Abstract
Objective
The purpose of this study was to validate the efficacy and safety of single-stage posterior instrumentation and anterior debridement for treatment of active spinal tuberculosis with kyphotic deformity.
Method
From January 2005 to January 2009, 13 males and 24 females were enrolled in this retrospective study. All patients underwent single-stage posterior instrumentation and fusion, combined with anterior radical debridement and bone grafting. Clinical and radiographic results were analysed.
Results
Patients were followed-up for 33.6 months on average. Bony fusion was achieved at six- to nine-month follow-up in all patients. The respective average kyphosis at the pre-operative and the last follow-up was 53.5° and 12.6°, with a mean correction of 40.9° (78.5%). Neurologic recovery averaged 1.5 grades on the Frankel scale. No recurrence of tuberculosis or instrumentation failure occurred.
Conclusion
Single-stage posterior instrumentation and anterior debridement with fusion was demonstrated to be a safe and effective method to achieve spinal decompression and kyphosis correction in patients with Pott’s disease.
Introduction
The past decade has witnessed an increasing incidence of spinal tuberculosis. Though many patients can be cured with chemotherapy, surgery is frequently imperative for spinal decompression [1]. Most patients experience recovery of neurologic function after anterior decompression combined with chemotherapy.
Tuberculosis of the thoracic and lumbar spine causing gross destruction of the anterior and middle columns often leads to kyphotic deformity [2, 3]. Rajasekaran and Shanmugasundaram [4, 5] reported that, among children treated conservatively, only 44% experienced improvement of the deformity, while in the remaining patients there was no change (17%) or deterioration (39%); in particular, kyphosis of more than 60° developed in 3–5% of cases. Thus, even after tuberculosis was cured, the deformity could progress. Moreover, if the destruction involves more than two vertebral bodies, severe kyphosis is frequently inevitable.
Kyphosis is not only a cosmetic problem, but also a threat to quality of life and survival. When greater than 60°, it can lead to delayed paralysis, after the original cause is cured [2]. Furthermore, cardiopulmonary dysfunction and the imbalance in the sagittal plane that is commonly seen in these patients make treatment challenging. The recovery of neurologic function is unpredictable even despite the deformity being corrected by surgery.
Therefore, for active tuberculosis with kyphosis, the two foremost objectives should be debridement and correction of deformity. Previous studies addressed these problems by anterior approaches [6–9], one- or two-stage combined anterior-posterior approach [10–14], or posterior approach alone [15, 16]. A mild kyphosis can be successfully corrected by the anterior approach alone [7–9, 17, 18], but for kyphosis of more than 30° the anterior approach by itself provides only limited correction, and hence the antero-posterior approach is warranted. The ideal strategy in the antero-posterior approach would be to achieve debridement and kyphosis correction in one stage. Nevertheless, a standardized surgical procedure has not been established. Points of controversy include whether the potential morbidity associated with both approaches being carried out in one procedure outweighs its benefit, whether the posterior approach should follow anterior debridement or vice versa, and the ideal instrumentation for posterior fixation [19, 20].
To our knowledge, there is no report of kyphosis correction in active tuberculous spondylitis by posterior transpedicular screw fixation followed by anterior surgery in one stage. The purpose of the present study was to present clinical and radiographic results of patients treated with such a procedure for Pott’s disease with a minimum two-year follow-up.
Materials and methods
Patient population
From January 2005 to January 2009, 37 patients diagnosed as active spinal tuberculosis of the thoracic and lumbar spine causing kyphosis of more than 30° were enrolled in this study. Patients with any of the following conditions were excluded: cervical tuberculosis, tuberculosis involving the posterior elements, leaping tuberculosis, and those who had previously undergone surgery for tuberculosis of the thoracic and lumbar spine. Diagnosis of active tuberculosis was made based on clinical symptoms, laboratory findings, and radiographic evidence. These included: (1) typical symptoms such as weight loss, low grade fever, night-sweats, and fatigue, or abscesses and fistulas; (2) significantly high erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP); and (3) production of sequestra and abscesses confirmed by computed tomography (CT) scanning, or abnormal signal intensity of the involved vertebrae, paravertebral abscesses or psoas abscesses. Patients who had one of these manifestations and with mycobacterium tuberculosis infection confirmed post-operative etiologically (acid-fast stain, bacterial cultures or polymerase chain reaction) or pathologically were defined as active tuberculosis. Written informed consent was obtained from all patients and the study protocol was approved by the Ethics Committee of the Second Xiangya Hospital.
