Abstract
Purpose
The purpose of this study was to present our experience in treating dorso-lumbar tuberculosis by one-stage posterior circumferential fusion and to compare this group with a historical group treated by anterior debridement followed by postero-lateral fusion and stabilization.
Methods
Between 2003 and 2008, 32 patients with active spinal tuberculosis were treated by one-stage posterior circumferential fusion and prospectively followed for a minimum of two years. Pain severity was measured using Visual Analogue Scale (VAS). Neurological assessment was done using the Frankel scale. The operative data, clinical, radiological, and functional outcomes were also compared to a similar group of 25 patients treated with anterior debridement and fusion, followed 10–14 days later by posterior stabilization and postero-lateral fusion.
Results
The mean operative time and duration of hospital stay were significantly longer in the two-stage group. The mean estimated blood loss was also larger, though insignificantly, in the two-stage group. The incidence of complications was significantly lower in the one-stage group. At final follow-up, all 34 patients with pre-operative neurological deficits showed at least one Frankel grade of neurological improvement, all 57 patients showed significant improvement of their VAS back pain score, the mean kyphotic angle has significantly improved, all patients achieved solid fusion and 43 (75.4%) patients returned to their pre-disease activity level or work.
Conclusion
Instrumented circumferential fusion, whether in one or two stages, is an effective treatment for dorso-lumbar tuberculosis. One-stage surgery, however, is advantageous because it has lower complication rate, shorter hospital stay, less operative time and blood loss.
Introduction
Tuberculosis of the spine is a very ancient disease. Several cases of Pott's disease of the spine were reported as early as the pre-dynastic time. The most authenticated case is that described in Nesparehan, a priest of Amun. It shows the typical collapse of a dorsal vertebra with angular kyphosis and a big abscess in the right iliac fossa [1].
Probably the first surgical attempts to treat Pott's disease of the spine were practiced late in the 19th century. Laminectomy to treat paraplegia and abscess drainage resulted in poor outcome probably because of absence of effective chemotherapeutics [2]. Hibbs and Albee introduced posterior spinal fusion to stabilize the spine and promote healing [3, 4].
After the introduction of effective chemotherapy against the Mycobacterium tuberculosis, more radical surgery was introduced. Ito et al. in1934 first described a radical operation for Pott’s disease, which involved costotransversectomy [5]. Capener in 1954 extended costotransversectomy by removal of the pedicle to allow decompression of the dural sac of any compressing bony spurs, granulation tissue, and bone or disc sequestra [6]. He called this operation lateral rhachotomy, which was later developed by Seddon and given the name antero-lateral rhachotomy [7].
Hodgson and his Hong Kong group are credited for development and popularization of anterior radical removal of the diseased area, decompression of the spinal cord and anterior spinal arthrodesis [8–10]. In recent years, many advocates of anterior surgery reported their experience in anterior radical debridement, strut bone graft with or without anterior instrumentation with good result as regards fusion rate and correction of acute local kyphosis [11–14]. However, long periods of immobilization, progressive kyphosis and graft failure are major drawbacks after anterior radical surgical treatment for tuberculosis of the spine. This persuaded some workers to do a second stage posterior fusion with instrumentation to avoid these complications [15]. This was further developed by doing anterior and posterior surgery in one operative session [16–22].
The senior author (GZS) began treating patients with Pott's disease both conservatively and surgically in 1965. Over the years, practically every described surgical method was used. The aim of this report is to present our experience in treating tuberculosis of the dorso-lumbar spine by circumferential fusion with instrumentation in a single stage through a posterior approach and to compare this group of patients with a historical group treated by staged anterior debridement and iliac bone grafting followed by postero-lateral fusion and pedicle screws stabilization.
Methods
This was a prospective study carried out between 2003 and 2008 on 32 patients suffering from active spinal tuberculosis. They were operated upon by one-stage surgery through a posterior midline incision. Surgery included excision of the diseased tissues, stabilization by pedicle screws, interbody fusion using iliac crest autograft with or without cage, and postero-lateral fusion. These patients were followed-up for a minimum of two years. The patients comprised 17 men and 15 women aged 19–70 years (mean, 45.7 years). The study protocol was approved by our institution ethics committee and all patients signed an informed consent.
There were 69 involved vertebral bodies (Table 1). Typically, the disease involved two adjacent vertebral bodies and the intervening disc (28 patients). In three patients, there was involvement of three adjacent vertebral bodies and two discs in between, while in one patient, there was involvement of four adjacent vertebral bodies and the discs in between. Ten patients had dorsal, 14 patients had dorsolumbar, and eight patients had lumbar involvement. Pain severity was measured using the Visual Analogue Scale (VAS). Neurological assessment was done using the Frankel scale [21]. Eighteen patients (56%) were neurologically free (Frankel E). Of the 14 patients with neurological deficits, one (3%) was Frankel B, ten (31%) were Frankel C, and three (9%) were Frankel D. None of the patients included had complete neurological deficit (Frankel A).
