Abstract
Purpose
We analysed delay in diagnosis (DID) and disease severity in patients with vertebral tuberculosis (TB) in India.
Methods
We interviewed 228 patients with vertebral TB and reviewed their diagnostic magnetic resonance images (MRIs). We examined patient characteristics at the time of presentation and associations between socioeconomic background, access to care, DID and radiographic disease severity at the time of diagnosis.
Results
The most common presenting symptom was localised back pain (84%), followed by fever (40%) and pain elsewhere (28%). The median DID was five months [interquartile range (IQR) 3–9]. In multivariate logistic regression, Muslim and older patients had a higher risk of extreme (more than ten months) DID [adjusted odds ratio (aOR) 2.91; 95% confidence interval (CI) 1.20–7.08 and 2.33; 95% CI 1.23–4.94, respectively]. One hundred and two patients (64%) had vertebral abscesses. Median local kyphotic deformity was 11.7° (IQR 0–18.5°). Fifty-four (34%) patients had radiologically severe disease at the time of diagnosis. Older patients and those with higher education were less likely to have severe disease at the time of diagnosis (aOR 0.32; 95% CI 0.13–0.76 and 0.20 95% CI 0.06–0.62, respectively). Patients who experienced extreme DID were more likely to have severe disease (aOR 2.67; 95% CI 1.05–6.99).
Conclusions
Most patients in this cohort experienced long delays in diagnosis, and such delay was significantly associated with the presence of severe disease. Clinicians in TB-endemic areas must consider vertebral TB early and obtain imaging in patients who complain of persistent back pain. Improved diagnostic criteria are needed to identify patients at higher risk of disease.
Introduction
Despite the availability of effective and inexpensive treatment, tuberculosis (TB) remains a major cause of morbidity and mortality worldwide. In 2009, there were an estimated 9.4 million cases of TB globally, resulting in 1.7 million deaths [1]. Skeletal TB accounts for approximately 2% of all TB cases, and vertebral TB (Pott’s disease) is the most common form, comprising over 50% of skeletal TB cases [2, 3]. Vertebral TB is an indolent disease, and, because confirmation of diagnosis requires biopsy or fine-needle aspiration, it is often diagnosed clinicoradiologically in endemic regions [4]. In the absence of spinal instability or spinal cord compression, medical therapy generally results in an excellent outcome [5], but complications can include severe kyphosis and neurological compromise. In such cases, invasive reconstructive surgery may be required to halt further deformity [6–9]. Spinal deformity results in significant medical and psychosocial comorbidity, leading to decreased productivity and quality of life [10]. Kyphosis affects the biomechanics of the spine and body, causing respiratory deficiencies, severe degenerative spinal stenosis and, occasionally, late-onset paraplegia [11]. Delay in diagnosis (DID) of vertebral TB is associated with an increased frequency of these complications [12] and a higher need for surgery [13]. DID is common because presenting symptoms are nonspecific, and plain X-rays are often normal early in the disease. Furthermore, public health infrastructure targets pulmonary TB, often ignoring extrapulmonary screening—especially in patients not infected with HIV [14]. Early diagnosis and medical management is the best way to avoid the additional morbidity, costs and risk of advanced vertebral TB [15].
Few studies have examined the causes of DID of vertebral TB [16, 17]. Given the progressive nature of the disease and the need for early diagnosis, identifying social or demographic factors that contribute to this delay could help TB programmes shorten the time to diagnosis. With this in mind, we conducted a cross-sectional study to characterise the demographic background, access to medical care and disease severity of patients presenting with vertebral TB. We hypothesised that patients who had a lower socioeconomic background and/or lower access to medical care would have both a longer DID and increased disease severity at the time of diagnosis.
Materials and methods
Objectives
The primary objective of this study was to identify risk factors for DID of vertebral TB. Secondary objectives included characterising the clinical presentation of the disease, describing patients’ socioeconomic characteristics and access to care and identifying clinical and demographic factors associated with severe disease at time of presentation.
Study population
Study participants were enrolled between January and June 2011 from two tertiary-care hospitals (one private – VIMHANS hospital, one public – Guru Teg Bahadur) in New Delhi, India. Patients who had been diagnosed with vertebral TB either clinicoradiologically, or pathologically via polymerase chain reaction (PCR), culture or histology, and who could provide their diagnostic imaging were invited to participate. Patients were recruited from inpatient wards, outpatient clinics, and TB clinics. All had either initiated or were planning to initiate antituberculosis therapy during the study period. The institutional review boards of the University College of Medical Sciences, Public Health Foundation of India, and Emory University approved the study. All participants provided written informed consent.
Definitions
DID was defined as the time between the initial onset of symptoms to the time of diagnosis. Extreme delay was defined as a delay of ten or more months. Based on modified criteria by Park et al. [15], severe disease was defined as involvement of more than three vertebral bodies, an angle of local kyphotic deformity 25° or more and/or an anterior height loss of 20% or more.
