Abstract
Spondylodiscitis affects children aged between two and eight years, and mainly involves the lumbar or lumbosacral spine. Diagnosis is difficult because the symptoms are not very specific and due to the children’s difficulty in communicating. Unlike adults, children have vascularised intervertebral discs, which explains the higher incidence of this disease in this age group. C-reactive protein, and blood and urine cultures are important laboratory tests. In most cases, fine needle or traditional biopsy helps identify the pathogen particularly in patients who do not respond to the antibiotic therapy test. Magnetic resonance imaging has high sensitivity and specificity in the investigation of pyogenic infection of the spine, particularly in the early stages, when these changes are not shown in other imaging tests. X-rays can take up to six weeks to show changes. The first radiographic sign of infection is the irregularity of the vertebral endplates in the infection area, followed by their erosion and that of the adjacent bone, decreased disc space, segmental collapse, loss of lordosis (in cases of low back involvement) and ultimately, permanent structural deformity. After eight to twelve weeks, local regeneration occurs, accompanied by bone sclerosis arising from the formation of new trabecular bone, replacing the necrotic cancellous bone. Effective treatment often leads to bone fusion of the affected disc space. However, when no therapy is adopted, total vertebral collapse can occur. The treatment involves immobilisation, antibiotic therapy, and surgical decompression in more advanced cases.
Introduction
Although spondylodiscitis has been known since ancient times [1], current knowledge of the disease is supported by inconsistent observational clinical studies—case series with no control group for comparison—due to the low incidence of the disease. Introduced to MeSH in 1989, the term discitis means inflammation of an intervertebral disc space which may lead to disc erosion. The search terms "Discitis" [MeSH] AND "Child" [MeSH] retrieved 131 studies in PubMed. None of the studies was a meta-analysis or a randomised clinical trial, notably because of the small number of cases and the lack of uniform evaluation criteria of the treatment outcomes, with patient-focused results.
Today, the aetiology is thought to be bacterial, especially in cases of pediatric discitis. Pyogenic infection of the spine was first reported by Lannelongue in 1879 [2]; however, spondylodiscitis was not defined as an independent entity until 1925, by Mayer [3]. Aetiologically, infections of the spine can be divided into pyogenic or unspecific, granulomatous or specific (tuberculosis, brucellosis or fungal) and parasitic. Among the pyogenic infections, the term spondylodiscitis covers vertebral osteomyelitis, spondylitis and discitis, which are different manifestations of the same pathological process [4].
Overall, the incidence of spondylodiscitis is approximately 1:250,000, corresponding to approximately 2–4% of infectious bone diseases [5, 6]. Unlike spondylodiscitis in adults, the incidence of which is higher after surgical procedures, paediatric spondylodiscitis is a rare disease with unknown incidence, due to the few quality case series available in the literature [7].
The average age for the diagnosis of discitis in children is approximately two to eight years and, although any level of the spine can be affected, the incidence of involvement of the lumbar or lumbo sacral region represents the majority of cases (75% of patients) [8]. The infection can occur in the thoracic, lumbar or sacral region, but it is more common in the lumbar region, particularly in children under five years of age [9, 10].
Spondylodiscitis is still a rare diagnosis, but in recent years its reported incidence has been increasing due to the availability of more effective diagnostic methods that enable earlier diagnoses [4]. From a clinical viewpoint, it is a disease with unspecific signs and symptoms, such as abdominal pain, fever and difficulty walking [11, 12]. Children’s inability to express themselves is also a relevant factor [13], often resulting in difficult and delayed diagnosis [9]. The onset of spondylodiscitis in children is basically different from that in adults, in terms of its course and severity. The clinical manifestation can often be frustrating, with unspecific symptoms, which are generally mild, making its diagnosis difficult [7].
Aetiopathogenesis
The pathogens can infect the spine from three sources: by haematogenous spread (arterial or venous—via the Batson plexus), by external direct inoculation, or by contiguous spread to the bone from adjacent tissues. The predominant form is blood haematogenous [4, 14].
Unlike adults, the intervertebral disc in children is vascularised [15, 16]. Vascularisation in children is made up of vessels across the cartilaginous vertebral plate and into the ring; after eight years of age, these vessels disappear, but a rich anastomotic network of vessels remains in the periphery of the disc. In adults, however, the disc is avascular, and intraosseous anastomoses occur at around 30 years of age [4, 13, 14, 16]. In children, the metaphysis of the vertebral body has a rich blood network, with an incomplete vascular ring that ends at the base of the pedicle. In other words, the dynamic understanding of the vascular physiology of the intervertebral disc explains the findings of clinical and imaging tests. The vascularised nature of the intervertebral disc in children explains the higher incidence of this disease in this age group. According to various studies, this fact explains the hypothesis of viral or bacterial aetiology, and also the satisfactory response to treatment with antibiotics [11–15]. The metaphyseal ring above and below two adjacent vertebrae presents common anastomosis through anastomoses of the posterior-lateral region of the disc [17]. In tuberculosis, the destruction starts with the vertebral body and spreads to the adjacent vertebra through the ligaments [18].
