Abstract
Purpose
The purpose of this study was to compare posterior and anterior surgical approach in combination with debridement, interbody autografting and instrumentation for thoracic and lumbar tuberculosis. These approaches were compared in terms of the operation duration, intraoperative blood loss, bony fusion, intraoperative and postoperative complications, neurological status and the angle of kyphosis.
Methods
Forty-seven patients with thoracic and lumbar tuberculosis who underwent either the posterior or the anterior approach in combination with debridement, interbody autografting and instrumentation from January 2004 to March 2010 were reviewed retrospectively. In group A (n = 25), the posterior approach was combined with debridement, interbody autografting and instrumentation. In group B (n = 22), the anterior approach was performed in addition to debridement, interbody autografting and instrumentation.
Results
All cases were followed up for 12–62 months. There was no statistically significant difference between groups in terms of the operation duration, intraoperative blood loss, bony fusion, intraoperative and postoperative complications, neurological status and the angle of kyphosis (p > 0.05). Good clinical outcomes were achieved in both groups.
Conclusions
The posterior approach combined with debridement, interbody autografting and instrumentation is an alternative procedure to treat thoracic and lumbar tuberculosis. The posterior approach is sufficient for lesion debridement. In addition, the posterior approach can maintain spinal stabilisation and prevent loss of corrected vertebral alignment as effectively as the anterior approach.
Introduction
Spinal tuberculosis is the most common form of extrapulmonary tuberculosis. Spinal tuberculosis can cause severe neurological deficits, kyphotic deformities and paraplegia. Chemotherapy and surgical procedures play a critical role in treating tuberculous spondylitis. Various surgical approaches, including the anterior and posterior approaches, have been widely used to treat spinal tuberculosis. The majority of researchers favour the anterior approach combined with a radical debridement and anterior fusion, because anterior radical surgical excision leads the surgeon directly to the lesion, provides optimal visualisation, decompresses the spinal cord directly and completely and prevents the possible progression of a kyphotic deformity [1–4]. However, there has been an important development in treating spinal tuberculosis in the past 20 years. The advent of diagnostic tools such as magnetic resonance imaging (MRI), computed tomography (CT)-guided biopsy and polymerase chain reaction (PCR) allow spinal tuberculosis to be diagnosed accurately. Posterior instrumentation has become popular as a technique to correct angular deformities and stabilise an unstable spine. Several authors have reported that the use of the posterior approach combined with debridement and instrumentation to treat spinal tuberculosis has led to favourable clinical outcomes [5–7]. However, the posterior approach remains controversial because it may affect posterior spinal column stability [8, 9].
In this study, we compared the posterior and anterior approaches combined with debridement, interbody autografting and instrumentation for thoracic and lumbar tuberculosis in terms of operation duration, blood loss, bony fusion, intraoperative and postoperative complications, neurological status and kyphosis angle.
Patients and methods
From January 2004 to March 2010, 47 consecutive patients with spinal tuberculosis who underwent the posterior or anterior approach combined with debridement, interbody autografting and instrumentation were enrolled in the study. In group A (n = 25), the posterior approach was combined with debridement, interbody autografting and instrumentation. In group B (n = 22), the anterior approach was combined with debridement, interbody autografting and instrumentation. Demographic characteristics for the two groups were similar (Table 1) , and no statistical difference was observed in age or gender distribution (p > 0.05). Ages ranged from 14 to 76 (mean 37.7) years. Seventeen male and thirty female patients were studied.
Table 1.