Data collection
All participants were given a standardised interview that included four sections. The first section recorded socioeconomic demographics of age, sex, city of permanent residence, religion, education level, total monthly household income and profession (see Appendix A for definitions). Section two detailed comorbidities, including tobacco and/or alcohol use. The third section detailed the vertebral TB diagnostic history. Patients were asked about their initial symptoms, type of health centre where they first presented, time from onset of their initial symptoms until their diagnosis of vertebral TB (and reason for delay, if any), time from diagnosis to initiation of treatment (and reason for delay, if any), duration of treatment and history of surgery for treatment of vertebral TB. The final section of the interview summarised each patient’s access to healthcare. Patients were asked about their general healthcare centre (public vs. private, distance, time and travel cost from their house) and whether or not they had health insurance.
Data analysis
All patients were included in the demographic DID analysis. Patients with an available magnetic resonance image (MRI) from the time of diagnosis were included in the analyses of disease severity; patients with X-rays or computed tomography (CT) scans only were excluded. Radiographs were analysed by the primary author (EK) who was blinded to participants’ survey results. Local kyphotic deformity angle (Cobb’s angle in the sagittal plane) and anterior percent height loss were measured using standardised definitions (see Appendix B for details) [18]. The affected vertebrae and the presence of an abscess (as determined by the official radiologist’s report) were recorded from the radiographs. Associations between outcomes (extreme DID and severe disease at presentation) and clinical and demographic variables were examined using logistic regression. For each outcome, all characteristics meeting the criterion of p < 0.2 on univariate analysis were included in a multivariate logistic regression model, which was run using a backwards stepwise technique. Finally, we examined the relationship between DID and presence of severe disease at presentation. Data were analysed using R (R 2008. R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient characteristics
We enrolled 228 patients with vertebral TB from the two study sites. Participant characteristics are listed in Table 1. There were 65 (29%) and 163 (71%) patients from Guru Teg Bahadur Hospital and VIMHANS Hospital, respectively. Many patients had high socioeconomic backgrounds, with 45% having either a graduate or postgraduate degree and 34% having a monthly household income of at least 30,000 Indian Rupees (approximately US $590). Patients reported relatively high access to care, with a median travel time of ten minutes to arrive at their primary health centre.
Table 1.
Patient demographics (n = 228)
| Number (%) | |
|---|---|
| Site | |
| Guru Teg Bahadur hospital | 65 (29) |
| VIMHANS hospital | 163 (71) |
| State of residence | |
| Delhi | 121 (53) |
| Haryana | 15 (7) |
| Uttar Pradesh | 70 (30) |
| Other | 22 (10) |
| Female | 120 (53) |
| Age, median years (IQR) | 34 (23–50) |
| <20 | 46 (20) |
| 20–29 | 59 (26) |
| 30–39 | 41 (18) |
| 40–49 | 29 (13) |
| >50 | 53 (23) |
| Median number of people in household (IQR) | 5 (4–6) |
| Religion | |
| Hindu | 179 (79) |
| Muslim | 29 (13) |
| Other | 20 (9) |
| Educational status | |
| Low | 53 (23) |
| Medium | 73 (32) |
| High | 102 (45) |
| Median monthly household income, rupees (IQR) | 15000 (7500–55000) |
| Low | 88 (39) |
| Medium | 48 (21) |
| High | 79 (35) |
| Unknown | 13 (6) |
| Current profession | |
| Unemployed | 31 (14) |
| Normal unemployed | 75 (33) |
| Student | 43 (19) |
| Semi skilled worker | 31 (14) |
| Professional | 48 (21) |
| Current or past tobacco use | 37 (16) |
| Current or past alcohol use | 37 (16) |
| History of pulmonary TB | 16 (7) |
| Health insurance | 57 (25) |
| General healthcare centre | |
| Government | 70 (31) |
| Private | 140 (62) |
| Other | 17 (7) |
| Median cost per visit of general healthcare, rupees (IQR) | 100 (50–300) |
| Median delay in diagnosis, months (IQR) | 5 (3–9) |
| Extreme delay (≥10 months) | 56 (25) |
| First health centre | |
| Government | 91 (40) |
| Private | 122 (54) |
| Other | 15 (7) |
| Median travel time to general healthcare centre, minutes (IQR) | 10 (5–16) |
| Median distance to general healthcare centre, km (IQR) | 1 (1–3) |
| Chief complaint | |
| Back pain | 192 (84) |
| Fever | 92 (40) |
| Pain elsewhere | 63 (28) |
| Sensory loss (numbness) | 19 (8) |
| Palpable mass | 17 (7) |
| Leg paraplegia (unable to walk but able to move the leg voluntarily) | 17 (7) |
| Leg paraplegia (flaccid paraplegia; no voluntary movements) | 8 (4) |
| Full body weakness | 6 (3) |
| Leg paraplegia (able to walk but spastic movements) | 6 (3) |
| Persistent cough | 6 (3) |
| Loss of appetite | 4 (2) |
| Unable to pass urine | 4 (2) |
| Difficulty swallowing | 3 (1) |
| Vomiting | 2 (<1) |
| Breathlessness | 1 (<1) |
| Dizziness | 1 (<1) |
| Excess sweating | 1 (<1) |
| Nausea | 1 (<1) |
| No mobility in arms | 1 (<1) |
| Seizure | 1 (<1) |
| Shivering | 1 (<1) |
| Slouch | 1 (<1) |
| Vertigo | 1 (<1) |
| Weight loss | 1 (<1) |
IQR interquartile range, TB tuberculosis
Patient presentation
The most common presenting symptom was localised back pain (192; 84%), followed by fever (92; 40%) and pain elsewhere (63; 28%) (Table 1). Most patients initially presented to a private (122; 54%) or government (91; 40%) clinic; the remainder (15; 7%) presented to a nonallopathic centre, including pharmacies and traditional healers. Thirty-one patients (14%) were diagnosed within one month of onset of the initial symptom, whereas 123 (54%) said they had been incorrectly diagnosed; 70 patients (31%) had not followed up with their physician after presentation, and four (2%) attributed the delay to the cost or distance of the health centre. At the time of interview, patients had been on antituberculosis therapy for a median duration of 8.5 months, and 41 (18%) had undergone surgery for vertebral TB.
