Abstract
Purpose
The focus of this study was to analyse the patient with disc space infection and the need for re-exploration.
Method
Thirty-five patients were analysed within the period from April 1992 and May 2011. The diagnosis was confirmed by the cardinal clinical features, raised erythrocyte sedimentation rate [ESR], raised C-reactive protein and MRI findings. All received 500-mg intravenous amikacin and one gram ceftriaxone at the time of anaesthetic induction and six hours after surgery.
Results
Age range was between 25–62 years. The appearance of symptoms was between four days and three weeks. Nine patients had silent chronic urinary tract infection. Twenty-nine patients had re-exploration while the others did well on conservative treatment. Neurological deficit was not recorded. All recovered well within six to nine months.
Conclusion
Re-exploration is recommended if no response is achieved after four day’s conservative treatment for or if the patient’s condition is critical.
Introduction
Postoperative disc space infection is relatively uncommon, and early diagnosis is difficult unless the surgeon is fully aware of this complication, which is associated with significant morbidity, increased cost, and poor long-term outcome. The incidence rate has been estimated to be below 1% [1].
The symptom may be regarded as a consequence of an unsatisfactory operation, recurring disc herniation or non pyogenic discitis. There are no haematological, biochemical, or imaging findings that are unequivocally diagnostic of this process [2]; for these reasons, the diagnosis is difficult and frequently delayed.
Staphylococcus is the most common aetiological agent of pyogenic discitis [3], followed by aerobic gram negative bacilli; other rare cases were fungal [4]: Clostridium perfringens [5], Haemophilus species [6] and Aspergillus fumigatus [7].
Early diagnosis, identification of the causal agent, and specific antimicrobial treatment is essential to avoid prolonged suffering and severe complications. The virulence of these infecting organisms, the host response and early surgical treatment, if required, influence treatment and determine outcome.
Prolonged and specific antibiotics given intravenously along with surgical treatment are probably the only ways to improve prognosis and avoid disability.
The aim of this study was to describe the cardinal clinical features of this usually missed condition and to support re-exploration rather than prolong conservative treatment.
Patients and methods
In a prospective study between April 1992 and May 2011, a total of 35 patients were diagnosed with postoperative intervertebral disc space infection. The diagnosis of pyogenic discitis was established on a clinical basis to be confirmed by investigation and clinical symptoms of back pain, radicular pain, muscle cramps, periodic attacks, no relief with rest, and sometimes associated with features of inflammatory disorders such as fever (above 38°C), with tenderness of the back on physical examination. Imaging studies (MRI) were compatible with disc space infection and microbiological evidence such as isolation of microorganisms, as were haematological investigations including erythrocyte sedimentation rate and CRP.
There were 26 males and nine females with a mean age of 44.5 years and range between 25–64 years. In 26 patients, the level was L4-L5, in three patients the level was L3-L4, while L5-S1 level was involved in six patients. Because we were fully aware of this condition delayed diagnosis was not encountered.
Diagnosis in the postoperative period was achieved between four days and three weeks. All patients were given prophylactic antibiotic at the time of anaesthetic induction (500 mg amikacin and one gm ceftriaxone). A careful observation in the postoperative period was made.
All had MRI once the diagnosis of disc space infection was suspected. All had blood tests including WBC and differential count, ESR and CRP. The clinical features were almost diagnostic. Once the suspicion is raised, intravenous antibiotics started immediately (vancomycine, ciproflacin) and oral rifampicine, with absolute bed rest, muscle relaxant and analgesia.
Results
The surgical procedures were fenestration in 19 patients, hemilaminectomy in eight patients, and eight patients had full laminectomy.
No relation was noticed between the size of the disc herniation and the incidence of infection, but prolonged surgery due to adhesion between the root, disc and dura or in bilateral exploration (right and left) was a factor leading to infection. Also, the incidence of infection was greater at the level L4-L5. Re-exploration was done from the posterior approach, and none of the cases studied required anterior fusion. All of the primary operations were first time surgical treatment.
Thirteen patients developed bony fusion at the site of infection. No specific underlying causes such as diabetes or immunodeficiency were noticed, but those with prolonged symptoms and severe pain for several months were more vulnerable.