There were 13 males and 24 females in our study, with an average age of 31.4 years (range, 6–62 years) and a mean disease course of ten months (range, 4–18 months). The main clinical presentations included back pain, limited spinal mobility, and a hump-shaped deformity. Eleven patients had a history of active pulmonary tuberculosis and six had palpable abscesses, although fistulas were not detected. Ten patients experienced weight loss and mild to moderate anaemia. Thirteen patients had a neurological deficit of varying degrees of severity, including unilateral or bilateral numbness and weakness of the lower extremity and walking disorders. Among these 13 patients, two demonstrated complete motor loss with preservation of some sensation function (Frankel Grade B), six had an incomplete loss of motor function (Frankel Grade C), and five had a mild defect of motor function (Frankel Grade D).
Radiographic evaluation
Kyphosis angle was measured according to the method of the British Medical Research Council [21]. Loss of vertebral body was assessed as described by Rajasekaran and Shanmugasundaram [4]. The most cephalad and caudad vertebral body involved were T3 and L4, respectively. The mean kyphosis of the patients was 53.5 ± 13.6° (range, 33–85°). Among the patients, 16 had thoracic spine involvement, with a mean kyphosis of 60.3 ± 14.7° (range, 40–85°). Nine cases had thoracolumbar segment involvement, with a mean kyphosis of 56.0 ± 10.2° (range, 47–68°). The remaining 12 patients had tuberculosis in their lumbar spine, with a mean kyphosis of 42.7 ± 5.9° (range, 33–52°). There were three patients who had a lesion involving one vertebral body, and 26 and five patients had lesions in two and three vertebral bodies, respectively. Three patients showed tuberculosis foci in more than three vertebral bodies (4, 5 and 6 vertebral bodies in each patient, respectively). The mean number of vertebral bodies involved was 2.3. Pre-operative CT or magnetic resonance imaging (MRI) revealed canal and paraspinal abscesses in 17 and 28 patients, respectively. Six patients demonstrated almost total loss of one vertebral body, or minimal residue of the dorsal vertebral body (Fig. 1). The mean vertebral loss in the patients was 1.2 (range, 0.7–2.9).
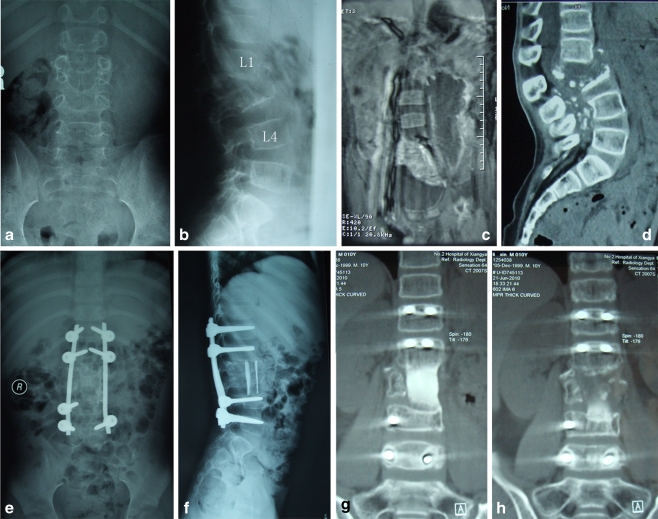
Fig. 1.
An eight-year-old boy. a,b Pre-operative X-ray indicating complete disappearance of L2 and destruction of a large part of L3 with a 33° kyphosis. c MRI demonstrates a huge flow injection abscess around the psoas. d A CT scan shows a total destruction of L3 except for an end plate, and protrusion of dead bone fragments and granulation tissue into the canal. e,f At the six-month follow-up, X-ray shows a complete correction of kyphosis, and perfect position of the strut grafts between L1 and L4. g,h At the 20-month follow-up, a CT scan shows a solid fusion with continuous bridging of the trabecular bone
Pre-operative preparation
After a clinical diagnosis of spinal tuberculosis was made and active pulmonary tuberculosis excluded, patients were treated with the HREZ chemotherapy regimen, consisting of isoniazid (300 mg/d), rifampicin (450 mg/d), ethambutol (750 mg/d) and pyrazinamide (1500 mg/d), for two to four weeks before surgery. Dosage was adjusted for children less than 14 years old according to body weight.
Operative indications
Indications for surgery were therapeutically refractory disease, panvertebral lesions, an expanding cold abscess, severe kyphosis or kyphosis likely to progress, and neurological deficit [1].
Operative technique
Briefly, for the posterior approach, patients were first placed in a prone position, and pedicle screws were placed into the first two normal vertebral bodies around the lesion. The cantilever beam technique was used for deformity correction. Ponte osteotomies were performed to increase flexibility in eight cases, as rigidity was found during surgery. After instrumentation and deformity correction, the anterior column was elongated and the height of collapsed vertebrae restored. No attempt at radical debridement or neural decompression was undertaken. The levels that were to be fused anteriorly were grafted using allogeneic bone after decortication of the laminae and transverse processes.