Delay in diagnosis
Median DID for all patients was five months (IQR 3–9). Once a diagnosis was made, however, almost all patients (96%) began treatment immediately. Fifty-six (25%) experienced an extreme DID (ten months or more) (Table 2). In univariate analyses, most patient demographics were not associated with extreme DID, although Muslim patients were significantly more likely to experience extreme DID compared with patients of other religions (OR 2.45; 95% CI 1.06–5.53). Low-income patients had a higher frequency of extreme DID compared with high-income patients (30% vs. 18%), but this did not reach statistical significance. Access to care, including distance to health centre and cost of attending visits, was not associated with extreme DID in univariate analysis. On multivariate analysis, Muslim religion and age over 30 years were independently associated with extreme DID, even after adjusting for potential confounders, including state of residence, income, alcohol use, cost of healthcare, first health centre and travel time [Muslim religion, adjusted odds ratio (aOR) 2.91; 95% confidence interval (CI) 1.20–7.08; age aOR 2.33; 95% CI 1.23–4.94] (Table 2).
Table 2.
Risk factors for extreme delay in diagnosis (n = 228)
| Nine months or less (%) | Ten months or more (%) | Crude OR (95% CI) | Adjusted OR (95% CI) | |
|---|---|---|---|---|
| Overall | 172 (75) | 56 (25) | – | – |
| Site | ||||
| Guru Teg Bahadur Hospital | 47 (72) | 18 (28) | 1.00 (ref) | – |
| VIMHANS Hospital | 125 (77) | 38 (23) | 0.79 (0.42–1.55) | – |
| State of residence | ||||
| Delhia | 98 (81) | 23 (19) | 1.00 (ref) | – |
| Haryanaa | 10 (67) | 5 (33) | 2.13 (0.62–6.63) | – |
| Uttar Pradesha | 49 (70) | 21 (30) | 1.83 (0.92–3.63) * | – |
| Othera | 15 (68) | 7 (32) | 1.99 (0.69–5.31) * | – |
| Sex | ||||
| Female | 94 (78) | 26 (22) | 1.00 (ref) | – |
| Male | 78 (72) | 30 (28) | 1.39 (0.76–2.56) | – |
| Age | ||||
| ≤30 | 84 (80) | 21 (20) | 1.00 (ref) | 1.00 (ref) |
| ≥ 31 | 88 (72) | 35 (28) | 1.59 (0.86–2.99) * | 2.33 (1.23–4.94) ** |
| Religion | ||||
| Hindu | 139 (78) | 40 (22) | 1.00 (ref) | 1.00 (ref) |
| Muslim | 17 (59) | 12 (41) | 2.45 (1.06–5.54) ** | 2.91 (1.20–7.08) ** |
| Other | 16 (80) | 4 (20) | 0.87 (0.24–2.53) | 0.71 (0.19–2.14) |
| Education statusb | ||||
| Low | 38 (72) | 15 (28) | 1.00 (ref) | – |
| Medium | 59 (81) | 14 (19) | 0.60 (0.26–1.39) | – |
| High | 75 (74) | 27 (26) | 0.91 (0.44–1.95) | – |
| Monthly incomeb | ||||
| Lowa | 62 (70) | 26 (30) | 1.00 (ref) | – |
| Mediuma | 35 (73) | 13 (27) | 0.89 (0.40–1.92) | – |
| Higha | 65 (82) | 14 (18) | 0.51 (0.24–1.06) * | – |
| Unknowna | 10 (77) | 3 (23) | 0.72 (0.15–2.56) | – |
| Current professionb | ||||
| Unemployed | 24 (77) | 7 (23) | 1.00 (ref) | – |
| Normal unemployed | 50 (67) | 25 (33) | 1.71 (0.67–4.79) | – |
| Student | 37 (86) | 6 (14) | 0.56 (0.16–1.87) | – |
| Semi-skilled | 22 (71) | 9 (29) | 1.40 (0.45–4.54) | – |
| Professional | 39 (81) | 9 (19) | 0.79 (0.26–2.48) | – |
| Tobacco use | ||||
| Current or past | 28 (76) | 9 (24) | 1.00 (ref) | – |
| Never | 144 (75) | 47 (25) | 1.02 (0.46–2.42) | – |
| Alcohol use | ||||
| Current or past | 31 (84) | 6 (16) | 1.00 (ref) | 1.00 (ref) |
| Never | 141 (74) | 50 (26) | 1.83 (0.77–5.10) * | 2.33 (0.91–6.80) |
| Health insurance | ||||
| No | 128 (75) | 43 (25) | 1.00 (ref) | – |
| Yes | 44 (77) | 13 (23) | 0.88 (0.42–1.75) | – |
| General healthcare centre | ||||
| Governmenta | 56 (80) | 14 (20) | 1.00 (ref) | – |
| Privatea | 105 (75) | 35 (25) | 1.33 (0.67–2.75) | – |
| Othera | 11 (61) | 7 (39) | 2.54 (0.81–7.74) * | – |
| Cost for general healthcare visit (rupees) | ||||
