Abstract
Purpose
The World Health Organisation has declared tuberculosis (TB) a global emergency and spinal tuberculosis is one of the most common forms. There is still controversy regarding optimum duration of treatment in osteoarticular tuberculosis due to the lack of well-defined criteria for the end point of treatment. Emergence of multi drug resistant tuberculosis, primarily due to use of poor drug regimens, further illustrates the need of newer and more effective diagnostic methods, particularly in developing countries.
Methods
This prospective clinical study to evaluate the role of technetium (99mTc)–ciprofloxacin scan as a tool to assess disease activity involved in 15 cases of TB spine with a mean age of 32.2 years (range 21–72). Following a clinico-radiological diagnosis, all patients were treated with standard anti tubercular treatment and a scan was done at zero, three and six months of treatment with tracer activity being recorded and compared in sequential scans along with a parallel evaluation of clinical and radiological profile at regular intervals.
Results
Out of 15 cases, nine had an initially positive bone scan. Two patients (22%) converted to negative scans at three months, whereas the remaining seven (78%) turned negative at six months. The end of six months treatment was also accompanied by clinico-radiological resolution in all cases.
Conclusion
In conclusion, technetium (99mTc)–ciprofloxacin scan could be a promising tool for monitoring disease activity in selected cases of tuberculosis spine as an alternative for therapeutic drug monitoring; however, due to the small sample size, studies with a large number of patients might be of help in defining these cases in a better way.
Introduction
Globally, tuberculosis is the most common infectious disease responsible for major morbidity [1]. One third of the world population is believed to be infected with Mycobacterium tuberculosis and there is enhanced susceptibility to TB infection in the HIV infected population. In addition, multi drug-resistant TB (MDR-TB) has been increasing in incidence, not only in developing countries but industrialised nations as well. In the World Health Organisation (WHO)–International Union Against Tuberculosis and Lung Disease (IUATLD) Working Group Global Anti-Tuberculosis Drug Resistance Surveillance, the incidence of MDR-TB was found to be 14%, of which primary multi-drug resistance was only 1.4%, indicating that MDR-TB is usually acquired as a result of poor chemotherapy. The global resurgence of TB and the rapid emergence of MDR-TB demands new protocols for efficacious treatment and clinical control of TB patients, particularly in developing countries.
Recently, we reported the use of the 99mTc–ciprofloxacin scan for monitoring disease activity in cases of extraspinal osteoarticular tuberculosis with high sensitivity and specificity; thus, we decided to study the course of tuberculosis spine with the help of sequential 99mTc–ciprofloxacin scans in an attempt to define an objective end point for cessation of antitubercular treatment in these cases.
Material and methods
This prospective study included 15 newly diagnosed cases of tuberculosis spine, aged between 21 to 72 years (mean 32.2 years). The purpose of the study was explained to each patient and a written informed consent was obtained. All new patients of tuberculosis spine planned for non operative treatment as per the criteria of middle path regimen [2] were included in this study. All patients with neurological involvement at the initial presentation secondary to their disease were excluded from this study.
Patients suspected for tuberculosis spine were evaluated thoroughly to establish a clinico-radiological diagnosis. All patients were investigated for estimation of haemoglobin level, total leucocyte count (TLC), differential leucocyte count (DLC), erythrocyte sedimentation rate (ESR), and chest X-ray. Histopathological diagnosis was established in seven cases. In two cases having superficial paraspinal abscesses, the aspirate was subjected to polymerase chain reaction, which proved positive for Mycobacterium tuberculosis. MRI of the spine was done in all cases and was taken as the supporting evidence of tuberculosis. MRI was repeated at the same interval as the scan for comparative purposes. All patients were given a brace suitable for the site of involvement for the period of antitubercular treatment [2].
