Abstract
Background
Infection after spinal fusion for scoliosis is a commonly reported complication. Although techniques in paediatric spinal fusion have improved with regard to infection prophylaxis, postoperative infection rates range from 0.4% to 8.7%.
Infection rates and causative factors
The rate of infection in surgery for adolescent idiopathic scoliosis (AIS) has ranged from 0.9% to 3%. The rate of infection in spinal surgery for deformity related to myelomeningocele has been reported to be from 8% to 24%. The rate of infection in spinal surgery for deformity related to cerebral palsy has been reported to be from 6.1% to 8.7%. Infection after spinal fusion for scoliosis related to a muscular dystrophy is generally less frequent. Despite a large number of cases and studies, the literature did not provide documentation of several factors that may be related to the occurrence of wound infection. The rate of wound infection after spine surgery is dependent on many factors, including the complexity of the procedure, health status of the patient, and potentially the experience and technique of the operating surgeon.
Treatment algorithm
The general algorithm for treatment depends on a variety of factors, including the delay from the index procedure, the infecting organism, the location and extent of the infection, the gross appearance of the fusion mass, and the surgical strategy used to correct the initial deformity. For infections that develop within the first 90 days after the index procedure all attempts to retain the instrumentation should be made. In late infections, the fusion mass must be carefully inspected before instrumentation removal is considered. Although fusion may appear to be solid both radiographically and intra-operatively, there still may be the possibility of loss of correction at last follow-up.
Conclusion
Deep wound infection after instrumented fusion of the spine remains a difficult and challenging clinical problem and entails substantial morbidity, cost, and recovery time for the patient. An aggressive approach to deep wound infection emphasising early irrigation and debridement allowed preservation of instrumentation and successful fusion in most cases. At the conclusion of treatment, patients can expect a medium-term clinical outcome similar to patients in whom infectious complication did not occur.
Introduction
Infection after spinal fusion for scoliosis is a commonly reported complication. Studies performed on adults report infection rates ranging from 9.3% to 20% [1, 2]. Although techniques in paediatric spinal fusion have improved with regard to infection prophylaxis, postoperative infections and wound complications remain, with current reported scoliosis infection rates ranging from 0.4% to 8.7% [3, 4].
According to the last SRS report [2], the overall rates of superficial and deep infection were 0.8% and 1.3%, respectively. Superficial and deep infections occurred in 0.8% and 1.2% of adult patients (n = 82,082), respectively, and in 1.0% and 1.7% of pediatric patients (n = 25,432).
In most cases, current treatment algorithms [4–8] are effective at eradicating the infection; chronic infection stemming from a postoperative spinal infection in a child is rare.
Infection rates related to specific diagnosis
Infection rates in scoliosis surgery associated with a specific underlying diagnosis have been reported in smaller studies. The largest diagnosis-specific studies have been in patients with adolescent idiopathic scoliosis (AIS). The rate of infection in surgery for AIS has ranged from 0.9% to 3% [3, 9, 10]. The largest study is based on self-reported cases from members of the Scoliosis Research Society, who reported a rate of infection of 1.1% in 6,334 patients [11].
Infection after spine fusion for neuromuscular scoliosis has been shown to range from 4.2% to 20.0% prevalence [12–14]. The term “neuromuscular scoliosis” covers a wide variety of conditions, each with its own rate of infection associated with spinal deformity surgery. Some of the factors that may account for the differences of infection rate between diagnoses are loss of sensation, loss of bowel and bladder control, previous surgical treatment of the spine, and altered soft tissue coverage. Patients without sensation in their lower extremities, buttocks, or lower back are at risk of the development of decubiti, which can, in turn, lead to an infection either by direct contamination or haematological spread. Those patients who lack bowel and bladder control risk seeding a wound with feces or urine. Furthermore, these patients develop frequent urinary tract infections, which can spread to implanted instrumentation or a surgical wound [15].