Demographic characteristics
| Group A (n = 25) | Group B (n = 22) | p value | |
|---|---|---|---|
| Age (mean) | 38.1 | 37.8 | p > 0.05 |
| Sex (M/F) | 10/15 | 7/15 | p > 0.05 |
| Spinal level (T:TL:L) | 7/10/8 | 6/10/6 |
T thoracic, TL thoracolumbar, L lumbar, M male, F female
All patients exhibited tuberculosis symptoms including fatigue, night sweats, low fever and weight loss, with a mean symptom duration of 16 (range 0.5–72) months. The patients were admitted to the hospital for dorsal spine pain or paraparesis. Upon admission, ten patients in group A exhibited neurological deficits with an American Spinal Injury Association (ASIA) grade of D, and 13 cases in group B exhibited neurological deficits with an ASIA grade of C or D. The average kyphosis angle of 12 cases in group A was 16.3°, whereas the average kyphosis angle of eight cases in group B was 24.4° . The diagnosis criteria of thoracic and lumbar tuberculosis included clinical presentation such as fatigue, night sweats, low-grade fever, weight loss, dorsal spine pain, paraparesis and gibbus; imaging of a destructive lesion by MRI, CT, plain radiographic films; and erythrocyte sedimentation rate ESR. The criteria of imaging diagnosis was followed as described by Desai and Buxi [10, 11]. A pathological examination such as CT-guided fine-needle aspiration cytology biopsy or excision biopsy of intraoperative lesions confirmed the diagnostic results. All patients received standard laboratory tests, including white and red blood cell count, ESR, blood chemistry profile, and Mantoux tuberculin skin test. An electrocardiogram and a radiograph of the chest, specific spinal lesions and any other suspected skeletal sites were conducted in all patients. MRI and CT imaging were performed in all cases. After the preliminary diagnosis of tuberculous spondylitis, all patients received standard antituberculous chemotherapy, including oral administration of rifampin (450 mg per day), isoniazid (300 mg per day), pyrazinamide (750 mg per day) or ethambutol (1,200 mg per day), and intramuscular streptomycins (0.75 g per day ). Chemotherapy lasted at least two weeks before the operation.
The indications for surgery included the presence of neurological deficits, spinal deformities, epidural abscesses compressing the dural sac, large paravertebral abscesses, radicular or dural compression caused by granulation tissue and abscesses, sequestrum or disc fragments resulting in neurological deficits or severe pain and a nondiagnostic biopsy specimen. In group A, all patients were treated using surgical debridement and internal fixation via a posterior approach. The surgery was performed under general endotracheal anaesthesia. First, patients were placed in the prone position, and a midline incision was made over the spinous process. Next, the fascia and muscle was subperiosteally stripped from the spinous processes and vertebra lamina to the facet joints and the pars interarticularis was exposed. Thirdly, a unilateral facetectomy and pediculectomy or bilateral facetectomy and pediculectomy were performed with debridement of the affected vertebral body, infected tissue, pus, granulation tissue, sequestrum and disc necrotic tissue using curettes. We performed debridement of the vertebral body and disc space through one or both pedicles according to the range of lesion. Fourthly, the affected spinal segments were stabilised using a transpedicular screw and rod system. When the screws could not be placed into the affected vertebra bilaterally, or when thoracolumbar junction involvement was present, two vertebrae above and one vertebra below the involved vertebra were incorporated into the instrumentation system to correct the kyphosis. Finally, an intervertebral bone autograft or a titanium cage with a cancellous bone from the iliac crest was used. The streptomycin (2–3 g) was sprayed onto the operation site, and a local drainage tube was inserted before the incision was closed. The anterior approach combined with debridement, bone autografting and instrumentation was performed in patients in group B, as described by Hodgson et al. [1]. A plate and screw system was used in nine cases, and a rod and screw system was used in 13 cases in group B.
Postoperative care
Blood pressure, respiration, pulse, amount of drainage and sense and motor responses of the lower extremities were monitored after the operation. The drainage tube was removed when the amount of drainage was less than ten ml per day after the operation. We recommended that all patients remained in the bracing apparatus for at least six month until bony fusion was observed on radiography. All patients received antituberculosis chemotherapy for 12–18 months and routine antibiotic treatment for five to seven days after the operation. Liver function and ESR rates were monitored carefully at regular intervals. A follow-up examination was performed at one, three, six, 12 and 18 months. Subsequent follow-ups were conducted at 12-month intervals. For the statistical analysis, chi-square test and t tests were used, and a p value <0.05 was considered statistically significant.