Table 1.
Demographic data of the patients
| Parameters | One stage, n = 32 | Two stages, n = 25 | P value |
|---|---|---|---|
| Gender (M/F) | 17/15 | 14/11 | 0.829 |
| Mean age in years (range) | 48.7 ± 13.6 (19–70) | 47.6 ± 13.5 (9–67) | 0.765 |
| Type of work | |||
| Manual (heavy) work | 17 (53.1%) | 11 (44%) | 0.520 |
| Mild to moderate | 5 (15.6%) | 7 (28%) | |
| Housewife | 10 (31.3%) | 7 (28%) | |
| Number of affected vertebrae | |||
| Two vertebrae and one disc | 28 (87.5%) | 22 (88%) | 0.646 |
| Three vertebrae and two discs | 3 (9.4%) | 3 (12%) | |
| Four vertebrae and three discs | 1 (3.1%) | 0 | |
| Preop. Frankel grade | |||
| Grade A | 0 | 0 | 0.165 |
| Grade B | 1(3.1%) | 3 (12%) | |
| Grade C | 8 (25%) | 11 (44%) | |
| Grade D | 8 (25%) | 3 (12%) | |
| Grade E | 15 (46.9%) | 8 (32%) | |
| Preop. degree of pain (VAS) | 8.5 ± 1.3 | 8.5 ± 1.1 | 0.953 |
| Incidence of comorbidities | |||
| None | 17 (53.1%) | 11 (44%) | - |
| Diabetes | 10 (31.2%) | 10 (40%) | |
| Renal impairment | 5 (15.6%) | 4 (16%) | |
| Cardiac affection | 2 (6.3%) | 1 (4%) | |
| Liver affection | 1 (3.1%) | 0 | |
| Respiratory affection | 0 | 1 (4%) | |
| Preop. kyphosis angle | 23.7° ± 4.5° | 21.7° ± 5.2° | 0.075 |
All patients had pre-operative antero-posterior and lateral radiographs and MRI. Multislice CT was also done when needed. The local kyphotic angle was measured using Cobb's technique as the angle between the superior endplate of the uppermost affected vertebral body and the inferior endplate of the lowermost involved vertebral body. Vertebral collapse, destruction, paravertebral and/or psoas abscess, and spinal cord compression due to abscess or bony debris were seen on MRI.
Inclusion criteria
Our surgical indications were as follows: (1) progressing neurological deficit due to neural compression despite antituberculous treatment, (2) severe persistent local pain not adequately responding to conservative treatment, and (3) marked vertebral destruction and/or collapse, to minimize the risk of development of subsequent deformity.
Exclusion criteria
Cases proven to be non-tuberculous by histopathological and bacteriological examination of biopsies taken during surgery were excluded.
Operative technique
Surgery starts by identification and exposure of the affected level. An adequate number of pedicle screws are then inserted proximally and distally based on the bone quality and the number of affected vertebrae, trying to save valuable motion segments without jeopardizing fixation adequacy, and the screws position is checked radiologically. A temporary stabilizing rod is always fixed on one side. In one level affection, a laminotomy and facetectomy are performed. The affected disc is then exposed and curetted with various curettes and shavers until bleeding bony surfaces are reached. A paravertebral or a psoas abscess usually drains during this step. Then the cavity created is measured and packed with appropriately sized autogenous tricortical iliac crest grafts together with an ample amount of cancellous bone chips. This is followed by the insertion of the second rod and application of compression to load the anterior column before final tightening of the locking screws. Inter-transverse fusion is finally performed (Fig. 1).
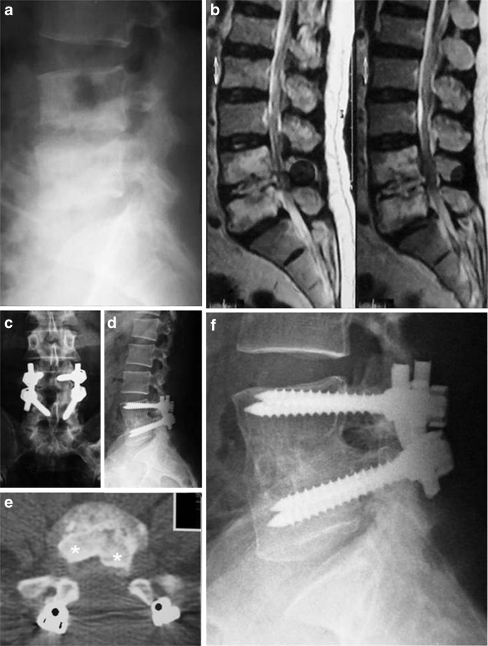
Fig. 1.