Previous retrospective studies reported specific rates of infection for each diagnosis, including scoliosis related to myelomeningocele, cerebral palsy (CP), muscle diseases, and spinal cord injury. The rate of infection in spinal surgery for deformity related to myelomeningocele has been reported to be from 8% to 24% [14, 16]. The rate of infection in spinal surgery for deformity related to cerebral palsy has been reported to be from 6.1% to 8.7% [17–19]. Infection after spinal fusion for scoliosis related to a muscular dystrophy is generally less frequent[20–22].
Factors associated with an increased risk of infection
Although there are studies which have examined deep wound infection and spine fusion surgery as well as risk factors for deep wound infection, there are limited studies evaluating clinical and radiographic factors associated with this complication. Despite a large number of cases, the SRS database [2] did not provide documentation of several factors that may be related to the occurrence of wound infection, including whether prophylactic antibiotics were administered, length of operative time, estimated blood loss, number of surgeons involved in the procedure, length of hospitalization, or patient comorbidities. In addition, there was no documentation of causative organisms, how the infection was managed, or outcomes.
The rate of wound infection after spine surgery is dependent on many factors, including the complexity of the procedure, health status of the patient, and potentially the experience and technique of the operating surgeon and facility related factors.
A recent study assessing complications after spine fusion reported increased complication among CP patients with baclofen pump, which was associated with re-operation and re-hospitalisation [23]. The development of delayed infection after spine fusion was also associated with multiple factors including past medical history and blood transfusion [15]. Increased risk of deep wound infection after spine fusion in CP patients has been observed in a retrospective case–control study to be related to severe cognitive impairment or mental retardation and to being an allograft recipient [14, 24].
The presence of metallic instrumentation may predispose to bacterial migration despite intra-operative antimicrobial coverage especially in “immunochallenged” patients such as patients with neuromuscular diseases. Many skin problems may be encountered in such patients including tape blisters, superficial suture reactions, skin breakdown from pressure sores, and prominent hardware [15].
It is immunologically sound to assume that children with excess body weight may have poor nutritional status, which may increase their risk of infection, and or poor wound healing, leading to deep wound infection. Therefore as fatty tissues provide more electrical resistance than the muscle, if electrocautery is used during surgery, and with uncoagulated blood vessels, it may result in haematoma, increasing the risk of infection in patients with higher mean weight [24–26].
Treatment algorithm
Currently, there is a general agreement that a draining spinal wound or haematoma necessitates operative irrigation and debridement. Intravenous or oral antibiotics are tailored to the results of intra-operative cultures [5, 27, 28].
Conservative management with antibiotic therapy alone is not attempted at our institution, considering the high risk of failure, presence of extensive instrumentation, and possible consequences of delay, including increasing severity of infection, clinically significant sepsis, and osteomyelitis [5, 29].
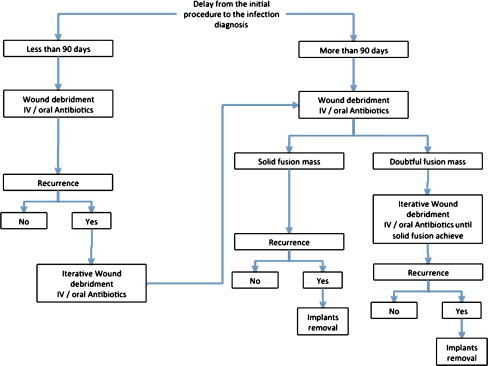
Our general algorithm for treatment has been largely dependent on a variety of factors, including the delay from the index procedure, the infecting organism, the location and extent of the infection, the gross appearance of the fusion mass, and the surgical strategy used to correct the initial deformity. In our experience, for infections that develop within the first 90 days after the index procedure all attempts to retain the instrumentation should be made.
Even if the infection cannot be completely cleared unless the instrumentation is removed, we recommend debridement without instrumentation removal in the early infections followed by suppressive antibiotics until adequate fusion is obtained (Fig. 1).