Severe back pain, muscle contraction, limited range of motion, spasticity, sciatica, inability to bend, positive Lasegue’s sign and refusal to do any movement were the significant clinical findings; also we noticed that shaking the patient’s bed induced pain.
Inflammatory signs in the spine were not recorded and the skin in the lumber region was normal; paravertebral abscess was confirmed by MRI only in five patients (14.3%).
No neurological deficit was detected either at the time of diagnosis nor at follow-up.
ESR was very significant for the diagnosis and follow-up ranging between 105–130 mm/hour and a raised C-reactive protein above 10 mg/l was a constant finding. The leukocytes and differential count were not useful and not significant.
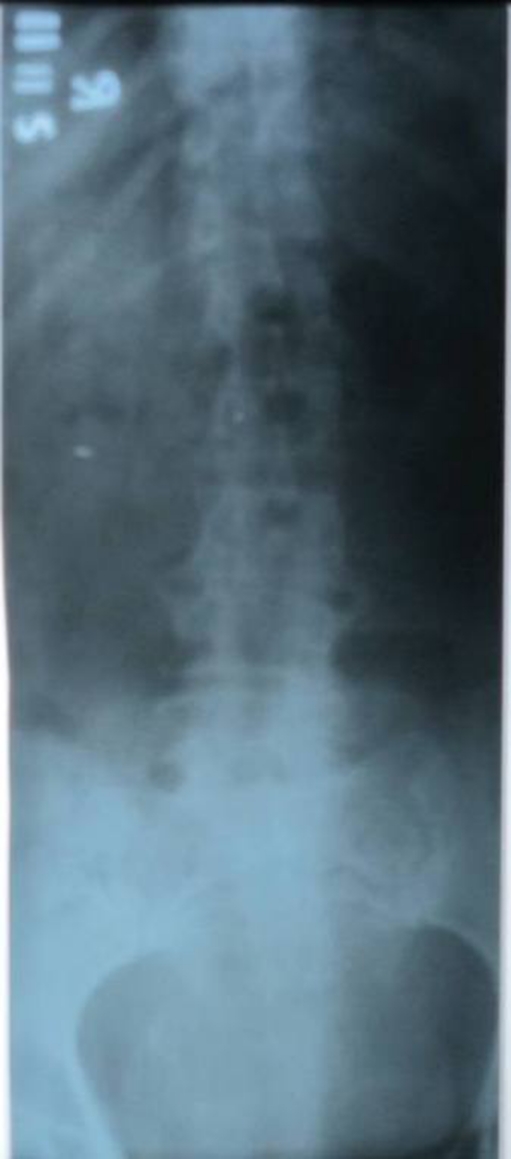
Blood culture was positive for Staphylococcus aureus in 13 patients (37.14%). Plain radiography was done in all patients but it did not support the diagnosis in the initial stages (Figs. 1, 2, 3, 4).
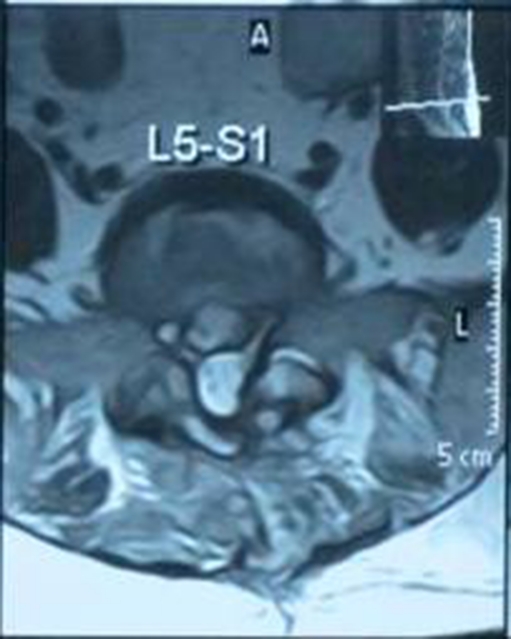
Fig.1.
