Abstract
Spinal tuberculosis (TB) produces neurological complications and grotesque spinal deformity, which in children increases even with treatment and after achieving healing. Long-standing, severe deformity leads to painful costo-pelvic impingement, respiratory distress, risk of developing late-onset paraplegia and consequent reduction in quality and longevity of life. The treatment objective is to avoid the sequelae of neural complications and achieve the healed status with a near-normal spine. In TB, the spine may become unstable if all three columns are diseased. Pathological fracture/dislocation of a diseased vertebral body may occur secondary to mechanical insult. Surgical decompression adds further instability, as part of the diseased vertebral body is excised. The insertion of a metallic implant is to provide mechanical stability and the use of an implant in tubercular infection is safe. Indications for instrumented stabilisation can be categorised as: (a) pan vertebral disease, in which all three columns are diseased; (b) long-segment disease, in which after surgical decompression a bone graft >5 cm is inserted with instrumentation to prevent graft-related complications and consequent progression of kyphosis and neural complications and (c) when surgical correction of a kyphosis is performed when both anterior decompression and posterior column shortening is required. The implant choice should be individualised according to the case. Pedicle screw fixation in kyphus correction in healed disease is a most suitable implant. Hartshill sublaminar wiring stabilisation in active disease is a suitable implant to stabilise the spine, taking purchase against healthy posterior complex of the vertebral body to save a segment.
Introduction
Spinal tuberculosis (TB) produces neurological complications and grotesque spinal deformity [1, 2] which is caused secondary to damage by infection. Even after achieving the healed status, spinal deformities continue to advance during growth due to the biomechanical stresses on the structurally weakened vertebral column and produce severe degenerative changes in the proximal and distal segments of the spine [1, 2]. The spinal cord undergoes intrinsic changes that produce late-onset paraplegia, with consequent poor chances of neural recovery after surgery [3]. In the pre-antibiotic era, the objective of treatment was to attain disease quiescence by natural immunity. With the introduction of antitubercular drugs, the treatment objective became achieving the healed status, but there was a resulting sequelae of kyphosis. Now, the objective is to cure the disease, with no sequelae of neural complications and an almost near-normal spine.
Kyphosis in spinal TB continues to deteriorate with conservative treatment in all cases and 3–5% of cases will have severe progression [4, 5]. 44% of children experience spinal deformity progression on prolonged follow-up despite having achieved disease healing [1, 4]. Some also develop neurological complications. This suggests that, besides the infective process, certain biomechanical considerations render the spine unstable and predisposes it to mechanical damage. The vertebral body or diseased segment of the spine is the weakest when treatment is started [6–8]. The infective process is halted under cover of anti-TB drugs and repair begins. The spine must be protected by suitable external braces until it attains structural strength. Surgically treatable lesions are debrided, and the resultant gap is grafted using a tricortical cortico-cancellous bone graft, which is weakest when first inserted. The reparative process, revascularisation and bone-graft incorporation takes several months. During this time, the spine needs to be protected either by an external corset or by instrumented stabilisation at the same time or during a second stage in order to obviate graft-related complications, such as slippage/breakage and consequent deterioration of kyphosis and neural complications [6]. The behaviour of the bone graft following uninstrumented anterior decompression in spinal TB was evaluated. The bone graft provides sufficient stability and structural support in only 41% of patients with a short defect. The need for an external splint was suggested when the bone graft exceeds 5 cm (two-disc heights) to prevent graft-related complications [1, 4]. Instrumented stabilisation also allows earlier ambulation and better rehabilitation, reduces morbidity and promotes a notable improvement in pain relief and self-confidence.
Spinal instability
The spine is considered unstable if two columns are disrupted, as in spinal trauma [9]. In vertebral fractures, the force of trauma acutely disrupts both columns, and the spine is maximally unstable on the day of trauma. Chronic inflammation of the two columns, as in spinal TB, may not always render the spine unstable because tissues show a concomitant healing response despite destruction by the infective process. Once a mechanical insult in the form of pathological fracture of a diseased vertebral body occurs, the spine becomes grossly unstable. Tubercular lesions in which all three columns are diseased are potentially unstable and unable to resist transitory stresses, which may lead to pathological subluxation/dislocation of the spinal column with resultant gross neural deficit. Similarly, laminectomy performed for a diseased vertebral body leads to increased neural deficit and consequent paraplegia [2].
Biomechanical stresses are more evident in tubercular lesions of junctional areas, such as the cervico-dorsal, dorso-lumbar, or lumbo-sacral spine, as they are the junction of a fixed and a mobile segment, and spinal curvature is changed from kyphosis to lordosis or vice versa [10]. Surgical decompression adds further instability, as part of the diseased vertebral body is excised to achieve spinal-cord decompression [2].
Metal and tuberculosis
Oga et.al observed no persistent or recurrent infection after surgery in patients with spinal TB treated with posterior spinal Instrumentation. The adherence property of the mycobacterium TB to stainless steel was evaluated experimentally. Few mycobacteria adhere to the stainless steel, whereas Staphylococcus heavily colonizes on stainless steel. Pyogenic organisms form a thick biofilm, whereas mycobacteria show a scanty biofilm. Hence, the use of implant in the presence of TB infection is theoretically safe [11].