Patients were then readjusted to a lateral decubitus position. The extrapleural or extraperitoneal anterior-lateral approach was taken and pleural or peritoneum injury was avoided. The tuberculous lesion, including paravertebral or psoas abscesses, collapsed vertebrae, and intervertebral discs, was thoroughly debrided until healthy bleeding margins were obtained. After sufficient spinal cord decompression was achieved, suitable strut grafts were used for stabilization. Tricortical rectangular grafts from the iliac crest were harvested from 17 patients, cortical allografts were used in nine patients (Fig. 2), and titanium mesh cages filled with morselized autogenous bone were used in another 11 patients. The choice of bone graft was made based on the involved levels and in consideration of the patient’s preference. Resected specimens were used for bacterial culture and for pathological diagnosis.
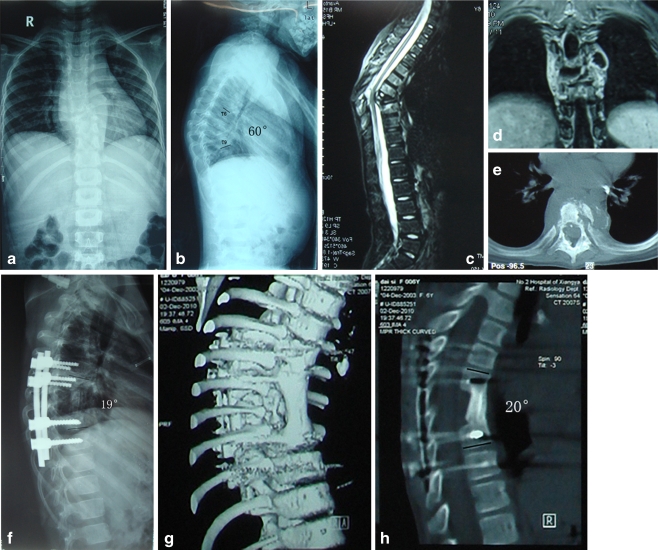
Fig. 2.
A six-year-old girl. a, b Pre-operative X-ray demonstrated destruction of T7 and T8, with a 60° kyphosis measured between T6 and T9. MRI reveals (c) protrusion of dead bone fragments and granulation tissue into the canal, leading to compression of the dural sac. d Pronounced paravertebral abscesses. e A cross-sectional CT scan shows motheaten bone destruction. f At the three-month follow-up, a lateral X-ray indicates that kyphosis was corrected to 19°. g,h At the nine-month follow-up, a CT scan shows successful fusion at the grafted site, and the kyphosis was 20°
Post-operative care
After surgery, patients resumed the oral HREZ chemotherapy. Six months later, pyrazinamide was discontinued. Patients received six- to 12-month regimens of the HRE chemotherapy (6HREZ/6-12HRE). Ambulation in a brace was allowed six to eight weeks after surgery. To monitor the activity of tuberculosis, routine blood tests, ESR, and CRP were ordered periodically. X-ray radiography was carried out at three, six and nine months, and then every year post-operatively to monitor for fusion of the bone graft, loss of correction, and instrumentation failure. Methods recommended by Lee et al. [22] were used to evaluate the fusion status. CT was done when there was uncertainty after X-ray. Patients who met the following criteria were considered to be healed: first, disappearance of clinical symptoms with the ability to return to normal activities; second, bony fusion achieved according to radiography; third, return of ESR and CRP to normal levels; and fourth, follow-up at one year post-operative showed no recurrence of tuberculosis.
Statistic analysis
The pre-operative Frankel grade and at the last follow-up were compared using the Wilcoxon signed rank test. Repeated measure analysis of the variance was used for comparison of kyphosis deformity before and after surgery, and at the last follow-up. The Student-Newman-Keuls test was used to compare the change of kyphosis angle and correction rate among different groups. Results were considered significant when P-values were less than 0.05.
Results
Spinal tuberculosis was confirmed in all 37 cases post-operatively, and 12 patients were positive for culture of mycobacterium. Patients were followed-up for at least 24 months, with an average of 33.6 months (range, 24–64 months). No recurrence was observed. The mean operation time was 330 min (range, 270–420 min) with an average blood loss of 950 mL (600–1800 mL). The average pre-operative ESR and CRP values were 49 mm/h (range, 27–98 mm/h) and 33 mg/L (13–62 mg/L), respectively, decreasing gradually three to six weeks post-operatively and returned to normal levels six months after surgery. Deterioration of neurologic status was not noted. Of the 13 cases that suffered from a neurological deficit pre-operatively, all experienced recovery post-operatively. At the last follow-up, Frankel grades C and D were observed in one patient each; all the rest demonstrated normal neurologic functions (Table 1). The average improvement of Frankel grade was 1.5, which was significantly better than pre-operative (P = 0.0002).
