Abstract
Purpose
There are few articles in the literature comparing outcomes between anterior and posterior instrumentation in the management of thoracic and lumbar spinal tuberculosis (TB).
Methods
Between January 2004 and December 2009, 217 adult patients, average age 39 (range 16–67) years with thoracic and lumbar spinal TB were treated by anterior radical debridement and fusion plus instrumentation, anterior radical debridement with fusion and posterior fusion with instrumentation, posterolateral debridement and fusion plus posterior instrumentation or transpedicular debridement and posterior fusion with instrumentation in a single- or two-stage procedure. We followed up 165 patients for 22–72 (mean 37) months. Of these, 138 underwent more than three weeks chemotherapy with isoniazid, rifampin, pyrazinamide and ethambutol, and the remaining 27 underwent operation for neurological impairment within six to 18 hours of the same chemotherapy regimen. In no case did relapse occur. Apart from eight patients with skip lesions treated by hybrid anterior and posterior instrumentation, anterior instrumentation was used in 74 patients (group A) and 83 patients (group B) were fixed posteriorly.
Results
In both groups, local symptoms were relieved significantly one to three weeks postoperatively; ten of 14 patients (71%) in group A and 14 of 19 (74%) in group B with neurological deficit had excellent or good clinical results (P > 0.05). Erythrocyte sedimentation rates (ESR) returned from 43.6 mm/h and 42.7 mm/h, respectively, preoperatively to normal levels eight to 12 weeks postoperatively. Kyphosis degree was corrected by a mean of 11.5° in group A and 12.6° in group B, respectively (P < 0.01). Correction loss was 6.8° in group A and 6.1° in group B at the last follow-up (P < 0.01). Fusion rates of the grafting bone were 92.5% and 91.8%, respectively, at final follow-up (P > 0.05). Severe complications did not occur.
Conclusion
These results suggest that both anterior and posterior instrumentation attain good results for correction of the deformity and maintaining correction, foci clearance, spinal-cord decompression and pain relief in the treatment of thoracic and lumbar spinal TB providing that the opeartive indication is accurately identified. However, the posterior approach may be superior to anterior instrumentation to correct deformity and maintain that correction.
Introduction
In developing countries, there is a still high incidence of tuberculosis (TB), and the spine is involved more often than other skeletal sites [1]. It is generally accepted that spinal TB is the most dangerous of any bone and joint TB because of its ability to cause bone destruction, deformity and paraplegia [2]. Antituberculous chemotherapy is the mainstay of spinal TB treatment. Radical debridement combined with fusion and instrumentation is used in patients with neurological deficit, caseous abscesses or sequestered bone formation, instability and a kyphotic angle over 30° [3]. However, controversy remains regarding the best surgical approach and instrumentation modality. The purpose of this paper is to discuss the outcome of anterior and posterior instrumentation under different surgical management techniques for spinal TB.
Materials and methods
A total of 217 consecutive adult patients, treated in our hospital during January 2004 and December 2009 for thoracic and lumbar spinal TB, were reviewed. Complete data were available for 165 (76%) patients, with an an average age of 39 (16–67) years. Apart from eight patients with skipped lesions, the thoracic spine was involved in 52 patients, the thoracolumbar spine (T11–L2) in 38 and the lumbar spine in 67. The pathogenic vertebral levels consisted of four contiguous vertebrae in 11 (thoracic), three 45, two in 84 and a lesion in one vertebra in 17. A definitive diagnosis was made by histological examination of tissue removed at surgery. Two patients with pyogenic infection in the lumbar spine that could not be proved histologically were excluded from this study.
All the patients had chemotherapy in conjunction with radical debridement of anterior pathological vertebrae, plus anterior or posterior instrumentation with fusion. Seventy-four patients (group A) who had no large paraspinal abscess, especially patients with thoracic TB, had anterior instrument fixation (Table 1). Patients underwent anterior debridement, spinal-cord decompression, distraction to correct kyphosis and titanium cage filled with morsellised rib bone or large autoiliac bone, with or without costal grafting with one-stage anterior plate or screw-rod instrumentation. If the remaining part of the vertebra was less than 50% of the original, internal fixation screws were placed in adjacent normal vertebral body. Eighty-three patients (group B) who had large paraspinal abscess or in whom radical debridement posterolaterally or posteriorly was possible, especially patients with lumbar TB, had posterior instrument fixation with simultaneous or staged anterior radical debridement or posterolateral or posterior radical debridement plus titanium cage filled with morsellised rib, large autoiliac or costal arch bone grafting [4]. Pedicle screws were placed in the vertebra following the procedure used in group A patients. The remaining eight patients with skipped types had hybrid combined anterior–posterior instrumentation.