Axial T2 showing extra dural abscess
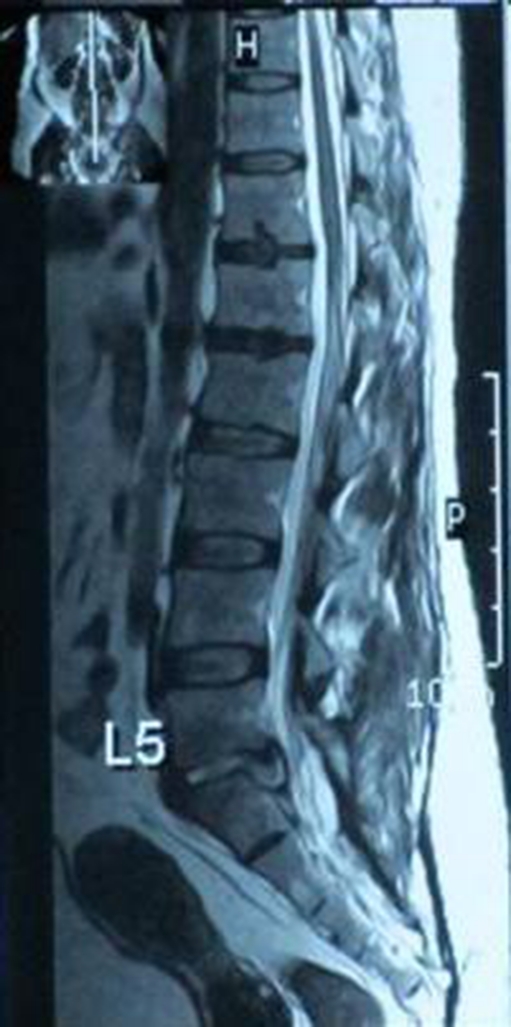
Fig. 2.
T2 sagittal showing disc space infection, with abscess collection pressing on the thecal sac
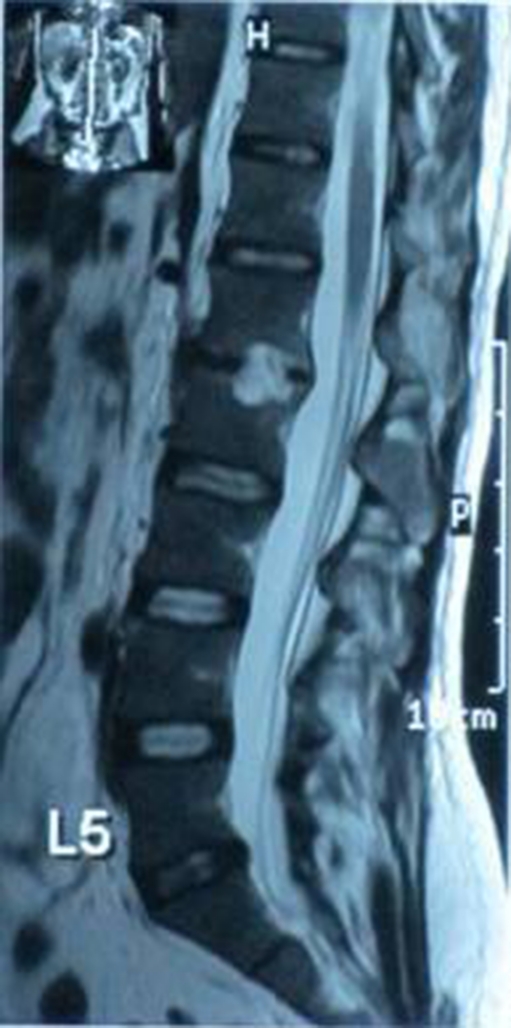
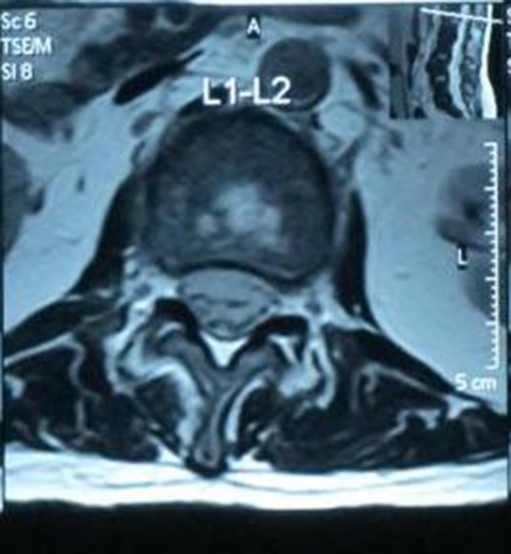
Fig. 3.
