Abstract
Purpose
Drug-resistant tuberculosis is a major public-health concern globally and can be difficult to manage clinically. Spinal tuberculosis is the most common manifestation of extrapulmonary tuberculosis. However, there have been few reports on the topic of drug-resistant spinal tuberculosis. The aim of this study was to investigate the clinical characteristics and drug susceptibility patterns and the outcomes of management with a combination of surgery and individualised chemotherapy, for drug-resistant spinal tuberculosis.
Methods
We retrospectively analysed 35 patients with drug-resistant tuberculous spondylitis. After surgery, individualised chemotherapy was tailored for each patient according to the drug-resistance profile and previous history of chemotherapy. The patients were followed up clinically and radiologically for an average period of 35.8 months.
Results
Among 35 drug-resistant spinal tuberculosis cases, 13 were retreatment cases. Twelve were multi-drug resistant tuberculosis (MDR-TB), and 23 were non-MDR-TB. The patients with MDR-TB and non-MDR-TB had undergone previous chemotherapy for an average of 14.50 ± 2.00 (0–60) months and 4.56 ± 1.54 (0–74) months, respectively. A total of 32 cases underwent open operations, and the other three had percutaneous drainage and local chemotherapy. Patients received individualised chemotherapy for an average of 23.6 months postoperatively. Local recurrence was observed in six patients. Thirty-three patients had been cured at the final follow-up, and the other two were still receiving chemotherapy.
Conclusions
Drug-resistant tuberculous spondylitis is mainly acquired through previous irregular chemotherapy and the spreading of drug-resistant strains. Management with a combination of surgery and individualised chemotherapy is feasible in the treatment of severe complications and the prevention of acquired drug resistance.
Introduction
Tuberculosis is a major public-health problem in developing countries, and there are some new challenges due to the emergence of HIV co-infection and drug-resistant tuberculosis [1]. The World Health Organisation has estimated that there are 440,000 multi-drug resistant tuberculosis (MDR-TB) cases, and 150,000 persons with MDR-TB died worldwide in 2009. China is one of the world’s 27 countries with the highest burden of MDR-TB and extensive drug-resistant tuberculosis (XDR-TB) [2]. Drug-resistant tuberculosis is becoming a serious challenge to the global control of the disease.
As the most common extrapulmonary form of tuberculosis, spinal tuberculosis is a growing hazard worldwide and has an aggressive behaviour of profound vertebral destruction and severe complications. The initial optimism with tuberculosis spondylitis lessens as we see the increased number of relapses and initial treatment failures owing to the emergence of drug-resistant tuberculosis [3]. However, drug-resistant tuberculosis of the spine has been received little attention in the literature. Our study aimed to investigate the clinical characteristics, drug susceptibility patterns, and the management with a combination of surgery and individualised chemotherapy of patients with drug-resistant spinal tuberculosis in Chongqing, China.
Materials and methods
Study population and data collection
A total of 249 patients with clinically and histologically proven spinal tuberculosis underwent surgery at Southwest Hospital between September 2005 and January 2010. Mycobacterium tuberculosis was cultured from specimens obtained from all patients (249/249). A total of 51% of cases (127/249) produced positive culture, which provided drug susceptibility testing (DST) later. Of them, 30.7% (39/127) had drug-resistant patterns. The following eligibility and exclusion criteria were set for the study subjects. The eligibility criteria were: DST showing resistance to at least one anti-tuberculosis drug and a minimum follow-up period of 18 months after the initiation of individualised chemotherapy. The exclusion criteria were: non-compliance with anti-tuberculosis chemotherapy and being lost to follow-up or death. Overall, 35 subjects meeting the eligibility criteria were included in the study. Two patients were lost to follow-up, and two patients were under chemotherapy for less than 18 months of follow-up and therefore were excluded from the study. Medical records were reviewed for patient demographics and clinical characteristics, anti-tuberculosis treatment history, DST profile, individualised treatment modalities and outcomes. Written informed consent was obtained from all patients.