Table 1.
Patient data
| Group | No. cases | Sex | Age (years) | Distribution of pathologic vertebrae | |||
|---|---|---|---|---|---|---|---|
| F | M | Thoracic | Thoracolumbar | Lumbar | |||
| A | 74 | 41 | 33 | 38.3 ± 1.3 | 39 | 27 | 8 |
| B | 83 | 51 | 32 | 39.8 ± 1.3 | 13 | 11 | 59 |
| Total | 157 | 92 | 65 | 39.1 ± 1.4 | 52 | 38 | 67 |
The standard four-drug therapy of isoniazid (INH) (5 mg/kg), rifampicin (10 mg/kg), ethambutol (15 mg/kg) and pyrazinamide (25 mg/kg) was administered postoperatively as first-line treatment, continued for three months and followed by three-drug antituberculous treatment (rifampicin/INH/ethambutol) for at least nine months. Patients were instructed to notice any adverse reaction of antituberculous agents. One or two weeks after surgery, patients were permitted to sit on the bed or walk supported by a thoracolumbosacral orthosis; this orthsis support was maintained for three months. All patients were X-rayed, and erythrocyte sedimentation rate (ESR), hepatic function, etc., were examined at one month intervals in the first three months, three month intervals in the next nine months, six month intervals in the second year and then once a year. Patients were then followed up for 22–72 (average 37) months. Kyphotic angle was calculated on lateral spinal X-ray by modified Konstam’s method [5]. The chi-square test was used for noncontinuous variables, and Student’s t test was used to analyse the statistical significance between groups. P < 0.05 was considered to be statistically significant.
Results
In group A, 70 (94.6%) patients had obtained excellent or good outcome at the last follow-up; Frankel grade decreased significantly after the surgical procedure. No patient deteriorated, including those with no neurological deficit preoperatively (Table 2). Mean ESR values were 43.6 ± 6.8 mm/h preoperatively and 83.5 ± 7.1 mm/h postoperatively, and returned to normal at the last follow-up. Follow-up radiographs demonstrated obvious bone-defect remineralisation, maintenance of spinal alignment and stabilisation of the involved segment in all patients (Fig. 1). The average time to bone union at the final follow-up was 5.6 ± 1.2 months. Mean kyphotic angle of 22.1° was reduced to 10.6° postoperatively. At the most recent follow-up, correction loss was 6.8° ± 1.9°. One patient had wound dehiscence that was sutured secondarily. A sinus was found in one patient two weeks postoperatively, and no local abscesses or sequestra were identified by computed tomography (CT) scan or magnetic resonance (MR) examinations. The sinus closed after two months of dressing change. Four (5.4%) patients had major drug complications; three had abnormal liver function combined with gastrointestinal tract reaction in two and with abnormal renal function in one. After changing rifampicin for rifapentine (twice a week), all patients gradually recovered.
Table 2.
Frankel grade of patients in groups A and B with neurological deficit before surgery and at last follow-up
| Preoperative Frankel grade | No (group a, group b) | Franke grade at last follow-up (group a, group b) | ||||
|---|---|---|---|---|---|---|
| A | B | C | D | E | ||
| C | 5,6 | 2,2 | 3,4 | |||
| D | 6,8 | 6,8 | ||||
| E | 3,5 | 3,5 | ||||
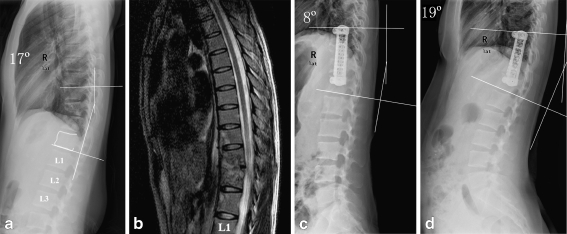
Fig. 1.
