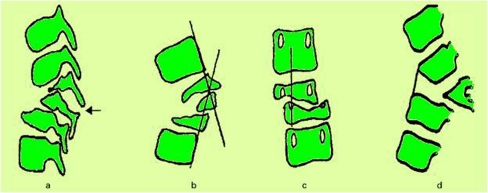
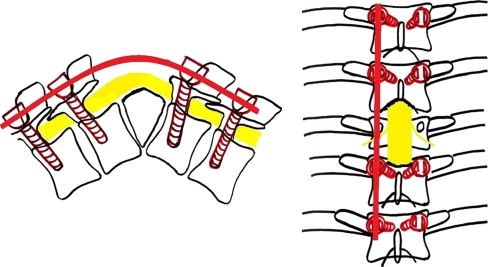
The “spine at risk” signs
Rajasekaran has described four simple radiological signs which reliably identify children who are at risk of severe deformity [7]. These signs are (a) “Separation of the facet joints” (b) “Retropulsion” (c) “Lateral translation and (d) “Toppling” (Fig. 3). All these signs represent the presence of spinal instability caused mainly due to the dislocation of the facet joints. Each of these signs is given a score of one. A score of three or more can accurately predict an increase in the kyphosis by more than 30° and a final deformity of more than 60° [7, 14].
Fig. 3.
“Spine at risk” radiological signs. (a) Separation of the facet joint, (b) Retropulsion, (c) Lateral translation, (d) Toppling
The practical value of these signs lies in the fact that they appear very early in the course of the disease, even during the active phase of infection [7]. This allows for early intervention in the form of surgery before the onset of progression of the deformity.
Surgery in active disease
There are numerous options for the surgical treatment of prevention of deformity.
Posterior (non-instrumented) only fusion surgery had a high failure rate especially when it was combined with laminectomy and is almost never performed nowadays [10]. Anterior debridement of the disease focus without insertion of a graft also has been shown to be ineffective in prevention of deformity [11]. Reports from Hong Kong (as a part of the MRC trials) emphasised the importance of anterior strut bone grafts following debridement to prevent deformity [12, 13]. However, other subsequent studies reported poor results with this technique mainly attributable to failure of the graft [11]. The need for additional stabilisation for protection of the grafts was evident.
The best form of stabilisation could be provided by instrumentation of the vertebral column however experience with pyogenic infections and the potential of the bacteria to survive around the metallic implants by creating a protective bio-film adherent to the implants made surgeons vary of introducing metallic foreign bodies adjacent to infected biological tissues. Oga et al. in 1993 demonstrated that bio-film formation and adherence of mycobacterium tuberculosis to the metallic implants is negligible making them susceptible to host defence mechanism and anti-tuberculous drugs [14]. Their findings removed the phobia of using instrumentation in active spinal tuberculosis [14, 15] leading many surgeons to use instrumentation posteriorly in addition to the anterior procedure [16–20] or anteriorly [21–24] with good results.
Later the outcomes of anterior only and combined anterior-posterior procedures for the treatment of spinal tuberculosis, with respect to degree of deformity correction, fusion and complications were found to be similar [16–18, 22, 25]. The widespread use of pedicle screw instrumentation and the development of the extended posterior approaches which allow the surgeon to perform anterior debridement and reconstruction from behind has led many surgeons to opt for posterior only surgery. It further avoids the possible hazards of violating the thoracic or abdominal cavities. Guven et al. [26] reported 98% cure rate with posterior spinal fusion and chemotherapy for ten to 12 months, without any debridement, in a cohort of 87 patients with ten years follow up.
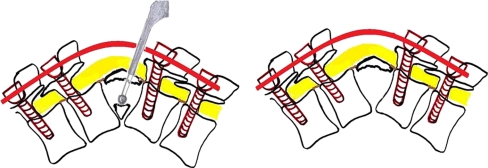
In the active stage of the disease when the deformity is mobile, it is the author’s routine practice to decompress and stabilise the spine using pedicle screw instrumentation through a posterior only approach. In lesser deformities (<30°) a primary posterior column shortening (Fig. 4) is performed to achieve correction, no spinal column distraction / lengthening is performed so as to allow for adequate interbody contact that facilitates good vertebral body healing. In patients with significant anterior column destruction in the thoracic region anterior column reconstruction is performed using Harm’s cylindrical mesh cage filled with bone graft. In the thoracolumbar and lumbar region if additional anterior column reconstruction is required an anterior approach is used.