| <50a | 54 (74) | 19 (26) | 1.00 (ref) | – |
| <50–100a | 27 (63) | 16 (37) | 1.68 (0.75–3.80) | – |
| <200a | 27 (79) | 7 (21) | 0.74 (0.26–1.91) | – |
| <300a | 20 (74) | 7 (26) | 0.99 (0.35–2.65) * | – |
| >300a | 44 (86) | 7 (14) | 0.45 (0.16–1.13) | – |
| Time to general healthcare centre | ||||
| <30 mina | 163 (76) | 52 (24) | 1.00 (ref) | – |
| >30 mina | 9 (69) | 4 (31) | 1.39 (0.37–4.47) | – |
| Distance to general healthcare centre | ||||
| 1 km | 91 (75) | 31 (25) | 1.00 (ref) | – |
| 2–3 km | 43 (75) | 14 (25) | 0.96 (0.45–1.95) | – |
| >4 km | 38 (78) | 11 (22) | 0.85 (0.37–1.82) | – |
| First healthcare centre | ||||
| Government | 66 (73) | 25 (27) | 1.00 (ref) | – |
| Private | 97 (80) | 25 (20) | 0.68 (0.36–1.29) | – |
| Other | 9 (60) | 6 (40) | 1.76 (0.54–5.40) | – |
aVariable included in the backwards stepwise multivariate logistic regression model but eliminated
bSee Appendix B for definitions of education, income and profession
* p < 0.20
** p < 0.05
*** p < 0.01
Radiographic findings and disease severity
Of the 228 participants, 161 (71%) had a diagnostic MRI available and were included in the radiographic analysis. Radiographic findings at the time of diagnosis are shown in Table 3. Fifty-four (34%) patients had radiologically severe disease at the time of diagnosis. In univariate analysis, patients with educational status, high monthly income, or medium monthly income were less likely to have severe disease at the time of diagnosis (Table 4). After adjustment for other covariates, older patients and those with higher education had a lower odds of having severe disease at the time of diagnosis (age aOR 0.32; 95% CI 0.13–0.65; high education aOR 0.20; 95% CI 0.06–0.62) (Table 4). Patients who experienced an extreme DID had a higher risk of having severe disease (aOR 2.67; 95% CI 1.05–6.99).
Table 3.
Radiographic characteristics (n = 161)
| Number (%) | |
|---|---|
| Number of vertebrae affected, median (IQR) | 2 (2–3) |
| Median local kyphotic deformity, degrees (IQR) | 11.7 (0–18.5) |
| Median anterior percent height loss (IQR) | 9.6 (3.9–22.8) |
| Abscess | 102 (64) |
| Location of infection | |
| Cervical | 19 (12) |
| Cervicothoracic | 5 (3) |
| Thoracic | 65 (40) |
| Thoracolumbar | 11 (7) |
| Lumbar | 47 (29) |
| Lumbosacral | 5 (3) |
| Sacrum | 7 (4) |
| Cervical, lumbar and sacrum | 1 (<1) |
| Thoracic and sacrum | 1 (<1) |
IQR interquartile range
Table 4.
Risk factors for severe disease at time of diagnosis (n = 161)
| Nonsevere (%) | Severe (%) | Crude OR (95% CI) | Adjusted OR (95% CI) | |
|---|---|---|---|---|
| Overall | 107 (66) | 54 (34) | – | – |
| Site | ||||
| Guru Teg Bahadur hospitala | 24 (48) | 26 (52) | 1.00 (ref) | – |
| VIMHANS hospitala | 83 (75) | 28 (25) | 0.31 (0.15–0.62) *** | – |
| State of residence | ||||
| Delhi | 56 (64) | 32 (36) | 1.00 (ref) | 1.00 (ref) |
| Haryana | 8 (89) | 1 (11) | 0.22 (0.01–1.27) * | 0.20 (0.01–1.53) |
| Uttar Pradesh | 35 (71) | 14 (29) | 0.70 (0.32–1.47) | 1.02 (0.37–2.77) |
| Other | 8 (53) | 7 (47) | 1.53 (0.49–4.66) | 4.33 (1.06–18.93) ** |
| Sex | ||||
| Female | 54 (68) | 26 (33) | 1.00 (ref) | – |
| Male | 53 (65) | 28 (35) | 1.10 (0.57–2.12) | – |
| Age | ||||
| ≤ 30 | 42 (55) | 34 (45) | 1.00 (ref) | 1.00 (ref) |
| ≥ 31 | 65 (76) | 20 (24) | 0.38 (0.20–0.74) *** | 0.32 (0.13–0.76) *** |
| Religion | ||||
| Hindu | 83 (65) | 44 (35) | 1.00 (ref) | 1.00 (ref) |
| Muslim | 9 (50) | 9 (50) | 1.89 (0.69–5.17) | 0.71 (0.20–2.43) |
| Other | 15 (94) | 1 (6) | 0.13 (0.01–0.65) ** | 0.01 (0.01–0.65) ** |
| Educational statusb | ||||
| Low | 19 (48) | 21 (53) | 1.00 (ref) | 1.00 (ref) |
| Medium | 32 (64) | 18 (36) | 0.51 (0.22–1.18) * | 0.41 (0.14–1.14) |