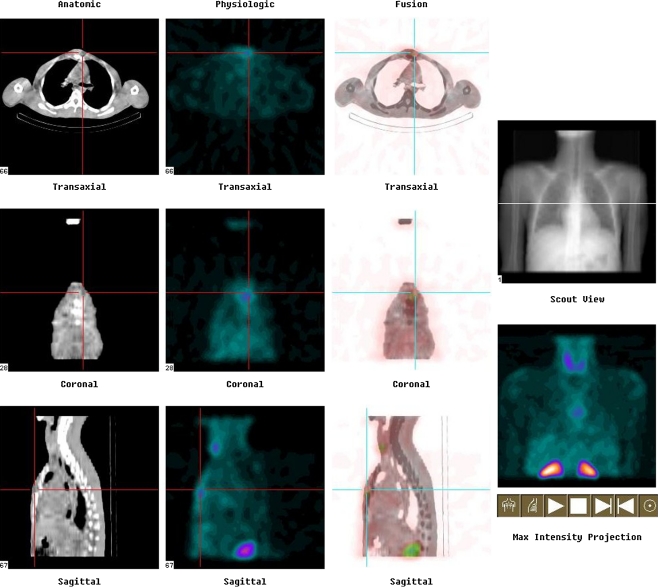
Patients thus diagnosed as having tuberculosis spine were then subjected to technetium 99m–ciprofloxacin (Diagnobact™) labelled bone scan [3] at the beginning of treatment, at three months of chemotherapy and after completion of six months of chemotherapy. The activity of tracer at the site was recorded and compared on sequential scans. Wherever required, SPECT-CT was done after one hour to correlate the functional image of SPECT with an anatomical image of CT (Fig. 1). For SPECT-CT imaging, step-and-shoot mode in 32 frames was employed. Each frame was of 20 seconds duration and was taken in 128 × 128 matrix size. In four and 24-hour scans, only spot images of the suspected area were taken.
Fig. 1.
Photograph showing sagittal and axial images of tracer uptake in a case of TB D4-5 on SPECT-CT
Recommended treatment regimens under the Revised National Tuberculosis Control Programme (RNTCP) of India were used. Follow-up was done at two weekly intervals during the intensive phase while at monthly intervals for the continuation phase of treatment. All patients were assessed for four quantitative (weight, ESR, pain, tenderness) and two qualitative parameters (fever and anorexia constitutional symptoms). Pain and tenderness were graded on a four-point scale [3]. Constitutional symptoms were described as either present or absent. The results were analysed using standard statistical tests.
Results
The most common site of involvement in our cases was dorsal spine in eight cases followed by lumbosacral spine in six cases. There was involvement of cervical spine in two cases and multifocal involvement of spine in one case involving all cervical, dorsal and lumbar regions. All patients had pain and tenderness at presentation which showed progressive improvement during treatment. The mean scores for pain and tenderness decreased from 3.73 and 3.89, respectively, at the time of first scan to 1.00 for tenderness and 1.2 for pain at third scan after six months of antitubercular treatment. Statistical analysis of both pain and tenderness scores using the ‘z’ test showed significant improvement with p value less than 0.05. All patients (100%) gained weight during the course of the treatment. Mean body weight was 58.6 kg at the time of commencing antitubercular treatment which increased to 60.7 kg after three months of treatment and 62.6 kg at six months of antitubercular treatment. All (100%) patients also showed an improvement in ESR during the course of treatment with mean values decreasing from 62 to 9.3 mm in the first hour at the end. All (100%) of our patients had constitutional symptoms in the form of anorexia and low grade fever at the time of presentation. All of these patients showed improvement in these symptoms within 12–14 weeks of starting the treatment. In our study constitutional symptoms were again the earliest parameter to show improvement with treatment.