Fig. 4.
Active tuberculosis D8-9, treated with a debridement, primary posterior column shortening and stabilisation through a posterior approach
The increased rate of failure of rib grafts and the morbidity associated with graft harvesting has led many surgeons to seek alternate materials for bridging the defects of the anterior column [27]. Titanium cages filled with cancellous bone grafts and fresh-frozen allografts from the humerus have been used with a high degree of success [10].
Surgery for established deformity
Surgery for established deformity is difficult, may have to be staged, and also is hazardous with a significant complication rate [6]. Even with a technique of staged procedures, Yau et al. reported mortality rate of 10% and the average amount of correction obtained by them was only 28% [6]. They advocated that corrective surgery be done only in patients in whom the deformity was severe, active disease still present, or, paraplegia or death from chest complications imminent.
Later refinement of surgical techniques, development of newer approaches and availability of rigid spinal instrumentation made single stage correction of established deformity relatively safe and a procedure with good outcome [28, 29]. ‘Transpedicular decancellation osteotomy’, ‘pedicle subtraction osteotomy’, ‘direct internal kyphectomy’ have been used to treat kyphosis in active as well as healed disease. Anil Jain et al. [28] reported kyphosis correction through an extra-pleural, antero-lateral (costo-transversectomy) approach with mean kyphosis correction of 27.3° and no persistent neurodeficits. Bezer et al. [29] used a ‘transpedicular decancellation osteotomy’ to correct post tuberculous deformity with no neural complications.
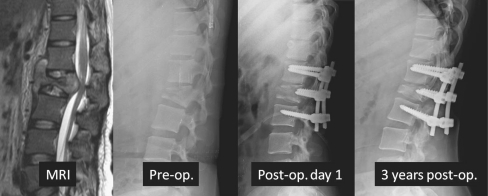
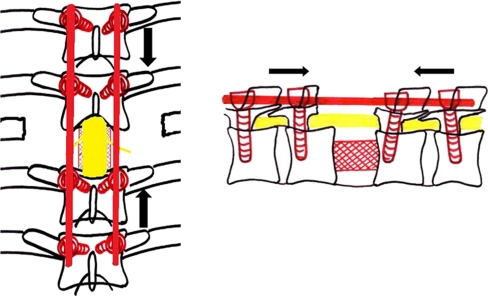
The author uses a single stage closing-opening wedge osteotomy to effectively and safely correct established deformity of more than 60° (Fig. 5). It is a circumspinal decompression and correction osteotomy using a single posterior approach for the correction of thoracic and thoracolumbar angular kyphotic deformity with or without neurological deficits.
Fig. 5.
Healed tuberculosis of the thoracolumbar deformity with a 102 degree kyphotic deformity. Posterior closing-opening wedge osteotomy was performed and deformity corrected to 48 degrees
Surgical procedure for closing-opening wedge osteotomy
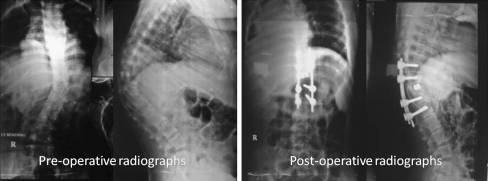
The procedure is a modification of the procedure described by Kawahara et al. [30] Using this procedure Rajasekaran et al. have reported good results at three years follow-up. Seventeen patients with a pre-operative kyphosis of mean 69.2 ± 25.1 degrees had a mean kyphosis correction of 56.8 ± 14.6% with an average blood loss of 820 ml. There were two major complications; one patient with pre-operative partial neurodeficit had neurological deterioration and another patient had implant failure requiring revision of the implants. All patients had significant improvement in pain and ODI scores at the last follow-up [31].
Procedure
Through a midline incision extending three or four vertebrae above and below the involved segment(s), the paraspinal muscles are dissected subperiosteally from the spinous processes and the laminae around the facet joints up to the tips of the transverse processes.