Results
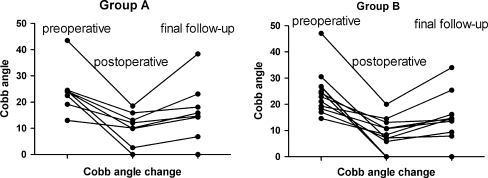
All cases were followed up for an average of 22.2 (range 12–62) months. In group A, the mean duration of surgery was 390.2 (range 240–840) min. Average blood loss during the surgery was 834.1 ml. Mean time spent in the hospital was 26.4 (range 14–45) days (Table 2). One patient suffered from internal fixation breakage due to failure of bony fusion one year after the operation. The remaining patients exhibited bony fusion (Fig. 1). The assessment of bony fusion was conducted as described by Moon et al. [12] (bone fusion standard: no loss of corrected angles, graft absorption, clear bone remodelling and clear bone hyperostosis). Thirteen cases exhibited a neurological deficit of grade C and D on the ASIA scale. As a result of the surgery, two patients improved two grades and 11 improved one grade (Table 3). Kyphosis angles were measured as described by Carman et al. [13] before and after the operation and at the final follow-up. The average preoperative Cobb angle was 24.4° (range 13–43.5°) in the eight patients with gibbus in group A, and the average postoperative Cobb angle was 10.3° (range 0–18.5°). At the final follow-up, the average Cobb angle was 16.4° (range 0–38.4°) for this group. An average loss of correction of 6.1° was observed at the final follow-up (Table 4). In group B, the mean duration of surgery was 428.6 (range135–860) min. Average blood loss during the surgery was 890.1 ml. The mean time spent in hospital was 25.5 (range 15–42) days (Table 2). Sinus formation was observed in three patients: in one patient two months and in two patients three months after the operation. For three patients, lesion debridement and sinus excision were performed. No breakage of internal fixation was observed, and all patients exhibited bone fusion (Fig. 2). Ten cases exhibited a neurological deficit of grade D on the ASIA scale; all patients improved by one grade (Table 3). The average preoperative Cobb angle was 16.3° (range 13–43.5°) in the 12 patients with gibbus. The average postoperative Cobb angle was 8.1° (range 0–20.0°). At the final follow-up, the average Cobb angle was 12.7° (range 0–34°). An average loss of correction of 4.6° was observed at the final follow-up. (Table 4).
Table 2.
Comparison between groups in terms of duration of operation, blood loss and time spent in the hospital (±standard deviation)
| Group A | Group B | P | |
|---|---|---|---|
| Duration of operation (min) | 390. ± 31.6 | 428.6 ± 41.6 | p > 0.05 |
| Blood loss (ml) | 858.1 ± 93.6 ml | 890.9 ± 115.6 | p > 0.05 |
| Time in hospital (d) | 26.4 ± 1.2 | 25.5 ± 1.4 | p > 0.05 |
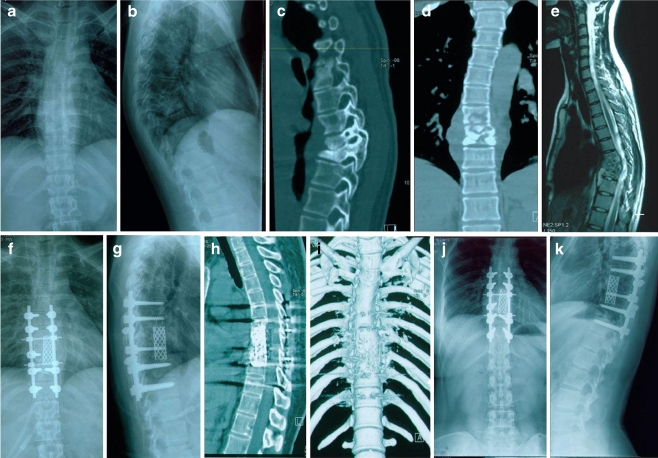
Fig. 1.
A 28-year-old female patient presented with kyphosis due to destructive tubercular spondylodiscitis at T7–9 with a paravertebral abscess (a–e). After posterior debridement, this large defect after the sagittal profile reconstruction and posterior instrumentation was bridged using a titanium cage and autologous bone grafting (f, g). Two years postoperatively, a mild kyphotic formation and bony fusion were observed (h–k)
Table 3.
