Abstract
Leukocyte telomere length (LTL) is a potential indicator of cellular aging; however, its relation to physical activity and sedentary behavior is unclear. The authors examined cross-sectionally associations among activity, sedentary behavior, and LTL among 7,813 women aged 43–70 years in the Nurses’ Health Study. Participants self-reported activity by questionnaire in 1988 and 1992 and sedentary behavior in 1992. Telomere length in peripheral blood leukocytes, collected in 1989–1990, was measured by quantitative polymerase chain reaction. The least-squares mean telomere length (z-score) was calculated after adjustment for age and other potential confounders. For total activity, moderately or highly active women had a 0.07-standard deviation (SD) increase in LTL (2-sided Ptrend = 0.02) compared with those least active. Greater moderate- or vigorous-intensity activity was also associated with increased LTL (SD = 0.11 for 2–4 vs. <1 hour/week and 0.04 for ≥7 vs. <1 hour/week; 2-sided Ptrend = 0.02). Specifically, calisthenics or aerobics was associated with increased LTL (SD = 0.10 for ≥2.5 vs. 0 hours/week; 2-sided Ptrend = 0.04). Associations remained after adjustment for body mass index. Other specific activities and sitting were unassociated with LTL. Although associations were modest, these findings suggest that even moderate amounts of activity may be associated with longer telomeres, warranting further investigation in large prospective studies.
Keywords: biological markers, cohort studies, epidemiology, exercise, physical activity, sedentary lifestyle, telomere
Telomeres are repetitive DNA-protein complexes that protect the ends of linear chromosomes and maintain genomic stability (1). Telomeres undergo erosion each time the cell divides; oxidative stress increases this attrition, and inflammation enhances leukocyte turnover (2). Thus, leukocyte telomere length (LTL) may reflect cumulative exposure to oxidative and inflammatory damage, serving as a potential indicator of cellular aging (2). Critically short telomeres may lead to chromosomal degradation, end-to-end fusion, and atypical recombination, processes implicated in disease development (3). Epidemiologic studies have shown links between shortened LTL and increased risk of age-related outcomes, such as cancer incidence and mortality (4–7), type 2 diabetes (8, 9), and cardiovascular disease (3, 10). Given that telomere shortening may underlie many diseases, it is important to identify modifiable factors that influence telomere dynamics.
Regular physical activity has been associated with decreased levels of oxidative stress and inflammation, and it helps to prevent chronic disease (11–13). Despite these benefits, increasing sedentary behavior is a major public health concern in the United States (13). Evidence from population-based studies on the relation between physical activity and telomere length has been limited and inconsistent (6, 14–19), and the potential role of moderate- or vigorous-intensity activity and specific types of activity is unclear. Moreover, previous studies have not evaluated the association between sedentary behavior and LTL. Obese individuals may possess shorter telomeres (20) and, as a sedentary lifestyle predicts obesity (21), sedentary behaviors may potentially influence telomere length.
To address these questions, we extended previous analyses of diet and other lifestyle factors and LTL in the Nurses’ Health Study (NHS) (15) to 7,813 women aged 43–70 years. We examined cross-sectionally the relation between physical activity and LTL and explored the potential role of moderate or vigorous and specific activities, as well as sedentary behaviors, in LTL dynamics.
MATERIALS AND METHODS
Study population
The NHS prospective cohort was established in 1976 when 121,700 female registered nurses in the United States aged 30–55 years completed and returned a mailed questionnaire. Detailed information on individual characteristics and new disease diagnoses has been updated biennially by questionnaire. From 1989 to 1990, 32,826 women provided blood samples and completed a questionnaire. Details of the NHS and blood collection methods have been described previously (22). Completion of the self-administered questionnaire and submission of a blood sample were considered to imply informed consent. The protocol for this study was approved by the human research committees at Brigham and Women’s Hospital, Boston, Massachusetts.
Among women who had previously provided a blood sample in the NHS, individuals were selected to participate in nested case-control studies of LTL and incident cancer or cardiovascular disease (23–25). In these studies, eligible cases were identified starting any time after blood collection in 1989–1990 up to June 1, 2008. Participants included in the current cross-sectional analysis comprised participants from the nested case-control studies. We included women selected as cases or controls because all participants were undiagnosed with the disease of interest at blood collection. The present analysis excluded women with missing activity and sedentary behavior information (n = 21) or LTL values (n = 47), LTL values generated by nonstandard assay conditions (n = 218), or extreme LTL values (n = 22). The final study population included 7,813 women (3,251 cases and 4,562 controls).