A 59-year-old farmer presented with persistent back pain, progressive weakness of his lower limbs, inability to walk unsupported and urinary retention. a Lateral X-ray shows reduction of L4-5 disc space, adjacent endplates rarefaction and erosion. b Sagittal MRI shows in addition high signal intensity of L4 and 5 vertebral bodies and the disc in between. c, d Five-year follow-up AP and lateral X-rays after one-stage circumferential fusion show solid fusion and maintenance of sagittal alignment. e Five-year follow-up CT demonstrates complete incorporation the two tricortical iliac grafts (star) and the bone chips inserted in the debrided disc space. f Close-up lateral view shows complete bone remodeling
In case of spondylodiscitis involving several vertebral bodies and discs, a laminectomy and facetectomy of all affected levels is performed after inserting the pedicle screws and the temporary stabilizing rod. The exiting nerve roots are identified, ligated and cut in the dorsal region, and protected and retracted in the lumbar region. The affected intervertebral discs are curetted as described. In the lumbar area, the transverse processes of the affected bodies are osteotomized at their bases and left in place. Using sharp osteotomes, the diseased parts of the affected vertebral bodies and their pedicles are osteotomized and excised in piecemeal fashion. Care is taken to avoid undue neural tissue retraction or injury by the instruments used. A long tricortical graft or a suitably sized cage packed with cancellous bone chips is inserted into the created cavity to rest on a healthy proximal and distal endplate (Fig. 2). The space around the strut graft or cage is also packed with cancellous bone chips. The other rod is then inserted, compression exerted on the screws to load the anterior column and the locking screws are tightened. In the area of the lumbar spine, inserting a long strut graft or cage can be tricky. It is best inserted from just above the most proximal exiting nerve root, slid downward and obliquely until it reaches the distal healthy endplate and then pushed forward underneath the proximal healthy endplate. Care should be taken to make sure the graft or the cage is very stable in position after applying compression to the pedicle screws; otherwise it is changed into a longer one. Then a formal inter-transverse fusion is performed as well. Recently, the use of expandable cages facilitated the cage insertion in the lumbar spine in between the exiting nerve roots (Fig. 3).
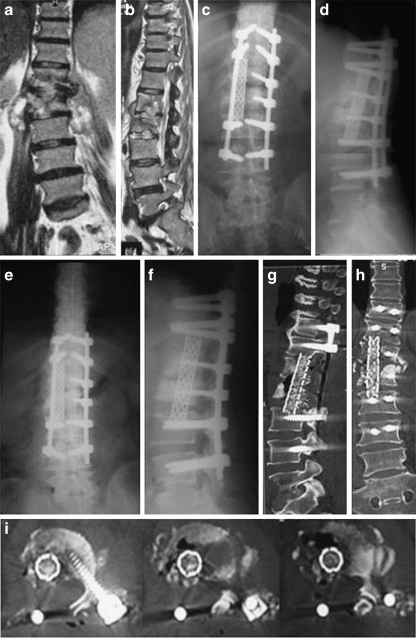
Fig. 2.
A 64-year-old housewife presented with chronic persistent back pain of more than four months, and inability to walk unsupported due to TB spondylodiscitis affecting L1 and L2 vertebrae. She was neurologically free. She has failed conservative treatment including antituberculous treatment and rigid bracing. a, b Coronal and sagittal MRIs show marked destruction and collapse. c, d Post-operative AP and lateral X-rays after one-stage posterior debridement and stabilization with interbody cylindrical cage filled with bone graft spanning the D12-L3 interval and pedicle screw. e, f Two-year follow-up AP and lateral X-rays. g, h, i Follow-up sagittal, coronal and axial CTs, respectively
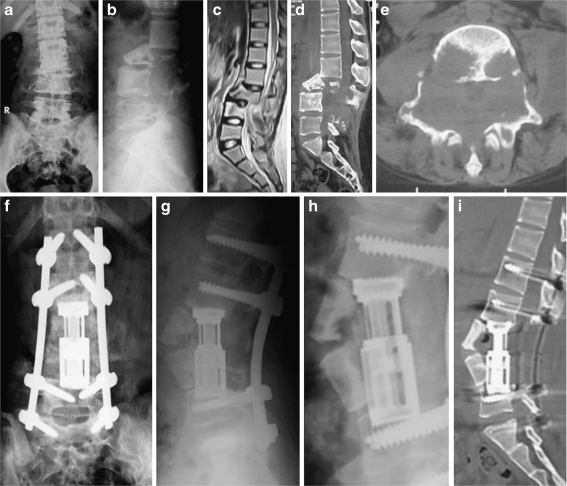
Fig. 3.