Recovery of neurological deficit American Spinal Injury Association (ASIA )
| Group (n) | Preoperative grade | Postoperative grade | Final follow-up |
|---|---|---|---|
| A 10 | D | E | E |
| B 2 | C | D | E |
| 11 | D | E | E |
Table 4.
Cobb angle preoperation, postoperation and at final follow-up (±standard deviation)
| Group | Preoperation | Postoperation | Final follow-up | Loss of correction |
|---|---|---|---|---|
| A | 24.4° ± 3.1° | 10.3° ± 2.2° | 16.4° ± 4.0° | 6.1° |
| B | 16.3° ± 2.6° | 8.1° ± 1.7° | 12.7° ± 2.9° | 4.6° |
Note: A statistically significant difference was found between the preoperative and postoperative values in these groups, p < 0.05
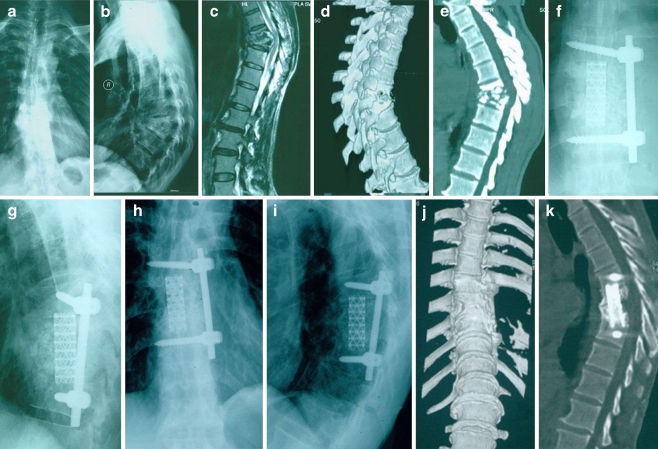
Fig. 2.
A 50-year-old male patient presented with kyphosis due to destructive tubercular spondylodiscitis at T8–9 with paravertebral abscess (a–e). After the anterior approach with derbridement, this large defect that occurred after a sagittal profile reconstruction and posterior instrumentation was bridged with a titanium cage and autologous bone grafting (f, g). Three years postoperatively, a loss of correction and a bony fusion were observed
The loss of kyphosis correction was present in both groups (Fig. 3).
Fig. 3.
Postoperative correction was successful, but loss of correction was observed at the final follow-up in both groups
Discussion
Surgical procedures play an important role in treating spinal tuberculosis. These surgical treatments should be based on the use of potent antitubercular drugs and modern diagnostic aids such as MRI. The main aims of surgical treatment are to stop the infectious process, remove necrotic tissue, restore circulation and decompress the spinal cord. In addition, these treatments aim at providing recovery of neurological deficit, restoration of spinal stability, prevention or correction of kyphosis deformity, pain relief and acceleration of the patients’ recovery process. Since Albee and Hibbs introduced posterior spinal fusion and potent antitubercular drugs were first used in spinal tuberculosis, various surgical treatments have been widely applied. Anterior and posterior approaches have mainly been used. However, the majority of surgeons favour the anterior approach because of concern over the safety of the posterior approach [8, 9]. The anterior surgical approach includes an anterolateral extrapleural approach and a transpleural anterior approach, which was developed by Hodgson et al. [1, 14] and has been used by many surgeons for spinal tuberculous lesions [2, 15–17]. The anterior approach is considered to be very important because it can provide the surgeon with direct access to the diseased vertebral segments to perform radical surgical debridement of the infected tissues. The Medical Research Council (MRC) of the United Kingdom reported that reduction in the size of late deformity is an advantage of anterior radical operations compared with conservative treatments involving the use of two or three antituberculous drugs with bed rest or immobilisation in brace and ambulant chemotherapy. The cure rate for conservative treatment and for anterior radical operation are 85% and 89.9%, respectively [18]. However, the anterior approach in combination with anterior instrumentation only provides partial spinal stability, and some authors have reported that it is necessary to add posterior stabilisation to restore spinal stability and correct kyphotic deformity [19, 20]. Huang et al. reported their clinical results in which cases of tubercular spine in children were surgically treated by anterior decompression, bone grafting, posterior instrumentation and fusion. In addition, the authors considered that the approach was feasible and effective [21]. On the contrary, Jain et al. considered that it is improper that all tubercular spines should be operated upon by the authors’ approach [22]. We think anterior decompression and posterior instrumentation of the tuberculous spine may be too drastic for elderly patients and children.