Fig. 1.
General algorithm of therapeutic strategy after diagnosis of postoperative spinal infection following children and adolescent deformity corrections
In late infections, the fusion mass must be carefully inspected before instrumentation removal is considered. If the infecting organism is S. epidermidis or methicilin-resistant S. aureus, the possibility of clearing the infection without removing the implants seems to be less likely [4, 8, 28, 30–32].
Need for implant removal
There is little previously reported data on the progression of spinal deformity after instrumentation removal for infection. Our experience with persistent infections has often led to removal of spinal implants. Very little literature is available regarding sequelae after implant removal[33–35]. Although fusion may appear to be solid both radiographically and intra-operatively, there still may be the possibility of loss of correction at the last follow-up. Although there are numerous reports of infection, there is very little literature available to help guide management of these patients. Controversies still exist regarding the retention of spinal implants, wound management, and the need for repeated irrigation and debridement surgeries [28, 36].
To our knowledge, the only published data are confined to patients with AIS. Rathjen et al. [33] reported on 43 patients with AIS and instrumentation removal for a variety of reasons at the Texas Scottish Rite Hospital. In three (7%) of the 43 patients, the coronal curves progressed 10° or more. Potter et al. [34] also reported on the magnitude of coronal curve progression (total progression, 9°) in 21 patients with AIS after infection.
Lastly, despite evidence of adequate fusion, patients with kyphotic deformity seem to progress more than those with a purely coronal deformity [33]. Caution should be taken when deciding to leave a previously instrumented kyphotic spine uninstrumented. On several occasions after debriding and removing instrumentation from kyphotic spines or spines with inadequate fusion masses, we have reinstrumented either in the same surgery or after a period of several weeks of intravenous antibiotics. However, currently, there are no clear guidelines to address such complex cases.
Neither theses studies nor others clearly define the likelihood of a child’s spinal deformity progressing after implant removal, though we suspect it will be very high. A future long-term follow-up study of the patients in whom implants were removed is mandatory to clarify the risk of progressive spinal deformity. In infected total joint replacements in adults, a common treatment strategy is removal of all implants at time of irrigation and debridement, followed by replacement of implants at a later date [37]. Perhaps this approach may be applicable to children with infected spine implants at risk for progressive deformity after implant removal.
Outcome
Interpretation of previous studies on outcomes of deep wound infection after spine surgery is difficult because of the variability in definition of infection, outcome instrument used, use of a control group, and/or sample size, leading to varying conclusions. Although some authors have stated that outcomes can be good to excellent with no long-term loss of function [7, 28, 33, 35, 38], Collins et al. reported that only 46% of patients had stable pain-free spines when managed with routine removal of implants in established fusions [39].
Especially in neuromuscular patients, deep wound infection is a severe and common complication of spine fusion[18, 40]. In children, an aggressive approach to deep wound infection emphasising early irrigation and debridement allowed preservation of instrumentation and successful fusion in most cases. At the end of treatment, patients can expect a medium-term clinical outcome similar to patients in whom this complication did not occur [30].
Conclusion
Deep wound infection after instrumented fusion of the spine remains a difficult and challenging clinical problem and entails substantial morbidity, cost, and recovery time for the patient. An aggressive approach to deep wound infection emphasising early irrigation and debridement allowed preservation of instrumentation and successful fusion in most cases. Late infections required treatment with implant removal and antibiotics but a long-term follow-up study of the patients in whom implants were removed is mandatory to clarify the risk of progressive spinal deformity. At the conclusion of treatment, patients can expect a medium-term clinical outcome similar to patients in whom infectious complication did not occur. Accurate information about clinical outcome after infection is useful for patients and surgeons in making informed choices regarding surgical care.