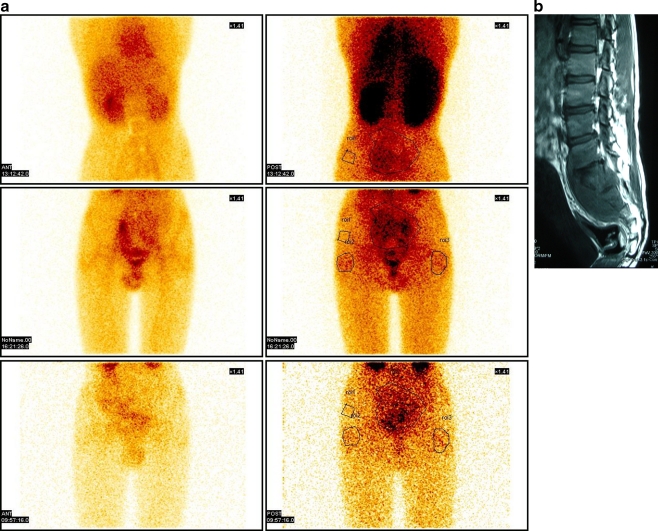
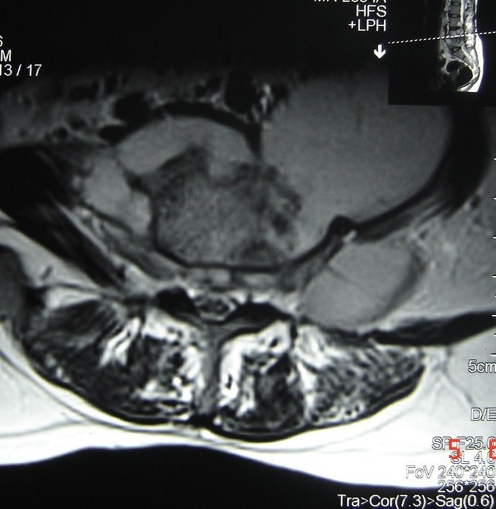
Out of 15 cases enrolled in the study, only nine had initial positive 99mTc bone scan. Two patients (22%) converted to negative scans at three months, whereas the remaining seven patients (78%) showed negative scans at six months. Though only qualitative analysis of scan results was done, there was a progressive diminution in scan activity during the sequential scans in those cases where first scan was positive (Fig. 2a, b). The end of six-month antitubercular treatment also coincided with clinicoradiological resolution in all cases. The initial scan was negative in the patient with multifocal involvement and in three patients with dorsal spine and two patients with lumbar involvement. All the patients with initial scan negative had larger paraspinal collections and greater bony destruction as evident on MRI scans (Fig. 3).
Fig. 2.
a, b Tracer uptake on Ciplox scan and its correlation with MRI in a case of TB L5-S3
Fig. 3.
Axial image of T1W MRI showing large paraspinal abscess and area of bony destruction
Discussion
Controversy surrounds the optimum duration of treatment in osteoarticular tuberculosis. While WHO continues to recommend the same regimens for extrapulmonary and pulmonary disease, the newer guidelines in the 2010 [4] edition refers to other experts [5] suggesting longer duration of treatment such as nine months because of difficulties of assessing treatment response in osteoarticular TB. In a recent study [6] to define the perspective of senior orthopaedic surgeons from India and other countries on the appropriate duration of antitubercular treatment in osteoarticular tuberculosis, there was no consensus.
Osteoarticular infections have always been difficult to treat, driving the orthopaedic surgeons to use a longer duration of antitubercular treatment. This clinical presumption is further confounded by the fact that, in contrast to pulmonary tuberculosis which has well-defined criteria for the initiation as well as follow-up of treatment in the form of sputum microscopy, no such test is available for osteoarticular tuberculosis. The emergence of MDR-TB further illustrates the need for new and effective diagnostic methods, particularly for use in developing countries, as the resistance in most of the cases is acquired as a result of poor regimens used to treat the infection.
Though TB is a great mimicker, the diagnosis of Pott’s spine in all our cases was made on clinico-radiological grounds consisting of a clinical presentation of pain overlying the affected vertebrae [7], weight loss [8], low-grade fever of varying duration and raised ESR. Plain radiographs of all these cases showed loss of vertebral height, disk space narrowing, erosions, blurring of the end plates, paravertebral abscesses, and soft tissue calcifications [9] in varying combinations. As MRI is a very good diagnostic modality for spinal TB, it was done in all the cases. It is useful in delineating paravertebral, epidural abscesses as well as in evaluating the extent of cord compression and the presence of intramedullary lesions, if any. However, characteristic findings of MRI in vertebral TB include decreased signal intensity on T1-weighted images of both vertebral bodies and disc spaces, but a signal intensity that is increased in the vertebral disc and markedly decreased in the vertebral bodies on T2-weighted images [10]. As radiological findings alone may be non-diagnostic and imaging studies are not fully reliable for differentiating spinal TB from other diseases, bacteriological and/or histological confirmation is needed [11] and was obtained in cases where it was possible.