Sagittal T2 showing L1-L2 disc space abscess collection
Fig. 4.
T2 axial showing disc space abscess
MRI was done in all patients with very significant findings: marked narrowing and obliteration of the specified disc, abnormal signal returned from disc, collection in the disc space and paraspinal area. Sometimes reherniation is seen clearly and specific post contrast enhancement with rim lination when there is abscess collection.
Only six patients improved on conservative treatment, the other 29 patients were re-explored after poor response to conservative treatment.
Operative findings and operative treatment
Twenty nine patients (82.86%) had re-exploration which revealed severe inflammatory reaction, necrotic material, and inflamed root and dura; hydrophilic herniated disc material was found pressing on the root in 16 patients (45.7%). All necrotic material was removed with the extruded disc material, and swabs taken for culture and sensitivity which was positive in 19 patients only (54.3%). The micro-organism was S. aureus sensitive to vancomycine, tobramycine, and rifampicine; antibiotics were given accordingly.
Transpedicular fixation was not required. Urine was also examined and cultured but only nine patients proved to have chronic urinary tract infection. Also, nine patients admitted to not having bowel motion seven days after surgery.
Twenty patients recovered fully within six months, four within seven months, six patients within eight months and five patients within nine months, with no disability or neurological deficit.
Discussion
The incidence of infection varies from 0.21% to 3.6% in association with all surgical procedures [8] but our incidence was [2.2%]. The risk is lower following discectomy if compared with spinal instrumentation and prolonged reconstructive spine surgery.
Our local theatre facilities are below European standards and, from all points of view, also the personal hygiene of our patients can be poor.
Serious risk factors were not recorded in the patients studied, and only nine patients (25.7%) had chronic urinary tract infection which may have played a role in the development of disc space infection. Infection occurs as a result of inoculation at the time of surgery.
Injury to the end plate, trauma to small vessels, haematoma collection and necrotic tissue caused by surgery provide ideal culture conditions for bacteria to grow in the disc space.
The mean age in this study was similar to that reported by other authors [9].
From the cases studied above, the cardinal clinical features, e.g. severe back pain, muscle spasm which is periodic in nature, and a raised temperature, were very helpful in the diagnosis, and this correlates with the study of Ahmed et al. [10].
None of our patients were diabetic, but Piotrowaski et al. [2] found that 26% of patients with postoperative spondylodiscitis had diabetes. The incidence of diabetes among all surgical patients is 6.8%.
Usually postoperative symptoms appear between six days and six months after surgery, but in our cases 26 patients were diagnosed within four days of surgery: four patients within ten days and five patients were diagnosed after three weeks.
Body temperature was not significantly high apart from two patients (5.7%) and this mirrors another study [11]. Also, motor and sensory signs were infrequent, which is in agreement with other studies [2, 12].
Paravertebral abscess was confirmed by MRI in five patients (14.3%), therefore an infrequent finding, as mentioned by another study [13].
Inflammatory signs in the spine were absent clinically and the skin looked normal, but this does not exclude a diagnosis of disc space infection. Furthermore, a negative culture does not exclude the diagnosis if the clinical features are highly suggestive of disc space infection. This is probably related to the antibiotics given as a prophylaxis.
Manuel et al. [14] consider blood culture very useful and believe it must always be done, while in our cases only 13 patients showed positive blood culture.
In our study S. aureus was the predominant organism to be found as noted in other studies [9, 15].
Rarely the causative organism was fungal [4], e.g. Clostridium perfringens [5], Haemophilus species [6], or Aspergillus fumigatus [7], and a very rare micro-organism was recorded as a case report. Thus it can be said that the micro-organism is staphylococcus unless proven otherwise.
Although ESR is considered a non specific parameter of inflammation, it was useful as a diagnostic and prognostic test in the cases studied; all patients had a very high ESR, making it a criterion for the diagnosis.