Extensive bone infarcts lead to the formation of cavitation and compression fractures, resulting in instability, deformity and risk of medullary compression. Failure to control the infection can cause paravertebral, psoas or epidural abscess, with risk of paraplegia, subdural abscess or meningitis [4].
Involvement of the posterior elements of the vertebrae is rare in the haematogenous form, due to the poor blood supply in this region [4]. Pyogenic spondylodiscitis from the haematogenous source particularly affects the lumbar spine, followed by the thoracic and cervical spine, reflecting the volume of blood flow. In tuberculosis, we observed involvement of the thoracic spine [4].
Direct inoculation is more common after surgery, punctures or epidural procedures, and is responsible for up to 25–30% of the cases in some series [4, 19]. Contiguous spread is rare and may be caused by an adjacent infectious focus, such as a retropharyngeal abscess [4].
Physical examination
The clinical symptoms are variable and often unspecific, which may delay the diagnosis [9, 14, 15, 17, 19]. Some studies report delayed diagnosis by up to three months in 50% of patients [14].
Children may have abdominal pain, low fever, difficulty walking, and irritability. Back pain is often the predominant complaint [14, 15, 17]. The back pain extends to the abdomen, hip, leg, scrotum or perineum, and is exacerbated by movement and worse at night. The child is usually not systemically ill. An important clinical sign is the "quarter sign"—difficulty or inability to bend over to pick up a coin—and irritation of the hip when held in extension (log-roll test) [19].
When the cervical spine is affected, manifestations may include dysphagia and stiff neck [4]. Contracture and spasm of paravertebral and psoas muscles occur, with limited mobility of the spine, which can be extremely painful and cause loss of the lumbar lordosis [4, 10, 14, 17, 20, 21]. Unspecific symptoms are also common, such as fatigue, loss of appetite, weight loss, and a lack of desire to play [4, 16]. Table 1 shows the unspecific clinical complaints of children with discitis seen at the Spine Clinic of Santa Casa de São Paulo. This characteristic often justifies the delayed diagnosis in these children. The most common complaints during the initial evaluation of the patient were difficulty walking and back pain. We found varied signs and symptoms in the medical records, including claudication, pain on palpation of the spine, abdominal pain, pain on moving the lower limb, correction of lumbar lordosis, and anterior trunk decompensation. No patient had neurological dysfunction, and two had fever of over 38°C [15].
Table 1.
Clinical signs and symptoms of patients with discitis seen at the Santa Casa de Misericórdia de São Paulo [16]
| Clinical signs and symptoms | No. of complaints | Percentage (%) |
|---|---|---|
| Difficulty walking | 4 | 15 |
| Lumbago | 4 | 15 |
| Claudication | 1 | 4 |
| Abdominal pain | 1 | 4 |
| Radiation to lower limbs | 1 | 4 |
| Back pain | 1 | 4 |
| Correction of lumbar lordosis | 3 | 12 |
| Decompensation of the trunk of the body | 1 | 4 |
| Pain on palpation of the spine | 8 | 31 |
| Fever | 2 | 7 |
| Neurological dysfunction | 0 | 0 |
| Total | 26 | 100 |
In our study, the average time to diagnosis, i.e. the time from the initial consultation to the diagnosis of discitis, was two days, with a range of zero to eight days. The average time from the onset of symptoms and seeking medical care was eight days, ranging from two to 20 days. We found that the initial diagnosis was incorrect in three patients. In these patients, the initial hypothesis was lumbar fracture, psoitis and urinary tract infection [15].
Neurological complications, such as medullary or spinal nerve compression and meningitis, occur in approximately 12% of the patients [14]. In general, these complications follow the initial pain, but they may also be the first manifestation [21]. Progression to weakness and paralysis suggests the formation of an epidural abscess or kyphotic collapse in the affected part of the spine [14, 22]. Predisposing factors include age (average age three years) [21], presence of comorbidities such as diabetes mellitus, cardiovascular disease, obesity, renal failure, chronic hepatitis, rheumatic disease, chronic use of steroids, cancer and AIDS [4, 19, 23]. The presence of previous genitourinary tract, respiratory tract or gastrointestinal infection is also observed [6, 22].
Tests
Laboratory tests
The laboratory variables to be determined include leucocyte count, C-reactive protein (CRP), and erythrocyte sedimentation rate (ESR). Usually, in patients with acute disease, a significant increase in the values of the inflammatory tests (C-reactive protein, CRP and ESR) is observed, while in patients with chronic infections, such as Pott’s disease or tuberculosis, these tests can show normal, or only slightly elevated results [4, 14].
The leucocyte count, often within the normal range, may be increased in 35% of cases. However, an increase above 12,000 cell/mm3 is rare. ESR also increases slightly, generally to around 40–85 mm/h (reference values between 0–20 mm/h) [4, 17]. CRP, despite being low in specificity, has higher clinical value compared with ESR, when the evolution of the treatment is monitored: an increased or decreased variation in its values is related to worsening or improvement of the clinical symptoms, respectively [23].
Blood and urine cultures are part of the laboratory tests. Blood cultures, usually obtained from two or three samples, are positive in 50% of the cases of unspecified discitis, and are an important guide to antibiotic therapy. In patients who have already begun taking antibiotics, the rate of identification of the pathogen decreases to around 15%. In these cases, the antibiotic therapy should be suspended for 72 hours before collecting new blood cultures [17, 23, 24].
