Abstract
Purpose
Multiplex Polymerase Chain Reaction (MPCR) is a technique in which two or more gene targets are amplified in a single reaction. This has increased sensitivity of diagnosis as a single gene target may be absent in some Mycobacterium tuberculosis strains.
Methods
MPCR using two target genes specific for Mycobacterium tuberculosis, that is, IS6110 and MPB 64, ZN staining and Mycobacterial culture were performed on synovial fluid/pus samples of 80 (three confirmed, 77 suspected) patients of osteoarticular tuberculosis and 25 non tuberculosis patients.
Results
MPCR had a sensitivity of 100% in confirmed cases and 81.8% in clinically suspected cases. AFB was positive in one patient and Mycobacterial culture was positive in three patients. MPCR also had 100% specificity; MPB64 was positive in five patients in which IS6110 was negative whereas IS6110 was positive in two patients in which MPB64 was negative.
Conclusions
MPCR is a sensitive and specific method for diagnosis of paucibacilliary conditions such as osteoarticular tuberculosis.
Introduction
Tuberculosis (TB) is still a major public health problem in the developing countries. The interaction of HIV with Mycobacterium tuberculosis has led to its resurgence even in developed nations, and has multiplied the burden of TB in developing countries [1]. India accounts for nearly one-third of global burden of TB [2]. Among the extra-pulmonary cases, osteoarticular involvement occurs in 5–10% of cases [3]. Prompt and accurate diagnosis and early treatment is required for better patient outcomes and to prevent further joint destruction. At present, the diagnosis of joint tuberculosis is largely based on clinical and imaging findings only, and these are not sensitive and specific enough to provide an early confirmed diagnosis of bone and joint TB [4]. Definitive diagnosis requires detection of tubercle bacilli, which can be done by smear microscopy (inexpensive but insensitive), while culture is considered to be the gold standard (takes six to eight weeks). The nucleic acid-based amplification test (NAA) has emerged as a promising tool for diagnosing extra-pulmonary TB reliably and quickly. A meta-analysis of NAA tests used in diagnosis of TB concludes that commercial tests yielded results with high specificity but low sensitivity, while heterogeneity and low diagnostic accuracy were a concern with the in-house PCR test [5].
Most of the PCR-based studies have used single target such as IS6110 or protein-b for amplification in diagnosis of tuberculosis [6]. Single gene target, however, may result in false negative results as some of these target genes may be absent in some of M. tuberculosis isolates [7, 8]. More reliable results would be obtained by using multiple target gene amplifications to cover for absence of some of them. In studies where two gene targets were amplified, the results with respect to sensitivity were much better [5]. Most studies have used IS6110 as a target for PCR based diagnosis of TB with varying degree of success. However, this IS6110 is absent in a proportion of M. tuberculosis isolates from India [9, 10]. An alternative approach is to use multiplex PCR (MPCR), in which several target genes are amplified simultaneously. Therefore, our study was undertaken to explore the utility of multiplex PCR using IS6110 and MPB64 genes as targets for rapid diagnosis of joint tuberculosis, as both of these are specific for the Mycobacterium TB complex. To the best of our knowledge, the use of multiplex PCR assay using these two targets together has not been done in osteoarticular TB.
Material and methods
A total of 105 specimens, received for acid fast staining and culture during the period of September 2008 to December 2009, were tested. Out of these 105 patients, three were confirmed OATB patients, 77 were suspected OATB patients and 25 were non TB patients, who acted as the control group. The relevant history and other details of patients were noted and they were divided into two groups on the basis of the following criteria.