Individualised surgery program
Individualised surgical procedures were performed according to the site of destruction, the complications and the general condition of the patients. A total of 32 patients who met the absolute surgical indications underwent focal debridement, fusion and instrumentation. The absolute surgical indications were severe deformity in nine patients, neurological deficit in 18 patients, and spinal instability in five patients. In addition, percutaneous catheter drainage and local chemotherapy were selected for the other thee patients without absolute indications (Table 1).
Table 1.
Surgical procedures performed for drug-resistant spinal tuberculosis (n = 35)
| Surgical procedure | Patients (n) | Percentage of total |
|---|---|---|
| Single-stage posterior focal debridement, fusion and instrumentation | 6 | 17% |
| Single-stage anterior focal debridement, fusion and instrumentation | 15 | 43% |
| Single-stage posterior instrumentation followed by anterior focal debridement | 8 | 23% |
| Two-stage posterior instrumentation followed by anterior focal debridement | 2 | 6% |
| Single-stage posterior debridement, closing wedge osteotomy, and instrumentation | 1 | 3% |
| CT-guided percutaneous catheter drainage and local chemotherapy | 2 | 6% |
| Percutaneous transforaminal endoscopic catheter drainage and local chemotherapy | 1 | 3% |
| Total | 35 | 100% |
The method for DST
Clinical specimens were obtained from patients during open operations or percutaneous drainage. The quality-assured culture and DST was carried out using the BACTEC MGIT 960 system (Becton-Dickinson, Sparks, MD, USA) and the proportion method on Lowenstein-Jensen medium at the Clinical Laboratory of the Chongqing Infectious Disease Medical Centre. For all drugs, except pyrazinamide, the following critical concentrations were used: 1 and 10 μg/ml isoniazid, 50 and 250 μg/ml rifampicin, 5 and 50 μg/ml ethambutol, 10 and 100 μg/ml streptomycin, 5 and 50 μg/ml levofloxacin, 1 and 10 μg/ml PAS, 25 and 100 μg/ml protionamide, 0.1 and 1 μg/ml pasiniazid, 50 and 250 μg/ml rifapentine, 10 and 100 μg/ml capreomycin, and 10 and 100 μg/ml amikacin. The result of DST is often reported within two months from primary cultures.
DST-guided individualised chemotherapy
While the results of DST were pending, it was necessary to institute empiric chemotherapy, depending on the previous treatment and contact history of the patient. The regimens were adjusted as necessary once the test results were available. Patients with DST results showing susceptibility to any first-line drug were treated with standard chemotherapy. If the result of DST showed resistance to any first-line drug, individualised chemotherapy was tailored for these patients based on their previous chemotherapy history and strain-susceptibility profile. There were several principles for the individualised chemotherapy [4, 5]: (a) any first line drug to which the isolate had proved to be sensitive; (b) an injectable drug for a minimum period of six months; (c) a quinolone; (d) the addition of any appropriate second-line drugs, such as ethionamide and cycloserine, to achieve a combination of four to five effective regimens; (e) other drugs, such as amoxicillin or clavulanate and clofazimine; and (f) the chemotherapy should be used for 18–24 months or longer. Among the 35 patients, seven cases with mono-resistance to levofloxacin or PAS acid were treated with standard regimens, while the other cases were adjusted to individualised regimens.
Follow-up index
The following indices were recorded preoperatively and at one, three, six, nine and 12 months of follow-up and every six months thereafter: (1) clinical presentation; (2) X-ray, CT and MRI scans; and (3) erythrocyte sedimentation rate (ESR), hepatic function and renal function. Fusion assessment was determined by X-ray, according to the criteria defined by Lee et al. [6]. Definitive fusion and probable fusion were classified as spinal fusion in this study. Additionally, the American Spinal Injury Association (ASIA) score system was used to evaluate neurological deficits.