Instrumented stabilisation is not used in all cases of spinal TB. Jain et al. analysed all articles in which instrumented stabilisation was reported over the last 20 years [2, 12]. Stabilisation was mostly performed to prevent on treatment the deterioration of kyphosis. There were 1,097 patients stabilised by either anterior or posterior instrumentation in 123 analysed series. Data was incomplete regarding the indication for instrumentation, choice of implant , number of vertebral bodies affected, pre-operative kyphosis and final kyphosis in the majority of the cases. Stabilisation was initially reported with Harrington distraction rods or sublaminar segmental wiring in the case of circumferential spine involvement or where laminectomy was performed in an anterior disease [13, 14]. In later series, posterior stabilisation by Harrington rods or Luque segmental wiring was reported to prevent graft related complications and eventual kyphosis progression in cases where pre-operative vertebral body loss was greater or the graft length after surgical debridement exceeded two disc heights [15, 16]. Anterior instrumentation (n = 635) was inserted in all segments of the spine. The mean vertebral body involvement was 25.35% (n = 161) cases, with >51% of cases having one vertebral body affected and 49% having two or more. Mean preoperative kyphosis was 25.35°, immediate postoperative kyphosis was 9.08° and final kyphosis was 12.97°. There was an overall 2.3° kyphosis progression after surgery [12].
Posterior instrumentation has been described after anterior decompression surgery as either a single- or two-stage procedure. Mean kyphosis correction achieved was 19.03° and at follow-up, correction loss was 2°. Overall, most of these cases involved only short-segment disease, with initial kyphosis of 30–35°, which could be reduced to 15–18° by instrumented stabilisation [12].
In the last 5 years, many articles have been published in which the principles of kyphosis correction in active and healed disease are described. MEDLINE and Google searches (2000–2011) were performed to identify review articles and case series published in English-language journals regarding instrumented stabilisation with the terms instrumented stabilisation, spinal TB, Pott’s disease." Studies with less than five cases were excluded. Additional studies were identified from review articles and articles cited in original papers. Abstracts, letters to the editor and unpublished data were not considered. Forty-one studies were identified that met the inclusion criteria. The spinal instrumentation used could be broadly categorised into the following four groups [2, 12].
Posterior instrumentation and anterior decompression performed as two separate approaches and one- or two-stage operations
Overall the mean preoperative kyphosis was corrected from 28.3° to 16.17° by posterior instrumentation and anterior debridement performed by two separate approaches and one-stage procedures in 668 patients [11, 17–35]. The correction attained in healed disease (n = 80) was reported in five series [17, 18, 25, 28, 31], whereas the remaining patients underwent instrumentation during active disease. In two of the series, a two-stage procedure was initially used, but as the surgeon gained expertise, the procedure was done in a single stage [17, 18]. Seventy-one patients (three series) underwent a two-stage procedure, with instrumentation performed after 2–3 weeks following debridement [22–24]. Kyphosis correction was maintained well in those with active disease. These patients required turning from the lateral to the prone position after anterior debridement for posterior instrumentation surgery.
However, if in situ posterior instrumentation is done first, then the degree of kyphosis correction will be less than desired [17, 18]. Erturer et al. reported simultaneous posterior–anterior–posterior surgery in a multilevel TB spondylitis associated with severe kyphosis. The authors used two approaches. Initially pedicle screws and rods were inserted via a midline posterior approach in the prone position, and the patient was then shifted to the lateral position for abscess drainage through an anterior exposure, from the right side during a thoracotomy for dorsal lesions and through the left side, via the thoracoabdominal approach, for dorsolumbar lesions. After debridement, the posterior incision was opened again and the kyphotic deformity corrected. A titanium mesh cage filled with spongious allograft bone chips was placed in the anterior corpectomy site, and appropriately contoured rods were placed posteriorly. Kyphosis correction was 35.1° [25]. Posterior instrumentation performed as a second stage following anterior decompression was associated with increased operating time, leading to greater blood loss, prolonged anesthesia and increased postoperative morbidity. None of the major complications related to posterior instrumentation were reported, such as loosening, breakage and implant prominence, apart from approach-related complications such as infection, skin necrosis and wound dehiscence. [11, 17–35].