Group 1: Osteoarticular TB group (N = 80) Confirmed OATB (n = 3): culture/smear positiveSuspected OATB (n = 77): culture and smear negative but suspected on the basis of imaging and clinical evaluation and response to anti-tubercular therapy
Group 2: Control group (N = 25) Patients with no history or evidence of tuberculosis
All the samples were subjected to three microbiological tests, namely, Ziehl-Neelsen staining (ZN), culture on Lowenstein-Jensen medium and multiplex PCR with IS6110 primers and MPB64 primers. The samples of the subjects were processed in a biosafety cabinet placed in a specially assigned room. At least 200–300 μl of specimens was aliquoted and stored at −20°C. PCR was done for only those specimens which were used for smear and culture examination for M. tuberculosis. Using 200 μl of centrifuge deposit for PCR, the rest of the deposit was used for acid fast microscopy by Ziehl-Neelsen method and culture was performed on two slopes of Lowenstein-Jensen medium. Multiplex PCR was standardised and found to have quantitative sensitivity to detect the DNA equivalent to two to three organisms. It tested positive with standard strain of M. tuberculosis, H37RV.
DNA was extracted according to the CTAB-phenol-chloroform extraction method. Briefly, 0.2 ml of sample was centrifuged at 10,000 rpm for ten minutes. The supernatant was discarded and pellet was suspended in 500 μl of TE Buffer (Tris-EDTA), 30 μl 10% SDS and 3 μl proteinase k (20 mg/ml), mixed and incubated at 37°C for one hour. After incubation, 100 μl of 5 M NaCl and 80 μl of high salt CTAB (cetyle-trimethyl ammonium bromide) were added and mixed followed by incubation at 65°C for ten minutes. An approximately equal volume (0.7–0.8 μl) of chloroform-isoamyl alcohol (24:1) was added, mixed thoroughly and centrifuged for five minutes at 10,000 rpm.
The aqueous viscous supernatant was carefully decanted and transferred to a new tube. An equal volume of phenol: chloroform- isoamylalcohol (25:24:1) was added followed by a five minute spin at 10,000 rpm. The supernatant was separated and then mixed with 0.6 volume of isopropanol to produce a precipitate. The precipitated nucleic acids were washed with 75% ethanol, dried and re-suspended in 100 μl of TE buffer.
Multiplex PCR (MPCR)
In each independent MPCR assay, test results were compared with the results for one positive and one negative control. The positive control included the DNA of H37Rv and negative control included the PCR grade water. Identification of M. tuberculosis was achieved using a specific pair of primers designed to amplify IS6110 and MPB64 in the M. tuberculosis complex and the expected band size was about 123 bp for IS611O and 240 bp for MPB64. The sequence of primers used for IS6110 was ISI: 5’-CCTGCGAGCGTAGGCGT 3 and IS2: 5’-CTCGTCCAGCGCCGCTTCGG 3’. Primers used for MPB64 were MPB1: 5’-TCC GCT GCC AGT CGT CTT CC-3’ and MPB2: 5’-GTC CTC GCG AGT CTA GGC CA-3’.
The following components were added to eppendrof (for 50 μl reaction): PCR buffer 10X, dNTPs (mix) 10 mM, primer IS1 (10 pm/μl), IS2(10 pm/μl), MPB1 (10 pm/μl) and MPB2 (10 pm/μl), Taq polymerase 5U/μl, DNA template and water. DNA amplification was performed for 40 cycles following an initial denaturation step at 94°C for five minutes in a thermo cycler by using the program: denaturation at 94°C for one minute, annealing at 65°C for 1.5 minutes, extension at 72°C for 1.5 min and final extension at 72°C for ten minutes.
The amplified product was stored at 4°C until the detection. For detection of amplification, samples were run on 1.5 % agarose gel stained with ethidium bromide. The stained gel was examined under UV light to look for bands 123 bp of IS611O and 240 bp of MPB64 using a molecular weight marker of 100 bp ladder. The samples showing the presence of these bands under ultraviolet transillumination were considered positive.
Statistical methods
The sensitivity, specificity, positive predictive value and the negative predictive value were calculated using the standard formulae. This work was part of a study approved by the Institute Ethics committee.