Various clinical criteria such as weight gain [4] and laboratory parameters such as ESR [12, 13] have been used in the past to help the clinician in making the diagnosis as well as following up the course of osteoarticular tuberculosis in the absence of any objective criteria. Both these criteria showed consistent and statistically significant improvement in all our cases also. Various serological investigations such as enzyme linked immunosorbent assay (ELISA) for antibody to mycobacterial antigen-6 [14] and polymerase chain reaction (PCR) [15–17] have also shown good sensitivity and specificity in establishing the diagnosis of skeletal tuberculosis, but they are not useful for following the course of disease. Though PCR has been reported to be useful for detection of Mycobacterium tuberculosis in extra pulmonary specimens, as was also evident in this case series where both the specimens came out to be positive, it is considered to be less appropriate for monitoring of antitubercular treatment as the majority of the samples from treated patients test positive despite sterile culture results [18].Even MRI may not be able to differentiate between residual infection and the post infection reparative process, once the treatment is completed [19, 20].
In a study using 99mTc–ciprofloxacin scan to detect tuberculosis in patients having a clinical suspicion of the disease, a high degree of correlation was found with the scan [21]. Recently we reported the successful use of 99mTc–ciprofloxacin scan in monitoring the disease activity in patients with extraspinal osteoarticular tuberculosis. In our study we found that Tc 99m scan was a promising tool for monitoring the drug response in cases of extra spinal skeletal tuberculosis almost akin to sputum microscopy for pulmonary tuberculosis.
This prospective cohort study was designed to evaluate the role of ciprofloxacin labelled with technetium (99mTc)–Diagnobact™ in cases of spinal tuberculosis as a tool to monitor the disease activity using sequential ciprofloxacin scans. Out of 15 cases, nine (60%) had initial positive 99mTc bone scan. All of the nine cases which had initial scan positive became scan negative by the end of six months of treatment, which was also accompanied by clinical as well as radiological resolution of disease process. Among 15 cases of spinal tuberculosis enrolled for this study, six (40%) had initial negative scans despite fulfilling the WHO criteria for the diagnosis.
The reason for this relatively high negative rate in cases of spinal tuberculosis is probably due to the decreased penetration of the radiotracer at the diseased vertebral level as a result of excessive fibrosis and decreased vascularity around the necrotic bone, which may retard the penetration and accumulation of the radiotracer as evident in large paraspinal collections and greater area of bony destruction seen in all such cases. A larger study with bigger cohorts may be able to further clarify these trends in spinal tuberculosis. It may be argued that the cases of spinal tuberculosis having first scan negative were not truly the cases of tubercular infection since histopathology was not done in any of these cases. However, all of these patients had classical clinical presentation with sign and symptoms suggestive of tuberculosis. The ESR was significantly raised in all cases. The radiological investigations including MRI done in all cases were suggestive of tuberculosis. Furthermore, two cases had superficial paraspinal abscesses which were aspirated and the PCR proved positive. All these cases showed good clinicoradiological and haematological response to antitubercular chemotherapy and recovered completely after six months of therapy.
In our studies, 99mTc–ciprofloxacin (Diagnobact™) has shown high sensitivity in cases of extra spinal tuberculosis but the same is not true in cases of spinal tuberculosis where its efficacy is lower. However, some patients are slow to respond to treatment, have drug resistant tuberculosis, or have concomitant disease states that can complicate the clinical situation. Such patients can be candidates for therapeutic drug monitoring, which has its own set of practical clinical problems [22]. Though measurement of serum level of various drugs or therapeutic drug monitoring can be of help in such cases, it is further confounded by the fact that patients responding well to ATT can have lower than expected levels of rifampin and isoniazid despite having adequate clinical response [23, 24]. Thus, patients at high risk of slow response such as those with DM [25] and concomitant positive scan at the first visit might benefit from this test to identify slow responders, which could then be considered for prolonged chemotherapy as all three patients with diabetes mellitus in our study showed positive scan at the first visit with progressive diminution of activity on sequential scans.