Posterior instrumentation with transpedicular or anterolateral anterior debridement
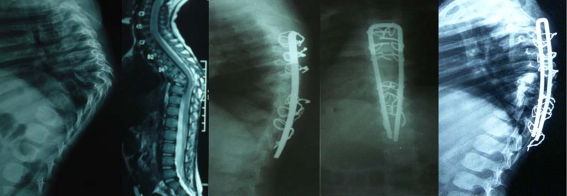
In this method, exposure of the spine is performed by midline posterior incision and pedicle screws are fixed [36–46]. Anterior decompression is performed anterolaterally after a costo-transversectomy or through the bed of the pedicles (Figs. 1, 2). These 11 series (n = 324) reported good kyphosis correction from mean pre-operative angle of 43.9° to post-operative 19.1°. Forty-four patients in three series [41, 43, 46] underwent transpedicular or anterolateral debridement, along with posterior instrumentation for active disease. The remainder had healed disease. Most of the recent studies used pedicle screw fixation; however Laheri et al. used the Hartshill system [36]. Complications were related to posterior instrumentation, such as loosening, breakage and prominence of the implant. There were also approach-related complications, such as infection, skin necrosis and wound dehisence. This approach is demanding and is associated with prolonged operative time and more extensive blood loss [36–46].
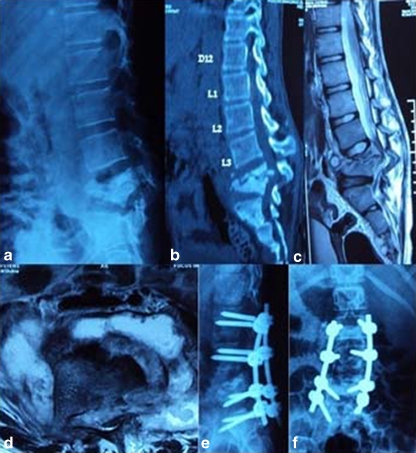
Fig. 1.
Pre-operative lateral (a) X-rays and sagittal reconstructed computed tomography (CT) (b), Midsaggitial T2-W1 (c) and axial (d) magnetic resonance image (MRI) of a 16-year old girl with Pott’s disease at L4–L5 showing reversal of normal lumbar lordosis to kyphosis of 50°. Immediate lateral (e) and anteroposterior (AP) (f) X-ray of the patient after anterior radical debridement and posterior instrumentation using pedicle screw fixation via the transpedicular approach
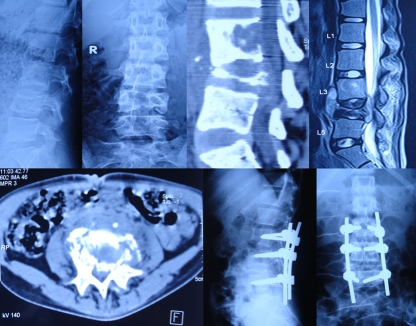
Fig. 2.
Pre-operative lateral (a) and anteroposterior (AP) (b) X-rays and midsagittal reconstructed computed tomography (CT) (c) and midsagittal T2-W1 magnetic resonance imaging (MRI) (d) and axial CT scan (e) of a 15-year old girl with spinal tuberculosis of L4–L5 showing reversal of normal lumbar lordosis to kyphosis of 30°. The lumbar kyphosis was corrected to 0° by pedicle subtraction osteotomy and stabilised with posterior pedicle screw fixation (f, g)
Posterior instrumentation and anterior decompression by single incision
Anterior decompression and concomitant posterior instrumentation with or without posterior column shortening was performed (Figs. 2, 3). The advantages suggested were simultaneous visualisation of anterior and posterior columns of the spine to achieve adequate decompression and posterior stabilisation [6, 10]. Of the cumulative 54 patients treated by this approach, 15 underwent instrumentation during active disease but had no kyphosis and 39 underwent kyphosis correction during active disease. The spine was instrumented with Hartshill rectangle and sublaminar wires. The mean pre-operative kyphosis of 51.6° was corrected to 27°. Complications were mainly approach-related, such as wound dehiscence at the T junction and wire breakage and loosening. [6, 10].
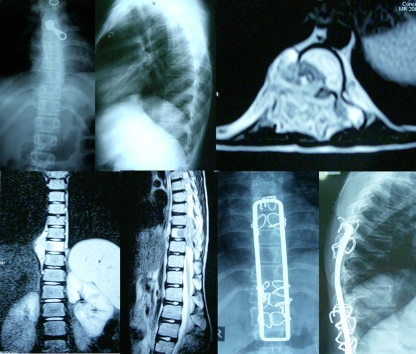
Fig. 3.