Results
The type of specimens received varied from synovial fluid (67; 83%) to pus (13; 17%) samples. Culture was positive from 2/13 pus samples and 1/67 synovial fluid samples. Out of 80 clinical TB specimens, one (1.25%) was AFB smear positive and 79 were AFB smear negative, confirming the paucibacilliary condition. In three culture positive confirmed cases of joint TB, all showed positive results using MPCR. In 77 clinically suspected cases of OATB, 66 (82.5%) were positive by MPCR (positivity was taken if band is present for any of two targets used or if bands are present for both of the targets as shown in Fig. 1). Out of 66 MPCR positive cases, MPB64 bands were present in 62 (80%) cases and IS6110 bands were present in 59 (73.75%) cases. There were five (6.2%) synovial fluid samples which were negative by IS6110 but were positive by MPB64 (Table 1). There were two cases which were IS6110 positive but were MPB64 negative. In the control group, all the tests were negative, thus giving specificity of 100% to MPCR. A final diagnosis of joint TB was made for 80 patients based on the results of culture, microscopy, MPCR and response to ATT. MPCR was positive in 66 out of 80 cases, while culture was positive in three cases and microscopy in one case. Thus, the sensitivities of MPCR, culture and microscopy were 82%, 3.39% and 1.25%, respectively. There were five cases (6.2%) which were IS6110 negative but MPB64 positive. An important finding was that by using MPCR, sensitivity increased to 82%, whereas sensitivity for MPB64 and IS6110 bands in isolation were 80% and 73.75%, respectively. In the non TB control group, all the tests were negative, thus giving the specificity of 100% (Table 2).
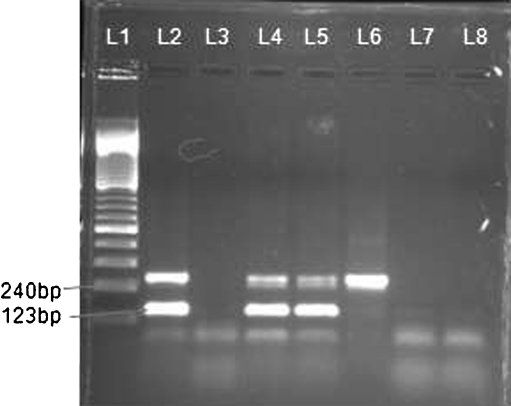
Fig. 1.
Shows the gel photograph of multiplex PCR. L1 shows the molecular marker, L2 shows positive control, L3 shows the negative control, L4 &L5 clinical samples positive with both markers i.e IS 6110 at 123 bp and MPB 64 at 240 bp. L6 shows clinical sample only positive with MPB 64. L7 &L8 shows negative clinical samples
Table 1.
Results of AFB smear, culture, and multiplex PCR
| Type | Subtype | No. of patients | Smear (+), N (%) | Culture (+), N (%) | mPCR, N (%) | IS6110 band (+), N (%) | MPB64 band (+), N (%) | Only IS6110 | Only MPB 64 |
|---|---|---|---|---|---|---|---|---|---|
| Group I | Confirmed OATB | 3 | 1 | 3 (100) | 3 (100) | 3 (100) | 43 (100) | - | - |
| Suspected OATB | 77 | - | - | 63 (81.8) | 56 (72) | 61 (79.22) | 2 (2.5) | 5 (6.4) | |
| Total | 80 | 1 | 3 (3.75) | 66 (82.5) | 59 (72.22) | 64 (80) | 2 (2.5) | 5 (6.2) | |
| Group II | Non-TB control group | 25 | - | - | - | - | - | - | - |
AFB acid fast bacilli, OATB osteoarticular tuberculosis, mPCR multiplex polymerase chain reaction
Table 2.