Biopsy of the vertebral body and/or disc space should be considered when no organism can be identified by less invasive techniques. CT-guided needle biopsy or fluoroscopy can be performed, with accuracy of up to 80% in the identification of the infection pathogen. Traditional open biopsies have 93.3% sensitivity in some case series, but with increased local morbidity [4, 14, 23]. Therefore, the possibility of using this diagnostic method in children who fail to respond to treatment with antibiotics is argued.
In addition to the bacteria cultures, fungi and mycobacteria should also be investigated when suspicion is high, as in cases of negative bacterial cultures or subacute infections [11, 14]. We reviewed the laboratory results of nine patients treated at our centre; the mean baseline ESR was 76.5 mm/hour, ranging from 20 to 98 mm/hour. The mean leucocyte count was 11,143 per mm3, ranging from 5,100 to 17,400 leucocytes per mm3. Blood culture and open biopsy of the lesion were performed in a patient undergoing hemilaminectomy with drainage of the epidural abscess shown on MRI. This patient had fever, leucocytosis and a symptom of intense back pain spreading to the lower limbs. The biopsy revealed chronic inflammation with recurrent outbreaks, but no bacterial growth was observed in the secretion culture. The Mantoux skin test (PPD) was requested for one patient who had a family history of tuberculosis [15]. The result was non-reactive.
Radiography
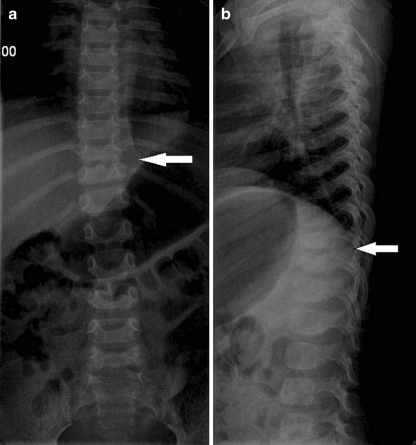
In the early stages of unspecified discitis, X-rays have very low sensitivity and specificity. The earliest radiographic sign is loss of definition and irregularity of the vertebral plateau, which can occur within two to eight weeks of the onset of symptoms. Discitis consists of four radiographic phases: the latent phase, when the radiographs are normal; the acute phase, which takes place within two to four weeks after the onset of the symptoms and is characterised by narrowing and erosion of the disc space (Fig. 1); the healing phase, which takes place within two to three months after the radiographic changes and is characterised by sclerosis of the contours of the vertebral bodies; and the late phase, characterised by narrowing of the affected disc space [12]. After a varying period of time (between eight and 12 weeks), local regeneration occurs with bone sclerosis due to the formation of new bone trabeculae replacing the necrotic cancellous bone. With the evolution of the symptoms, erosion of the endplates and adjacent bone is visible, leading to a decrease in the disc space, segmental collapse, loss of lordosis in cases where the back is affected, and finally, permanent structural deformation. Effective treatment often leads to bone fusion of the affected disc space; however, total vertebral collapse can occur when no therapy is adopted [4, 7, 8, 16, 20].
Fig. 1.
Initial X-rays, two months of back pain symptoms, showing D10–D11 narrowing space. a AP view. b Lateral view
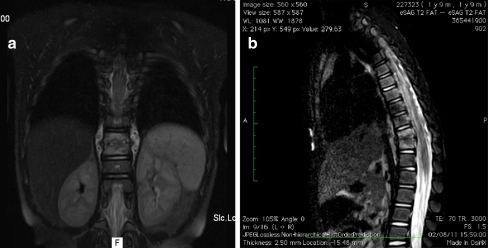
In the diagnosis of spondylodiscitis, in the absence of radiographic findings indicative of the disease, bone scintigraphy is a sensitive test that can be carried out to check for infection. Magnetic resonance imaging (MRI), however, is the most useful imaging method for the investigation of pyogenic infection of the spine, particularly in the early stages, when other types of imaging, such as X-rays, do not yet reveal changes, or when they reveal unspecific changes, such as scintigraphy [18] (see Figs. 2, 3, 4).
Fig. 2.
Same patient as in Fig. 1 with MRI showing D10–D11 compromised. a AP view. b Lateral view
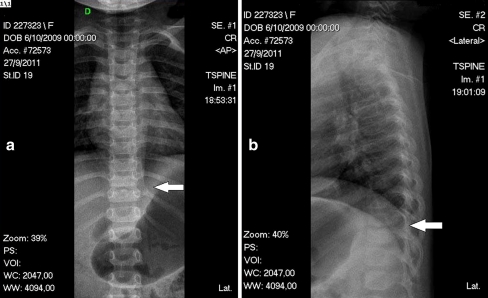
Fig. 3.
Same patient as in Figs. 1 and 2, X-rays after seven weeks of antibiotic plus brace treatment. a AP view. b Lateral view
Fig. 4.