Pre-operative T2-W1 anteroposterior (AP) (a) and lateral (b) X-rays, along with axial (c), coronal (d) and midsagittal T2-W1 (e) magnetic resonance image (MRI) of an 18-year-old woman with Pott’s disease showing panvertebral involvement of the D 7 vertebra and destruction of the right pedicle. Postoperative AP (f) and lateral (g) X-rays after anterolateral decompression and debridement and posterior instrumentation by Hartshill fixation and sublaminar wires
Anterior instramentation stabilisation
Anterior instrumentation with plate or rods are described, with suggestion that this approach decreases operating time, blood loss and postoperative morbidity and are performed with a single approach [21, 33–35, 47–55]. In the anterior instrumentation group, there was only one series(n = 22) [55], and in that series, anterior instrumentation was done during healed disease in only six cases; 811 patients were operated upon during active disease stage. It is difficult to eradicate the fear that infection will persist beneath the metal if it is placed in the area of infection. Benli et al. compared results of anterior plate and rod systems after debridement and fusion and found that there were no significant differences between the two systems in terms of sagittal alignment reconstruction and fusion rate [21]. Dual-rod designs may offer greater adjustability and control over screw placement as well as increased load sharing, but possibly at the expense of rigidity. Plate systems are designed to be stiffer and less prone to fatigue failure, but there are theoretical concerns and unanswered questions regarding the risk of pseudoarthrosis and device-related osteopaenia with very rigid spinal implants [21]. The authors reported no significant differences between the two instrumentation systems in terms of sagittal alignment, reconstruction and fusion rate [21, 33–35, 47–55]. The limitation with these studies was the limited kyphosis correction of 15–20° from 30°. Cumulative pre-operative kyphosis in the anterior instrumentation group (n = 811) was 23°, which was corrected to 9.7°. Complications associated with anterior instrumentation are cage dislodgement, major vessel injury, screw displacement and injury to visceral organs such as oesophageal perforation and Zenker’s diverticulum due to anterior screws [21, 33–35, 47–55].
Indications for instrumented stabilisation
Overall, after review of the literature and the author’s personal experience, the indications for instrumented stabilisation are [2, 6, 10, 12]:
panvertebral disease;
long-segment disease;
surgical correction of kyphosis;
miscellaneous.
Panvertebral disease
The spine is potentially unstable in TB lesions of all three columns, with consequent risk of pathological subluxation/dislocation and paraplegia. It can be suspected radiologically by absence of a pedicle shadow and minimal scoliosis in anteroposterior (AP) x-rays, along with vertebrae body (VB) disease [56]. Prophylactic stabilisation is indicated in such cases, with anterior decompression if pathological dislocation of the spine has occurred. (Fig. 3).
Long-segment disease
Instrumented stabilisation is indicated to provide mechanical stability to anterior bone graft in a spinal TB lesion with disease of four or more vertebral bodies (length of bone graft >4–5 cm) and anterior decompression and fusion is contemplated (Fig. 4).
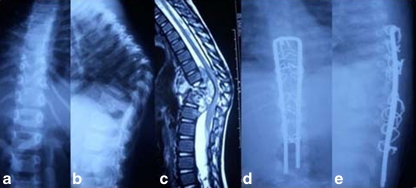
Fig. 4.
Preoperative anterorposterior (AP) (a) and lateral (b) X-rays, along with midsagittal T2-W1 magnetic resonance image (MRI) (c) of a 10-year-old boy with long-segment disease with dorsal spine kyphosis of 70°. Postoperative AP (d) and lateral (e) X-rays after kyphosis correction by sequential anterior decompression using corpectomy, shortening of the posterior column, posterior instrumentation using Hartshill’s system and sublaminar wires and anterior and posterior bone grafting
Kyphosis correction
Kyphosis correction in active disease can be performed by anterior corpectomy, posterior column shortening, anterior fusion, posterior fusion and posterior instrumentation (Fig. 5).
Fig. 5.
Pre-operative lateral (a) X-rays and midsagittal T2-W1 magnetic resonance imaging (MRI) (b) of a 6-year-year-old girl with dorsal spine kyphosis of 60°. Postoperative lateral (c) and anteroposterior (AP) (d) X-rays after kyphosis correction using sequential anterior decompression via corpectomy, shortening of the posterior column, posterior instrumentation using an open Hartshill system with sublaminar wires, and anterior and posterior bone grafting. At 18 months of follow-up, lateral (e) X-ray of the same patient showing migration of the Hartshill system proximally, indicating that it does not hamper the child’s growth
Miscellaneous indications
In cervical-spine disease, anterior decompression, bone grafting and anterior cervical plate fixation is adequate following lesion debridement. Many studies describe bone defects after anterior debridement that are bridged by a titanium cage filled with bone graft on the supposition that the cage resists sinking in VB disease with consequent kyphosis increase. However, insertion of tricortical corticocancellous iliac crest graft has also been reported, with minimal sinking in diseased VB when concomitant posterior instrumented stabilisation has been done.
Implant choice
Implant choice should be individualised according to the case. The anterior plate or rods and screws can be used in short-segment disease. As healthy vertebral bodies are necessary above and below the diseased segment to acquire purchase, this system can be used in mild to moderate kyphosis only. Anterior instrumentation can only be used when disease affects the anterior and middle columns only and the posterior column is healthy. In panvertebral disease, anterior instrumentation alone does not provide mechanical stability. Hence, stabilisation by posterior instrumentation is indicated. Regional osteopaenia is the essential feature of the TB lesion. Hence, the screw should span the healthy vertebral body, with good bone stock to provide mechanical stability.
Anterior instrumentation appears to be more advantageous than posterior instrumentation, as both instrumentation and grafting are done as single-stage surgery through the same incision, minimising total blood loss and surgery time with no risk of the graft slipping out due to turning the patient for posterior instrumentation. It also prevents fusing an unnecessarily large number of levels. However, the problems with this approach are lack of adequate space to insert the anterior implants and the possible problem of prominent hardware impaling the great vessels, particularly in the thoracic spine. Further, retrieval of dislodged anterior implant is more risky in view of scarring adjacent to major vessels [57, 58].