Sensitivity and specificity of PCR test compared to culture / AFB smear
| Test | Test results | OATB control groups | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | |
|---|---|---|---|---|---|---|---|
| (N = 80) | (N = 25) | ||||||
| Multiplex PCR | Positive | 66 | - | ||||
| Negative | 14 | 25 | 82.5 | 100 | 100 | 64.1 | |
| MPB64 | Positive | 64 | - | ||||
| Negative | 16 | 25 | 80 | 100 | 100 | 60.97 | |
| IS6110 | Positive | 59 | - | ||||
| Negative | 21 | 25 | 73.75 | 100 | 100 | 54.34 | |
| Culture | Positive | 3 | - | ||||
| Negative | 77 | 25 | 3.89 | 100 | 100 | 27.17 | |
| Microscopy | Positive | 1 | - | ||||
| Negative | 79 | 25 | 1.25 | 100 | 100 | 24.03 | |
AFB acid fast bacilli, OATB osteoarticular tuberculosis, PCR polymerase chain reaction, PPV positive predictive value, NPV negative predictive value
Discussion
Osteoarticular TB is a paucibacilliary condition, which requires early diagnosis and prompt treatment to decrease the sequelae associated with this condition. In this study, culture for MTB complex was found to be positive from 15.38% of pus samples and from one synovial fluid sample. Vikas et al. [11] also showed that culture positivity was more from pus samples as compared to synovial fluid samples and this is an issue in joint TB, as tubercular arthritis is the more common presentation. Another reason for low culture positivity maybe the presence of non-viable mycobacteria in clinical samples, as many patients are referred to tertiary care centres after the institution of anti tubercular treatment at the referring hospital.
In an attempt to increase diagnostic accuracy, we performed MPCR using two targets, i.e. IS6110 and MPB64, together for diagnosis of Mycobacterium tuberculosis complex. We were able to pick up those cases which were missed by IS6110 or vice versa, as the use of two targets together increased the sensitivity of NAA test. Most previous studies have used IS6110 as a single target, because IS6110 is present in multiple copies in the M. tuberculosis genome, and can give higher sensitivity [12, 13]. It has however been shown that there are M. tuberculosis strains originating from India, which do not contain IS6110 [12]; its utility in isolation in the diagnosis of TBM thus becomes suspect [14]. To overcome this shortcoming we prospectively evaluated MPCR using two different genes for rapid diagnosis of joint TB, and MPCR positivity was shown to be 82.5%, with 100% positivity in culture confirmed OATB cases.
Some studies have assessed amplification of multiple gene targets from the same samples, but this was using CSF [15]; an increase in sensitivity was noted when all these assays were analysed together [16]. A study by Hulder et al. [17] reported an increase in sensitivity of PCR if two targets were used for diagnosis of TBM. Our study is unique in the fact that both genes were amplified together from one sample and we were able to diagnose the cases which were missed by IS6110 alone or by MPB64 in isolation. Other studies which have evaluated two or three targets had put up separate PCRs for each reaction, which increases the cost of the test and also the chances of cross contamination. This method of using multiplex PCR in one sample reduces errors, as well as cost, and increases the sensitivity of the test, which is doubly important in osteoarticular TB where there is doubtful diagnosis. Additionally we were also able to pick up 5% of cases of joint TB, in which IS6110 was missing, and the test would have come as false negative; these were diagnosed by MPB64 as amplification for both targets had been carried out at the same time.
In MPCR, sensitivity of MPB64 was 100% in confirmed joint TB cases and 80% in suspected cases while the specificity was 100%. Results of our study were similar to another study by Titov et al. [18], in which only MPB64 was used as target; they showed PCR positivity in 8/8 samples from bone and joint tuberculosis. IS6110 was positive in 100% of confirmed OATB cases and 72.72% of suspected cases. Studies have evaluated IS6110 PCR, and sensitivity of this has been documented as 61.7% (Tiwari et al.) [19] to 73% (Pandey et al.) [20]. The reason for low sensitivity in many studies could be due to low volume of sample available, insufficient lysis of cells, loss of DNA during purification or different target used for amplification [20]. In our study, the reasons for PCR negativity in few clinically suspected joint TB cases could be the presence of the low number of bacteria, poor lyses of bacteria in the samples, presence of PCR inhibitors in the samples or even institution of antituberculous therapy prior to coming to the hospital, although this has not been extensively studied. Sometimes the tough cell wall of M. tuberculosis makes the isolation of target DNA difficult, or there may be presence of inhibitors of PCR which give false negative results [21]. Thus early identification will help in institution of prompt treatment preventing joint damage, more so when some techniques like technetium-99 m-labelled ciprofloxacin (99m Tc) scan are emerging as a promising tool for monitoring drug response [22].