Results
Demographic and clinical characteristics
Among 35 patients, 17 were female and 18 were male. The median age was 36.5 (range, 4–62) years old. One patient had involvement of 13 vertebral body levels, three had involvement of six levels, five had involvement of four levels, six had involvement of three levels, 16 had involvement of two levels, and four had involvement of a single level. A total of 62.9% (22/35) of the patients were primary cases, and the remaining 37.1% (13/35) were retreatment cases. Among the retreatment patients, 61.5% (8/13) had received previous irregular chemotherapy for a mean period of 30.5 months (range, four to 74 months). Eighteen (55%) subjects had neurological deficits, and the ASIA classifications were: grade B, three cases; grade C, six cases; and grade D, nine cases. Kyphotic deformity was present in eight subjects, and the average kyphosis angle was 44.2º (range, 32.6–100.8º). None of the patients was co-infected with HIV (Table 2).
Table 2.
Demographic and clinical characteristics of the study population
| Characteristic | Value (N = 35) |
|---|---|
| Male/female | 18/17 |
| Age, y; median (range) | 36.5 (4–62) |
| HIV co-infection | 0 (0%) |
| Other sites of tuberculosis | 9 (25.7%) |
| Pulmonary tuberculosis | 9 (25.7%) |
| Tuberculous pleuritis | 2 (5.7%) |
| Tuberculous peritonitis | 1 (2.9%) |
| Tuberculous pericarditis | 1 2.9%) |
| Renal tuberculosis | 1 (2.9%) |
| Other osteoarticular tuberculosis | 2 (5.7%) |
| Paravertebral cold abscess | 27 (77.1%) |
| Previous surgery for spinal TB | 7 (20.0%) |
| Weight loss > 5 kg | 12 (34.3%) |
Values given as n (%) unless otherwise noted
Drug-resistance patterns and anti-tuberculosis treatment history
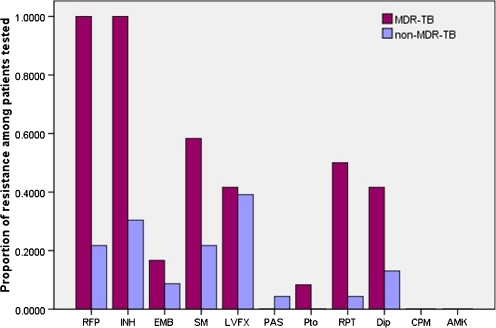
Of all the patients, 12 cases (34.3%) were classified as having MDR-TB, and 23 cases (65.7%) had non-MDR-TB. The rates of resistance to all drugs were 54.3% to isoniazid, 48.6% to rifampicin, 34.3% to streptomycin, 25.7% to pasiniazid, 20.0% to rifapentine, 11.4% to ethambutal, 4.0% to levofloxacin, 2.9% to PAS, 2.9% to protionamide, 0% to capreomycin, and 0% to amikacin (Fig. 1). Mono-drug resistance was found in 16 cases, resistance to two drugs was found in seven cases and to three drugs in five cases, four drugs in two cases, five drugs in two cases, six drugs in two cases, and eight drugs in one case. The mean turn-around time of DST was 44.8 (range, 36–62) days. The mean delay in diagnosis for spinal tuberculosis (the time interval between the onset of symptoms and establishing spinal tuberculosis clinically) was 8.74 ± 7.28 (range, one to 35) months, and the mean delay in diagnosis for drug-resistant spinal TB (the time interval between making the diagnosis of spinal tuberculosis and DST demonstrating drug-resistant patterns) was 8.43 ± 2.12 (range, 1.2–75) months. The proportions of retreatment among MDR-TB and non-MDR-TB cases were 66.7% (8/12) and 21.7% (5/23), respectively. The patients with MDR-TB and non-MDR-TB had undergone previous chemotherapy for mean periods of 14.50 ± 2.00 (range, 0–60) months and 4.56 ± 1.54 (range, 0–74) months, respectively.
Fig. 1.