A 27-year-old girl presented with back pain, leg numbness and inability to walk unsupported due to TB spondylitis affecting L3 and L4 vertebrae. She was neurologically free. a, b AP and lateral X-rays show marked destruction and collapse and subluxation. c Sagittal MRI. d, e Sagittal and axial CT. f, g AP and lateral plain X-rays taken 6 months following a one-stage posterior debridement, stabilization with an expandable cage and pedicle screws post-operatively show excellent restoration of alignment and advancing fusion. h Magnified lateral X-ray showing the expandable cage filled and surrounded by bone graft spanning the interval between L2 and L5 vertebrae. i Six-month follow-up sagittal CT
A biopsy and a sample for bacteriological diagnosis acquired during surgery confirmed the diagnosis in all cases. On the second post-operative day, ambulant patients were allowed out of bed, while those with neurological deficits started active rehabilitation. No external support was used in any patient. Following the most recent recommendation by the Medical Research Council [23], all patients were also treated medically with a three-drug combination (rifampicin, isoniazide and ethambutol) for a period of six to nine months according to their clinical and laboratory outcomes. All patients were followed-up for a mean period of 39 months (range, 24–72 months).
The recorded clinical and radiological outcomes were compared to a historical group of 25 patients, who were treated earlier by the same surgeons with anterior debridement and fusion with iliac crest graft, followed 10–14 days later by posterior stabilization and posterolateral fusion (Fig. 4). Table 1 summarizes the clinical data of the patients.
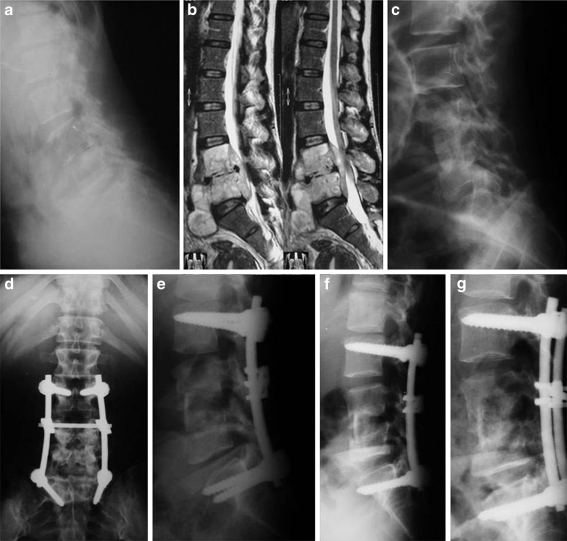
Fig. 4.
A 43-year-old farmer presented with chronic persistent back pain, progressive weakness of his lower limbs and inability to walk unsupported due to L4-5 TB spondylodiscitis. a Lateral X-ray shows reduction of L4-5 disc height with endplate rarefaction and erosion. b Sagittal MRI shows L4-5 spondylodiscitis with large prevertebral and epidural abscesses. c Post-operative lateral X-ray following a first stage anterior retroperitoneal debridement and tricortical iliac crest graft show significant reduction of the local kyphosis from 15° to (−10°). d, e Two-month follow-up AP and lateral X-rays following second stage posterior stabilization and fusion. f One-year follow-up lateral X-ray. g Five-year follow-up lateral X-ray with bone remodeling 5° loss of correction
Statistical analysis
Statistical analysis of the data was performed using SPSS 17.0 software (SPSS Inc., Chicago, IL). Continuous variables (such as age, VAS, kyphosis angle, blood loss, operative time, duration of hospital stay, and follow-up duration) were compared using a two-sample t-test. Probability values of less than 0.05 were considered to be significant. Grouped variables (such as gender, type of work, neurological status, number of affected vertebrae, incidence of co-morbidities, and incidence of operative complications) were evaluated using a Pearson chi-square test; values of less than 0.05 were considered significant. Statistical evaluation of all the demographic data of patients was performed and showed no statistical difference between the two groups.
Results
The mean operative time was 156 ± 30 minutes (range, 110–240 minutes) in the one-stage group and 262. ± 62 minutes (range, 180–360 minutes) in the combined two-stage group (P = 0.001). The mean estimated blood loss was 794 ± 243 ml (range, 500–1500 ml) in the one-stage group and 900 ± 256 ml (range, 650–1800 ml) in the two-stage group with no statistical difference between both groups. Table 2 summarizes the results.
Table 2.