References
- 1.MacEwen GD. Operative treatment of scoliosis in cerebral palsy. Reconstr Surg Traumatol. 1972;13:58–67. [PubMed] [Google Scholar]
- 2.Sansur CA, Smith JS, Coe JD, et al. Scoliosis research society morbidity and mortality of adult scoliosis surgery. Spine (Phila Pa 1976) 2011;36:E593–E597. doi: 10.1097/BRS.0b013e3182059bfd. [DOI] [PubMed] [Google Scholar]
- 3.Ho C, Sucato DJ, Richards BS. Risk factors for the development of delayed infections following posterior spinal fusion and instrumentation in adolescent idiopathic scoliosis patients. Spine (Phila Pa 1976) 2007;32:2272–2277. doi: 10.1097/BRS.0b013e31814b1c0b. [DOI] [PubMed] [Google Scholar]
- 4.Viola RW, King HA, Adler SM, et al. Delayed infection after elective spinal instrumentation and fusion. A retrospective analysis of eight cases. Spine (Phila Pa 1976) 1997;22:2444–2450. doi: 10.1097/00007632-199710150-00023. [DOI] [PubMed] [Google Scholar]
- 5.Kuo CH, Wang ST, Yu WK, et al. Postoperative spinal deep wound infection: a six-year review of 3230 selective procedures. J Chin Med Assoc. 2004;67:398–402. [PubMed] [Google Scholar]
- 6.Li S, Zhang J, Li J, et al. Wound infection after scoliosis surgery: an analysis of 15 cases. Chin Med Sci J. 2002;17:193–198. [PubMed] [Google Scholar]
- 7.Weinstein MA, McCabe JP, Cammisa FP., Jr Postoperative spinal wound infection: a review of 2,391 consecutive index procedures. J Spinal Disord. 2000;13:422–426. doi: 10.1097/00002517-200010000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Richards BS. Delayed infections following posterior spinal instrumentation for the treatment of idiopathic scoliosis. J Bone Joint Surg Am. 1995;77:524–529. doi: 10.2106/00004623-199504000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Coe JD, Arlet V, Donaldson W, et al. Complications in spinal fusion for adolescent idiopathic scoliosis in the new millennium. A report of the Scoliosis Research Society Morbidity and Mortality Committee. Spine (Phila Pa 1976) 2006;31:345–349. doi: 10.1097/01.brs.0000197188.76369.13. [DOI] [PubMed] [Google Scholar]
- 10.Guigui P, Blamoutier A. Complications of surgical treatment of spinal deformities: a prospective multicentric study of 3311 patients. Rev Chir Orthop Repar Appar Mot. 2005;91:314–327. doi: 10.1016/S0035-1040(05)84329-6. [DOI] [PubMed] [Google Scholar]
- 11.Sansur CA, Reames DL, Smith JS, et al. Morbidity and mortality in the surgical treatment of 10,242 adults with spondylolisthesis. J Neurosurg Spine. 2010;13:589–593. doi: 10.3171/2010.5.SPINE09529. [DOI] [PubMed] [Google Scholar]
- 12.Tsirikos AI, Lipton G, Chang WN, et al. Surgical correction of scoliosis in pediatric patients with cerebral palsy using the unit rod instrumentation. Spine (Phila Pa 1976) 2008;33:1133–1140. doi: 10.1097/BRS.0b013e31816f63cf. [DOI] [PubMed] [Google Scholar]
- 13.Sponseller PD. Pediatric revision spinal deformity surgery: issues and complications. Spine (Phila Pa 1976) 2010;35:2205–2210. doi: 10.1097/BRS.0b013e3181e7d675. [DOI] [PubMed] [Google Scholar]
- 14.Sponseller PD, LaPorte DM, Hungerford MW, et al. Deep wound infections after neuromuscular scoliosis surgery: a multicenter study of risk factors and treatment outcomes. Spine (Phila Pa 1976) 2000;25:2461–2466. doi: 10.1097/00007632-200010010-00007. [DOI] [PubMed] [Google Scholar]