a–d A 33-year-old woman with spinal tuberculosis (TB) in the T9–12 vertebrae, especially T10–11. Three mobile segments were instrumented anteriorly, and 9° correction of kyphosis deformity was obtained after anterior radical debridement and titanium cage filled with morsellised rib-bone grafting and instrumentation in the sagittal plane. There was an 11° loss of correction in local kyphosis angle at the last visit. Preoperative a lateral radiograph and b sagittal magnetic resonance image (MRI), and c postoperative lateral and d 12-month follow up lateral radiographs
In group B, 78 (94.0%) patients obtained excellent or good outcome at final follow-up. No patients with neurological deficit preoperatively deteriorated postoperatively (Table 2). Mean ESR values were 42.7 ± 7.3 mm/h preoperatively and 81.8 ± 6.7 mm/h postoperatively and returned to normal at the last follow-up. Follow-up plain radiographs showed good posterolateral fusion masses, maintenance of spinal alignment and stabilisation of the involved segment in all cases (Fig. 2). The average time to bone union was 5.4 ± 1.5 months at the last follow-up. The average kyphotic angle of 7.4° was reduced to −6.2° postoperatively. At the most recent follow-up, correction loss was 6.1° ± 1.3°. One patient had dehiscence that was sutured secondarily. Two sinuses were found in two patients two and four weeks, respectively, after the operation, and no local abscesses or sequestra were identified by CT scan or MR examinations. The sinuses were closed after two months of dressing change. Five (6.0%) patients had major drug complications: three of them had abnormal liver function, two of those had combined gastrointestinal tract reaction, and the other two patients had abnormal renal function. After adjusting the chemotherapy regimen, all patients gradually recovered. Surgical results of each group are described in (Table 3).
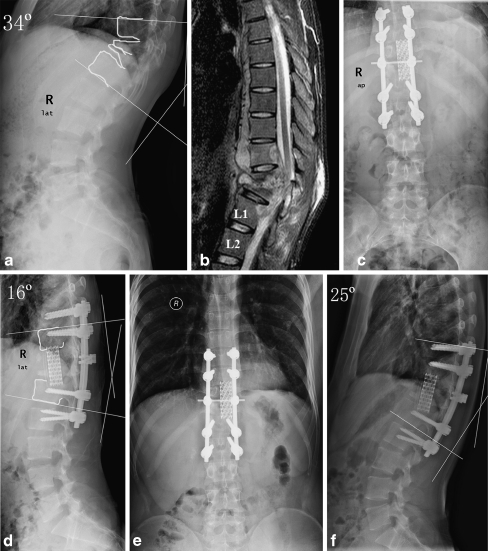
Fig. 2.
a–f A 23-year-old man with spinal tuberculosis (TB) in the T10–L1 vertebrae, especially in T11–12. Three mobile segments were instrumented posteriorly, and 18° correction of kyphosis deformity was obtained after anterior radical debridement and titanium cage filled with morsellised rib-bone grafting and posterior instrumentation in the sagittal plane. There was a 9° loss of correction in local kyphosis angle at the last visit. Preoperative a lateral radiograph and b sagittal magnetic resonance image (MRI), postoperative c anteroposterior and d lateral radiographs and12-month control e anteroposterior and f lateral radiographs
Table 3.
Surgical result of thoracic and lumbar spinal tuberculosis treated by anterior and posterior instrumentation
| Group A | Group B | P value | |
|---|---|---|---|
| No. patients | 74 | 83 | – |
| Erythrocyte sedimentation rate | |||
| Preoperative (mm/h) | 43.6 ± 6.8 | 42.7 ± 7.3 | >0.05 |
| Postoperative (mm/h) | 83.5 ± 7.1 | 81.8 ± 6.7 | >0.05 |
| 12 weeks postoperative (mm/h) | Normal | Normal | – |
| Excellent (normal life and work activities) and good (physical activities slightly limited ) results at final follow-up | 70 | 78 | >0.05 |
| No symptoms or signs of tuberculosis at final follow-up | 74 | 83 | >0.05 |
| No abnormal radiological findings of spinal tuberculosis at final follow-up | 74 | 83 | >0.05 |
| Fusion level | 4.11 ± 0.67 | 4.21 ± 1.06 | >0.05 |
| Fusion time of bone graft (months) | 5.6 ± 1.2 | 5.4 ± 1.5 | >0.05 |
| Average kyphosis angle (pre/postoperatively (°) | 22.1/10.6* | 7.4/-6.2* | <0.01/<0.01 |
| Average correction (°) | 11.5 ± 2.7 | 12.6 ± 1.2 | <0.01 |
| Average loss of correction, final follow-up (°) | 6.8 ± 1.9** | 6.1 ± 1.3** | <0.01 |
| Wound dehiscence | 1 | 1 | >0.05 |
| Sinus | 1 | 2 | >0.05 |
| Drug complications (liver and renal lesion; gastrointestinal tract reactions) | 4 | 5 | >0.05 |
*Statistically significant differences between preoperative and postoperative average kyphosis angle (Student’s t test, P < 0.01)
**Statistically significant differences between postoperative average kyphosis angle and that at last follow-up (Student’s t test, P < 0.01)
Discussion
Importance of instrumentation in surgical treatment of thoracic and lumbar spinal TB