Assessment of physical activity and sedentary behavior
Detailed information on physical activity during the past year was assessed by questionnaire in 1988 and updated in 1992. Participants reported their average weekly time spent on any of 8 activities: walking or hiking, jogging, running, bicycling, lap swimming, playing tennis, calisthenics/aerobics/aerobic dance/rowing machine, and playing squash or racquetball. Participants also reported their usual walking pace and the number of flights of stairs climbed daily. Our primary analyses used activity reported in 1988—the assessment closest to the time of blood collection. To incorporate the frequency, duration, and intensity of activity, we calculated the total metabolic equivalent (MET) hours of activity/week (MET-hours/week) (26). Moderate- or vigorous-intensity activity was defined as any activity requiring a typical energy expenditure of 3 METs or greater (13). Sedentary behavior was assessed in 1992, when participants reported their average weekly time spent sitting at work or away from home or while driving, sitting at home watching television or movies, and other sitting at home (e.g., reading, meal times, at desk).
The reproducibility and validity of these questions have been described elsewhere (27). Briefly, in a similar population of NHS II participants (n = 151), the correlation over a 1-year period between activity reported by questionnaire and that assessed by past-week recalls was 0.79, and the correlation between moderate or vigorous activity reported by questionnaire and that assessed by activity diaries was 0.62. In the NHS, physical activity was significantly associated with decreased risk of breast (28) and colorectal (29) cancers, coronary heart disease (30), and type 2 diabetes (31), while sedentary behaviors increased risk of type 2 diabetes and obesity (21).
Assessment of covariates
We collected information on factors potentially associated with physical activity or sedentary behavior and telomere length. From the questionnaire completed at blood collection, we assessed age, body mass index (calculated as weight (kg)/height (m)2), and various blood collection variables. From NHS questionnaires administered at approximately the time of collection, we assessed various anthropometric, reproductive, and lifestyle factors, as well as the presence of chronic diseases. Using a validated semiquantitative food frequency questionnaire (32) administered in 1990, we assessed intakes of dietary factors.
Measurement of relative leukocyte telomere length
Genomic DNA was extracted from peripheral blood leukocytes by using the QiAmp 96-spin blood protocol (Qiagen, Valencia, California). We assessed relative LTL using quantitative polymerase chain reaction (33). The average relative LTL was calculated as the telomere repeat copy number/single-gene (36B4) copy number (T/S) exponentiated ratio (34). Laboratory technicians masked to participant characteristics assayed each sample in triplicate. Quality control samples were interspersed on each plate to assess variability. In all nested case-control studies, coefficients of variation for the telomere and single-gene assay were less than 4%, and coefficients of variation for the T/S exponentiated ratio were less than 17%. Although this assay provides a relative measurement of telomere length, T/S ratios correlate well with absolute telomere lengths determined by Southern blot (r = 0.82; P < 0.0001) (34).
Statistical analysis
Multivariate linear regression was used to examine age-adjusted associations between characteristics of the study population and total physical activity reported in 1988. The distribution of relative LTL values varied slightly across nested case-control sets. Thus, among participants in each set, we calculated the natural logarithm of LTL to improve normality, identified outlying LTL values using the generalized extreme studentized deviate many-outlier procedure (35), and computed z-scores of LTL.
We examined age-adjusted correlations between various factors and LTL using Spearman partial rank correlation coefficients. To examine the association among physical activity, sedentary behavior, and LTL, we estimated adjusted least-squares mean LTL (z-scores) by category of activity or sedentary behavior using 3 generalized linear models. In the first model, we adjusted for age in years. In the second model, we additionally adjusted for pack-years of smoking, menopausal status, postmenopausal hormone use, case status, easy walking in analyses of moderate or vigorous activity, other activities in analyses of specific activities, and total activity in analyses of sedentary behavior. In the final model, we additionally adjusted for body mass index at blood collection to assess its influence on these relations. We also considered the following factors potentially associated with physical activity or sedentary behavior and telomere length: blood collection variables (e.g., fasting status, time and date of blood collection), nested case-control set, intakes of dietary factors (e.g., total energy, alcohol, polyunsaturated fatty acids, fat, fiber, linoleic acid), oral contraceptive use, body mass index at age 18 years, diabetes, hypertension, waist and hip measurements, and occupational status. As results were similar after including these variables, we omitted them from the final models. We tested for linear trend across categories by including activity or sedentary behavior categories as an ordinal predictor in multivariate linear regression models. Preliminary evidence suggested a potential U-shaped relation between activity and LTL; we evaluated departures from linearity by including a second-order activity term in polynomial regression models and conducted an F test to assess model fit.