This fact was also mentioned by other authors Dall et al. [9] and Dauch [12]. A mild rise in ESR (not reaching 60 mm/hour) was recorded in some of our patients without disc space infection, and this has been mentioned by Bircher et al. [16].
We found CRP (a rising titre) very useful to support the clinical diagnosis and also for follow-up, although the CRP value is non specific. We agree with Rawling et al. [17] in considering CRP more informative than ESR because a rise in CRP value is very rare in the absence of disc space infection.
We did not find plain radiographs useful in detecting early changes but they may be useful in the late stage. Manuel et al. [14] reported that 12.9% of his patients showed normal radiographs.
We have no experience with bone scan because it is not available in our facility. Manuel et al. [14] consider bone scans not useful.
MRI has higher sensitivity and specificity than other imaging. In our cases, MRI was very useful even in the early stages of the disease. Bed rest was useful, and “pantaloon” spica was also very useful, but is cumbersome and poorly tolerated in hot and humid weather.
The author believes that not all antibiotics are effective in disc space infection; some such as cefazolin are useless [18]. Thus, positively charged and broad spectrum antistaph antibiotics are the drugs of choice. The effective antibiotics in this study were vancomycin, ciprafloxacin and rifampicin given in the same time for several weeks depending on the patient’s general condition and local response.
Diclofenac sodium was the analgesia preferred by most of the patients studied; muscle relaxant was also very useful.
Re-exploration was performed through posterior approach even in patients with paraspinal abscess which resolve with antibiotics and time.
Surgical management must be individualised in the short term; the surgical outcome is superior to conservative treatment. Surgery helps to obtain an adequate amount of culture and biopsy material in addition to clearly showing the site of lesion, nevertheless a high dose trial of antibiotics may help in a few cases.
If abscess is confirmed by MRI, the answer is always reoperation.
The prognosis is related to early detection, early surgical intervention if required, identification of the micro-organism, initiation of specific treatment and the duration of treatment which is tailored to the patients’ condition.
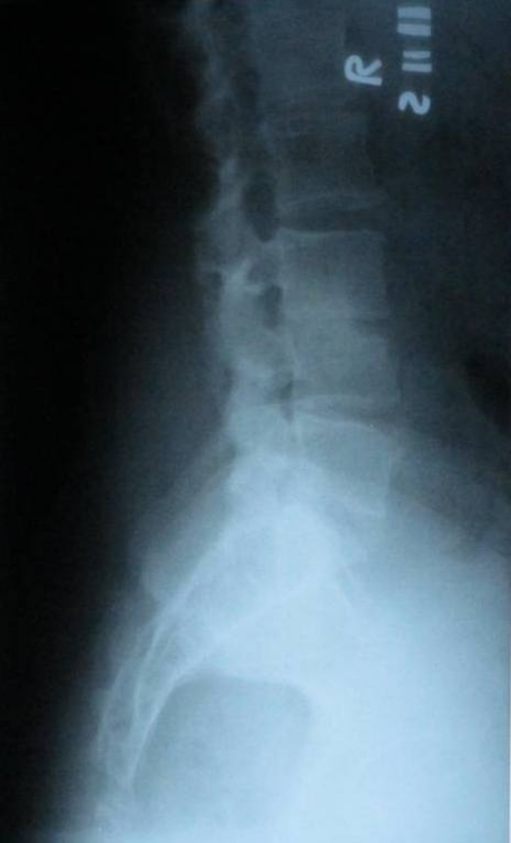
The good late outcome probably was related to the development of bony fusion between vertebrae, though the fusion was only proved by radiography in 13 patients (Fig. 5 and 6).
Fig. 5.
Plain radiography L3-L4 showing obliteration and narrowing of disc space due to bony fusion
Fig. 6.
Plain radiography showing obliteration of disc space L3-L4 due to bony fusion
Conclusions
If postoperative disc space infection is suspected on a clinical basis, urgent action should be taken, and then ESR, CRP, blood and wound culture and MRI should be performed.
Intravenous antistaphylococcal antibiotics are the drugs of choice in addition to absolute bed rest, muscle relaxant and analgesia, to be followed by careful observation for the response.
Early re-exploration is superior to prolonged conservative treatment.