Table 1.
Neurologic recovery according to Frankel grade
| Pre-surgery | Final follow-up | |||||
|---|---|---|---|---|---|---|
| A | B | C | D | E | ||
| A | 0 | |||||
| B | 2 | 1 | 1 | |||
| C | 6 | 1 | 5 | |||
| D | 5 | 5 | ||||
| E | 24 | 24 | ||||
Pre-operative kyphosis was 53.5° on average and became 11.0° after surgery, with a correction rate of 81.6%. All the patients demonstrated solid fusion at six- to nine-month follow-up. The average kyphosis at the last follow-up was 12.6°; loss of correction was 1.6° on average. The stable kyphosis correction was 40.9° on average, with a correction rate of 78.5%. Table 2 lists the kyphosis correction in patients according to pathological regions and bone graft materials.
Table 2.
Kyphosis correction post-surgery and during follow-up by pathological regions and bone substitute materials
| Parameter | The number of affected vertebrae | Pre-operative kyphosis angle (°) | Post-operation | Follow-up | |||||
|---|---|---|---|---|---|---|---|---|---|
| Kyphosis angle (°) | Angle correction (°) | Correction rate (%) | Kyphosis angle (°) | Angle correction (°) | Correction rate (%) | ||||
| Pathological region | Thoracic | 2.8 | 60.3 ± 14.7 | 17.7 ± 9.3 | 42.6 ± 8.1 | 72.0 ± 9.6 | 19.2 ± 9.1 | 41.1 ± 8.3 | 69.3 ± 9.1 |
| Thoracolumbar | 1.9 | 56.0 ± 10.2 | 8.7 ± 2.7 | 47.3 ± 9.2 | 84.5 ± 4.7 | 11.4 ± 3.4 | 44.6 ± 9.6 | 79.4 ± 6.1 | |
| Lumbar | 1.9 | 42.7 ± 5.9 | 3.8 ± 5.7 | 38.9 ± 4.5 | 92.3 ± 13.6 | 4.7 ± 5.5 | 38.0 ± 4.6 | 90.1 ± 13.1 | |
| Bone substitute material | Iliac crest | 1.9 | 49.9 ± 7.0 | 8.4 ± 4.6 | 41.5 ± 5.3 | 83.7 ± 7.9 | 10.5 ± 5.1 | 39.4 ± 4.9 | 79.6 ± 8.4 |
| Allogeneic bone | 1.9 | 47.2 ± 9.7 | 8.0 ± 4.7 | 39.2 ± 8.6 | 83.4 ± 10.3 | 9.1 ± 4.8 | 38.1 ± 9.0 | 80.9 ± 11.1 | |
| Titanium mesh | 3.2 | 64.3 ± 18.1 | 17.5 ± 13.9 | 46.8 ± 9.4 | 76.9 ± 20.8 | 18.7 ± 13.9 | 45.6 ± 9.1 | 74.9 ± 20.1 | |
| Mean | 2.3 | 53.5 ± 13.6 | 11.0 ± 9.3 | 42.5 ± 7.9 | 81.6 ± 13.5 | 12.6 ± 9.3 | 40.9 ± 7.8 | 78.5 ± 13.3 | |
During follow-up loss of correction was small, although significant, judging from the time effect (P = 0.0003). Whereas, neither (time*bone substitute material), (time* pathological region), nor (time* Pathological region* Bone substitute material) was significant, implying that there was no difference in consolidation time and loss of correction between iliac crest, allogeneic bone and titanium mesh. Also, different pathological regions do not influence the loss of correction (Table 3).
Table 3.
Repeated measures ANOVA for change in kyphosis angle during post-operative follow-up
| Variance source | SS | DF | Mean square | F value | P value |
|---|---|---|---|---|---|
| Bone substitute material | 170.166738 | 2 | 85.083369 | 1.45 | 0.2521 |
| Pathological region | 2218.433123 | 2 | 1109.216561 | 18.88 | <.0001 |
| Pathological region * bone substitute material | 1006.085862 | 4 | 251.521465 | 4.28 | 0.0079 |
| Error (group) | 1645.114286 | 28 | 58.754082 | ||
| Time | 32.37942740 | 1 | 32.37942740 | 16.96 | 0.0003 |
| Time* bone substitute material | 2.89302129 | 2 | 1.44651065 | 0.76 | 0.4781 |
| Time* pathological region | 3.24431829 | 2 | 1.62215915 | 0.85 | 0.4382 |
| Time* pathological region* bone substitute material | 2.25220911 | 4 | 0.56305228 | 0.29 | 0.8787 |
| Error (time) | 53.44761905 | 28 | 1.90884354 |
SS sum of squares, DF degree of freedom
There were four peri-operative complications (10.8%). One was superficial infection around the posterior incision. One case with pleural effusion was cured by closed drainage for five days. Another patient who suffered from refractory intercostal neuralgia was relieved by nonsteroidal anti-inflammatory drugs and hyperbaric oxygen for three weeks. In one patient, the donor site of an iliac crest bone graft healed by second intention. No severe neurologic complication was observed.