| High | 56 (79) | 15 (21) | 0.24 (0.10–0.56) *** | 0.20 (0.06–0.62) *** |
| Monthly incomeb | ||||
| Lowa | 31 (50) | 31 (50) | 1.00 (ref) | – |
| Mediuma | 26 (72) | 10 (28) | 0.38 (0.15–0.91) ** | – |
| Higha | 47 (82) | 10 (18) | 0.21 (0.09–0.48) *** | – |
| Unknowna | 3 (50) | 3 (50) | 1.00 (0.17–5.77) | – |
| Current professionb | ||||
| Unemployed | 13 (59) | 9 (41) | 1.00 (ref) | – |
| Normal unemployed | 37 (70) | 16 (30) | 0.62 (0.22–1.78) | – |
| Student | 16 (57) | 12 (43) | 1.08 (0.35–3.41) | – |
| Semi skilled | 17 (68) | 8 (32) | 0.68 (0.20–2.25) | – |
| Professional | 24 (73) | 9 (27) | 0.54 (0.17–1.70) | – |
| Tobacco use | ||||
| Current or past | 20 (71) | 8 (29) | 1.00 (ref) | – |
| Never | 87 (65) | 46 (35) | 1.32 (0.56–3.40) | – |
| Alcohol use | ||||
| Current or pasta | 22 (79) | 6 (21) | 1.00 (ref) | – |
| Nevera | 85 (64) | 48 (36) | 2.07 (0.83–5.94) * | – |
| Health insurance | ||||
| No | 78 (64) | 44 (36) | 1.00 (ref) | – |
| Yes | 29 (74) | 10 (26) | 0.61 (0.26–1.34) | – |
| General healthcare center | ||||
| Government | 24 (57) | 18 (43) | 1.00 (ref) | 1.00 (ref) |
| Private | 73 (69) | 33 (31) | 0.60 (0.29–1.27) * | 0.46 (0.15–1.30) |
| Other | 10 (77) | 3 (23) | 0.40 (0.08–1.53) * | 0.17 (0.03–0.87) ** |
| Cost for general healthcare visit (rupees) | ||||
| <50a | 36 (71) | 15 (29) | 1.00 (ref) | – |
| <50–100a | 18 (56) | 14 (44) | 1.87 (0.74–4.74) * | – |
| <200a | 16 (67) | 8 (33) | 1.20 (0.41–3.37) | – |
| <300a | 16 (67) | 8 (33) | 1.20 (0.41–3.37) | – |
| >300a | 21 (70) | 9 (30) | 1.03 (0.37–2.73) | – |
| Delay in diagnosis | ||||
| <9 months | 85 (70) | 36 (30) | 1.00 (ref) | 1.00 (ref) |
| >10 months | 22 (55) | 18 (45) | 1.93 (0.92–4.03) * | 2.67 (1.05–6.99) ** |
| First health center | ||||
| Government | 37 (65) | 20 (35) | 1.00 (ref) | – |
| Private | 57 (63) | 34 (37) | 1.10 (0.56–2.22) | – |
| Other | 13 (100) | 0 (0) | 0.00 | – |
| Time to general healthcare center | ||||
| <30 mina | 104 (68) | 48 (32) | 1.00 (ref) | – |
| >30 mina | 3 (33) | 6 (67) | 4.33 (1.10–21.22) ** | – |
| Distance to general healthcare center | ||||
| 1 km | 55 (64) | 31 (36) | 1.00 (ref) | 1.00 (ref) |
| 2–3 km | 38 (85) | 7 (16) | 0.33 (0.12–0.78) ** | 0.48 (0.16–1.36) |
| >4 km | 14 (47) | 16 (53) | 2.03 (0.88–4.76) * | 2.44 (0.79–7.80) |
a Variable was included in the backwards stepwise multivariate logistic regression model but eliminated
b See appendix B for definitions of education, income, and profession
* p < 0.20
** p < 0.05
*** p < 0.01
Discussion
We found an increased risk of severe vertebral TB disease in patients who experience a long DID. To our knowledge, this is the first study examining this association in vertebral TB, but our findings are consistent with earlier reports of pulmonary TB that show worse outcomes associated with delay [19] and emphasise the importance of making an early diagnosis. In vertebral TB, late initiation of chemotherapy provides the infection more time to weaken the vertebral column, leading to kyphotic deformities. If diagnoses are made earlier, potential complications, such as paralysis, may be prevented [12], and the extra costs and morbidity associated with surgical treatment may be avoided. However, a majority of patients with vertebral TB in New Delhi experience unacceptably long delays in diagnosis compared with patients in other TB-endemic countries. The average DID in our cohort was approximately twice the delay reported in two earlier studies [13, 20]. Practitioners in endemic areas such as India need to maintain a higher suspicion of this disease and provide greater scrutiny of patients who present repeatedly for back pain.