- 15.Mohamed Ali MH, Koutharawu DN, Miller F, et al. Operative and clinical markers of deep wound infection after spine fusion in children with cerebral palsy. J Pediatr Orthop. 2010;30:851–857. doi: 10.1097/BPO.0b013e3181f59f3f. [DOI] [PubMed] [Google Scholar]
- 16.Benson ER, Thomson JD, Smith BG, et al. Results and morbidity in a consecutive series of patients undergoing spinal fusion for neuromuscular scoliosis. Spine (Phila Pa 1976) 1998;23:2308–2317. doi: 10.1097/00007632-199811010-00012. [DOI] [PubMed] [Google Scholar]
- 17.Modi HN, Suh SW, Yang JH, et al. Surgical complications in neuromuscular scoliosis operated with posterior-only approach using pedicle screw fixation. Scoliosis. 2009;4:11. doi: 10.1186/1748-7161-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thacker M, Hui JH, Wong HK, et al. Spinal fusion and instrumentation for paediatric neuromuscular scoliosis: retrospective review. J Orthop Surg (Hong Kong) 2002;10:144–151. doi: 10.1177/230949900201000207. [DOI] [PubMed] [Google Scholar]
- 19.Szoke G, Lipton G, Miller F, et al. Wound infection after spinal fusion in children with cerebral palsy. J Pediatr Orthop. 1998;18:727–733. doi: 10.1097/00004694-199811000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Modi HN, Suh SW, Song HR, et al. Treatment of neuromuscular scoliosis with posterior-only pedicle screw fixation. J Orthop Surg Res. 2008;3:23. doi: 10.1186/1749-799X-3-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramirez N, Richards BS, Warren PD, et al. Complications after posterior spinal fusion in Duchenne's muscular dystrophy. J Pediatr Orthop. 1997;17:109–114. doi: 10.1097/00004694-199701000-00023. [DOI] [PubMed] [Google Scholar]
- 22.Shapiro F, Sethna N, Colan S, et al. Spinal fusion in Duchenne muscular dystrophy: a multidisciplinary approach. Muscle Nerve. 1992;15:604–614. doi: 10.1002/mus.880150512. [DOI] [PubMed] [Google Scholar]
- 23.Caird MS, Palanca AA, Garton H, et al. Outcomes of posterior spinal fusion and instrumentation in patients with continuous intrathecal baclofen infusion pumps. Spine (Phila Pa 1976) 2008;33:E94–E99. doi: 10.1097/BRS.0b013e3181642aae. [DOI] [PubMed] [Google Scholar]
- 24.Lipton GE, Miller F, Dabney KW, et al. Factors predicting postoperative complications following spinal fusions in children with cerebral palsy. J Spinal Disord. 1999;12:197–205. [PubMed] [Google Scholar]
- 25.Hatlen T, Song K, Shurtleff D, et al. Contributory factors to postoperative spinal fusion complications for children with myelomeningocele. Spine (Phila Pa 1976) 2010;35:1294–1299. doi: 10.1097/BRS.0b013e3181bf8efe. [DOI] [PubMed] [Google Scholar]
- 26.Jevsevar DS, Karlin LI. The relationship between preoperative nutritional status and complications after an operation for scoliosis in patients who have cerebral palsy. J Bone Joint Surg Am. 1993;75:880–884. doi: 10.2106/00004623-199306000-00008. [DOI] [PubMed] [Google Scholar]
- 27.Mok JM, Cloyd JM, Bradford DS, et al. Reoperation after primary fusion for adult spinal deformity: rate, reason, and timing. Spine (Phila Pa 1976) 2009;34:832–839. doi: 10.1097/BRS.0b013e31819f2080. [DOI] [PubMed] [Google Scholar]