The posterior pedicle screw can be applied, ensuring insertion into relatively healthy vertebrae. Otherwise, it may cut through when corrective forces are applied to correct kyphosis in active disease. When correcting kyphosis in healed disease, pedicle screws in two vertebrae on either side is adequate. Hartshill instrumentation can be applied, gaining purchase against a healthy posterior complex spanning between one healthy segment above and below the diseased segment. Isolated use of cages has been described in a disease focus with the aim of preventing bone graft sinking or dislodgement. The risk of sinking/dislodgement can be minimised if tricortical corticocancellous bone graft is used. Dislodgement of cages has been reported, and there are greater risks of this occurring when they are placed in diseased vertebral bodies. Also, they may damage major vessels in their vicinity [57, 58]. Retrieving dislodged cages is dangerous and risky. Isolated insertion of cages should be avoided; whenever one is placed, however, it should be supported by anterior–posterior instrumentation.
Conclusion
Instrumented stabilisation is safe in spinal TB. It is indicated in selected cases to prevent during treatment (with or without surgery) kyphosis deterioration and graft-related complications, such as graft slippage or breakage with consequent kyphosis progression and neural deterioration. Panvertebral disease, long-segment TB lesions and kyphosis correction are the most appropriate indications for instrumented stabilisation. Posterior surgery only is gaining acceptance. Pedicle screw fixation for kyphosis correction in healed TB disease is the most suitable implant. Hartshill sublaminar wiring stabilisation in active disease is a suitable implant to stabilise the spine, ensuring purchase against the healthy posterior complex of the vertebral body to save a segment. The low cost of the Hardsill system also supports its use in low-income countries where facilities are scarce and spinal TB is prevalent.
References
- 1.Rajasekaran S. The problem of deformity in spinal tuberculosis. Clin Orthop Relat Res. 2002;398:85–92. doi: 10.1097/00003086-200205000-00012. [DOI] [PubMed] [Google Scholar]
- 2.Jain AK. Tuberculosis of spine (2010) A fresh look at an old disease. J Bone Joint Surg Br. 2010;92:905–913. doi: 10.1302/0301-620X.92B7.24668. [DOI] [PubMed] [Google Scholar]
- 3.Tuli SM. Severe kyphotic deformity in tuberculosis of the spine. Int Orthop. 1995;19:327–331. doi: 10.1007/BF00181121. [DOI] [PubMed] [Google Scholar]
- 4.Rajasekaran S, Shanmugasundaram TK. Prediction of the angle of gibbus deformity in tuberculosis of the spine. J Bone Joint Surg Am. 1987;69:503–509. [PubMed] [Google Scholar]
- 5.Guven O. Severe kypootic deformity in tuberculosis spine. Int Orthop. 1996;20:271. [PubMed] [Google Scholar]
- 6.Jain AK, Dhammi IK, Prashad B, Sinha S, Mishra P. Simultaneous anterior decompression and posterior instrumentation of the tuberculosis spine using an anterolateral extrapleural approach. J Bone Joint Surg Br. 2008;90:1477–1481. doi: 10.1302/0301-620X.90B11.20972. [DOI] [PubMed] [Google Scholar]
- 7.Altman GT, Altman DT, Frankovitch KF. Anterior and posterior fusion for children with tuberculosis of spine. Clin Orthop. 1996;325:225–231. doi: 10.1097/00003086-199604000-00027. [DOI] [PubMed] [Google Scholar]
- 8.Mehta JS, Bhojraj SY. Tuberculosis of thoracic spine a classification based on the selection of surgical strategies. J Bone Joint Surg Br. 2001;83B:859–863. doi: 10.1302/0301-620X.83B6.11142. [DOI] [PubMed] [Google Scholar]
- 9.Jain AK, Dhammi IK. Tuberculosis of the spine: a review. Clin Orthop Relat Res. 2007;460:39–49. doi: 10.1097/BLO.0b013e318073bd29. [DOI] [PubMed] [Google Scholar]
- 10.Jain AK, Maheshwari AV, Jena S. Kyphus correction in spinal tuberculosis. Clin Orthop Relat Res. 2007;460:117–123. doi: 10.1097/BLO.0b013e318073bd29. [DOI] [PubMed] [Google Scholar]
- 11.Oga M, Arizono T, Takasita M, Sugioka Y. Evaluation of the risk of instrumentation as a foreign body in spinal tuberculosis: Clinical and biologic study. Spine. 1993;18:1890–1894. doi: 10.1097/00007632-199310000-00028. [DOI] [PubMed] [Google Scholar]