Discussion
For active spinal tuberculosis, anterior debridement is imperative. Anterior debridement, strut grafting fusion and fixation, has become the standard for surgical treatment of spinal tuberculosis [6–9]. The disadvantages of the anterior approach used alone include insufficient kyphosis correction and post-operative loss of correction. For mild kyphosis, it has been associated with long-term loss of deformity correction [23], although the problem can be alleviated by anterior instrumentation [7]. Most reports focus application of anterior surgery on patients with deformities less than 30°, as it has limited correction capacity on kyphosis of greater severity [7–9, 17, 18]. Furthermore, it is not suited for patients with multiple-level involvement.
Other surgeons attempted to address this challenge with single-stage posterior debridement and fixation [15, 16, 24]. This strategy can only be used with localized foci of no more than three levels and without extensive formation of abscesses. Additionally, posterior debridement bears the potential risk of tuberculosis spread to the posterior healthy regions, infection, and fistulas.
Thus, anterior debridement combined with posterior fusion and fixation was developed [3, 10, 12, 13, 25–27], which enables immediate and sufficient stabilization after kyphosis correction. Table 4 summarizes published reports focused on treatment of active spinal tuberculosis with kyphosis of more than 30°. Sundararaj et al. [13] claimed that for short-term active tuberculosis, posterior correction was relatively easy to achieve and hence could be done first. Whereas for most patients, they chose to debride anteriorly first, gradually distract anterior columns for correction of kyphosis, and then follow by posterior fixation. A correction of 9.06° was achieved, which was similar to the result (9˚) of Huang et al. [25].
Table 4.
References focused on kyphosis (>30°) correction in active spinal tuberculosis
| References | No. of patients | Stage | Surgical approach | Type of instrumentation | Operation time (min) | Blood loss (ml) | Follow-up (months) | Kyphosis angle (Pre) (°) | Kyphosis angle (Pos.) (°) | Final follow-up (°) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Children | Adults | ||||||||||
| Louw, 1990 [10] | 9 | 10 | One 6 | Ant – Post. | PS. H. W. | NA | Ant. 674, Post. 556, | 25.5 | 56 | 27 | 30 |
| Two 13 | One 878 | ||||||||||
| Altman, 1996 [29] | 5 | None | Two | Ant – Post. | No instrument | NA | NA | 12.8 years | 100 | 71.2 | 74.8 |
| Al-Sebai, 2001 [3] | 6 | 8 | O / T | Ant – Post. | PS. H. | NA | NA | 40 | 50 | 19 | NA |
| Chen, 2002 [12] | 1 | 28 | Two | Ant – Post. | PS. H. W. | NA | NA | 4.7 years | 34.6 | 14.9 | 17.3 |
| Sundararaj, 2003 [13] | 77 | One 70 | Ant – Post. | PS. H. W. | 5 hours for one stage | 1000 ml for one stage | 36.7 | Dorsal 26.93 | Dorsal 15.86 | Dorsal 20.25 | |
| Dorsolumbar 32.4 | Dorsolumbar 14.6 | Dorsolumbar 17.4 | |||||||||
| Two 7 | Lumbar 15.36 | Lumbar −1.28 | Lumbar 2.24 | ||||||||
| Lumbosacral −14 | Lumbosacral −28 | Lumbosacral −14.33 | |||||||||
| Mukhtar, 2003 [27] | 22 | One 12 | Ant – Post. 9 | PS. H. W. | NA | NA | >18 | 42 | 15 | 18 | |
| Two 10 | Post – Ant. 13 | ||||||||||
| Zhang, 2007 [26] | None | 23 | One | Ant – Post. | PS. H. | 172 | 1100 | 42 | 44 | 20 | 24 |
| Huang, 2009 [25] | 15 | None | One | Ant – Post. | PS. | 263 | 650 | 30.3 | 36 | 23 | 27 |
| Jain. 2008 [20] | 38 | One | Anterolateral Extrapleural | W. | 3.5 hours | 1175 | 32.9 | 49.08 | 22.5 | 24 | |
| Deng, 2009 [15] | 34 | One | Post. | PS. | 208 | 410 | 4.5 years | 57.8 | NA | 11.4 | |
| Gokce, 2008 [24] | None | 12 | One | Post. | PS. | 4.6 hours | 1900 | 62 | 51.1 | 23.2 | 28.6 |
| Laheri, 2001 [16] | 10 | 18 | One | Post. | PS. H. W. | NA | 850 | 5.8 years | 63.5 | 23.5 | 26.7 |
| Hirakawa, 2010 [30] | None | 10 | Two | Post – Ant. | PS. H. W. | Post. 219±66; Ant. 289±157 | Post. 627±444; Ant. 627±444 | 48.2 | 35.6±39.2 | 26.2±35.5 | 31.3±38.7 |
| Moon, 1995 [28] | 5 | 39 | One 19 | Post – Ant. | PS. H. W. | NA | NA | 18 | Adult 37 | Adult 16 | Adult 18 |
| Two 25 | Child 55 | Child 28 | Child 31 | ||||||||