Another case of spondylodiscitis showing absence of radiographic changes in the acute phase of the disease
MRI has high sensitivity (96%) and specificity (94%), and because it is not invasive, it is the test of choice for identifying a spinal infection [7, 11, 18, 20, 23]. It should be noted that in the early stages, the differential diagnosis between unspecific infection (pyogenic discitis) and specific infection (spinal tuberculosis) can be difficult. The distinction should be guided by the laboratory data, the aspects shown in the images, and the evolution of the patient’s clinical symptoms [7, 18].
Bone scintigraphy with technetium-99 m pyrophosphate is highly sensitive, and shows positivity within one to two days of the onset of the infection (Fig. 5). The drawbacks of the technique, however, include low specificity, insufficient spatial resolution of the affected site, and the length of time it takes to carry out the test. Scintigraphy with leucocytes marked with indium increases the specificity of the tests for pyogenic discitis. However, the high accuracy of the MRI has led to scintigraphy falling into disuse [16, 17, 19].
Fig. 5.
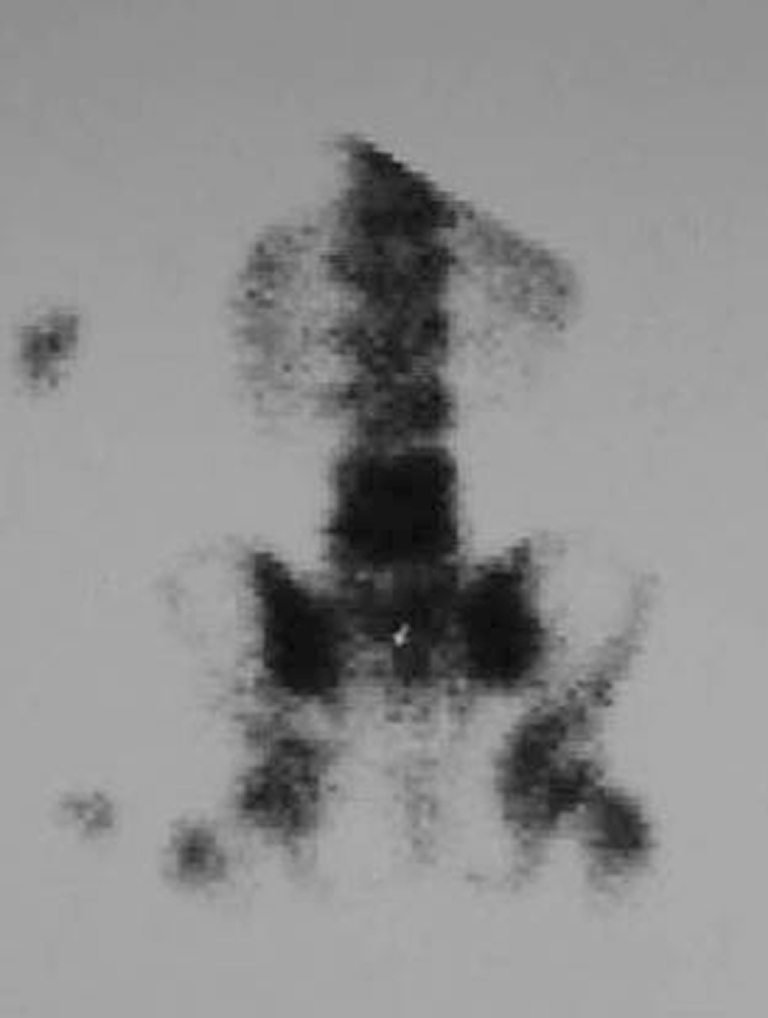
Scintigraphy provides diagnosis at an earlier stage
Computed tomography (CT) scan is easy to perform, faster and more affordable than MRI. It is particularly useful for pre-operative planning, and is of fundamental help in percutaneous procedures, such as needle biopsies. The areas of erosion of the endplates that appear early on in the affected vertebral levels are easier to detect by CT scan than by the images from plain radiographs, and are suggestive of granulomatous or specific infection, such as tuberculosis [17, 19].