In exploratory analyses, we evaluated whether associations differed by categories of age (<60, ≥60 years), body mass index (18.5–<25, ≥25 kg/m2), smoking (ever, never), or weight change between 1988 and date of blood collection (weight loss >2 kg, weight maintenance or gain). We used F tests to compare nested models with and without interaction terms between activity or sedentary behavior and these variables.
P values were 2 tailed, and P ≤ 0.05 was considered significant. All statistical analyses used SAS, version 9.1, software (SAS Institute, Inc., Cary, North Carolina).
RESULTS
The mean age of participants was 59 years. More active women were slightly older at blood collection and at menarche, more likely to currently use postmenopausal hormones, and less likely to be current smokers; they had slightly lower body mass index at age 18 years and higher intake of total energy, and they spent less time sitting (Table 1). As expected, these women also had lower values of anthropometric variables measured at blood collection, including body mass index, waist/hip ratio, and weight gain since age 18 years. Increased age was correlated with shorter telomeres in our population (rs = −0.09, P < 0.001) (Table 2). After adjustment for age, LTL was inversely correlated with pack-years of smoking (rs = −0.04, P < 0.001) and anthropometric measures (body mass index: rs = −0.04, P < 0.001; waist/hip ratio: rs = −0.03, P = 0.04; waist: rs = −0.04, P = 0.002). LTL was uncorrelated with intakes of total energy, alcohol, caffeine, and other sources of antioxidants.
Table 1.
Age and Age-adjusted Characteristics of 7,813 Women in the Nurses’ Health Study by Total Physical Activity Reported in 1988a,b
| Characteristic | Total Activity in 1988, MET-hours/weekc |
P Valued | |||||
| <3 (n = 1,605) |
9–<18 (n = 1,680) |
≥27 (n = 1,337) |
|||||
| % | Mean (SD) | % | Mean (SD) | % | Mean (SD) | ||
| Age at blood collection, yearse | 58.5 (6.7) | 59.1 (6.6) | 59.5 (6.5) | <0.001 | |||
| Ever oral contraceptive use | 42.8 | 44.2 | 41.8 | 0.57 | |||
| Nulliparous | 5.4 | 6.0 | 7.2 | 0.33 | |||
| Parity, no. of childrenf | 3.2 (1.7) | 3.1 (1.7) | 3.0 (1.7) | 0.08 | |||
| Age at menarche, years | 12.5 (1.4) | 12.6 (1.3) | 12.7 (1.5) | 0.01 | |||
| Postmenopausal | 83.5 | 82.1 | 81.6 | 0.08 | |||
| Age at menopause, years | 50.2 (7.7) | 50.5 (7.7) | 50.1 (7.3) | 0.74 | |||
| Postmenopausal hormone use | |||||||
| Never | 45.9 | 40.3 | 40.1 | <0.001 | |||
| Past | 17.0 | 16.5 | 15.0 | 0.18 | |||
| Current | 36.6 | 42.6 | 44.1 | <0.001 | |||
| Smoking status | |||||||
| Never | 43.9 | 45.3 | 41.3 | 0.40 | |||
| Past | 35.9 | 40.7 | 46.5 | <0.001 | |||
| Current | 20.1 | 13.9 | 11.7 | <0.001 | |||
| Body mass index at age 18 yearsg | 21.6 (3.3) | 21.4 (2.9) | 21.3 (2.7) | 0.04 | |||
| Body mass index at blood collectiong | 26.6 (5.7) | 25.3 (4.5) | 24.8 (4.4) | <0.001 | |||
| Waist circumference, cm | 62.8 (36.5) | 62.6 (33.0) | 64.2 (31.1) | 0.31 | |||
| Waist/hip ratio | 0.8 (0.1) | 0.8 (0.1) | 0.8 (0.08) | <0.001 | |||
| Weight gain since age 18 years, kg | 13.3 (13.1) | 10.5 (11.2) | 9.4 (11.1) | <0.001 | |||
| Energy intake, kcal/day | 1,754.5 (529.4) | 1,765.2 (508.4) | 1,797.9 (518.7) | 0.008 | |||
| Total sitting in 1992, hours/week | 39.0 (23.7) | 38.3 (22.6) | 35.7 (22.0) | <0.001 | |||
| Total activity in 1988, MET-hours/week | 1.4 (0.9) | 12.9 (2.7) | 46.0 (18.4) | ||||
Abbreviations: MET-hours/week, metabolic equivalent hours of activity/week; SD, standard deviation.
Values are standardized to the age distribution of the study population at blood collection.