Footnotes
Conflict of interest The author declares no conflict of interest.
References
- 1.Ford LT, Key JA (1955) Postoperative infection of intervertebral disc space. South Med J 48(12):1295–1303 [DOI] [PubMed]
- 2.Piotrowaski WP, Krombholz MA, Muhl B. Spondylodiscitis after lumbar disc surgery. Neurosurg Rev. 1994;17:189–193. doi: 10.1007/BF00418428. [DOI] [PubMed] [Google Scholar]
- 3.Osenbach RK, Hitchon PW, Menezes AH. Diagnosis and management of pyogenic vertebral osteomyelitis in adult. Surg. Neurol. 1990;33:266–275. doi: 10.1016/0090-3019(90)90047-S. [DOI] [PubMed] [Google Scholar]
- 4.Williams et al. (1999) Fungal spinal osteomyelitis in immunocompromised patients. Am J Neuroradiol 20:381–385 [PMC free article] [PubMed]
- 5.Beguiritain JL, Depablos J, Lombark R, Gomez A (1986) Discitis due to clostridium perfringens. Spine 11(2):170–172 [DOI] [PubMed]
- 6.Stephanian E, Coffey RJ, Segal R. Intervertebral disc space infection and osteomyelitis due to Hemophilus species. Spinal disorders. 1989;2(2):114–119. [PubMed] [Google Scholar]
- 7.Lang EW, Pitts LH. Intervertebral disc space infection caused by Aspergillus fumigatus. Eur Spine J. 1996;5(3):207–9. doi: 10.1007/BF00395517. [DOI] [PubMed] [Google Scholar]
- 8.El-Gindi S, Aref S, Salama M et al. (1976) Infection of intervertebral discs after operation. J Bone Joint Surg Br 58:114–116 [DOI] [PubMed]
- 9.Dall BE, Rowe DE, Odette WG, Battes DH. Postoperative discitis: diagnosis and management. Clin Orthop Relat Res. 1987;224:138–46. [PubMed] [Google Scholar]
- 10.Mumtaz A, Muhammed Y. Lumbar discitis, prevalence and management after lumbar disc surgery. Professional Medicine Journal. 2010;17(4):628–632. [Google Scholar]
- 11.Puranen J, Makela J, Lahde S. Postoperative intervertebral discitis. Acta Orthop Scand. 1984;55:461–5. doi: 10.3109/17453678408992395. [DOI] [PubMed] [Google Scholar]
- 12.Dauch WA (1986) Infection of the intervertebral space following conventional and microsurgical operation. Acta-Neurochir (Wien) 82:43–49 [DOI] [PubMed]
- 13.Dietze DB, Fessler RG, Jacon RP. Primary reconstruction for spinal infection. J Neurosurg. 1997;86:981–9. doi: 10.3171/jns.1997.86.6.0981. [DOI] [PubMed] [Google Scholar]
- 14.Manuel E, et al. Postoperative spondylodiskitis: etiology, clinical findings, prognosis, and comparison with nonoperative pyogenic spondylodiskitis. Clinical Infectious Disease. 1999;29:339–45. doi: 10.1086/520212. [DOI] [PubMed] [Google Scholar]
- 15.Sapico FL, Montgomerie JZ (1979) Pyogenic vertebral osteomyelitis, report of nine cases and review of the literature. Rev Infect Dis 1:754–756 [DOI] [PubMed]
- 16.Bircher MD, Tasker T, Crawshaw C, Mulholland RC. Discitis following lumbar surgery. Spine. 1988;13:98–102. doi: 10.1097/00007632-198801000-00023. [DOI] [PubMed] [Google Scholar]
- 17.Rawling CE, Wilkins RH, Gallis HA, Goldner JR, Francis R. (1983). Postoperative intervertebral disc space infection. Neurosurgery 13:371–375 [DOI] [PubMed]
- 18.Rebecca W, Ramzi R, Robert F, Robert M. Preventing and treating discitis: Cephazolin penetration in ovine lumbar intervertebral disc. Eur Spine J. 2006;15(9):1397–1403. doi: 10.1007/s00586-006-0144-6. [DOI] [PMC free article] [PubMed] [Google Scholar]