- 12.Jain AK, Dhammi IK, Jain S, Mishra P. Kyphosis in spinal tuberculosis – prevention and correction. Indian J Orthop. 2010;44(2):127–136. doi: 10.4103/0019-5413.61893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harda BE. The classic wiring of the vertebrae as a means of immobilization in fractures and Pott’s disease. Clin Orthop. 1975;112:4–8. [PubMed] [Google Scholar]
- 14.Harrington RR. The history and development of harrington instrumentation. Clin Orthop. 1988;227:3. [PubMed] [Google Scholar]
- 15.Luque E. The anatomical basis and development of segmental spinal instrumentation. Spine. 1982;7:256–259. doi: 10.1097/00007632-198205000-00010. [DOI] [PubMed] [Google Scholar]
- 16.Panjabi M, Abumi K, Durancean J, Crisco J. Biomechanicl evaluation of spinal fixation devices - stability provided by eight internal fixation devices. Spine. 1988;13:1135–1140. doi: 10.1097/00007632-198810000-00014. [DOI] [PubMed] [Google Scholar]
- 17.Moon MS, Woo YK, Lee KS, Ha KY, Kim SS, Sun DH. Posterior instrumentation and anterior interbody fusion for tuberculous kyphosis of dorsal and lumbar spines. Spine. 1995;20:1910–1916. doi: 10.1097/00007632-199509000-00013. [DOI] [PubMed] [Google Scholar]
- 18.Louw JA. Spinal tuberculosis with neurological deficit: Treatment with anterior vascularised rib grafts, posterior osteotomies and fusion. J Bone Joint Surg Br. 1990;72:686–693. doi: 10.1302/0301-620X.72B4.2380228. [DOI] [PubMed] [Google Scholar]
- 19.Sundararaj GD, Behera S, Ravi V, Venkatesh K, Cherian VM, Lee V. Role of posterior stabilisation in the management of tuberculosis of the dorsal and lumbar spine. J Bone Joint Surg Br. 2003;85:100–106. doi: 10.1302/0301-620X.85B1.13300. [DOI] [PubMed] [Google Scholar]
- 20.Wang B, Ozawa H, Tanaka Y, Matsumoto F, Aizawa T, Kokubun S. One-stage lateral rhachotomy and posterior spinal fusion with compression hooks for Pott’s paralysis in the elderly. J Orthop Surg. 2006;14(3):310–314. doi: 10.1177/230949900601400314. [DOI] [PubMed] [Google Scholar]
- 21.Benli IT, Kis M, Akalın S, Citak M, Kanevetci S, Duman E. The results of anterior radical debridement and anterior instrumentation in Pott's disease and comparison with other surgical techniques. Kobe J Med Sci. 2000;46:39–68. [PubMed] [Google Scholar]
- 22.Wen-Jer C, Chi-Chuan W, Chi-Hsiung J, Lih-Huei C, Chi-Chien N, Po-Liang L. Combined anterior and posterior aurgeries in the treatment of spinal tuberculous spondylitis. Clin Orthop Relat Res. 2002;398:50–59. doi: 10.1097/00003086-200205000-00008. [DOI] [PubMed] [Google Scholar]
- 23.Hirakawa A, Miyamoto K, Masuda T, Fukuta S, Hosoe H, Iinuma N, Iwai C, Nishimoto H, Shimizu K. Surgical outcome of 2-stage (posterior and anterior) surgical treatment using spinal instrumentation for tuberculous spondylitis. J Spinal Disord Tech. 2010;23(2):133–138. doi: 10.1097/BSD.0b013e31819a870f. [DOI] [PubMed] [Google Scholar]
- 24.Talu U, Gogus A, Ozturk C, Hamzaoglu A, Domanic U. The role of posterior instrumentation and fusion after anterior radical debridement and fusion in the surgical treatment of spinal tuberculosis: experience of 127 cases. J Spinal Disord Tech. 2006;19(8):554–559. doi: 10.1097/01.bsd.0000211202.93125.c7. [DOI] [PubMed] [Google Scholar]
- 25.Erturer E, Tezer M, Aydogan M, Mirzanlı C, Ozturk I. The results of simultaneous posterior-anterior-posterior surgery in multilevel tuberculosis spondylitis associated with severe kyphosis. Eur Spine J. 2010;19(12):2209–2215. doi: 10.1007/s00586-010-1481-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mukhtara AM, Farghalyb MM, Shaban H. Ahmeda surgical treatment of thoracic and lumbar tuberculosis by snterior Interbody fusion and posterior instrumentation. Med Princ Pract. 2003;12:92–96. doi: 10.1159/000069113. [DOI] [PubMed] [Google Scholar]
- 27.Karaeminogullari O, Aydinli U, Ozerdemoglu R, Ozturk C. Tuberculosis of the lumbar spine: outcomes after combined treatment of two-drug therapy and surgery. Spine. 2007;30(1):55. doi: 10.3928/01477447-20070101-15. [DOI] [PubMed] [Google Scholar]