In conclusion, though highly reliable for extra spinal tuberculosis, Tc99m may not be a useful marker for monitoring the disease activity in all cases of spinal tuberculosis, but can be a useful clinical test to assess the adequacy of response to treatment in selected cases such as those with diabetes mellitus which are at high risk for slow response. However, due to small sample size, studies with large numbers of patients might be of help to identify such cases in a better way. Progressive diminution of scan intensity during the chemotherapy is also likely to be a subject of interest in further studies wherein response to treatment could be quantified in relation to duration of chemotherapy.
Acknowledgments
Conflict of interest statement The authors declare that they have no conflict of interest.
References
- 1.World Health Organization (2008) Global tuberculosis control: surveillance, planning, financing. Publication no. WHO/HTM/TB/2008.393. World Health Organization, Geneva, Switzerland
- 2.Tuli SM (2004) Tuberculosis of the skeletal system, 3rd edn. Jaypee Brothers, New Delhi
- 3.Bhardwaj V, Agrawal M, Suri T, Sural S, Kashyap R, Dhal A (2010) Evaluation of adequacy of short-course chemotherapy for extraspinal osteoarticular tuberculosis using (99 m)Tc ciprofloxacin scan. Int Orthop. Nov 30 (Epub) [DOI] [PMC free article] [PubMed]
- 4.WHO (2010) Treatment of tuberculosis, 2010. http://whqlibdoc.who.int/publications/2010/9789241547833_eng.pdf. Accessed 21 June 2011
- 5.American Thoracic Society, CDC, Infectious Diseases Society of America Treatment of tuberculosis. Morbidity and Mortality Weekly Report. Recommendations and Reports. 2003;52(RR-11):1–77. [PubMed] [Google Scholar]
- 6.Agarwal A, Arora A, Kumar S. A survey of prescribing pattern for osteoarticular tuberculosis: orthopaedic surgeons' and infectious disease experts' perspective. Indian J Tuberc. 2009;56(4):201–205. [PubMed] [Google Scholar]
- 7.Azzam NI, Tammawy M. Tuberculous spondylitis in adults: diagnosis and treatment. Br J Neurosurg. 1988;2:85–91. doi: 10.3109/02688698808999663. [DOI] [PubMed] [Google Scholar]
- 8.Pertuiset E, Johann B, Liote F. Spinal tuberculosis in adults. A study of 103 cases in a developed country. Medicine. 1999;78:309–320. doi: 10.1097/00005792-199909000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Moore SL, Rafii M. Imaging of musculoskeletal and spinal tuberculosis. Radiol Clin North America. 2001;39:329–342. doi: 10.1016/S0033-8389(05)70280-3. [DOI] [PubMed] [Google Scholar]
- 10.Kelft E, Vyve M, Parizel PM, Selosse P, Schepper A. MR imaging of tuberculous spondylitis. JBR-BTR. 1992;75:202–204. [PubMed] [Google Scholar]
- 11.Ahmadi J, Bajaj A, Destian S. Spinal tuberculosis: atypical observations at MR imaging. Radiology. 1993;189:489–493. doi: 10.1148/radiology.189.2.8210378. [DOI] [PubMed] [Google Scholar]
- 12.Yoon HJ, Song YG, Park WI, Choi JP, Chang KH, Kim JM. Clinical manifestations and diagnosis of extrapulmonary tuberculosis. Yonsei Med J. 2004;45(3):453–461. doi: 10.3349/ymj.2004.45.3.453. [DOI] [PubMed] [Google Scholar]
- 13.Martini M, Cuahes M. Bone and joint tuberculosis: a review of 652 cases. Orthopedics. 1988;11:861–866. doi: 10.3928/0147-7447-19880601-04. [DOI] [PubMed] [Google Scholar]