Among the variables tested, we found few risk factors associated with increased DID. This is contrary to prior studies of pulmonary TB from India, which found longer DID in patients that had to travel further for healthcare, alcoholic patients and patients who received care from government institutions [21]. We did find an association between travel time and DID on univariate analysis, but this was not significant on multivariate analysis. In our cohort, Muslim patients experienced longer delays in diagnosis, even when adjusted for income, education and state of origin [22]. Healthcare disparities affecting minorities are often multifactorial, involving the healthcare system, the provider, and the patients themselves [23]. Further research is required to clarify the mechanisms driving the association between religion and diagnostic delay.
Many previous reports found high rates of TB in patients with lower education and income [24], but our study demonstrates that vertebral TB is still prevalent in the upper strata of society. These patients, particularly those with higher educational backgrounds, had less severe disease at the time of diagnosis. Although they experienced no difference in DID, patients with higher education may have presented to their healthcare centre sooner. This would have shortened the time between symptom onset and eventual diagnosis and may have contributed to the lower disease severity. Our study did not measure such patient-driven delay, as we felt this would introduce an unacceptably high level of recall bias.
We found that older age was associated with a longer DID. Because back pain is such a frequent complaint of older patients in the primary care setting, physicians may be less likely to pursue additional testing in this population, thus delaying the diagnosis for those with vertebral TB. Interestingly, however, despite the differences in DID, younger age was associated with an increased disease severity at the time of diagnosis. Prior reports show that kyphotic deformities continue to progress in younger patients [6], and this may explain why, in our study population, age was more important than DID in driving disease severity. The strong association between age and disease severity has been attributed to the large cartilaginous proportion of the vertebral body in children compared with adults and the alteration of the anterior growth plate of the vertebrae affecting the normal growth spurt [11]. In a separate analysis, we stratified patients by ages younger than ten, 11–60, and older than 61; results confirmed that children (age under ten) had more severe disease at the time of diagnosis and the elderly (age over 60) had less severe disease (data not shown).
A major difficulty in making a timely diagnosis is the lack of specific clinical diagnostic criteria for vertebral TB. Eighty-four percent of patients in our study noted back pain as their initial symptom, but there were few other complaints that were specific for this disease. Development of diagnostic criteria for early suspicion of the disease is an important area for future research. It has been suggested that the diagnosis of vertebral TB could be improved if patients with persistent lower back pain were kept under observation with sequential X-rays every six weeks to monitor for any demineralisation or subtle reduction of disc space [11]. This strategy, however, has not yet been tested prospectively.
Our study has many strengths, including the large and ethnically diverse cohort, as well as participation of both public and private hospitals. Because of the cross-sectional design, however, our data is subject to recall bias. Patient-reported “misdiagnosis” could not be confirmed as patients’ prior medical records were not available. For radiological analysis, our measure for local kyphotic deformity did not account for the natural lordosis in the cervical and lumbar spine. In addition, we did not have have radiographic information for 24% of our participants, which could have introduced selection bias. We found no statistically significant differences, however, between demographics of patients who did and did not have an MRI (data not shown). Because we interviewed primarily patients attending follow-up visits in the clinic, we may have selected a more adherent population, inadvertently excluding those who defaulted on treatment. Lastly, patients were not followed prospectively, so we are unable to examine the effects of socioeconomic factors and diagnostic delay on final treatment outcomes.
In conclusion, vertebral TB is an indolent disease that requires significant time to develop clinical and radiological signs and often months to diagnose. Special attention needs to be directed to patients in TB-endemic areas who present with repeated complaints of back pain. Given our findings that delay is associated with more severe disease at the time of diagnosis, better diagnostic criteria are needed to guide cost- and time-effective screening for patients in whom there is a suspicion of vertebral TB. Early diagnosis and treatment of vertebral TB may prevent the development of severe disease and associated complications, thus reducing medical costs and the possibility of future disability.
Acknowledgements
The authors thank Drs. S.M. Tuli, Oheneba Boachie, Rick Hodes and Ellie Schoenbaum for their guidance in the development of this project, Drs. D. Prabhakaran, N. Tandon, V. Narayan and M. Ali for their support throughout the project, the Center for Chronic Disease Control in New Delhi, and Meredith Blevins for her biostatics consultations. JCMB is supported by the National Institutes of Health (K23 AI083088). This work was supported by the National Institutes of Health Office of the Director, Fogarty International Center, Office of AIDS Research, National Cancer Center, National Eye Institute, National Heart, Blood, and Lung Institute, National Institute of Dental and Craniofacial Research, National Institute On Drug Abuse, National Institute of Mental Health, National Institute of Allergy and Infectious Diseases, and National Institutes of Health Office of Women’s Health and Research through the Fogarty International Clinical Research Scholars and Fellows Program at Vanderbilt University (R24 TW007988) and the American Relief and Recovery Act.
Conflict of interest The authors have no financial disclosures or other conflicts of interest.