- 28.Hong-Qi Z, Yu-Xiang W, Chao-feng G, Zhao D, Deng A, Jian-Huang Wu, Liu J-Y. One-stage posterior focus debridement, fusion, and instrumentation in the surgical treatment of cervicothoracic spinal tuberculosis with kyphosis in children: a preliminary report. Childs Nerv Syst. 2010;27(5):735–742. doi: 10.1007/s00381-010-1319-3. [DOI] [PubMed] [Google Scholar]
- 29.Li J, Lü GH, Wang XB, Wang B, Lu C, Deng YW. One-stage combined anterior and posterior strategy in treating active tuberculosis of thoracic and lumbar spine complicated with severe kyphotic deformity. Zhonghua Wai Ke Za Zhi. 2010;48(8):597–600. [PubMed] [Google Scholar]
- 30.Gu XF, Cheng L, Zhou YY. Radical debridement and single stage posterior spinal fusion and instrumentation for the treatment of thoracic-lumber tuberculosis. Zhonghua Yi Xue Za Zhi. 2009;89(41):2898–2901. [PubMed] [Google Scholar]
- 31.Zaveri GR, Mehta SS. Surgical treatment of lumbar tuberculous spondylodiscitis by transforaminal lumbar interbody fusion (TLIF) and posterior instrumentation. J Spinal Disord Tech. 2009;22(4):257–262. doi: 10.1097/BSD.0b013e31818859d0. [DOI] [PubMed] [Google Scholar]
- 32.Feng D, Kang J, Hou Z. Combined anterior and posterior surgeries for lumbarsacral junction tuberculosis. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2008;22(4):408–410. [PubMed] [Google Scholar]
- 33.Yu FY, Ma YZ, Chen X, Li HW. Surgical treatment of previously treated thoracic and thoracolumbar spinal tuberculosis. Zhonghua Yi Xue Za Zhi. 2010;90(27):1877–1881. [PubMed] [Google Scholar]
- 34.Zheng QX, Pan HT, Guo XD, Wu YC, Wu HB. Anterior radical debridement and bone grafting with one-stage instrumentation anteriorly or posteriorly for the treatment of thoracic and lumbar spinal Tuberculosis. Zhonghua Jie He He Hu Xi Za Zhi. 2008;31(2):99–102. [PubMed] [Google Scholar]
- 35.Güzey FK, Emel E, Bas NS, Hacisalihoglu S, Seyithanoglu MH, Karacor SE, Ozkan N, Alatas I, Sel B. Thoracic and lumbar tuberculous spondylitis treated by posterior debridement, graft placement, and instrumentation: a retrospective analysis in 19 cases. J Neurosurg Spine. 2005;3(6):450–458. doi: 10.3171/spi.2005.3.6.0450. [DOI] [PubMed] [Google Scholar]
- 36.Laheri VJ, Badhe NP. Dewnany GT (2001) Single stage decompression, anterior interbody fusion and posterior instrumentation for tuberculous kyphosis of the dorso-lumbar spine. Spinal Cord. 2001;39:429–436. doi: 10.1038/sj.sc.3101185. [DOI] [PubMed] [Google Scholar]
- 37.Lee SH, Sung JK, Park YM. Single-stage transpedicular decompression and posterior instrumentation in treatment of thoracic and thoracolumbar spinal tuberculosis: A retrospective case series. J Spinal Disord Tech. 2006;19:595–602. doi: 10.1097/01.bsd.0000211241.06588.7b. [DOI] [PubMed] [Google Scholar]
- 38.Gokce A, Ozturkmen Y, Mutlu S, Caniklioglu M. Spinal osteotomy: Correcting sagittal balance in tuberculous spondylitis. J Spinal Disord Tech. 2008;21:484–488. doi: 10.1097/BSD.0b013e3181586023. [DOI] [PubMed] [Google Scholar]
- 39.Bezer M, Kucukdurmaz F, Guven O. Transpedicular decancellation osteotomy in the treatment of posttuberculous kyphosis. J Spinal Disord Tech. 2007;20:209–215. doi: 10.1097/01.bsd.0000211271.89485.f1. [DOI] [PubMed] [Google Scholar]
- 40.Kalra KP, Dhar SB, Shetty G, Dhariwal Q. Pedicle subtraction osteotomy for rigid post-tuberculous kyphosis. J Bone Joint Surg Br. 2006;88:925–927. doi: 10.1302/0301-620X.88B7.17366. [DOI] [PubMed] [Google Scholar]
- 41.Guven O, Kumano K, Yalcin S, Karahan M, Tsuji S. A single stage posterior approach and rigid fixation for preventing kyphosis in the treatment of spinal tuberculosis. Spine. 1994;19:1039–1043. doi: 10.1097/00007632-199405000-00007. [DOI] [PubMed] [Google Scholar]