Many surgeons report that the anterior approach is unnecessary for spinal tuberculosis because the spontaneous anterior fusion of the vertebral body can occur without treatment. This surgery was considered too drastic by some authors because it may necessitate division of the diaphragm and segmental vessels [23, 24]. Surgeons supporting the posterior approaches report that modern posterior spinal instrumentation can provide rigid fixation and stability [6, 7]. Additionally, correcting angular deformity is safer and more effective with posterior instrumentation and fusion, especially with transpedicular instrumentation or anterior decompression plus posterior fixation. Guzey et al. [6] reported that 19 patients with single segmental tuberculous spondylitis underwent posterior debridement, graft placement and instrumentation: one patient died of a myocardial infarction, and one patient experienced a single broken pedicle screw after three months. The mean angulation in the 13 patients with kyphotic deformities was 18.2° (range 5–42°) preoperatively; this angle was reduced to 17.3° (range 0°–42°) after surgery. Thus, the authors reported that the posterior approach was sufficient for infection debridement and spinal stabilisation in patients with thoracic and lumbar tuberculous spondylitis. However, Jain and Tuli [8, 9] think that posterior approaches combined with lesion debridement are unsafe because the posterior approach may destroy the healthy posterior spinal column
Some authors make a classification to guide spinal tuberculosis treatment. Oguz et al. make a new classification to guide surgical treatment of spinal tuberculosis [25]. However, Samuel et al. consider that new classification did not include the posterior spinal tuberculosus and that the classification proposed by Mehta and Bhojraj seemed more reasonable and inclusive [4, 26]. Because of the complexity of spinal tuberculosis, there is not one classification serving all purposes of treating spinal tuberculosis.
In the literature, there is one report comparing the anterior approach with the posterior approach for spinal tuberculosis. Lee et al. [5] reported their experiences with the anterior or posterior approaches combined with internal fixation for thoracolumbar tuberculosis. They concluded that there was no difference in outcomes between groups and that the posterior approach was useful for treating early-diagnosed thoracic and thoracolumbar spinal tuberculosis for the following indications: (1) <50% collapse of the vertebral body, and (2) mild kyphosis (<30°). In our study, we did not observe a statistically significant difference in operation duration, amount of blood loss, loss of correction and hospitalisation time between the anterior and posterior approaches. Although there was loss of correction in both groups, spinal instability was not observed at follow-up. The main cause of loss of correction is that the anterior and middle columns of the spine are mildly compressed because of new bone formation and osteolytic bone destruction during bone repair. Another explanation is that we often use short-segment fixation for the affected level. Loss of correction does not affect bony fusion because the correction loss is mild. All patients in both groups exhibited improved neurological condition at the final follow-up examination. During the follow-up period, results of the posterior and anterior approaches for thoracic and lumbar tuberculosis were acceptable. In group A, broken internal fixation was observed in one case, which was resolved as follows: We removed the broken internal fixation, performed the posterior approach combined with lesion debridement, filled the path of the pedicle screw and disc space with autologous bone and used the posterior pedicle screws and rod system in situ. Therefore, we did not extend the instrumentation. In group B, sinus formation was found in three cases. We considered that the tuberculosis infection was not well controlled, which caused reactivation. We performed lesion debridement, removed the graft, performed autograft again through the original incision, excised the sinus and kept the drainage tube in place for at least three days. We did not remove the instrumentation because it was not loose. In our research, fewer complications occurred in group A than in group B, but this finding may be attributable to the small sample size. This was an uncontrolled, retrospective study of clinical outcomes of the posterior and anterior approaches combined with debridement, interbody autografting and instrumentation for patients with thoracic and lumbar tuberculosis. We conclude that the posterior approach combined with debridement, interbody graft and instrumentation can be applied in most patients with thoracic and lumbar tuberculosis. In our experience, the indication for the anterior approach is that the lesion exists in the anterior and middle column of the thoracic and lumbar vertebra, whereas the indication for the posterior approach is that the lesion exists in the anterior, middle and posterior thoracic and lumbar vertebra. When anterior vertebral blood vessels are affected by the tuberculosis lesion and no clear space can be observed between lesion and blood vessels on MRI, the anterior approach is recommended. The potency of antituberculous drug treatments and surgeon’s experience are important factors in the success of the procedure, irrespective of the approach.