Pedicle screws are then inserted into normal vertebrae two or three above and below the lesion. The target vertebra is identified, and a bilateral laminectomy is then performed at the involved level, with partial laminectomy and facetectomy performed at adjacent levels. A temporary rod is applied to maintain spinal stability while performing the wedge resection of the anterior column (Fig. 6).
Fig. 6.
Laminectomy completed and a temporary rod inserted to stabilise the spine while decompression is carried out
The pedicles and intercostal nerve roots are then identified. Usually it is possible to gain sufficient exposure of the fusion mass by gentle retraction of the nerve roots. In patients with more than 60° deformity there is usually crowding of nerve roots. In the thoracic region, two or three nerve roots can be safely sacrificed by ligating about 3–4 cms away from the intervertebral foramen. The spinal branch of the segmental artery, which runs along with the nerve root, should also be ligated and divided.
The ribs at the affected level are transected 3–4 cm lateral to the costotransverse joint, and the pleura is bluntly separated from the ribs. The rib heads at the corresponding level, and the transverse process, are then carefully excised, extra-pleurally. The pedicle of the affected level is excised and the nerve roots of the affected level are retracted to make the room for the operative procedure of anterior decompression or wedge osteotomy. Blunt dissection is followed anteriorly on both sides through the plane between the pleura and the vertebral body. The aorta is carefully dissected anteriorly away from the anterior aspect of the vertebral body with a spatula and dissecting fingers. While the integrity of the endplates of adjacent vertebrae is maintained, the vertebral body is carefully removed using a curette, rongeur, or high-speed burr in a wedge shape approximating the angular deformity, keeping the posterior vertebral cortex. A second temporary rod is then attached to the screws on the opposite side. The initial rod is removed and the decompression procedure repeated on that side (Figs. 7 and 8). At the end of the decompression, a circumferential exposure of the dural tube is obtained. Then, the posterior vertebral cortex at the apex of deformity is finally drilled out under direct vision using a diamond burr and 1 mm Kerrison’s rongeur from the lateral direction. The adjacent discs are removed using a curette. Using contoured rods, shortening is carefully done to produce a concertina collapse of the wedge and correction of the deformity. At the first evidence of kinking of the cord or a concertina type of ballooning of the dura, the shortening is stopped. This is considered the end point of the correction possible by closing the wedge, and further correction is attempted by opening the wedge anteriorly. The anterior defect is then measured and a vertebral spacer, such as Harms’ titanium mesh cylinder with autograft inside, is carefully inserted into the intervertebral gap. The placement of the graft requires some attention since translation of the vertebral column is frequent. The screws are then compressed posteriorly (Fig. 9) so that the graft is secured in its place. A wake up test is performed to ensure neurological stability.
Fig. 7.
Rib and transverse process resected and vertebral body resection performed unilaterally. The rod is switched contra-laterally and similar procedure is performed
Fig. 8.
Vertebral resection is performed with a high speed burr and curettes. The posterior cortex is the last to be excised as it protects the cord against inadvertent damage
Fig. 9.
Compression of the screws to achieve correction
The incision is closed in layers over drains. The patient is allowed to ambulate one week after surgery with a thoracolumbosacral orthosis. The patient is maintained in an orthosis until the follow up radiographs shows adequate healing and fusion.
Conclusion
Prevention of deformity should be the primary aim in the treatment of spinal tuberculosis as the availability of potent antituberculous drugs has made uncomplicated tuberculosis a medical disease. The severity of deformity in spinal tuberculosis depends on the extent of vertebral destruction, level of lesion and age of the patient with more severe deformities seen in children and in lesions involving the thoracolumbar spine. In children deformity may continue to progress during growth even after the disease is cured and they should be followed up until completion of growth. The presence of two or more “spine at risk” radiological signs or “pre-treatment” deformities of 30 degrees are harbingers of severe late collapse especially in children. Surgical procedures performed in the active stage to prevent deformity are simpler and have less morbidity compared to surgical correction of established deformities. There are numerous surgical procedures available for prevention and correction of deformities from which the surgeon may choose one depending upon his preference and expertise. With the refinement of surgical technique and rigid spinal instrumentation providing three column support, a posterior only approach for prevention and correction of deformity in spinal tuberculosis is now possible.