O / T one stage or two stage, Ant anterior, Post posterior, PS pedicle screws, H hooks, W sublaminar wires, NA not mentioned
Louw et al. [10] treated 19 patients with neurologic deficit by the Kalafong procedure. If the surgery was done in two stages, a strut graft was immediately inserted after anterior debridement, and the posterior procedure was performed 10–14 days later. This does not alter the length of the anterior column, because the anterior strut graft acts as a pivot. However, as a one-stage procedure, anterior debridement was still carried out first, but the posterior procedure was completed before the rib graft was inserted. The aim of the one-stage procedure was to obtain maximum correction of the kyphosis by elongation of the anterior column. An average kyphosis of 56° was reduced to 30° at the latest follow-up.
The one-stage procedure described previously carries a risk of neurologic injury when the patient’s position is changed for the posterior approach, as anterior debridement without immediate structure graft causes spinal instability [24, 25]. Jain et al. [20] proposed an anterolateral extrapleural approach to complete the anterior debridement, posterior fixation, and anterior grafting procedure in one incision. The weakness is the use of sublaminar wires, which provide only semi-rigid fixation and is inadequate to resist axial loads.
For patients with multiple-level tuberculosis lesions causing evident kyphosis and with extensive abscesses (especially flow injection abscesses), we prefer posterior pedicle screw instrumentation for reduction and fixation, followed by immediate anterior debridement and strut grafting. The aim of posterior surgery is kyphosis correction without exposure of the lesion, minimizing the risk of tuberculosis spread. Patients with a history longer than six months tend to have facet fusion leading to rigid deformity that cannot be reduced by fixation alone and sometimes even causes the pull-out of screws. Thus, Ponte osteotomies should be performed to facilitate correction. We believe the advantages of posterior pedicle screw instrumentation are multifold: sufficient kyphosis correction can be accomplished, exposure of tuberculosis foci posteriorly is avoided, three-column spinal fusion can prevent the imbalanced spinal growth of children, and long-term correction can be maintained.
We achieved a mean kyphosis correction of 40.9°, with a correction rate of 78.5%, higher than previously reported by Jain et al. (51.1%) [20], Louw et al. (44.8%) [10] and Moon et al. (56.7%) [28]. During follow-up, the correction was satisfactorily preserved with loss of correction of 1.6° on average, which was better than reported by Sundararaj et al. (4.64°) [13], Louw et al. (3.3°) [10] and Moon et al. (3°) [28]. Sundararaj et al. [13] observed subsidence and slippage of bone grafts in 6.5% of patients. In our study, graft-related problems were not seen, which we believe is attributable to the solid fixation of the posterior instrumentation.
Another controversial matter is whether the posterior fixation should be performed before or after anterior debridement. Although Jain et al. [20] considered that posterior fixation without prior anterior debridement was a risk for increased neurologic injury, we reasoned that when posterior correction was performed first, further spinal shortening was prevented; the anterior compression caused by the tuberculosis focus (including abscess, caseous tissue and ruptured discs) should not pose instant threats neurologically. Moreover, with a one-stage procedure, decompression can be done immediately, thereby eliminating risk factors for spinal injury. No incidence of neurologic exacerbation occurred in our study and all patients experienced improvement of their neurologic function, proving the safety of this strategy.
In conclusion, our study demonstrated the clinical efficacy and safety of one-stage posterior fixation and anterior debridement in patients with Pott’s disease. We showed that the procedure achieved effective kyphosis correction and thorough debridement. Serious complications were not seen, indicating that prolonged anaesthesia and surgery were not associated with increased morbidity.