Appendix A
| Variable | Options | Level |
|---|---|---|
| Educational status | Illiterate, literate with no formal education | Low |
| Up to primary school (up to class IV), secondary school (ITI course, class XII/X or intermediate) | Medium | |
| Graduate (B.A/B.Sc/B.Com/Diploma etc.), professional degree/post graduate | High | |
| Monthly income | Refuse/Don't know | Unknown |
| <10,000 | Low | |
| 10,001–30,000 | Medium | |
| >30,001 | High | |
| Current profession | Unemployed | Unemployed |
| Housewife/retired | Normal unemployed | |
| Student | Student | |
| Unskilled manual labourer, landless labourer | Low | |
| Skilled manual labourer, small business owner, small farmer, marginal landowner, rickshaw driver, army jawan, carpenter, fitter | Medium | |
| Professional, big business, landlord, university teacher, class IAS/services officer, lawyer, trained, clerical, medium business owner, middle level farmer, teacher, maintenance (in charge), personnel manager | High |
Appendix B
Anterior vertebral body percent loss [25]
- For a lesion affecting a single vertebra:
- The heights of the normal vertebra above [A] and below [B] the lesion are measured
- The average of these values is taken; this value is the presumed normal height of the diseased vertebra in the lesion C = [(A + B)/2]
- The actual height of the diseased vertebra is measured on the x-ray [D]
- Anterior vertebral body percent loss is calculated by taking the difference of the measured height [D] from the presumed normal height [C] and dividing this difference by [C]
- For a lesion affecting multiple vertebrae:
- The heights of the normal vertebra above and below the lesion are measured and the average is taken (as in the single lesion case)
- This number is multiplied by the number of vertebrae affected; this value is the presumed normal height of the diseased vertebrae in the lesion
- The actual height of the diseased segment is measured
- Anterior vertebral body percent loss is then calculated as in a single lesion
Local kyphotic deformity (Konstam’s angle, or cobb’s angle in the sagital plane) [18, 26][27]
A straight line is drawn through the superior surface of the first normal vertebra cranially from the lesion
A second line is drawn through the inferior surface of the first normal vertebra caudally from the lesion
These two lines cross and form an acute angle which is the local kyphotic deformity
In mild angles, perpendiculars can be drawn, with angle a measured at their intersection (angle A in diagrams below)
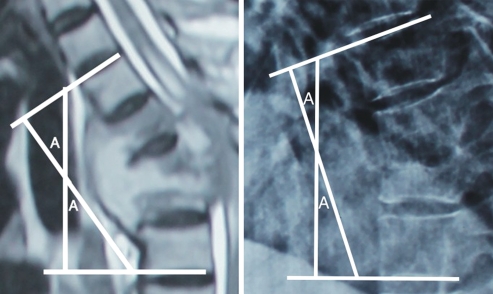
If multiple levels are affected non-consecutively, there will be an equal number of Konstam’s angles
If multiple levels are affected consecutively, there will be a single Konstam’s angle measured from the first normal vertebrae above and below the lesion.
Contributor Information
Eli Kamara, Phone: +1-516-5267840, Email: eli.kamara@gmail.com.
Anil K. Jain, Phone: +91-11-22586262, Email: dranilkjain@gmail.com
References
- 1.Global Tuberculosis Control: WHO report 2010 (2010). World Health Organization, Geneva
- 2.Sharma SK, Mohan A. Extrapulmonary tuberculosis. Indian J Med Res. 2004;120(4):316–353. [PubMed] [Google Scholar]
- 3.Sandher DS, Al-Jibury M, Paton RW, Ormerod LP. Bone and joint tuberculosis: cases in Blackburn between 1988 and 2005. J Bone Joint Surg Br. 2007;89(10):1379–1381. doi: 10.1302/0301-620X.89B10.18943. [DOI] [PubMed] [Google Scholar]
- 4.Rasit A. The Role of Polymerase Chain Reaction (PCR) in Diagnosis of Spine Tuberculosis after Pre-operative Anti-tuberculosis Treatment. Malaysian Orthopaedic Journal. 2011;5(1):8–12. doi: 10.5704/MOJ.1103.002. [DOI] [Google Scholar]
- 5.Nene A, Bhojraj S. Results of nonsurgical treatment of thoracic spinal tuberculosis in adults. Spine J. 2005;5(1):79–84. doi: 10.1016/j.spinee.2004.05.255. [DOI] [PubMed] [Google Scholar]
- 6.Rajasekaran S. The problem of deformity in spinal tuberculosis. Clin Orthop Relat Res. 2002;398:85–92. doi: 10.1097/00003086-200205000-00012. [DOI] [PubMed] [Google Scholar]
- 7.Jain AK. Tuberculosis of the spine: a fresh look at an old disease. J Bone Joint Surg Br. 2010;92(7):905–913. doi: 10.1302/0301-620X.92B7.24668. [DOI] [PubMed] [Google Scholar]
- 8.Boachie-Adjei O, Squillante RG. Tuberculosis of the spine. Orthop Clin North Am. 1996;27(1):95–103. [PubMed] [Google Scholar]
- 9.Turgut M. Spinal tuberculosis (Pott's disease): its clinical presentation, surgical management, and outcome. A survey study on 694 patients. Neurosurg Rev. 2001;24(1):8–13. doi: 10.1007/PL00011973. [DOI] [PubMed] [Google Scholar]