- 28.Rihn JA, Lee JY, Ward WT. Infection after the surgical treatment of adolescent idiopathic scoliosis: evaluation of the diagnosis, treatment, and impact on clinical outcomes. Spine (Phila Pa 1976) 2008;33:289–294. doi: 10.1097/BRS.0b013e318162016e. [DOI] [PubMed] [Google Scholar]
- 29.Clark CE, Shufflebarger HL. Late-developing infection in instrumented idiopathic scoliosis. Spine (Phila Pa 1976) 1999;24:1909–1912. doi: 10.1097/00007632-199909150-00008. [DOI] [PubMed] [Google Scholar]
- 30.Mok JM, Guillaume TJ, Talu U, et al. Clinical outcome of deep wound infection after instrumented posterior spinal fusion: a matched cohort analysis. Spine (Phila Pa 1976) 2009;34:578–583. doi: 10.1097/BRS.0b013e31819a827c. [DOI] [PubMed] [Google Scholar]
- 31.Malamo-Lada H, Zarkotou O, Nikolaides N, et al. Wound infections following posterior spinal instrumentation for paralytic scoliosis. Clin Microbiol Infect. 1999;5:135–139. doi: 10.1111/j.1469-0691.1999.tb00526.x. [DOI] [PubMed] [Google Scholar]
- 32.Richards BS, Herring JA, Johnston CE, et al. Treatment of adolescent idiopathic scoliosis using Texas Scottish Rite Hospital instrumentation. Spine (Phila Pa 1976) 1994;19:1598–1605. doi: 10.1097/00007632-199407001-00008. [DOI] [PubMed] [Google Scholar]
- 33.Rathjen K, Wood M, McClung A, et al. Clinical and radiographic results after implant removal in idiopathic scoliosis. Spine (Phila Pa 1976) 2007;32:2184–2188. doi: 10.1097/BRS.0b013e31814b88a5. [DOI] [PubMed] [Google Scholar]
- 34.Potter BK, Kirk KL, Shah SA, et al. Loss of coronal correction following instrumentation removal in adolescent idiopathic scoliosis. Spine (Phila Pa 1976) 2006;31:67–72. doi: 10.1097/01.brs.0000192721.51511.fe. [DOI] [PubMed] [Google Scholar]
- 35.Muschik M, Luck W, Schlenzka D. Implant removal for late-developing infection after instrumented posterior spinal fusion for scoliosis: reinstrumentation reduces loss of correction. A retrospective analysis of 45 cases. Eur Spine J. 2004;13:645–651. doi: 10.1007/s00586-004-0694-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rhee MA, Klerk LW, Verhaar JA. Vacuum-assisted wound closure of deep infections after instrumented spinal fusion in six children with neuromuscular scoliosis. Spine J. 2007;7:596–600. doi: 10.1016/j.spinee.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 37.Lentino JR. Prosthetic joint infections: bane of orthopedists, challenge for infectious disease specialists. Clin Infect Dis. 2003;36:1157–1161. doi: 10.1086/374554. [DOI] [PubMed] [Google Scholar]
- 38.Gitelman A, Joseph SA, Jr, Carrion W, et al. Results and morbidity in a consecutive series of patients undergoing spinal fusion with iliac screws for neuromuscular scoliosis. Orthopedics. 2008;12:31. doi: 10.3928/01477447-20081201-08. [DOI] [PubMed] [Google Scholar]
- 39.Collins I, Wilson-MacDonald J, Chami G, et al. The diagnosis and management of infection following instrumented spinal fusion. Eur Spine J. 2008;17:445–450. doi: 10.1007/s00586-007-0559-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Auerbach JD, Spiegel DA, Zgonis MH, et al. The correction of pelvic obliquity in patients with cerebral palsy and neuromuscular scoliosis: is there a benefit of anterior release prior to posterior spinal arthrodesis? Spine (Phila Pa 1976) 2009;34:E766–E774. doi: 10.1097/BRS.0b013e3181b4d558. [DOI] [PubMed] [Google Scholar]