The aims of treating spinal TB are to eradicate the infection, prevent or treat neurological deficits, correct kyphosis deformities, achieve normal sagittal contours of the spinal column and achieve unrestricted mobilisation and full patients’ activities of daily living as soon as possible [6]. To patients with severe spinal TB, vertebrae collapse resulting in spinal instability can undoubtedly increase kyphosis deformity and prolong the conservative treatment period and bed-rest time if chemotherapy alone is adopted. Kim et al. [7] reported 140 patients who were treated with radical anterior surgery in 1993. They obtained 51% initial correction of kyphosis, but the rate of correction dropped to 7.5% by the second year follow-up. In Benli et al’s. series, 72 adult patients with different surgical procedures were assessed. Eight patients underwent anterior debridement and fusion only, leading to an 8.6% correction rate and an average correction loss of 23.6° during follow-up. This compares with 76.8% average correction and 2.5° correction loss in 11 patients who had posterior instrumentation following anterior radical surgery [8]. Therefore, it is agreed that the insertion of strut grafts in the space after lesion debridement provides insufficient support anteriorly [9]. Efficacy and safety have been certified by both clinical and microbiological results, which suggest that there were no persisting or recurring cases of infection after instrumentation surgery, that there was little Mycobacterium tuberculosis adherent to the metal and only a little adherent biofilm was observed [10].
Lee et al. [11] reported that transpedicular instrumentation provided rapid relief of instability catch and excellent prevention of late angular deformity in patients with limited spinal-bone destruction. Chen et al. [12] extended the use of this technique in 12 patients with advanced spinal TB. It can be concluded from our study and other reports that early reconstruction of spinal instability plays an important role in treating active spinal TB [13]. Apart from increasing the stability of the spinal column, instrumentation should also help encourage neurological recovery, as rigid stabilisation of the spine has been shown experimentally to promote neurological recovery [14]. Our results confirm that neurological deficits clinically improved at least one grade according to the Frankel grading system after surgery in both anterior and posterior instrumentation groups. Lee et al. [15] and Broner et al. [16] reported that the immobilisation effect achieved through posterior instrumentation during the operation might also be useful in suppressing infection and that a relatively stable internal environment can prevent TB recurrence [17]. In our series, both anterior and posterior instrumentation arrested infection and promoted lesion healing, which is demonstrated by a significant decrease in ESR eight to 12 weeks after the procedures and satisfying rate of disease recurrence.
Option of instrumentation modality in the surgical treatment of thoracic and lumbar spinal TB
To date, consensus as to whether anterior or posterior instrumentation should be adopted in the treatment of spinal TB is not available in the literature [15, 18–20]. Many authors tend to emphasise the importance of tailoring management options to the needs of the individual patient [13]. Deciding upon the appropriate surgical procedure should be determined by the position and extent of foci; however, radical debridement remains the key [21]. In cases of incomplete focal excision, sinuses may appear, and there is the likelihood of failure of both bone grafting and internal fixation.
The surgical procedures we used in the series of thoracic and lumbar TB reported here and their corresponding indications are summarised as follows:
Anterior radical debridement and strut grafting with instrumentation is indicated in all patients—especially those with thoracic TB and those already treated—but is dangerous in patients with lumbarsacral spinal TB due to the complicated anatomy.
Posterolateral decompression and strut grafting with posterior instrumentation is indicated in patients with thoracic or thoracolumbar spinal TB whose lesion are located in the posterior part of the vertebral body or who are not fit for thoracotomy or are infirm or elderly.
Single-stage transpedicular decompression and posterior instrumentation is especially indicated in patients with lower lumbar spinal TB whose foci are located in the posterior part of the vertebral body, with compressed nerve roots and spinal cord, resulting in spinal stenosis, but without obvious caseous abscesses or sequestra in the anterior part of the vertebra body.