Summary of results
| Parameters | One stage, n = 32 | Two stages, n = 25 | P value |
|---|---|---|---|
| Follow-up duration (years) | 4.6 ± 1.7 | 6.1 ± 2.2 | 0.006 |
| Mean operative time (minutes) | 156 ± 30 | 262. ± 62 | 0.001 |
| Mean blood loss (ml) | 794 ± 243 | 900 ± 256 | 0.116 |
| Incidence of complications | 4 (12.5%) | 12 (48%) | 0.003 |
| Complications | |||
| Donor site problems | 1 (3.1%) | 7 (28%) | - |
| Dural tear | 2 (6.3%) | 0 | |
| Anterior graft dislodgement (revision) | 0 | 2 (8%) | |
| Incisional hernia | 0 | 1 (4%) | |
| DVT | 0 | 1 (4%) | |
| Wound hematoma | 1 (3.1%) | 0 | |
| Paralytic ileus | 0 | 1 (4%) | |
| Hospital stay (days) | 2.6 ± 0.9 | 15.6 ±1.5 | 0.001 |
| Follow-up neurological function | |||
| Grade D | 4 (12.5%) | 5 (20%) | 0.485 |
| Grade E | 28 (87.5%) | 20 (80%) | |
| Mean neurological improvement | 1.58 ± 0.9 | 1.7 ± −0.58 | 0.665 |
| Follow-up back pain score (VAS) | 1.8 ± 1.1 | 1.8 ± 1 | 0.986 |
| Mean back pain improvement (VAS) | 6.63 | 6.64 | 0.347 |
| Return to work | |||
| Same work or activity level | 24 (75%) | 19 (76%) | 0.972 |
| Lighter work | 7 (21.9% | 5 (20%) | |
| Retired | 1 (3.1%) | 1 (4%) | |
| Post-operative kyphosis angle | 3.8° ± 3.4° | to 3.4° ± 6.4° | 0.469 |
| Follow-up kyphosis angle | 4.9° ± 1.5° | 4.2° ± 2.2° | 0.567 |
| Fusion rate | 100% | 100% | - |
A significant difference in the duration of hospital stay was observed between both operative groups (Table 2). The mean hospital stay was 2.6 ± 0.9 days in the one-stage group, and 15.6 ± 1.5 days in the two-stage group (P = 0.001). The difference is mainly due to the time lag waiting for the patients' recovery between performing the two stages of the surgery.
All 34 patients with pre-operative neurological deficits showed at least one Frankel grade of neurological improvement at final follow-up observation, with no significant difference observed between the two operative groups. The remaining 23 patients who were neurologically free did not demonstrate any neurological deterioration (Table 2).
All patients showed significant improvement of their VAS back pain score (2 or more grades) at follow-up (Table 2). The mean pre-operative VAS pain score decreased from 8.5 ± 1.3 to 1.8 ± 1.1 in the one-stage group, while in the two-stage group it improved from 8.5 ± 1.1 to 1.8 ± 1 at the latest follow-up, with no significant difference observed between both groups.
At two years follow-up, 43 (75.4%) of the 57 patients returned to their pre-disease activity level or work, 12 patients (21.1%) resumed a lighter work, while two (3.5%) retired, with no difference between the two groups (Table 2).
The mean pre-operative kyphotic angle was 23.7° ± 4.5° in the one-stage group and 21.7° ± 5.2° in the two-stage group. Post-operatively, this has significantly improved to 3.8° ± 3.4° in the one-stage group and to 3.4° ± 6.4° in two-stage group, with no significant difference between the two groups (Table 2). At the latest follow up, the mean angle was 4.9° ± 1.5° in the one-stage group and 4.2° ± 2.2° in the two-stage group. All patients achieved solid fusion.
The incidence of complications was significantly lower in the one-stage group (p = 0.003) when compared with the two-stage group (Table 2). Complications in the one-stage group included incidental dural tear (2 patients) that was recognized intra-operatively and successfully repaired, wound hematoma that was drained (1 patient), and donor site discomfort (1 patients). Complications in the two-stage group included persistent anterior donor site discomfort (7 patients), incisional hernia (1 patient), DVT (1 patient), paralytic ileus (1 patient) and anterior bone graft dislodgement (2 patients), that were revised 2 days after the anterior debridement stage (1 patient), and 2 days after the posterior stabilization-fusion stage (1 patient).
Discussion
Tuberculosis almost always affects the anterior column of the spine, namely, the disc and the adjacent vertebral bodies; therefore, the traditional and logical thinking has long been to use an anterior surgical approach to reach the spine to evacuate an abscess, excise the diseased tissues, decompress the neural tissues, and to insert a bone graft to correct kyphosis, achieve solid fusion and minimize disease recurrence [12]. The introduction of anterior fixation devices has helped to enhance stability, minimize graft dislodgement, and to allow early mobilization and rehabilitation of patients and contribute to overall better outcome [11–15]. However, because osteoporosis is a constant feature of the disease, these fixation devices were frequently not adequate to stabilize the spine and prevent late deformation. Many authors suggested the prolonged use of external support or the addition of posterior fusion in a second stage surgery, what constitutes two-stage circumferential fusion [16, 17].