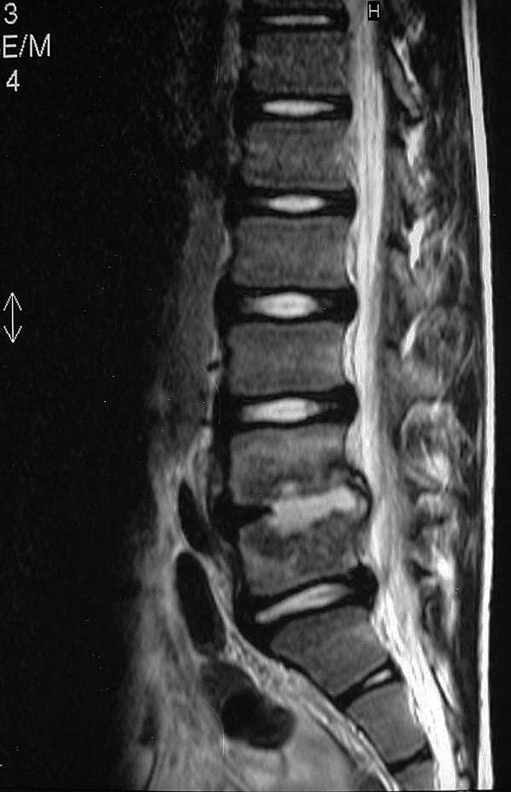
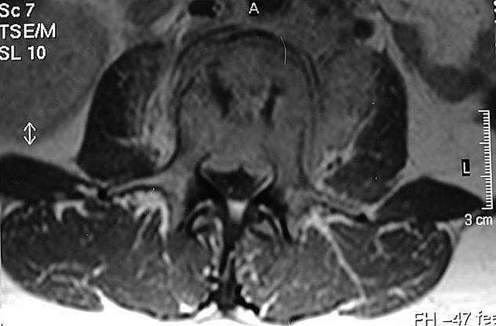
Finally, MRI is the gold standard complementary imaging test for the diagnosis and monitoring of pyogenic spondylodiscitis. MRI suggests the diagnosis in 36–55% of the cases within less than two weeks of the onset of symptoms. After two weeks, spondylodiscitis can be accurately diagnosed in up to 76% of the cases. The findings are characteristic and can be seen in the MRI images in the early stages: the presence of vascularised fibrous tissue, fatty transformation of the bone marrow, subchondral fibrosis, and bone sclerosis can be easily observed. In the T1-weighted sequences, hyposignals can be observed in the bone marrow, with hypersignal in the T2 sequences. The intervertebral disc also has a hypersignal in the T2 sequence, due to the increase of local fluid related to inflammatory oedema (Figs. 6 and 7). The use of Gadolinium contrast can further improve the visualisation of the affected vertebral endplates [19, 20].
Fig. 6.
T2-weighted sagittal MRI shows a signal change in the intervertebral disc
Fig. 7.
Note the fluid collection in the epidural space
Care should be taken in interpreting the MRI images after resolution of the pathological condition. The signs of acute infection (hyposignal in T1 and hypersignal in T2) may remain; in this case, clinical correlation is needed to define the exacerbation or recurrence of the disease. The presence of abundant paravertebral abscess is a sign observed in spondylodiscitis by Pott’s disease, according to the majority of the case series investigated [19].
Conservative treatment
The goal of treatment is to eliminate the infection and restore and preserve the function and structure of the spine, relieving the pain. In children, conservative treatment is usually sufficient, with most of the patients’ symptoms improving satisfactorily and without sequelae. Therefore, conservative treatment is the treatment of choice in this population, despite the radiographic changes in the follow-up of these children. The principles of conservative treatment include the establishment of an accurate microbiological diagnosis, treatment with specific antibiotics, spinal immobilisation, and care when checking the clinical and radiographic evidence of instability of the spine, progression of the infection or neurological deterioration [4, 14, 16, 17, 19].
While awaiting the results of the laboratory tests and infection culture, the spine must be immobilised with a plaster or lumbo sacral orthosis. Rest and spinal immobilisation are important, particularly where there is intense pain or risk of spinal instability [4, 13, 16, 17].
Immobilisation should also be performed for cervical spondylodiscitis by orthosis or a halo brace in cases of severe tuberculous spondylodiscitis. For thoracic or lumbar lesions, the infection should be treated with bed rest until the symptoms of pain and spasms subside. The patient is then placed in a thoracic-lumbo-sacral orthosis (TLSO) for thoracic and lumbar infections or a lumbo-sacral orthosis (LSO) for lumbo sacral infections. Upper thoracic lesions should be immobilised with a TLSO extended to the cervix. Spinal immobilisation and restricted activities should be maintained for 10–12 weeks, or until evidence of clinical and laboratory resolution [14].
Since the advent of antibiotics, the mortality rate has decreased from 25–56% to less than 5%. Blood tests should be performed on three separate occasions for blood cultures, if possible during peaks of fever and chills after discontinuation of any antipyretic agent. When these tests are negative and no clinical and laboratory response to the therapy is observed, percutaneous biopsy of the affected disc is indicated. Blood cultures should be collected routinely after the procedure [4, 15, 21]. However, antimicrobial treatment should not be initiated until the organism has been identified, except in patients at risk, such as those with neutropenia or severe sepsis, according to some authors [4, 14]. Given the lack of specificity and poor biopsy results, the therapeutic test is indicated according to the clinical, laboratory and imaging history of the cases.
Some studies advocate that when spondylodiscitis is suspected in children, antibiotic treatment should be empirical, based on the assessment of the probable organism and the patient’s risk factors. As the most commonly isolated organisms are Staphylococcus aureus and Streptococcus, the recommended antibiotics are a combination of third-generation cephalosporins and oxacillin/clindamycin [13, 21]. Salmonella can be responsible for infection in immunosuppressed patients and in children with sickle-cell anaemia, and requires specific treatment [6].
Some authors, who question the bacterial aetiology, contest the need of treatment with antibiotics due to the fact that spontaneous resolution of the disc inflammation has been observed in some cases without antibiotics [16].
The total duration of antimicrobial therapy remains unclear [4, 10, 14, 19]. The recommendations on the duration of antibiotic therapy in the treatment of spondylodiscitis in children are controversial in the literature, ranging from one week to three weeks intravenously, followed by supplementary oral therapy until resolution of the inflammation and clinical improvement. The total treatment can last from two weeks to six months, according to the patient’s response [10, 11, 13, 17, 21].