- 10.Ettinger B, Block JE, Smith R, Cummings SR, Harris ST, Genant HK. An examination of the association between vertebral deformities, physical disabilities and psychosocial problems. Maturitas. 1988;10(4):283–296. doi: 10.1016/0378-5122(88)90064-3. [DOI] [PubMed] [Google Scholar]
- 11.Jain AK, Dhammi IK, Jain S, Mishra P. Kyphosis in spinal tuberculosis - Prevention and correction. Indian J Orthop. 2010;44(2):127–136. doi: 10.4103/0019-5413.61893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jain AK. Treatment of tuberculosis of the spine with neurologic complications. Clin Orthop Relat Res. 2002;398:75–84. doi: 10.1097/00003086-200205000-00011. [DOI] [PubMed] [Google Scholar]
- 13.Su SH, Tsai WC, Lin CY, Lin WR, Chen TC, Lu PL, Huang PM, Tsai JR, Wang YL, Feng MC, Wang TP, Chen YH. Clinical features and outcomes of spinal tuberculosis in southern Taiwan. J Microbiol Immunol Infect. 2010;43(4):291–300. doi: 10.1016/S1684-1182(10)60046-1. [DOI] [PubMed] [Google Scholar]
- 14.Pertuiset E, Beaudreuil J, Liote F, Horusitzky A, Kemiche F, Richette P, Clerc-Wyel D, Cerf-Payrastre I, Dorfmann H, Glowinski J, Crouzet J, Bardin T, Meyer O, Dryll A, Ziza JM, Kahn MF, Kuntz D. Spinal tuberculosis in adults. A study of 103 cases in a developed country, 1980–1994. Medicine (Baltimore) 1999;78(5):309–320. doi: 10.1097/00005792-199909000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Park DW, Sohn JW, Kim EH, Cho DI, Lee JH, Kim KT, Ha KY, Jeon CH, Shim DM, Lee JS, Lee JB, Chun BC, Kim MJ. Outcome and management of spinal tuberculosis according to the severity of disease: a retrospective study of 137 adult patients at Korean teaching hospitals. Spine (Phila Pa 1976) 2007;32(4):E130–135. doi: 10.1097/01.brs.0000255216.54085.21. [DOI] [PubMed] [Google Scholar]
- 16.Storla DG, Yimer S, Bjune GA. A systematic review of delay in the diagnosis and treatment of tuberculosis. BMC Public Health. 2008;8:15. doi: 10.1186/1471-2458-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cormican L, Hammal R, Messenger J, Milburn HJ. Current difficulties in the diagnosis and management of spinal tuberculosis. Postgrad Med J. 2006;82(963):46–51. doi: 10.1136/pgmj.2005.032862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keynan O, Fisher CG, Vaccaro A, Fehlings MG, Oner FC, Dietz J, Kwon B, Rampersaud R, Bono C, France J, Dvorak M. Radiographic measurement parameters in thoracolumbar fractures: a systematic review and consensus statement of the spine trauma study group. Spine (Phila Pa 1976) 2006;31(5):E156–165. doi: 10.1097/01.brs.0000201261.94907.0d. [DOI] [PubMed] [Google Scholar]
- 19.Lienhardt C, Rowley J, Manneh K, Lahai G, Needham D, Milligan P, McAdam KP. Factors affecting time delay to treatment in a tuberculosis control programme in a sub-Saharan African country: the experience of The Gambia. Int J Tuberc Lung Dis. 2001;5(3):233–239. [PubMed] [Google Scholar]
- 20.Weng CY, Chi CY, Shih PJ, Ho CM, Lin PC, Chou CH, Wang JH, Ho MW. Spinal tuberculosis in non-HIV-infected patients: 10 year experience of a medical center in central Taiwan. J Microbiol Immunol Infect. 2010;43(6):464–469. doi: 10.1016/S1684-1182(10)60072-2. [DOI] [PubMed] [Google Scholar]
- 21.Rajeswari R, Chandrasekaran V, Suhadev M, Sivasubramaniam S, Sudha G, Renu G. Factors associated with patient and health system delays in the diagnosis of tuberculosis in South India. Int J Tuberc Lung Dis. 2002;6(9):789–795. [PubMed] [Google Scholar]
- 22.Census of India (2001). Accessed 10/23/11
- 23.Cooper LA, Hill MN, Powe NR. Designing and evaluating interventions to eliminate racial and ethnic disparities in health care. J Gen Intern Med. 2002;17(6):477–486. doi: 10.1046/j.1525-1497.2002.10633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muniyandi M, Ramachandran R, Balasubramanian R, Narayanan PR. Socio-economic dimensions of tuberculosis control: review of studies over two decades from Tuberculosis Research Center. J Commun Dis. 2006;38(3):204–215. [PubMed] [Google Scholar]
- 25.Jain AK, Aggarwal PK, Arora A, Singh S. Behaviour of the kyphotic angle in spinal tuberculosis. Int Orthop. 2004;28(2):110–114. doi: 10.1007/s00264-003-0516-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Konstam PG, Blesovsky A. The ambulant treatment of spinal tuberculosis. Br J Surg. 1962;50:26–38. doi: 10.1002/bjs.18005021908. [DOI] [PubMed] [Google Scholar]
- 27.Jutte P, Wuite S, The B, Altena R, Veldhuizen A. Prediction of deformity in spinal tuberculosis. Clin Orthop Relat Res. 2007;455:196–201. doi: 10.1097/01.blo.0000246559.27596.33. [DOI] [PubMed] [Google Scholar]