Proportion of resistance to anti-tuberculosis drugs among MDR-TB and non-MDR-TB patients. Number of patients was 35. MDR-TB multi-drug resistant tuberculosis, Non-MDR-TB non-multi-drug resistant tuberculosis, INH isoniazid, RFP rifampin, EMB ethambutol, SM streptomycin, LVFX levofloxacin, PAS para-aminosalicylic acid, AMK amikacin, CPM capreomycin Pto protionamide, Dip pasiniazid, RPT rifapentine
Treatment outcomes
The 35 patients received individualised chemotherapy for an average of 23.6 (range, 18–29) months. The mean follow-up duration was 35.8 (range, 18–89) months. Of all patients, spinal tuberculosis was completely cured in 33 patients, and two were still receiving treatment. Follow-up radiography proved that definitive fusion was achieved in 32 patients and probable fusion in three patients. The average fusion period was 9.2 (range, six to 17) months. The average kyphosis angle was 14.8º (range, −6.25 to 36.7º) postoperatively, and there was no significant loss of correction at the final follow-up. The ASIA scores of the 18 cases with neurological deficits pre- and postoperatively are shown in Table 3. The mean ESR was 6.24 mm/h (range, 0–32 mm/h) at the final follow-up. Five patients had gastrointestinal side effects from chemotherapy, one had mild urticaria, and two had drug hepatitis. Among them, three required a modification of regimen, and the other five tolerated the 18–24 months of chemotherapy well, after symptomatic management.
Table 3.
1Pre- and postoperative neurological status by ASIA score system
| Preoperative | Number of cases | Postoperative | ||||
|---|---|---|---|---|---|---|
| A | B | C | D | E | ||
| A | 0 | 0 | 0 | 0 | 0 | 0 |
| B | 3 | 0 | 0 | 0 | 3 | 0 |
| C | 6 | 0 | 0 | 0 | 2 | 4 |
| D | 9 | 0 | 0 | 0 | 1 | 8 |
| E | 17 | 0 | 0 | 0 | 0 | 17 |
Local recurrence was observed in six (17.14%) patients postoperatively, when the results of DST had not yet been revealed (Fig. 2). Among them, four presented with sinus formation at the incision, one with progressive neurological deficit and one with sinus formation and tuberculous peritonitis. After focal re-debridement, excision of the sinus and individualised chemotherapy, all of the six cases achieved complete cures without recurrence.
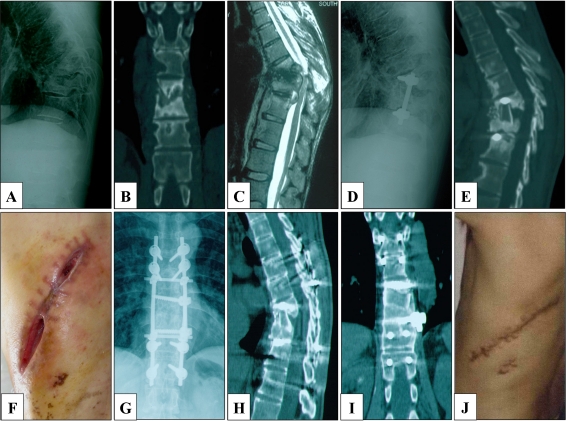
Fig. 2.
Male patient, 35 years old. The patient presented with a persistent backache for 1 year and was admitted to our clinic in 2007. a, b Preoperative radiograph and CT showed destruction of T8-11 vertebrae. c MRI additionally showed paravertebral abscess formation and compression of the spinal cord. The patient underwent one-stage anterior debridement, autograft fusion and instrumentation after admission. However, the patient refused to undergo DST for economic reasons. He received a combination of rifampicin and isoniazid, provided by the local Centre for Disease Control for free, after discharge from the hospital. d, e At a four-month follow-up, radiography and CT revealed the failure of the fusion and instrumentation. f Severe incision dehiscence was also observed. The patient received a repeated, single-stage anterior radical debridement, posterior autograft fusion and instrumentation. Forty-three days postoperatively, the result of DST demonstrated that the M. tuberculosis strain was resistant to isoniazid. Then the patient received a modified regimen, including pyrazinamide, ethambutol, rifapentine, levofloxacin and streptomycin, for 24 months. Streptomycin was discontinued after six months of treatment. g, h, i Three-year follow-up radiographs and CT showed the infected site had healed, and satisfactory bony fusion was achieved. j The sinus tract achieved complete cure, and the ESR was decreased to 2 mm/h
Discussion
Tuberculosis of the spine is an ancient disease, and its dismal outcomes in the pre-antibiotic era have improved significantly due to potent antitubercular drugs and advances in surgical management [7]. However, spinal tuberculosis is still a life-threatening disease owing to the emergence of drug-resistant strains. Despite this, there have been few studies on the topic of the clinical characteristics and drug-resistance profiles, or the treatment outcomes, of drug-resistant spinal tuberculosis.