References
- 1.Jain AK. Tuberculosis of the spine: a fresh look at an old disease. J Bone Joint Surg Br. 2010;92:905–913. doi: 10.1302/0301-620X.92B7.24668. [DOI] [PubMed] [Google Scholar]
- 2.Tuli SM. Severe kyphotic deformity in tuberculosis of the spine. Int Orthop. 1995;19:327–331. doi: 10.1007/BF00181121. [DOI] [PubMed] [Google Scholar]
- 3.Al-Sebai MW, Al-Khawashki H, Al-Arabi K, Khan F. Operative treatment of progressive deformity in spinal tuberculosis. Int Orthop. 2001;25:322–325. doi: 10.1007/s002640100259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rajasekaran S, Shanmugasundaram TK. Prediction of the angle of Gibbus deformity in tuberculosis of the spine. J Bone Joint Surg Am. 1987;69:503–509. [PubMed] [Google Scholar]
- 5.Rajasekaran S. The natural history of post-tubercular kyphosis in children. Radiological signs which predict late increase in deformity. J Bone Joint Surg Br. 2001;83:954–962. doi: 10.1302/0301-620X.83B7.12170. [DOI] [PubMed] [Google Scholar]
- 6.Upadhyay SS, Sell P, Saji MJ, Sell B, Hsu LC. Surgical management of spinal tuberculosis in adults. Hong Kong operation compared with debridement surgery for short and long term outcome of deformity. Clin Orthop Relat Res. 1994;302:173–182. [PubMed] [Google Scholar]
- 7.Dai LY, Jiang LS, Wang W, Cui YM. Single-stage anterior autogenous bone grafting and instrumentation in the surgical management of spinal tuberculosis. Spine (Phila Pa 1976) 2005;30:2342–2349. doi: 10.1097/01.brs.0000182109.36973.93. [DOI] [PubMed] [Google Scholar]
- 8.Jin D, Qu D, Chen J, Zhang H. One-stage anterior interbody autografting and instrumentation in primary surgical management of thoracolumbar spinal tuberculosis. Eur Spine J. 2004;13:114–121. doi: 10.1007/s00586-003-0661-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benli IT, Kaya A, Acaroglu E. Anterior instrumentation in tuberculous spondylitis: is it effective and safe. Clin Orthop Relat Res. 2007;460:108–116. doi: 10.1097/BLO.0b013e318065b70d. [DOI] [PubMed] [Google Scholar]
- 10.Louw JA. Spinal tuberculosis with neurological deficit. Treatment with anterior vascularised rib grafts, posterior osteotomies and fusion. J Bone Joint Surg Br. 1990;72:686–693. doi: 10.1302/0301-620X.72B4.2380228. [DOI] [PubMed] [Google Scholar]
- 11.Lee TC, Lu K, Yang LC, Huang HY, Liang CL. Transpedicular instrumentation as an adjunct in the treatment of thoracolumbar and lumbar spine tuberculosis with early stage bone destruction. J Neurosurg. 1999;91:163–169. doi: 10.3171/spi.1999.91.2.0163. [DOI] [PubMed] [Google Scholar]
- 12.Chen WJ, Wu CC, Jung CH, Chen LH, Niu CC, Lai PL. Combined anterior and posterior surgeries in the treatment of spinal tuberculous spondylitis. Clin Orthop Relat Res. 2002;398:50–59. doi: 10.1097/00003086-200205000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Sundararaj GD, Behera S, Ravi V, Venkatesh K, Cherian VM, Lee V. Role of posterior stabilisation in the management of tuberculosis of the dorsal and lumbar spine. J Bone Joint Surg Br. 2003;85:100–106. doi: 10.1302/0301-620X.85B1.13300. [DOI] [PubMed] [Google Scholar]
- 14.Klockner C, Valencia R. Sagittal alignment after anterior debridement and fusion with or without additional posterior instrumentation in the treatment of pyogenic and tuberculous spondylodiscitis. Spine (Phila Pa 1976) 2003;28:1036–1042. doi: 10.1097/01.BRS.0000061991.11489.7F. [DOI] [PubMed] [Google Scholar]
- 15.Deng Y, Lv G, An HS. En bloc spondylectomy for the treatment of spinal tuberculosis with fixed and sharply angulated kyphotic deformity. Spine (Phila Pa 1976) 2009;34:2140–2146. doi: 10.1097/BRS.0b013e3181b34ce7. [DOI] [PubMed] [Google Scholar]
- 16.Laheri VJ, Badhe NP, Dewnany GT. Single stage decompression, anterior interbody fusion and posterior instrumentation for tuberculous kyphosis of the dorso-lumbar spine. Spinal Cord. 2001;39:429–436. doi: 10.1038/sj.sc.3101185. [DOI] [PubMed] [Google Scholar]