Acknowledgements
The authors thank professor Wei Sun for his help in the preparation of this article.
Reference
- 1.Hodgson A-R, Stock FE, Fang HSY, Ong GB. Anterior spinal fusion. The operative approach and pathological findings in 412 patients with Pott’s disease of the spine. Br J Surg. 1960;48:172–178. doi: 10.1002/bjs.18004820819. [DOI] [PubMed] [Google Scholar]
- 2.Kirkaldy-Willis W-H, Thomas TG. Anterior Approaches in the Diagnosis and Treatment of Infections of the Vertebral Bodies. J Bone Joint Surg Am. 1965;47:87–110. [PubMed] [Google Scholar]
- 3.Cameron JA, Robinson CE, Robertson DE. The radical treatment of Pott’s disease and Pott’s paraplegia by extirpation of the diseased area and anterior spinal fusion. Am Rev Respir Dis. 1962;86:76–80. doi: 10.1164/arrd.1962.86.1.76. [DOI] [PubMed] [Google Scholar]
- 4.Mehta JS, Bhojraj SY. Tuberculosis of the thoracic spine. A classification based on the selection of surgical strategies. J Bone Joint Surg Br. 2001;83(6):859–863. doi: 10.1302/0301-620X.83B6.11142. [DOI] [PubMed] [Google Scholar]
- 5.Lee SH, Sung JK, Park YM. Single-stage transpedicular decompression and posterior instrumentation in treatment of thoracic and thoracolumbar spinal tuberculosis: a retrospective case series. J Spinal Disord Tech. 2006;19(8):595–602. doi: 10.1097/01.bsd.0000211241.06588.7b. [DOI] [PubMed] [Google Scholar]
- 6.Guzey FK, Emel E, Bas NS, Hacisalihoglu S, Seyithanoglu MH, Karacor SE, Ozkan N, Alatas I, Sel B. Thoracic and lumbar tuberculous spondylitis treated by posterior debridement, graft placement, and instrumentation: a retrospective analysis in 19 cases. J Neurosurg Spine. 2005;3(6):450–458. doi: 10.3171/spi.2005.3.6.0450. [DOI] [PubMed] [Google Scholar]
- 7.Lee JS, Moon KP, Kim SJ, Suh KT. Posterior lumbar interbody fusion and posterior instrumentation in the surgical management of lumbar tuberculous spondylitis. J Bone Joint Surg Br. 2007;89(2):210–214. doi: 10.2106/JBJS.F.00437. [DOI] [PubMed] [Google Scholar]
- 8.Tuli SM. Tuberculosis of the spine: a historical review. Clin Orthop Relat Res. 2007;460:29–38. doi: 10.1097/BLO.0b013e318065b75e. [DOI] [PubMed] [Google Scholar]
- 9.Jain AK (2010) Tuberculosis of the spine: a fresh look at an old disease. J Bone Joint Surg Br 92(7):905–913 [DOI] [PubMed]
- 10.Desai SS. Early diagnosis of spinal tuberculosis by MRI. J Bone Joint Surg Br. 1994;76(6):863–869. [PubMed] [Google Scholar]
- 11.Buxi TB, Doda SS, Mathur RK, Maini PS, Nijhawan VK, Sogani S, Agarwal HN, Madan VS, Byotra SP. Computed tomography in spinal tuberculosis. Comput Med Imaging Graph. 1991;15(6):379–388. doi: 10.1016/0895-6111(91)90163-P. [DOI] [PubMed] [Google Scholar]
- 12.Moon MS, Woo YK, Lee KS, Ha KY, Kim SS, Sun DH. Posterior instrumentation and anterior interbody fusion for tuberculous kyphosis of dorsal and lumbar spines. Spine. 1976;20(17):1910–1916. doi: 10.1097/00007632-199509000-00013. [DOI] [PubMed] [Google Scholar]
- 13.Carman DL, Browne RH, Birch JG. Measurement of scoliosis and kyphosis radiographs. Intraobserver and interobserver variation. J Bone Joint Surg Am. 1990;72(3):328–333. [PubMed] [Google Scholar]