- 42.Hang QS, Zheng C, Hu Y, Xianoling Y, Huazi Xu, Zhang G, Wang Q. One-stage surgical management for children with spinal tuberculosis by anterior decompression and posterior instrumentation. Int Orthop (SICOT) 2009;33(5):1385–1390. doi: 10.1007/s00264-009-0758-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rajasekaran S, Vijay K, Shetty AP. Single-stage closing-opening wedge osteotomy of spine to correct severe post-tubercular kyphotic deformities of the spine: a 3-year follow-up of 17 patients. Eur Spine J. 2010;19(4):583–592. doi: 10.1007/s00586-009-1234-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bezer M, Kucukdurmaz F, Aydin N, Kocaoglu B, Guven O. Tuberculous spondylitis of the lumbosacral region: long-term follow-up of patients treated by chemotherapy, transpedicular drainage, posterior instrumentation, and fusion. J Spinal Disord Tech. 2005;18(5):425–429. doi: 10.1097/01.bsd.0000171627.11171.6f. [DOI] [PubMed] [Google Scholar]
- 45.Gong K, Wang Z, Luo Z (2010) Single-stage posterior debridement and transforaminal lumbar interbody fusion with autogenous bone grafting and posterior instrumentation in the surgical management of lumbar tuberculosis. Arch Orthop Trauma Surg. Jun 17. [Epub ahead of print] [DOI] [PubMed]
- 46.Güven O, Bezer M, Aydin N, Ketenci IE. Treatment strategy in tuberculous spondylitis: long-term follow-up results of 55 patients. Acta Orthop Traumatol Turc. 2008;42(5):334–343. [PubMed] [Google Scholar]
- 47.Yilmaz C, Selek HY, Gürkan I, Erdemli B, Korkusuz Z. Anterior instrumentation for the treatment of spinal tuberculosis. J Bone Joint Surg [Am] 1999;81-B:1261–1267. doi: 10.2106/00004623-199909000-00007. [DOI] [PubMed] [Google Scholar]
- 48.Ozdemir HM, Us AK. Ogün T (2003) The role of anterior spinal instrumentation and allograft fibula for the treatment of pott disease. Spine. 2003;28:474–479. doi: 10.1097/01.BRS.0000048666.17934.17. [DOI] [PubMed] [Google Scholar]
- 49.Christodoulou AG, Givissis P, Karataglis D, Symeonidis PD, Pournaras J. Treatment of tuberulosis spondylitis with anterior stabilization and titanium cage. Clin Orthop. 2006;444:60–65. doi: 10.1097/01.blo.0000201175.87635.28. [DOI] [PubMed] [Google Scholar]
- 50.Dai LY, Jiang LS, Wang W, Cui YM. Single-stage anterior autogenous bone grafting and instrumentation in the surgical management of thoracolumbar spinal tuberculosis. Spine. 2005;30:2342–2349. doi: 10.1097/01.brs.0000182109.36973.93. [DOI] [PubMed] [Google Scholar]
- 51.Benli IT, Alanay A, Akalin A, Acaraoglu KE. Comparision of anterior instrumentation systems and the results of minimum 5 years follow up in the treatment of tuberculosis spondylitis. Kobe J Med Sci. 2004;50(5–6):167–180. [PubMed] [Google Scholar]
- 52.Benli IT, Aydın E, Kis M, Akalın S, Tuzuner M, Baz AB. The results of anterior instrumentation in vertebral tuberculosis. J Turkish Spine Surg. 1996;7(3):98–101. [Google Scholar]
- 53.Benli IT, Acaroglu E, Akalin S, Kis M, Duman E, Un A. Anterior radical debridement and anterior instrumentation in tubercolous spondylitis. Eur Spine J. 2003;12:224–234. doi: 10.1007/s00586-002-0403-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Benli IT, Akalin S, Kis M, Citak M, Kurtulus B, Duman E. The results of anterior fusion and Cotrel – Dubousset – Hopf instrumentation in idiopathic scoliosis. Eur Spine J. 2000;9(6):505–515. doi: 10.1007/s005860000176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Halit C, Ramazan AK, Osman NT, Cengiz T, Brahim C, Yunus A. A long-term follow-up study of anterior tibial allografting and instrumentation in the management of thoracolumbar tuberculous spondylitis. J Neurosurg Spine. 2008;8:30–38. doi: 10.3171/SPI-08/01/030. [DOI] [PubMed] [Google Scholar]
- 56.Jain AK. Treatment of tuberculosis of the spine with neurologic complications. Clin Orthop. 2002;398:75–84. doi: 10.1097/00003086-200205000-00011. [DOI] [PubMed] [Google Scholar]
- 57.Chen CL, Chou CW, Su WW, Cheng CY, Yu CT. Dislodged upper thoracic cage in the gastrointestinal tract: a case report and literature reviews. Spine. 2008;33(21):E802–E806. doi: 10.1097/BRS.0b013e3181878791. [DOI] [PubMed] [Google Scholar]
- 58.Korovessis P, Petsinis G, Koureas G, Iliopoulos P, Zacharatos S. Anterior surgery with insertion of titanium mesh cage and posterior instrumented fusion performed sequentially on the same day under one anesthesia for septic spondylitis of thoracolumbar spine: is the use of titanium mesh cages safe? Spine. 2006;31(9):1014–1019. doi: 10.1097/01.brs.0000215049.08622.9d. [DOI] [PubMed] [Google Scholar]