In this study, we have evaluated multiplex PCR using two genes together, i.e. IS6110 and MPB64, showing a sensitivity of 82.82% and specificity of 100%; culture positivity in the same samples was only seen in 3.89%. We thus conclude that MPCR by using more than one target gene increases the diagnostic yield in paucibacilliary conditions like osteoarticular TB, as those cases which are likely to be missed due to absence of the IS6110 sequence in the Indian population would also be detected. The use of the same sample for multiple genes targeting also reduces the cost.
Acknowledgments
Conflict of interest None.
References
- 1.WHO (2006) Global tuberculosis control. Surveillance, planning, financing. World Health Organization, Geneva, Switzerland, p 242
- 2.WHO (2009) The WHO's report on global TB. World Health Organization, Geneva, Switzerland
- 3.Titov AG, Vyshnevskaya EB, Mazurenko SI, Santavirta S, Konttinen YT. Use of polymerase chain reaction to diagnose tuberculous arthritis from joint tissues and synovial fluid. Arch Pathol Lab Med. 2004;128:205–209. doi: 10.5858/2004-128-205-UOPCRT. [DOI] [PubMed] [Google Scholar]
- 4.Sequeira W, Co H, Block JA. Osteoarticular tuberculosis: current diagnosis and treatment. Am J Ther. 2000;7:393–398. doi: 10.1097/00045391-200007060-00009. [DOI] [PubMed] [Google Scholar]
- 5.Pai M, Flores LL, Pai N, Hubbard A, Riley LM, Colford JM. Diagnostic accuracy of nucleic acid amplification tests for tuberculous meningitis: a systematic review and meta-analysis. Lancet Infect Dis. 2003;3:633–643. doi: 10.1016/S1473-3099(03)00772-2. [DOI] [PubMed] [Google Scholar]
- 6.Sharma K, Sharma A, Singh M, Ray P, Dandora R, Sharma SK, Modi M, Prabhakar S, Sharma M. Evaluation of PCR using protein b primers for rapid diagnosis of tuberculous meningitis. Neurol India. 2010;58:727–731. doi: 10.4103/0028-3886.72189. [DOI] [PubMed] [Google Scholar]
- 7.Jonas V, Alden MJ, Curry JI, Kamisango K, Knott CA, Lankford R, Wolfe JM, Moore DF. Detection and identification of Mycobacterium tuberculosis directly from sputum sediments by amplification of rRNA. J Clin Microbiol. 1993;31:2410–2416. doi: 10.1128/jcm.31.9.2410-2416.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Noel AB, Lecossier D, Nassif X, Birgite G, Frebault VL, Hance AJ. Rapid diagnosis of tuberculosis by amplification of Mycobacterial DNA in clinical samples. Lancet. 1989;2:1069–1071. doi: 10.1016/S0140-6736(89)91082-9. [DOI] [PubMed] [Google Scholar]
- 9.Narayanan S, Girish C, Mahadevan S, Rajajee S. Evaluation of PCR using TRC4 and IS6110 primers in detection of tuberculous meningitis. J Clin Microbiol. 2002;39:2006–2008. doi: 10.1128/JCM.39.5.2006-2008.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Radhakrishnan I, Kumar RA, Mundayoor S. Implication of low frequency of IS6110 in fingerprinting field isolates of Mycobacterium tuberculosis from Kerala. India J Clin Microbiol. 2001;39:1683. doi: 10.1128/JCM.39.4.1683.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vikas A, Shenai S, Mohrir G, Deshmukh M, Bhaduri A, et al. Osteoarticular tuberculosis—diagnostic solution in a disease endemic region. J Infect Dev Ctries. 2009;3:511–516. doi: 10.3855/jidc.469. [DOI] [PubMed] [Google Scholar]