- 14.Hodgson AR, Stock FE. Anterior spinal fusion. A preliminary communication on the radical treatment of Pott’s disease and Pott’s paraplegia. 1956. Clin Orthop Relat Res. 1994;300:16–23. [PubMed] [Google Scholar]
- 15.Kohli SB. Radical surgical approach to spinal tuberculosis. J Bone Joint Surg Br. 1967;49(4):668–673. [PubMed] [Google Scholar]
- 16.Zhao J, Lian XF, Hou TS, Ma H, Chen ZM. Anterior debridement and bone grafting of spinal tuberculosis with one-stage instrumentation anteriorly or posteriorly. Int Orthop. 2007;31(6):859–863. doi: 10.1007/s00264-006-0253-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jin D, Qu D, Chen J, Zhang H. One-stage anterior interbody autografting and instrumentation in primary surgical management of thoracolumbar spinal tuberculosis. Eur Spine J. 2004;13(2):114–121. doi: 10.1007/s00586-003-0661-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anonymous (1998) A 15-year assessment of controlled trials of the management of tuberculosis of the spine in Korea and Hong Kong. Thirteenth Report of the Medical Research Council Working Party on Tuberculosis of the Spine. J Bone Joint Surg Br 80(3):456–462 [DOI] [PubMed]
- 19.Sundararaj GD. Simultaneous anterior decompression and posterior instrumentation of the tuberculous spine using an anterolateral extrapleural approach. J Bone Joint Surg Br. 2009;91(5):702–703. doi: 10.1302/0301-620X.91B5.22532. [DOI] [PubMed] [Google Scholar]
- 20.Jain AK, Dhammi IK, Prashad B, Sinha S, Mishra P. Simultaneous anterior decompression and posterior instrumentation of the tuberculous spine using an anterolateral extrapleural approach. J Bone Joint Surg Br. 2008;90(11):1477–1481. doi: 10.1302/0301-620X.90B11.20972. [DOI] [PubMed] [Google Scholar]
- 21.Huang QS, Zheng C, Hu Y, Yin X, Xu H, Zhang G, Wang Q. One-stage surgical management for children with spinal tuberculosis by anterior decompression and posterior instrumentation. Int Orthop. 2009;33(5):13851390. doi: 10.1007/s00264-009-0758-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jain S (2010) Comment on Huang et al. One-stage surgical management for children with spinal tuberculosis by anterior decompression and posterior instrumentation. Int Orthop 34 (5):769770;– author relply 771. doi:10.1007/s00264-010-0975-y [DOI] [PMC free article] [PubMed]
- 23.Guven O, Kumano K, Yalcin S, Karahan M, Tsuji S. A single stage posterior approach and rigid fixation for preventing kyphosis in the treatment of spinal tuberculosis. Spine. 1976;19(9):1039–1043. doi: 10.1097/00007632-199405000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Garst RJ. Tuberculosis of the spine: a review of 236 operated cases in an underdeveloped region from 1954 to 1964. J Spinal Disord. 1992;5(3):286–300. doi: 10.1097/00002517-199209000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Oguz E, Sehirlioglu A, Altinmakas M, Ozturk C, Komurcu M, Solakoglu C, Vaccaro AR. A new classification and guide for surgical treatment of spinal tuberculosis. Int Orthop. 2008;32(1):127133. doi: 10.1007/s00264-006-0278-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Samuel S (2010) Comment on Oguz et al. A new classification and guide for surgical treatment of spinal tuberculosis. Int Orthop 34 (4):613. doi:10.1007/s00264-009-0939-2 [DOI] [PMC free article] [PubMed]