The criteria for the discontinuation of the antimicrobial treatment include resolution of the symptoms, and normalisation of ESR and CRP. It has been proposed that a weekly reduction of 50% in CRP represents adequate progression [4, 10, 17, 19].
Surgery
Surgery is rarely indicated for disc infection in children. Treatment with antibiotics, followed by orthosis when applicable, is safe and effective for most infections [2, 4, 8, 15, 21].
Surgical decompression is indicated for more advanced infections. Surgical intervention may also be necessary in cases of neurological deficit due to medullary or root compression, major destruction of the vertebral bodies, progressing to rapid transformation of the deformity into kyphosis, spinal instability, or failure of conservative treatment [14].
The objectives of surgery include removing the septic focus and, at the same time, detecting the pathogen and stabilising the infected area of the spine, thereby achieving fused vertebrae. This procedure provides a more reliable means of controlling the infection [13, 14, 22]. Another reason is to treat the spinal deformity with or without decompression of the spinal canal.
There is a variety of surgical approaches, and the choice depends on the characteristics of the patient and the surgical experience of each department. In general, only the anterior vertebral elements are involved in the infection [11, 25, 26]. Some degree of stability is usually maintained by the intact posterior elements, preventing significant subluxation. Therefore, decompressive laminectomy alone can further destabilise the spine and result in an increase in neurological deficit. This procedure is only indicated in cases of epidural abscess, in the rare situation when they are located in the dorsal area. In some cases, an infection located in the disc space with involvement of the spinal canal can be treated by a posterior-lateral debridement by means of costotransversectomy as described by Capener [4, 14].
The use of autologous bone graft after adequate debridement is still defended. The most common iliac crest grafts include tricortical, rib and fibula. Due to a reluctance to use implants because of the fear of perpetuating the infection, prolonged bed rest becomes necessary, despite the surgery [14, 22, 26]. Occasionally, immobilisation may be necessary to provide stability and minimise the risk of future deformity [21].
The implantation of osteosynthesis material in an infected area can lead to microbial colonisation of the metal surface and persistent infection. This risk is reduced when complete debridement is associated with the use of antibiotics. Titanium implants are now more commonly used, and do not appear to be associated with an increased rate of recurrence [14, 22, 27].
Surgery has an important role in relieving pain, correcting deformities and neural involvement, and restoring function, but it is the exception rather than the rule, in cases of paediatric discitis [2, 4, 8, 20].
Outcomes
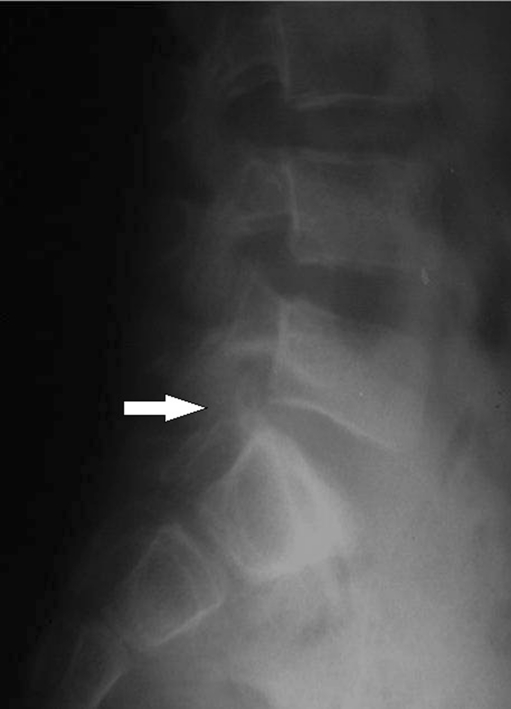
Once the symptoms are resolved, patients should be followed-up for one to two years. Radiographic assessment can show a decrease in disc space (Fig. 8) and vertebra magna, and even spontaneous fusion of the vertebrae leading to a "block vertebra" with or without narrowing of the spinal canal. Clinically, the symptoms can become chronic, with decreased mobility and mild back pain [9, 10].
Fig. 8.