This retrospective study demonstrated that 27 (77.1%) cases were less than 45 years old, and nine (25.7%) cases were comorbid with other sites of tuberculosis. Therefore, with respect to age, these patients were young but had already lost their capacity to work, becoming a heavy burden on society. Additionally, drug-resistant tuberculosis of the spine is associated with a decreased probability of cure and a high risk of severe complications. The rates of kyphosis (28.6%) and neurological deficits (51.4%) were higher than that reported by Pawar et al. [3]; the reason for this finding may be due to the more prolonged diagnostic delay of spinal tuberculosis in our series. Another point of interest was that the 35 cases were all HIV-negative, which suggested that there was no clear association between HIV and drug-resistant tuberculosis in this study.
The development of drug-resistant spinal tuberculosis is mainly acquired through previous improper anti-tuberculosis regimens, poor patient compliance, a prolonged diagnosis of drug resistance (a mean period of 8.43 ± 2.12 months in this study), and the spreading of drug-resistant strains. Because DST for tuberculosis spondylitis is not routinely undertaken in resource-limited countries, most settings in China use a standardised or empirical regimen, without the assessment of drug susceptibility. In our study, the proportion of resistance to first-line drugs and some second-line drugs (pasiniazid, rifapentine and levofloxacin) was much greater than that of other drugs, which indirectly demonstrated that standardised or empirical regimens consisting of these drugs have been widely used in this area. Of the 12 MDR-TB cases, 67% were retreatment cases and had received a mean duration of 14.50 months of chemotherapy before admission to our department, and there was only one patient who had a history of pulmonary tuberculosis and had received DST previously. Moreover, 40% of the MDR-TB cases were resistant to quinolones, and the development of XDR-TB is inevitable when injectable agents are improperly selected for spontaneously occurring resistant mutations [8]. Although the proportion of retreatment (22%) in non-MDR-TB cases was lower than that in MDR-TB cases, 50% of the non-MDR-TB patients were resistant to rifampicin or isoniazid, and the development of MDR-TB was also unavoidable when such first-line drugs were improperly used. This study also demonstrated that 63% (22/35) of the patients were new and usually regarded as primary resistance. Therefore, the spread of drug-resistant strains greatly contributed to the emergence of drug-resistance in the local area. Moreover, the other 37% (13/35) were retreatment cases. Among them, 61.5% (8/13) had at some point received irregular chemotherapy. In our experience, improper anti-tuberculosis chemotherapy is proven to be the main cause of acquired drug resistance [2, 3, 5, 9]. Thus, the DST should be carried out on all of the initial and retreatment spinal tuberculosis.
Optimal management of drug-resistant tuberculosis of the spine relies first on the early detection of such patients. Because of the difficulty in obtaining repeated focal samples, such as pus or granulomas, to isolate the organism, the signs of the emergence of drug-resistant spinal tuberculosis should be considered clinically before the result of DST is demonstrated by a lack of clinical or radiological improvement, by the appearance of a new lesion or a sinus formation or by rapid and profound vertebral destruction after chemotherapy for three to five months [7]. Delayed individualised treatment may result in acquired drug resistance. Therefore, there is an urgent need to include cultures and DST in the clinical pathway for the treatment of spinal tuberculosis. The methods of DST using the BACTEC MGIT 960 system can provide definitive results [10], but they usually require at least 36 days to produce the strain-susceptibility profile, leading to inadequate treatment and further acquired resistance. In our study, all of the six local recurrences occurred before culture results and DST were available; the reason for this outcome may have been improper chemotherapy over this period. Therefore, developing new, rapid and accurate molecular DST methods, such as commercial INNO-LiPA, Genotype MDR-TBplus and Xpert MTB/RIF, is essential for the early diagnosis of drug-resistant tuberculosis, and some of these methods are also suitable for resource-poor countries [11–14]. However, most of the current rapid diagnosis systems were designed for rifampicin and isoniazid, and there is still no molecular DST method to detect simultaneously all of the first- and second-line drugs.