- 17.Cavusoglu H, Kaya RA, Turkmenoglu ON, Tuncer C, Colak I, Aydin Y. A long-term follow-up study of anterior tibial allografting and instrumentation in the management of thoracolumbar tuberculous spondylitis. J Neurosurg Spine. 2008;8:30–38. doi: 10.3171/SPI-08/01/030. [DOI] [PubMed] [Google Scholar]
- 18.Ozdemir HM, Us AK, Ogun T. The role of anterior spinal instrumentation and allograft fibula for the treatment of Pott disease. Spine (Phila Pa 1976) 2003;28:474–479. doi: 10.1097/01.BRS.0000048666.17934.17. [DOI] [PubMed] [Google Scholar]
- 19.Sundararaj GD. Simultaneous anterior decompression and posterior instrumentation of the tuberculous spine using an anterolateral extrapleural approach. J Bone Joint Surg Br. 2009;91:702. doi: 10.1302/0301-620X.91B5.22532. [DOI] [PubMed] [Google Scholar]
- 20.Jain AK, Dhammi IK, Prashad B, Sinha S, Mishra P. Simultaneous anterior decompression and posterior instrumentation of the tuberculous spine using an anterolateral extrapleural approach. J Bone Joint Surg Br. 2008;90:1477–1481. doi: 10.1302/0301-620X.90B11.20972. [DOI] [PubMed] [Google Scholar]
- 21.[No authors listed] (1973) A controlled trial of ambulant out-patient treatment and in-patient rest in bed in the management of tuberculosis of the spine in young Korean patients on standard chemotherapy a study in Masan, Korea. First report of the Medical Research Council Working Party on Tuberculosis of the Spine. J Bone Joint Surg Br 55:678–697 [PubMed]
- 22.Lee CK, Vessa P, Lee JK. Chronic disabling low back pain syndrome caused by internal disc derangements. The results of disc excision and posterior lumbar interbody fusion. Spine (Phila Pa 1976) 1995;20:356–361. doi: 10.1097/00007632-199502000-00018. [DOI] [PubMed] [Google Scholar]
- 23.Schulitz KP, Kothe R, Leong JC, Wehling P. Growth changes of solidly fused kyphotic bloc after surgery for tuberculosis. Comparison of four procedures. Spine (Phila Pa 1976) 1997;22:1150–1155. doi: 10.1097/00007632-199705150-00016. [DOI] [PubMed] [Google Scholar]
- 24.Gokce A, Ozturkmen Y, Mutlu S, Caniklioglu M. Spinal osteotomy: correcting sagittal balance in tuberculous spondylitis. J Spinal Disord Tech. 2008;21:484–488. doi: 10.1097/BSD.0b013e3181586023. [DOI] [PubMed] [Google Scholar]
- 25.Huang QS, Zheng C, Hu Y, Yin X, Xu H, Zhang G, Wang Q. One-stage surgical management for children with spinal tuberculosis by anterior decompression and posterior instrumentation. Int Orthop. 2009;33:1385–1390. doi: 10.1007/s00264-009-0758-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang HQ, Guo CF, Xiao XG, Long WR, Deng ZS, Chen J. One-stage surgical management for multilevel tuberculous spondylitis of the upper thoracic region by anterior decompression, strut autografting, posterior instrumentation, and fusion. J Spinal Disord Tech. 2007;20:263–267. doi: 10.1097/01.bsd.0000211281.68400.1b. [DOI] [PubMed] [Google Scholar]
- 27.Mukhtar AM, Farghaly MM, Ahmed SH. Surgical treatment of thoracic and lumbar tuberculosis by anterior interbody fusion and posterior instrumentation. Med Princ Pract. 2003;12:92–96. doi: 10.1159/000069113. [DOI] [PubMed] [Google Scholar]
- 28.Moon MS, Woo YK, Lee KS, Ha KY, Kim SS, Sun DH. Posterior instrumentation and anterior interbody fusion for tuberculous kyphosis of dorsal and lumbar spines. Spine (Phila Pa 1976) 1995;20:1910–1916. doi: 10.1097/00007632-199509000-00013. [DOI] [PubMed] [Google Scholar]
- 29.Altman GT, Altman DT, Frankovitch KF. Anterior and posterior fusion for children with tuberculosis of the spine. Clin Orthop Relat Res. 1996;325:225–231. doi: 10.1097/00003086-199604000-00027. [DOI] [PubMed] [Google Scholar]
- 30.Hirakawa A, Miyamoto K, Masuda T, Fukuta S, Hosoe H, Iinuma N, Iwai C, Nishimoto H, Shimizu K. Surgical outcome of 2-stage (posterior and anterior) surgical treatment using spinal instrumentation for tuberculous spondylitis. J Spinal Disord Tech. 2010;23:133–138. doi: 10.1097/BSD.0b013e31819a870f. [DOI] [PubMed] [Google Scholar]