Anterior radical debridement and strut grafting with posterior instrumentation is indicated in patients with severe destruction by the lesion, resulting in the impossibility of anterior instrumentation, or in patients with severe lower lumbar kyphosis that requires lordosis correction and restoration, or those in whom initial anterior instrumentation failed.
Lee et al. [15] suggested that single-stage transpedicular decompression and posterior instrumentation is useful for treating early-diagnosed thoracic and thoracolumbar spinal TB. However, in our experience, this procedure may be more applicable for TB of the lower lumbar spinal region, where there is no spinal cord but cauda equina, instead, therefore presenting less operative risk. Jin et al. [2] advocated that anterior interbody autografting and instrumentation is indicated in patients who need anterior radical surgery, direct spinal-cord decompression and early reconstruction of spinal stability. Our experience suggests that it should be borne in mind that anterior instrumentation is dangerous to patients with large paravertebral abscess, whereas posterior instrumentation may be preferable because in such cases, M. tuberculosis is more or less adherent to the metal cage and thus forms a colony, which leads to failure of anterior instrumentation.
Comparison between outcome of anterior and posterior instrumentation in surgical treatment of thoracic and lumbar spinal TB
There are few articles in the literature comparing outcomes between anterior and posterior instrumentation in surgical management of thoracic and lumbar spinal TB. We feel the reasons may be as the follows: (1) Anterior and posterior instrumentation, respectively, have their surgical indications; therefore their outcomes cannot be compared because patients require different levels of treatment. (2) It is generally assumed that anterior instrumentation allows ideal correction of kyphosis deformity and maintenance of correction as well as posterior instrumentation. Nevertheless, we did this research because we believe our results will help determine which instrumentation modality—anterior or posterior—is best indicated for each individual patient.
Güven et al. [22] reported a series of ten cases in which posterior instrumentation was used and found there was a 3.4° loss in the correction of local kyphosis. Domaniç et al. [23] reported that series with anterior debridement, kyphosis correction was more successful in patients who underwent additional posterior Cotrel-Dubousset (CD) instrumentation. Moon et al. [24] and Chen et al. [25] respectively reported 44 and 29 patients with spinal TB who were treated by anterior radical surgery combined with posterior instrumentation and fusion. They achieved remarkable kyphosis correction, and loss of correction after surgery was negligible (1–3°). Conversely, Benli et al. [26] observed that anterior instrumentation increased the rate of kyphosis correction (79.7 ± 20.1%) and was effective in maintaining it, with an average loss of 1.1° ± 1.7°. In the report of Jin et al. [2], a mean of 18° of kyphosis correction was achieved in adult patients after anterior instrumentation surgery during the follow-up period. Zhao et al. [27] found kyphotic deformity was corrected by an average of about 16° using anterior instrumentation and fusion, and in the follow-up period, correction loss was 1° (6.3%). According to Karaeminogullari et al. [6], the mean correction loss at final follow-up (mean five years) following anterior radical debridement and fusion or posterolateral debridement, fusion and instrumentation alone was 45–50%, whereas the corresponding finding for anterior radical debridement and fusion plus posterior instrumentation and fusion was 12%. Lee et al. [15] reported that loss of kyphotic correction angle and loss of correction were statically significant (P < 0.05) following both anterior instrumentation and fusion and single-stage transpedicular decompression and posterior instrumentation. Kim et al. [28] operated on 21 patients with Pott’s disease using anterior instrumentation and found that, although a 67.7% correction (11.3°) was achieved initially, 83% correction (9.4°) was lost at the latest follow-up.
On the basis of these reports and our finding, that both anterior and posterior instrumentation can obtain good results in correcting the deformity and maintaining that correction, foci clearance, spinal cord decompression and pain relief in the treatment of thoracic and lumbar spinal TB providing that the operative indication for instrumentation is correctly chosen. Furthermore, we found that posterior may be superior to anterior instrumentation for correcting kyphosis deformity and maintaining that correction in the thoracic and lumbar spine. In our series, correction loss following anterior instrumentation was 6.8° ± 1.9°, which compares with 9.4° loss in Kim et al’s. series [28]. Correction loss following posterior instrumentation in our study was 6.1° ± 1.3°, which compares with 3.4° loss in Güven et al’s. series [22]. The reason for this might be that pedicle screws cross the vertebral body pedicle, the strongest part of the vertebral body, providing 3D correction and stabilisation, which is much stronger than anterior instrumentation. Although anterior instrumentation is used more often in the thoracic region, which has support of the bony thorax, the screw fixed in the vertebral body cannot provide the same strength as the pedicle screw. Another disadvantage of anterior instrumentation is that inclusion of an unnecessarily large number of levels into the fusion cannot be avoided when the extent of vertebral body destruction exceeds 50%, otherwise the screw would become loose if placed in the pathological vertebral body [22]. This would result in an unacceptable excessive loss of spinal movement. Nevertheless, posterior instrumentation can overcome the shortcomings of anterior instrumentation because only the pathological segments are fused, as the pedicle screws are placed in adjacent normal vertebral bodies and can be removed postoperatively without leading to excessive movement loss in the spine.