- 12.Rafi A, Naghily B. Efficiency of polymerase chain reaction for the diagnosis of tuberculous meningitis. Southeast Asian J Trop Med Public Health. 2003;32:357–360. [PubMed] [Google Scholar]
- 13.Nguyen LN, Kox LF, Pham LD, Kuijper S, Kolk AH. The potential contribution of the polymerase chain reaction to the diagnosis of tuberculous meningitis. Arch Neural. 1996;53:771–776. doi: 10.1001/archneur.1996.00550080093017. [DOI] [PubMed] [Google Scholar]
- 14.Donald PR, Victor TC, Jordaan AM, Schoeman JF, Heldon PD. Polymerase chain reaction in the diagnosis of tuberculous meningitis. Scand J Infect Dis. 1993;25:613–617. doi: 10.3109/00365549309008550. [DOI] [PubMed] [Google Scholar]
- 15.Bhigjee AI, Padayachee R, Paruk H, Hallwith-Pillay KD, Maris S, Connoly C. Diagnosis of tuberculous meningitis: clinical and laboratory parameters. Int J Infect Dis. 2007;11:348–354. doi: 10.1016/j.ijid.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 16.Rafi W, Venkataswamy MM, Ravi V, Chandramuki A. Rapid diagnosis of tuberculous meningitis : a comparative evaluation of in-house PCR assays involving three mycobacterial DNA sequences, IS6110, MPB64 and 65 KDa antigen. J Neurol Sci. 2007;252:163–168. doi: 10.1016/j.jns.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 17.Halder S, Sharma N, Gupta VK, Tyagi JS. Efficient diagnosis of tuberculous meningitis by detection of Mycobacterium tuberculosis DNA in cerebrospinal fluid filtrates using PCR. J Med Microbiol. 2009;58:616–624. doi: 10.1099/jmm.0.006015-0. [DOI] [PubMed] [Google Scholar]
- 18.Titov AG, Vyshnevskaya EB, Mazurenko SI, Santavirta S, Konttinen YT. Use of polymerase chain reaction to diagnose tuberculous arthritis from joint tissues and synovial fluid. Arch Pathol Lab Med. 2004;128:205–209. doi: 10.5858/2004-128-205-UOPCRT. [DOI] [PubMed] [Google Scholar]
- 19.Tiwar V, Jain A, Verma RK. Application of enzyme amplified mycobacterial DNA detection in the diagnosis of pulmonary and extrapulmonary tuberculosis. Indian J Med Res. 2003;118:224–228. [PubMed] [Google Scholar]
- 20.Pandey V, Chawla K, Acharya K, Rao S, Rao S. The role of polymerase chain reaction in the management of osteoarticular tuberculosis. Int Orthop. 2009;33:801–805. doi: 10.1007/s00264-007-0485-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Noordohek GT, Kolk AH, Bjune G, Catty D, Dale JW, Fine PE, et al. Sensitivity and specificity of PCR for detection of Mycobacterium tuberculosis: a blind comparison study among laboratories. J Clin Microbiol. 1994;32:277–284. doi: 10.1128/jcm.32.2.277-284.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhardwaj V, Agrawal M, Suri T, Sural S, Kashyap R , Dhal A (2010) Evaluation of adequacy of short-course chemotherapy for extraspinal osteoarticular tuberculosis using 99mTc ciprofloxacin scan. Int Orthop [Epub ahead of print] Nov 30. doi:10.1007/s00264-010-1162) [DOI] [PMC free article] [PubMed]