Although there is little evidence to guide surgeons in treating drug-resistant tuberculosis of the spine effectively, this investigation demonstrated that it could be cured with a combination of the available drugs and surgery. There are several principles for the chemotherapy of patients with drug-resistant spinal tuberculosis (vide ut supra), and the chemotherapy program for drug-resistant spinal tuberculosis should be administered by specialised physicians. Due to the complexity of the disease, side effect monitoring and directly observed therapy should be guaranteed for good treatment compliance. A total of 33 of the 35 cases in our series achieved a cure after 18–24 months of individualised chemotherapy, while the other two were still undergoing treatment, which suggested that a satisfactory clinical outcome could be achieved with DST-guided chemotherapy in patients with drug-resistant spinal tuberculosis.
In our experience, spinal instability, severe deformity and progressive paraplegia are absolute surgical indications for spinal tuberculosis [15]. Patients with absolute indications often require surgical treatment to eradicate the foci thoroughly and to prevent the development of neurological deficits and deformity and, if it exists, to manage drug resistance. Furthermore, CT-guided percutaneous biopsy, catheter drainage and local chemotherapy are alternative methods for patients with relative indications (massive cold abscesses and nonresponse to conservative treatment), and these processes can obtain the focal tissue needed to perform cultures and DST, as well as decrease the patient’s burden of Mycobacterium tuberculosis strains [16, 17]. In the investigation, three cases without severe complications accepted percutaneous catheter drainage and local chemotherapy, while the other cases that met the absolute indications underwent one-stage or two-stage focal debridement, fusion and instrumentation, according to the particular condition of each patient. At the final follow-up, all of the patients achieved a good outcome in the following parameters: healing of disease, deformity correction and maintenance, improvement in neurology, and bony fusion of affected segments.
To summarize, drug resistant tuberculosis spondylitis is an aggressive disease. As we battle it, DST with anti-tuberculosis drugs, in combination with surgery and individualised chemotherapy, and regular monitoring of adverse effects are important, not only for therapeutic success, but also for the prevention of acquired drug resistance. The limitations of this study are its retrospective nature and the small number of subjects. Long-term follow-up of a prospective cohort study is needed in the future.
Acknowledgement
Appreciation is extended to Dr. Min Zhong, Dr. Yonglin Chen, Dr. Feng Wu and Chongqing Infecious Disease Medical Center, Chongqing, China for performing the drug susceptibility testing and data collection.
Footnotes
Litao Li and Zehua Zhang contributed equally to this work.
Contributor Information
Litao Li, Phone: +86-23-65340297, Email: lltop_1@sina.com.
Jianzhong Xu, Email: xjzslw@163.com.