Finally, we again emphasise that the antituberculous chemotherapeutic regimen is the most important element in treating spinal TB and in guaranteeing operative success. We suggest the standard chemotherapy protocol with a total course of treatment lasting for at least 12 months (3HRZE/9HRE), because multicentre prospective control studies are still lacking regarding the efficacy of short-course antituberculous chemotherapy. It should be noted that many recurrences of spinal TB are related to an inadequate chemotherapy protocol.
Footnotes
The Co-first authors are Yuan Zheng Ma and Xu Cui
References
- 1.Nagashima H, Yamane K, Nishi T, et al. Recent trends in spinal infections: retrospective analysis of patients treated during the past 50 years. Int Orthop. 2010;34:395–399. doi: 10.1007/s00264-009-0741-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jin D, Dongbin Qu, Chen J, et al. One-stage anterior interbody autografting and instrumentation in primary surgical management of thoracolumbar spinal tuberculosis. Eur Spine J. 2004;13:114–121. doi: 10.1007/s00586-003-0661-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Swanson AN, Pappou IP, Cammisa FP, et al. Chronic infections of the spine: surgical indications and treatments. Clin Orthop Relat Res. 2006;444:100–106. doi: 10.1097/01.blo.0000203447.44146.55. [DOI] [PubMed] [Google Scholar]
- 4.Huang Qi-Shan, Zheng C, Yuezheng Hu, et al. One-stage surgical management for children with spinal tuberculosis by anterior decompression and posterior instrumentation. Int Orthop. 2009;33:1385–1390. doi: 10.1007/s00264-009-0758-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rajasekaran S, Shanmugasundaram TK. Prediction of the angle of gibbus deformity in tuberculosis of the spine. J Bone Joint Surg Am. 1987;69:503–508. [PubMed] [Google Scholar]
- 6.Karaeminogullari O, Aydinli U, Ozerdemoglu R, et al. Tuberculosis of the Lumbar Spine: Outcomes After Combined Treatment of Two-drug Therapy and Surgery. Orthopedics. 2007;30(1):55–59. doi: 10.3928/01477447-20070101-15. [DOI] [PubMed] [Google Scholar]
- 7.Kim BJ, Ko HS, Lim Y, et al. The clinical study of the tuberculous spondylitis. J Korean Orthop Assoc. 1993;28:2221–2232. [Google Scholar]
- 8.Benli IT, Kıs M, Akalın S, et al. The results of anterior radical debridement and anterior instrumentation in Pott’s disease and comparison with other surgical techniques. Kobe J Med Sci. 2000;46:39–68. [PubMed] [Google Scholar]
- 9.Faraj AA. Anterior instrumentation for the treatment of spinal tuberculosis (letter) J Bone Joint Surg Am. 2001;83A:463. doi: 10.2106/00004623-200103000-00027. [DOI] [PubMed] [Google Scholar]
- 10.Oga M, Arizono T, Takasita M, et al. Evaluation of the risk of instrumentation as a foreign body in the spinal tuberculosis: clinical and biologic study. Spine. 1993;18:1890–1894. doi: 10.1097/00007632-199310000-00028. [DOI] [PubMed] [Google Scholar]
- 11.Lee TC, Lu K, Yang LC, et al. Transpedicular instrumentation as an adjunct in the treatment of thoracolumbar and lumbar spine tuberculosis with early stage bone destruction. J Neurosurg. 1999;91:163–169. doi: 10.3171/spi.1999.91.2.0163. [DOI] [PubMed] [Google Scholar]
- 12.Chen YC, Chang MC, Wang ST, et al. One-stage posterior surgery for treatment of advanced spinal tuberculosis. J Chin Med Assoc. 2003;66:411–417. [PubMed] [Google Scholar]