- 14.Maekura R, Nakagawa M, Nakamura Y, Hiraga T, Yamamura Y, Ito M, Ueda E, Yano S, He H, Oka S, et al. Clinical evaluation of rapid serodiagnosis of pulmonary tuberculosis by ELISA with cord factor (trehalose-6,6'-dimycolate) as antigen purified from Mycobacterium tuberculosis. Am Rev Respir Dis. 1993;148:997–1001. doi: 10.1164/ajrccm/148.4_Pt_1.997. [DOI] [PubMed] [Google Scholar]
- 15.Chiac P, Yen TSB, You JB, Maa JS, Fiss EH, Chang CH. Detection and identification of Mycobacterium tuberculosis by DNA amplification. J Clin Microb. 1990;28:1877–1880. doi: 10.1128/jcm.28.9.1877-1880.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noel AB, Lecossier D, Nassif X, Birgite G, Frebault VL, Hance AJ. Rapid diagnosis of tuberculosis by amplification of mycobacterial DNA in clinical samples. Lancet. 1989;2(8671):1069–1071. doi: 10.1016/S0140-6736(89)91082-9. [DOI] [PubMed] [Google Scholar]
- 17.Pandey V, Chawla K, Acharya K, Rao S, Rao S. The role of polymerase chain reaction in the management of osteoarticular tuberculosis. Int Orthop. 2009;33(3):801–805. doi: 10.1007/s00264-007-0485-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tortoli E, Lavinia F, Simonetti MT. Evaluation of a commercial ligase chain reaction kit (Abbott LCx) for direct detection of Mycobacterium tuberculosis in pulmonary and extrapulmonary specimens. J Clin Microbiol. 1997;35(9):2424–2426. doi: 10.1128/jcm.35.9.2424-2426.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soler R, Rodriguez E, Remuinan C, Santos M. MRI of musculoskeletal extraspinal tuberculosis. J Computer Assisted Tomography. 2001;25(2):177–183. doi: 10.1097/00004728-200103000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Vuyst D, Vanhoenacker F, Gielen J, Bernaerts A, Schepper AM. Imaging features of musculoskeletal tuberculosis. Eur Radiol. 2003;13:1809–1819. doi: 10.1007/s00330-002-1609-6. [DOI] [PubMed] [Google Scholar]
- 21.Sharma R, Tewari KN, Bhatnagar A, Mondal A, Mishra AK, Singh AK, Chopra MK, Rawat H, Kashyap R, Tripathi RP. Tc-99 m ciprofloxacin scans for detection of tubercular bone infection. Clin Nucl Med. 2007;32(5):367–370. doi: 10.1097/01.rlu.0000259322.31974.e8. [DOI] [PubMed] [Google Scholar]
- 22.Peloquin CA. Therapeutic drug monitoring in the treatment of tuberculosis. Drugs. 2002;62(15):2169–2183. doi: 10.2165/00003495-200262150-00001. [DOI] [PubMed] [Google Scholar]
- 23.Chideya S, Winston CA, Peloquin CA, Bradford WZ, Hopewell PC, Wells CD, et al. Isoniazid, rifampin, ethambutol and pyrazinamide pharmacokinetics and treatment outcomes among predominately HIV-infected cohort of adults with tuberculosis from Botswana. Clin Infect Dis. 2009;48:1685–1694. doi: 10.1086/599040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang KC, Leung CC, Yew WW, Kam KM, Yip CW, Ma CH, et al. Peak plasma rifampicin level in tuberculosis patients with slow culture conversion. Eur J Clin Microbiol Infect Dis. 2008;27:467–472. doi: 10.1007/s10096-007-0454-6. [DOI] [PubMed] [Google Scholar]
- 25.Heysell SK, Moore JL, Keller SJ, Houpt ER. Therapeutic drug monitoring for slow response to tuberculosis treatment in a state control program, Virginia, USA. Emerg Infect Dis. 2010;16(10):1546–1553. doi: 10.3201/eid1610.100374. [DOI] [PMC free article] [PubMed] [Google Scholar]