References
- 1.Caminero JA, Sotgiu G, Zumla A, Migliori GB. Best drug treatment for multidrug resistant and extensively drug-resistant tuberculosis. Lancet Infect Dis. 2010;10(9):621–629. doi: 10.1016/S1473-3099(10)70139-0. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization (2010) Global tuberculosis control 2010. IOP Publishing PhysicsWeb. http://www.who.int/tb/publications/global_report/en/. Accessed July 2010
- 3.Pawar UM, Kundnani V, Agashe V, Nene A. Multidrug-resistant tuberculosis of the spine-is it the beginning of the end? A study of twenty-five culture proven multidrug-resistant tuberculosis spine patients. Spine (Phila Pa 1976) 2009;34(22):806–810. doi: 10.1097/BRS.0b013e3181af7797. [DOI] [PubMed] [Google Scholar]
- 4.Mukherjee JS, Rich ML, Socci AR, Joseph JK, Alcántara Virú F, Shin SS, Furin JJ, Becerra MC, Barry DJ, Kim JY, Bayona J, Farmer P, Smith Fawzi MC, Seung KJ. Programmes and principles in treatment of multi-drug resistant tuberculosis. Lancet. 2004;363(9407):474–481. doi: 10.1016/S0140-6736(04)15496-2. [DOI] [PubMed] [Google Scholar]
- 5.Loddenkemper R, Sagebiel D, Brende A. Strategies against multi-drug resistant tuberculosis. Eur Respir J. 2002;36(Suppl):66–77. doi: 10.1183/09031936.02.00401302. [DOI] [PubMed] [Google Scholar]
- 6.Lee CK, Vessa Paul, Lee JK. Chronic disabling low back pain syndrome caused by internal disc derangements: the results of disc excision and posterior lumbar interbody fusion. Spine. 1995;20(3):356–360. doi: 10.1097/00007632-199502000-00018. [DOI] [PubMed] [Google Scholar]
- 7.Jain AK. Tuberculosis of the spine: a fresh look at an old disease. J Bone Joint Surg Br. 2010;92(B):905–913. doi: 10.1302/0301-620X.92B7.24668. [DOI] [PubMed] [Google Scholar]
- 8.Jassal M, Bishai WR. Extensively drug-resistant tuberculosis. Lancet Infect Dis. 2009;9:19–30. doi: 10.1016/S1473-3099(08)70260-3. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization (2010) Multidrug and extensively drug-resistant TB (M/XDR-TB): 2010 global report on surveillance and response. IOP Publishing PhysicsWeb. http://www.who.int/tb/publications/mdr_surveillance/en/. Accessed March 2010
- 10.Rüsch S, Gaby E, Casal M. Multicenter laboratory validation of the BACTEC MGIT 960 technique for testing susceptibilities of mycobacterium tuberculosis to classical second-line drugs and newer antimicrobials. J Clin Microbiol. 2006;44(3):688–692. doi: 10.1128/JCM.44.3.688-692.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yao C, Zhu T, Li Y, Zhang L, Zhang B, Huang J, Fu W. Detection of rpoB, katG and inhA gene mutations in Mycobacterium tuberculosis clinical isolates from Chongqing as determined by microarray. Clin Microbiol Infect. 2010;16(11):1639–1643. doi: 10.1111/j.1469-0691.2010.03267.x. [DOI] [PubMed] [Google Scholar]
- 12.Catharina C, Boehme PN. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med. 2010;363(11):1005–1015. doi: 10.1056/NEJMoa0907847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Catharina C, Boehme MP, Nicol PN. Feasibility, diagnostic accuracy, and effectiveness of decentralised use of the Xpert MTB/RIF test for diagnosis of tuberculosis and multidrug resistance: a multicentre implementation study. Lancet. 2011;377(9776):1495–1505. doi: 10.1016/S0140-6736(11)60438-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bazira J, Asiimwe BB, Joloba ML (2010) Use of the GenoType® MTBDRplus assay to assess drug resistance of Mycobacterium tuberculosis isolates from patients in rural Uganda. BMC Clin Pathol 10(5) [DOI] [PMC free article] [PubMed]
- 15.Xu JZ. The surgical indication and methods for spinal tuberculosis. Chin J Spine Spinal Cord. 2006;16(12):889–890. [Google Scholar]
- 16.Dinc H, Ahmetoglu A, Baykal S, Sari A, Sayil O. Image-guided percutaneous drainage of tuberculous iliopsoas and spondylodiskitic abscesses: midterm results. Radiology. 2002;225(2):353–358. doi: 10.1148/radiol.2252011443. [DOI] [PubMed] [Google Scholar]
- 17.Nagashima H, Yamane K, Nishi T, Nanjo Y, Teshima R. Recent trends in spinal infections: retrospective analysis of patients treated during the past 50 years. Int Orthop. 2010;34(3):395–399. doi: 10.1007/s00264-009-0741-1. [DOI] [PMC free article] [PubMed] [Google Scholar]