References
- 1.Moon MS, Lee MK. The changes of the kyphosis of the tuberculous spine in children following ambulant treatment. Korean Orthop Assoc. 1971;6:203–208. [Google Scholar]
- 2.Parathasarathy R, Sriram K, Satha T, et al. Short course chemotherapy for tuberculosis of the spine: a comparison between ambulant treatment and radical surgery: ten year report. J Bone Joint Surg. 1999;81B:464–471. doi: 10.1302/0301-620X.81B3.9043. [DOI] [PubMed] [Google Scholar]
- 3.Moon MS. Tuberculosis of the spine, controversies and a new challenge. Spine. 1997;22:1791–1797. doi: 10.1097/00007632-199708010-00022. [DOI] [PubMed] [Google Scholar]
- 4.Moon MS, Kim I, Woo YK, Park YO. Conservative treatment of tuberculosis of the thoracic and lumbar spine in adults and children. Int Orthop. 1987;11:315–322. doi: 10.1007/BF00271307. [DOI] [PubMed] [Google Scholar]
- 5.Tuli SM. Severe kyphotic deformity in tuberculosis of the spine. Int Orthop. 1995;19:327–331. doi: 10.1007/BF00181121. [DOI] [PubMed] [Google Scholar]
- 6.Yau ACMC, Hsu LCS, O’Brien JP, Hodgson AR. Tuberculosis kyphosis-correction with spinal osteotomy, halo-pelvic distraction and anterior and posterior fusion. J Bone Joint Surg. 1974;56A:1419–1434. [PubMed] [Google Scholar]
- 7.Rajasekaran S. The natural history of post-tubercular kyphosis in children: radiological signs which predict late increase in deformity. J Bone Joint Surg. 2001;83B:954–962. doi: 10.1302/0301-620X.83B7.12170. [DOI] [PubMed] [Google Scholar]
- 8.Rajasekaran S (1999) A longitudinal study on the progress of deformity in children with spinal tuberculosis. PhD Thesis. Chennai, India, Tamilnadu Dr.MGR Medical University
- 9.Rajasekaran S. Buckling collapse of the spine in childhood spinal tuberculosis. Clin Orthop Relat Res. 2007;460:86–92. doi: 10.1097/BLO.0b013e31806a9172. [DOI] [PubMed] [Google Scholar]
- 10.Rand C, Smith MA. Anterior spinal tuberculosis: paraplegia following laminectomy. Ann R Coll Surg Engl. 1989;71(2):105–109. [PMC free article] [PubMed] [Google Scholar]
- 11.Upadhyay SS, Saji MJ, Sell P, et al. The effect of age on the change in deformity after radical resection and anterior arthrodesis for tuberculosis of the spine. J Bone Joint Surg. 1994;76A:701–708. doi: 10.2106/00004623-199405000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Hodgson AR, Stock FE. Anterior spinal fusion for the treatment of tuberculosis of the spine. J Bone Joint Surg. 1960;42A:295–310. [Google Scholar]
- 13.Medical Research Council A 10 years assessment of a controlled trial comparing debridement and anterior spinal fusion in the management of tuberculosis of the spine in patients on standard chemotherapy in Hong Kong: VIII report. J Bone Joint Surg. 1982;64B:393–398. doi: 10.1302/0301-620X.64B4.7047536. [DOI] [PubMed] [Google Scholar]
- 14.Oga M, Arizono T. Evaluation of the risk of instrumentation as a foreign body in spinal tuberculosis: clinical and biologic study. Spine. 1993;18(13):1890–1894. doi: 10.1097/00007632-199310000-00028. [DOI] [PubMed] [Google Scholar]
- 15.Ha K-Y, Ryoo SJ, et al. Adherence and biofilm formation of staphylococcus epidermidis and mycobacterium tuberculosis on various spinal implants. Spine. 2004;30:38–43. doi: 10.1097/01.brs.0000147801.63304.8a. [DOI] [PubMed] [Google Scholar]
- 16.Chen WJ, Wu CC, Jung CH, et al. Combined anterior and posterior surgeries in the treatment of spinal tuberculous spondylitis. Clin Orthop Relat Res. 2002;398:50–59. doi: 10.1097/00003086-200205000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Sunderaraj GD, Behera S. Role of posterior stabilisation in the management of tuberculosis of the dorsal and lumbar spine. J Bone Joint Surg Br. 2003;85-B:100–106. doi: 10.1302/0301-620X.85B1.13300. [DOI] [PubMed] [Google Scholar]