- 13.Yilmaz C, Selek HY, Gurkan I, et al. Anterior instrumentation for the treatment of spinal tuberculosis. J Bone Joint Surg Am. 1997;81A:1261–1267. doi: 10.2106/00004623-199909000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Al-Sebai MW, Al-Khawashki H, Al-Arabi K, et al. Operative treatment of progressive deformity in spinal tuberculosis. Int Orthop. 2001;25:322–325. doi: 10.1007/s002640100259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee Sun-Ho, Sung J-K, Park Y-M. Single-stage Transpedicular Decompression and Posterior Instrumentation in Treatment of Thoracic and Thoracolumbar Spinal Tuberculosis (A Retrospective Case Series) J Spinal Disord Tech. 2006;19:595–602. doi: 10.1097/01.bsd.0000211241.06588.7b. [DOI] [PubMed] [Google Scholar]
- 16.Broner FA, Garland DE, Zigler JE. Spinal infection in the immunocompromised host. Orthop Clin North Am. 1996;27:37–46. [PubMed] [Google Scholar]
- 17.Altman GT, Altman DT, Frankovitch KF. Anterior and posterior fusion for children with tuberculosis of the spine. Clin Orthop. 1996;325:225–231. doi: 10.1097/00003086-199604000-00027. [DOI] [PubMed] [Google Scholar]
- 18.Talu U, Gogus A, Ozturk C. The role of posterior instrumentation and fusion after anterior radical debridement and fusion in the surgical treatment of spinal tuberculosis: experience of 127 cases. J Spinal Disord Tech. 2006;19:554–559. doi: 10.1097/01.bsd.0000211202.93125.c7. [DOI] [PubMed] [Google Scholar]
- 19.Issack PS, Boachie-Adjei O (2011) Surgical correction of kyphotic deformity in spinal tuberculosis. Int Orthop Jun 15 (Epub ahead of print) - doi:10.1007/s00264-011-1292-9 [DOI] [PMC free article] [PubMed]
- 20.Jain Ak, Jain S (2011) Instrumented stabilization in spinal tuberculosis. Int Orthop Jul 1, (Epub ahead of print) - doi:10.1007/s00264-011-1296-5 [DOI] [PMC free article] [PubMed]
- 21.Wang Z, Ge Z, Jin W, et al. Treatment of spinal tuberculosis with ultrashort-course chemotherapy in conjunction with partial excision of pathologic vertebrae. Spine J. 2007;7:671–681. doi: 10.1016/j.spinee.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 22.Güven O, Kumano K, Yalcin S, et al. A single stage posterior approach and rigid fixation for preventing kyphosis in the treatment of spinal tuberculosis. Spine. 1994;19:1039–1043. doi: 10.1097/00007632-199405000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Domanic U, Hamzaoglu A, Sar C, Yavuzer Y. Posterior fusion and instrumentation after anterior radical debridement and fusion in the surgical treatment of Pott’s disease. J Turkish Spine Surg. 1993;4:16–19. [Google Scholar]
- 24.Moon MS, Woo YK, Lee KS, et al. Posterior instrumentation and anterior interbody fusion for tuberculous kyphosis of dorsal and lumbar spines. Spine. 1995;20:1910–1916. doi: 10.1097/00007632-199509000-00013. [DOI] [PubMed] [Google Scholar]
- 25.Chen WJ, Wu CC, Jung CH, Chen LH, et al. Combined anterior and posterior surgeries in the treatment of spinal tuberculous spondylitis. Clin Orthop Relat Res. 2002;398:50–59. doi: 10.1097/00003086-200205000-00008. [DOI] [PubMed] [Google Scholar]
- 26.Teoman Benli İ, Acaroğlu E, Akalin S, et al. Anterior radical debridement and anterior instrumentation in tuberculosis spondylitis. Eur Spine J. 2003;12:224–234. doi: 10.1007/s00586-002-0403-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao J, Lian XF, Hou TS, et al. Anterior debridement and bone grafting of spinal tuberculosis with one-stage instrumentation anteriorly or posteriorly. Int Orthop. 2007;31:859–863. doi: 10.1007/s00264-006-0253-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim KS, Ko SH, Youm KS, et al. Anterior spinal instrumentation in treatment of spinal tuberculosis. J Korean Orthop Assoc. 1998;33:1560–1568. [Google Scholar]