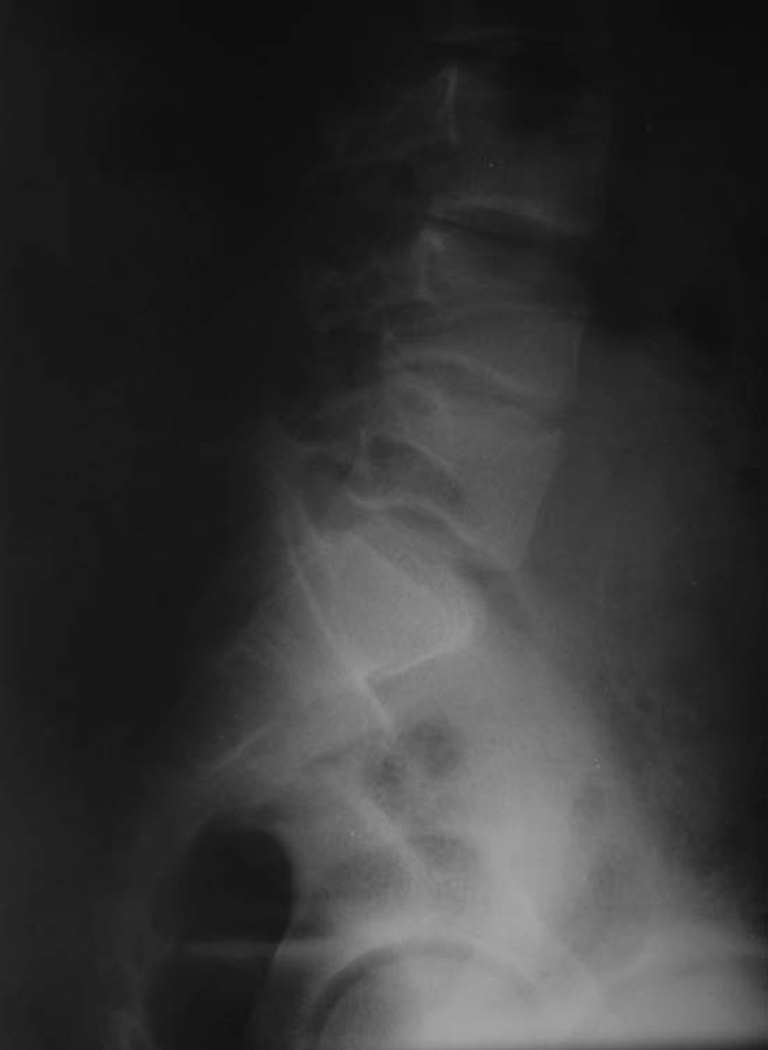
The decrease in disc space is evident after five years of treatment. Patient is now asymptomatic
Conclusion
In summary, discitis is an uncommon disease, defined as an unspecific inflammatory process of the intervertebral disc. From a clinical point of view, it is a disease that affects particularly children, often with unspecific signs and symptoms such as abdominal pain, fever and difficulty walking, resulting in difficult and delayed diagnosis [1–3]. A definitive diagnosis is based on narrowing of the intervertebral disc space, shown in plain radiographs of the spine, associated with fever, leucocytosis and an increased erythrocyte sedimentation rate. The culture of the material collected (open or closed biopsy of the lesion) reveals the aetiology in less than 50% of cases [2, 5, 11, 25, 26]. The bacterium most commonly involved is Staphylococcus aureus [2–5]. Radiographic changes can be seen within two to three weeks of the onset of the disease. Thus, a high level of suspicion is needed before indicating subsidiary auxiliary tests that could reveal the structural change of the intervertebral disc at an early stage. Several authors have reported that bone scintigraphy and, currently, magnetic resonance imaging (MRI) are valuable for early diagnosis. Trauma or the presence of a bacterial or viral agent in the intervertebral disc have been indicated, by different researchers, as the cause of this disease. As the aetiology is still unknown, and is still being debated in the literature, there is no consensus on the best therapy to adopt [2, 4, 5, 7, 8, 11, 25, 26]. Despite the various treatment options reported in the literature, in the vast majority of cases, the disease in children clears up satisfactorily and without sequelae [11, 15, 26].
References
- 1.Tayles N, Buckley HR. Leprosy and tuberculosis in iron age Southeast Asia? Am J Phys Anthropol. 2004;125(2):239–256. doi: 10.1002/ajpa.10378. [DOI] [PubMed] [Google Scholar]
- 2.Kulowski J. Pyogenic osteomyelitis of the spine: an analysis and discussion of 102 cases. J Bone Joint Surg Am. 1936;18:343–364. [Google Scholar]
- 3.Mayer L. An unusual case of infection of the spine. J Bone Joint Surg Am. 1925;7:957–968. [Google Scholar]
- 4.Gouliouris T, Aliyu SH, Brown NM. Spondylodiscitis: update on diagnosis and management. J Antimicrob Chemother. 2010;65(Suppl 3):iii11–iii24. doi: 10.1093/jac/dkq303. [DOI] [PubMed] [Google Scholar]
- 5.Digby JM, Kersley JB. Pyogenic non-tuberculous spinal infection: an analysis of thirty cases. J Bone Joint Surg Br. 1979;61(1):47–55. doi: 10.1302/0301-620X.61B1.370121. [DOI] [PubMed] [Google Scholar]
- 6.Govender S. Spinal infections. J Bone Joint Surg Br. 2005;87(11):1454–1458. doi: 10.1302/0301-620X.87B11.16294. [DOI] [PubMed] [Google Scholar]
- 7.Cushing A. Diskitis in children. Clin Infect Dis. 1993;17(1):1–6. doi: 10.1093/clinids/17.1.1. [DOI] [PubMed] [Google Scholar]
- 8.Fernandez M, Carrol CL, Baker CJ. Discitis and vertebral osteomyelitis in children: an 18-year review. Pediatrics. 2000;105(6):1299–1304. doi: 10.1542/peds.105.6.1299. [DOI] [PubMed] [Google Scholar]
- 9.Chandrasenan J, Klezl Z, Bommireddy R, Calthorpe D. Spondylodiscitis in children: a retrospective series. J Bone Joint Surg Br. 2011;93(8):1122–1125. doi: 10.1302/0301-620X.93B8.25588. [DOI] [PubMed] [Google Scholar]
- 10.Early SD, Kay RM, Tolo VT. Childhood diskitis. J Am Acad Orthop Surg. 2003;11(6):413–420. doi: 10.5435/00124635-200311000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Brown R, Hussain M, McHugh K, Novelli V, Jones D. Discitis in young children. J Bone Joint Surg Br. 2001;83(1):106–111. doi: 10.1302/0301-620X.83B1.10865. [DOI] [PubMed] [Google Scholar]