- 18.Klockner C, Velencia R. Sagittal alignment after anterior debridement and fusion with or without additional posterior instrumention in the treatment of pyogenic and tuberculous spondylodiscitis. Spine. 2003;28(10):1036–1042. doi: 10.1097/01.BRS.0000061991.11489.7F. [DOI] [PubMed] [Google Scholar]
- 19.Moon MS, Woo YK, Lee KS, et al. Posterior instrumentation and anterior interbody fusion for tuberculous kyphosis of dorsal and lumbar spines. Spine. 1995;20:1910–1916. doi: 10.1097/00007632-199509000-00013. [DOI] [PubMed] [Google Scholar]
- 20.Talu U, Gogus A. The role of posterior instrumentation and fusion after anterior radical debridement and fusion in the surgical treatment of spinal tuberculosis: experience of 127 cases. J Spinal Disord Tech. 2006;19:554–559. doi: 10.1097/01.bsd.0000211202.93125.c7. [DOI] [PubMed] [Google Scholar]
- 21.Benli IT, Acaroglu E. Anterior radical debridement and anterior instrumentation in tuberculosis spondylitis. Eur Spine J. 2003;12(2):224–234. doi: 10.1007/s00586-002-0403-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benli IT, Alanay A, Akalin S, et al. Comparison of anterior instrumentation systems and the results of minimum 5 year follow-up in the treatment of tuberculosis spondylitis. Kobe J Med Sci. 2004;50(6):167–180. [PubMed] [Google Scholar]
- 23.Ozdemir HM, Kemal US. The role of anterior spinal instrumentation and allograft fibula for the treatment of Pott disease. Spine. 2003;28(5):474–479. doi: 10.1097/01.BRS.0000048666.17934.17. [DOI] [PubMed] [Google Scholar]
- 24.Yilmaz G, Selek HY, et al. Anterior instrumentation for the treatment of spinal tuberculosis. J Bone Joint Surg. 1999;81-A(9):1261–1267. doi: 10.2106/00004623-199909000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Dai LY, Jiang LS, Wang W, et al. Single-stage anterior autogenous bone grafting and instrumentation in the surgical management of spinal tuberculosis. Spine. 2005;30:2342–2349. doi: 10.1097/01.brs.0000182109.36973.93. [DOI] [PubMed] [Google Scholar]
- 26.Guven O, Kumano K, Yalcin S, et al. A single stage posterior approach and rigid fixation for preventing kyphosis in the treatment of spinal tuberculosis. Spine. 1994;19:1039–1043. doi: 10.1097/00007632-199405000-00007. [DOI] [PubMed] [Google Scholar]
- 27.Govender S, Parbhoo AH. Support of the anterior column with allografts in tuberculosis of the spine. J Bone Joint Surg. 1999;81B:106–109. doi: 10.1302/0301-620X.81B1.9316. [DOI] [PubMed] [Google Scholar]
- 28.Jain AK, Aditya VM. Kyphus correction in spinal tuberculosis. Clin Orthop Relat Res. 2007;460:117–123. doi: 10.1097/BLO.0b013e318073bd29. [DOI] [PubMed] [Google Scholar]
- 29.Bezer M, Kucukdurmaz F. Transpedicular decancellation osteotomy in the treatment of posttuberculous kyphosis. J Spinal Disord Tech. 2007;20:209–215. doi: 10.1097/01.bsd.0000211271.89485.f1. [DOI] [PubMed] [Google Scholar]
- 30.Kawahara N, Tomita K, Hisatoshi B, et al. Closing–opening wedge osteotomy to correct angular kyphotic deformity by a single posterior approach. Spine. 2001;26:391–402. doi: 10.1097/00007632-200102150-00016. [DOI] [PubMed] [Google Scholar]
- 31.Rajasekaran S, Vijay K, Shetty AP. Single-stage closing-opening wedge osteotomy of spine to correct severe post-tubercular kyphotic deformities of the spine: a 3-year follow-up of 17 patients. Eur Spine J. 2010;19(4):583–592. doi: 10.1007/s00586-009-1234-z. [DOI] [PMC free article] [PubMed] [Google Scholar]