In circumferential fusion in two- or one-stage, anterior lumbar inter-body fusion, whether by iliac autograft alone or with cage, restores stability to the weight bearing anterior column. Postero-lateral fusion restores stability to the posterior columns and completes the circumferential fusion [24]. Pedicle screws fixation provides immediate stability to the spine and avoids prolonged recumbency or use of braces.
Circumferential fusion is widely practised for degenerative lumbar disc disease and spondylolisthesis. Reported fusion rates vary between 92% and 100% [25–28]. There are few reports on circumferential fusion for tuberculosis. Wang et al. reported a 100% fusion rate after one-stage lateral rachotomy and posterior instrumentation [16]. Other workers also reported similar results after anterior and posterior fusion [16, 20, 29]. In this report, circumferential fusion in two or one stage resulted in 100% fusion rate.
In this study, circumferential fusion in two or one stage resulted in clinical and functional improvement in both groups. At final follow-up all patients with pre-operative neurological deficits showed neurological improvement; all patients had significant improvement of their VAS back pain score. Kyphosis has significantly improved in both groups with no difference between them. At two years follow-up, 43 (75.4%) of the 57 patients returned to their pre-disease activity level or work, 12 patients (21.1%) resumed a lighter work, while two (3.5%) retired, with no difference between the two groups.
Findings in this study are in favour of one-stage posterior circumferential fusion. The mean operative time and duration of hospital stay are significantly shorter, and accordingly less costly, than in the two-stage surgery. Also, the mean estimated blood loss was smaller. Similar findings were reported by other workers [30]. In this study the incidence of complications was significantly lower in the one-stage group when compared with the two-stage group. An advantage of one-stage posterior circumferential fusion is avoiding thoracotomy and thoraco-abdominal approaches, which exert considerable stress on the lungs especially so in the elderly who often suffer from impaired pulmonary function [18, 31–33]. Furthermore, anterior approaches may pose a risk of great vessels and internal organ injury. The retroperitoneal approach requires careful protection of the ureter, lumbo-sacral plexus, and sympathetic chain [34]. Similar series have reported on major vascular injuries [35], deep venous thrombosis [11, 36], pneumothorax, haemothorax, atelectasis and pneumonia [11, 36, 37], urinary tract infections [11], secondary non-specific infection [36–38] occasionally necessitating re-debridement and implant removal [35], hardware failure or breakage [12, 38], and death due to pulmonary insufficiency [11, 38].
In this study, the incidence of donor site problems was significantly higher (28%) in the two-stage group than in the one-stage group (3.1%). In one-stage posterior surgery the iliac bone graft is obtained from the posterior iliac crest, frequently though the same incision, while in two-stage surgery bone graft is obtained from the anterior iliac crest for the anterior surgery and from the posterior crest for the posterior surgery. The wound and the subsequent scar of the anterior donor site lie in a sodden skin crease in patients who have pendulous abdomen. This may explain the higher incidence of donor site problems in two-stage surgery when compared with one stage.
In one-stage circumferential fusion, the posterior elements of the vertebrae are removed. Some authors consider this type of fusion a 270° procedure, and not a 360° as in two-stage procedures. However, the fusion rates in both techniques appeared comparable in spondylolisthesis [24]. In this report both techniques resulted in bony union in all cases.