- 12.Crawford AH, Kucharzyk DW, Ruda R, Smitherman HC., Jr Diskitis in children. Clin Orthop Relat Res. 1991;266:70–79. [PubMed] [Google Scholar]
- 13.Waizy H, Heckel M, Seller K, Schroten H, Wild A. Remodeling of the spine in spondylodiscitis of children at the age of 3 years or younger. Arch Orthop Trauma Surg. 2007;127(6):403–407. doi: 10.1007/s00402-007-0316-9. [DOI] [PubMed] [Google Scholar]
- 14.Skaf GS, Domloj NT, Fehlings MG, Bouclaous CH, Sabbagh AS, Kanafani ZA, et al. Pyogenic spondylodiscitis: an overview. J Infect Public Health. 2010;3(1):5–16. doi: 10.1016/j.jiph.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 15.Avanzi O, Chih LY, Meves R, Mattos C. Tratamento da discite na criança [Treatment of discitis in the child] Rev Assoc Med Bras. 2005;5(2):113–116. doi: 10.1590/S0104-42302005000200019. [DOI] [PubMed] [Google Scholar]
- 16.Kayser R, Mahlfeld K, Greulich M, Grasshoff H. Spondylodiscitis in childhood: results of a long-term study. Spine (Phila Pa 1976) 2005;30(3):318–323. doi: 10.1097/01.brs.0000152097.57891.98. [DOI] [PubMed] [Google Scholar]
- 17.Garron E, Viehweger E, Launay F, Guillaume JM, Jouve JL, Bollini G. Nontuberculous spondylodiscitis in children. J Pediatr Orthoped. 2002;22(3):321–328. doi: 10.1097/01241398-200205000-00010. [DOI] [PubMed] [Google Scholar]
- 18.Couto BB, Umeta RSG, Caffaro MFS, Meves R, Landim E, Avanzi O. Análise radiológica comparativa entre espondilodiscite tuberculosa e inespecífica [Comparative radiological analysis between spondylodiscitis tuberculosis and nonspecific] Coluna/Columna. 2010;9(4):394–400. doi: 10.1590/S1808-18512010000400009. [DOI] [Google Scholar]
- 19.Sobottke R, Seifert H, Fätkenheuer G, Schmidt M, Gossmann A, Eysel P. Current diagnosis and treatment of spondylodiscitis. Dtsch Arztebl Int. 2008;105(10):181–187. doi: 10.3238/arztebl.2008.0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gabriel KR, Crawford AH. Magnetic resonance imaging in child who had clinical signs of discitis. Report of a case. J Bone Joint Surg Am. 1988;70(6):938–941. [PubMed] [Google Scholar]
- 21.Dormans JP, Moroz L. Infection and tumors of the spine in children. J Bone Joint Surg Am. 2007;89(Suppl 1):79–97. doi: 10.2106/JBJS.F.00475. [DOI] [PubMed] [Google Scholar]
- 22.Hadjipavlou AG, Mader JT, Necessary JT, Muffoletto AJ. Hematogenous pyogenic spinal infections and their surgical management. Spine (Phila Pa 1976) 2000;25(13):1668–1679. doi: 10.1097/00007632-200007010-00010. [DOI] [PubMed] [Google Scholar]
- 23.Kim CJ, Song KH, Jeon JH, Park WB, Park SW, Kim HB, et al. A comparative study of pyogenic and tuberculous spondylodiscitis. Spine (Phila Pa 1976) 2010;35(21):E1096–E1100. doi: 10.1097/BRS.0b013e3181e04dd3. [DOI] [PubMed] [Google Scholar]
- 24.McCarthy JJ, Dormans JP, Kozin SH, Pizzutillo PD. Musculoskeletal infections in children: basic treatment principles and recent advancements. J Bone Joint Surg Am. 2004;86(4):850–863. [PubMed] [Google Scholar]
- 25.Rudert M, Tillmann B. Lymph and blood supply of the human intervertebral disc. Cadaver study of correlations to discitis. Acta Orthop Scand. 1993;64(1):37–40. doi: 10.3109/17453679308994524. [DOI] [PubMed] [Google Scholar]
- 26.Whalen JL, Parke WW, Mazur JM, Stauffer ES. The intrinsic vasculature of developing vertebral end plates and its nutritive significance to the intervertebral discs. J Pediatr Orthop. 1985;5(4):403–410. doi: 10.1097/01241398-198507000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Chen WH, Jiang LS, Dai LY. Surgical treatment of pyogenic vertebral osteomyelitis with spinal instrumentation. Eur Spine J. 2007;16(9):1307–1316. doi: 10.1007/s00586-006-0251-4. [DOI] [PMC free article] [PubMed] [Google Scholar]