On the basis of the results of this study, it is concluded that circumferential fusion, in two or one stage, is an effective line of treatment for tuberculosis of the dorso-lumbar spine. One-stage surgery however, is advantageous because it has lower complication rate, as well as shorter hospital stay, operative time and mean blood loss.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Nunn JF. Ancient Egyptian medicine. London: British Museum Press; 1996. [PubMed] [Google Scholar]
- 2.Roaf R, Kirkaldy-Willis WH, Cathro AJM. Surgical treatment of bone and joint tuberculosis. Edinburgh: E & S Livingstone; 1959. [Google Scholar]
- 3.Hibbs RA. An operation for progressive spinal deformities. NY Med J. 1911;93:1013. [Google Scholar]
- 4.Albee FH. Transplantation of a portion of the tibia into the spine for Pott's disease: a preliminary report. JAMA. 1911;57:885. doi: 10.1001/jama.1911.04260090107012. [DOI] [PubMed] [Google Scholar]
- 5.Ito H, Tsuchiya J, Asami G. A new radical operation for Pott’s disease. J Bone Jt Surg Br. 1934;16:499–515. [Google Scholar]
- 6.Capener N. The evolution of lateral rhachotomy. J Bone Jt Surg Br. 1954;36:173–179. doi: 10.1302/0301-620X.36B2.173. [DOI] [PubMed] [Google Scholar]
- 7.Seddon HJ. Pott's paraplegia. In: Platt H, editor. Modern trends in orthopaedics (second series) London: Butterworth; 1956. [Google Scholar]
- 8.Hodgson AR, Stock FE. Anterior spinal fusion a preliminary communication on the radical treatment of Pott's disease and Pott's paraplegia. Br J Surg. 1956;44:266. doi: 10.1002/bjs.18004418508. [DOI] [PubMed] [Google Scholar]
- 9.Hodgson AR, Stock F, Hodgson AR, Stock FE. Anterior spine fusion for the treatment of tuberculosis of the spine: the operative findings and results of treatment of the first one hundred cases. J Bone Jt Surg. 1960;42 A:295. [Google Scholar]
- 10.Korkusuz F, Islam C, Korkusuz Z. Prevention of postoperative late kyphosis in Pott's disease by anterior decompression and intervertebral grafting. World J Surg. 1997;21:524–528. doi: 10.1007/PL00012280. [DOI] [PubMed] [Google Scholar]
- 11.Yilmaz C, Selek HY, Gurkan I, Erdemli B, Korkusuz Z. Anterior instrumentation for the treatment of spinal tuberculosis. J Bone Jt Surg. 1999;81A:1261. doi: 10.2106/00004623-199909000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Benli IT, Kiş M, Akalin S, Citak M, Kanevetçi S, Duman E. The results of anterior radical debridement and anterior instrumentation in Pott's disease and comparison with other surgical techniques. Kobe J Med Sci. Apr. 2000;46(1–2):39–68. [PubMed] [Google Scholar]
- 13.Benli IT, Alanay A, Akalin S, Kiş M, Acaroğlu E, Ateş B, Aydin E. Comparison of anterior instrumentation systems and the results of minimum 5 years follow-up in the treatment of tuberculosis spondylitis. Kobe J Med Sci. 2004;50(5–6):167–180. [PubMed] [Google Scholar]
- 14.Jin D, Qu D, Chen J, Zhang H. One-stage anterior interbody autografting and instrumentation in primary surgical management of thoracolumbar spinal tuberculosis. Eur Spine J Mar. 2004;13(2):114–121. doi: 10.1007/s00586-003-0661-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Talu U, Gogus A, Ozturk C, Hamzaoglu A, Domanic U. The role of posterior instrumentation and fusion after anterior radical debridement and fusion in the surgical treatment of spinal tuberculosis: experience of 127 cases. J Spinal Disord Tech. 2006;19(8):554–559. doi: 10.1097/01.bsd.0000211202.93125.c7. [DOI] [PubMed] [Google Scholar]
- 16.Altman GT, Altman DT, Frankovitch KF (1996) Anterior and posterior fusion for children with tuberculosis of the spine. Clin Orthop Relat Res Apr (325):225–31 [DOI] [PubMed]
- 17.Wang B, Ozawa H, Tanaka Y, Matsumoto F, Aizawa T, Kokubun S. One-stage lateral rhachotomy and posterior spinal fusion with compression hooks for Pott’s paralysis in the elderly. J Orthop Surg. 2006;14(3):310–314. doi: 10.1177/230949900601400314. [DOI] [PubMed] [Google Scholar]
- 18.Jain AK, Dhammi IK, Prashad B, Sinha S, Mishra P. Simultaneous anterior decompression and posterior instrumentation of the tuberculous spine using an anterolateral extrapleural approach. J Bone Jt Surg Br. 2008;90(11):1477–1481. doi: 10.1302/0301-620X.90B11.20972. [DOI] [PubMed] [Google Scholar]
- 19.Huang QS, Zheng C, Hu Y, Yin X, Xu H, Zhang G, Wang Q. One-stage surgical management for children with spinal tuberculosis by anterior decompression and posterior instrumentation. Int Orthop Oct. 2009;33(5):1385–1390. doi: 10.1007/s00264-009-0758-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ye M, Li JQ, Zou Y, Wang JG, Wang K, Zhou DS. One stage anterior and posterior fusion and posterior fixation for the treatment of thoracic and lumbar spinal tuberculosis. Zhongguo Gu Shang. 2009;22(1):23–25. [PubMed] [Google Scholar]
- 21.Jain AK, Jain S (2011) Instrumented stabilization in spinal tuberculosis. Int Orthop. Jul 1. [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 22.Frankel HL, Hancock DO, Hyslop G, Melzak J, Michaelis LS, Ungar GH, Vernon JD, Walsh JJ. The value of postural reduction in the initial management of closed injuries of the spine with paraplegia and tetraplegia. Paraplegia. 1969;7:179–192. doi: 10.1038/sc.1969.30. [DOI] [PubMed] [Google Scholar]
- 23.Medical Research Council Five-year assessment of controlled trials of short-course chemotherapy regimens of 6, 9 or 18 months' duration for spinal tuberculosis in patients ambulatory from the start or undergoing radical surgery. Fourteenth report of the medical research council working party on tuberculosis of the spine. Int Orthop. 1999;23:73–81. doi: 10.1007/s002640050311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heary RF, Bono CM (2002) Circumferential fusion for spondylolisthesis in the lumbar spine. Neurosurg Focus 13(1):E3 [PubMed]
- 25.Christensen FB, Hansen ES, Eiskjaer SP, Høy K, Helmig P, Neumann P, Niedermann B, Bünger CE (2002) Circumferential lumbar spinal fusion with Brantigan cage versus posterolateral fusion with titanium Cotrel-Dubousset instrumentation: a prospective, randomized clinical study of 146 patients. Spine (Phila Pa 1976) 27(23):2674–2683 [DOI] [PubMed]
- 26.Madan SS, Boeree NR. Comparison of instrumented anterior interbody fusion with instrumented circumferential lumbar fusion. Eur Spine J. 2003;12:567–575. doi: 10.1007/s00586-002-0516-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gertzbein SD, Betz R, Clements D, et al. Semirigid instrumentation in the management of lumbar spinal conditions combined with circumferential fusion. A multicenter study. Spine. 1996;21:1918–1926. doi: 10.1097/00007632-199608150-00018. [DOI] [PubMed] [Google Scholar]
- 28.Gertzbein SD, Hollopeter M, Hall SD. Analysis of circumferential lumbar fusion outcome in the treatment of degenerative disc disease of the lumbar spine. J Spinal Disord. 1998;11:472–478. doi: 10.1097/00002517-199812000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Qu DB, Jin DD, Chen JT, Feng L, Jiang JM, Wang JX. One-stage surgical management for spinal tuberculosis. Zhonghua Yi Xue Za Zhi. 2003;83(2):110–3. [PubMed] [Google Scholar]
- 30.He Q, Xu J (2011) Comparison between the antero-posterior and anterior approaches for treating L5-S1 vertebral tuberculosis. Int Orthop. Jul 7. [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 31.Jain AK. Treatment of tuberculosis of the spine with neurologic complications. Clin Orthop Relat Res. 398;398:75–84. doi: 10.1097/00003086-200205000-00011. [DOI] [PubMed] [Google Scholar]
- 32.Mehta JS, Bhojraj SY. Tuberculosis of the thoracic spine. A classification based on the selection of surgical strategies. J Bone Joint Surg Br. 2001;83:859–863. doi: 10.1302/0301-620X.83B6.11142. [DOI] [PubMed] [Google Scholar]
- 33.Guven O, Kumano K, Yalcin S, Karahan M, Tsuji S. A single stage posterior approach and rigid fixation for preventing kyphosis in the treatment of spinal tuberculosis. Spine. 1994;19:1039–1043. doi: 10.1097/00007632-199405000-00007. [DOI] [PubMed] [Google Scholar]
- 34.Niemeyer T, Bövingloh AS, Halm H, Liljenqvist U. Results after anterior–posterior lumbar spinal fusion: 2–5 years follow-up. Int Orthop. 2004;28:298–302. doi: 10.1007/s00264-004-0577-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Benli IT, Acaroğlu E, Akalin S, Kiş M, Duman E, Un A. Anterior radical debridement and anterior instrumentation in tuberculosis spondylitis. Eur Spine J. 2003;12:224–234. doi: 10.1007/s00586-002-0403-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Govender S. The outcome of allografts and anterior instrumentation in spinal tuberculosis. Clin Orthop Relat Res. 2002;398:60–66. doi: 10.1097/00003086-200205000-00009. [DOI] [PubMed] [Google Scholar]
- 37.Christodoulou AG, Givissis P, Karataglis D, Symeonidis PD, Pournaras J. Treatment of tuberculous spondylitis with anterior stabilization and titanium cage. Clin Orthop Relat Res. 2006;444:60–65. doi: 10.1097/01.blo.0000201175.87635.28. [DOI] [PubMed] [Google Scholar]
- 38.Özdemir HM, Us AK, Oğün T. The role of anterior spinal instrumentation and allograft fibula for the treatment of pott disease. Spine. 2003;28:474–479. doi: 10.1097/01.BRS.0000048666.17934.17. [DOI] [PubMed] [Google Scholar]