Values may not add to 100% because of missing data.
Lowest, middle, and highest categories of total activity are presented.
Two-sided P values were calculated by using multivariate linear regression adjusted for age.
Value not age adjusted.
Among parous women.
Body mass index: weight (kg)/height (m)2.
Table 2.
Age-adjusted Correlations Between Relative Telomere Length (z-score) and Various Characteristics of 7,813 Women in the Nurses’ Health Study, United States, 1989–1990
| Characteristic | No. | rsa | P Valueb |
| Age at blood collection, yearsc | 7,813 | −0.09 | <0.001 |
| Pack-years of smoking | 7,684 | −0.04 | <0.001 |
| Body mass index at age 18 yearsd | 7,444 | −0.01 | 0.29 |
| Body mass index at blood collectiond | 7,797 | −0.04 | <0.001 |
| Waist/hip ratio | 5,614 | −0.03 | 0.04 |
| Waist circumference, cm | 5,634 | −0.04 | 0.002 |
| Weight change since age 18 years, kg | 7,454 | −0.02 | 0.04 |
| Age at menarche, years | 7,756 | 0.01 | 0.30 |
| Duration of postmenopausal hormone use | 6,486 | 0.0007 | 0.96 |
| Total energy intake, kcal/day | 7,406 | −0.005 | 0.69 |
| Alcohol consumption, g/day | 7,406 | 0.002 | 0.85 |
| Fruit and vegetable intake, g/day | 7,165 | 0.003 | 0.79 |
| Caffeine intake, mg/day | 7,406 | 0.006 | 0.58 |
| Vitamin A intake, IU/day | 7,406 | 0.01 | 0.25 |
| Vitamin E intake, mg/day | 7,406 | 0.01 | 0.26 |
rs = Spearman’s partial rank correlation coefficient.
P values are 2 sided.
Value not age adjusted.
Body mass index: weight (kg)/height (m)2.
Total MET-hours/week of activity was positively associated with LTL (Table 3). The age-adjusted least-squares mean LTL (z-score) across categories of total activity (<3, 3–<9, 9–<18, 18–<27, ≥27 MET-hours/week) was −0.04, −0.02, 0.04, 0.05, and 0.03, respectively (Ptrend = 0.02). Further adjustment for smoking and other variables did not appreciably alter the findings. Compared with the least active women, those moderately or highly active had a 0.07-standard deviation (SD) increase in LTL (Ptrend = 0.02). Moderate- or vigorous-intensity activity was positively associated with LTL (Table 4). The multivariate-adjusted least-squares mean LTL across categories (<1, 1–<2, 2–<4, 4–<7, ≥7 hours/week) was −0.005, 0.01, 0.10, 0.07, and 0.03, respectively (Ptrend = 0.02). There was no evidence of a nonlinear relation between total activity (P = 0.34) or moderate or vigorous activity (P = 0.42) and LTL.
Table 3.
Estimated Least-Squares Mean Telomere Length (z-score) and 95% Confidence Interval by Total Physical Activity Reported in 1988, Nurses’ Health Study, United States
| Total Activity in 1988, MET-hours/week |
Ptrenda | ||||||||||
| <3 (n = 1,605) |
3–<9 (n = 2,005) |
9–<18 (n = 1,680) |
18–<27 (n = 1,037) |
≥27 (n = 1,337) |
|||||||
| LTL | 95% CI | LTL | 95% CI | LTL | 95% CI | LTL | 95% CI | LTL | 95% CI | ||
| Age adjusted | −0.04 | −0.09, 0.01 | −0.02 | −0.06, 0.03 | 0.04 | −0.008, 0.09 | 0.05 | −0.01, 0.11 | 0.03 | −0.02, 0.08 | 0.02 |
| Multivariateb | −0.02 | −0.09, 0.05 | 0.000 | −0.07, 0.07 | 0.05 | −0.01, 0.12 | 0.06 | −0.01, 0.14 | 0.05 | −0.03, 0.12 | 0.02 |
| Multivariate + body mass indexc,d | −0.01 | −0.08, 0.06 | −0.001 | −0.07, 0.06 | 0.05 | −0.02, 0.12 | 0.06 | −0.02, 0.13 | 0.04 | −0.03, 0.11 | 0.05 |
Abbreviations: CI, confidence interval; LTL, leukocyte telomere length; MET-hours/week, metabolic equivalent hours of activity/week.
P values are 2 sided.
Adjusted for age in years (continuous), pack-years of smoking (0, >0–<20, 20–<40, ≥40, missing), menopausal status (pre-, post-, dubious), postmenopausal hormone use (never, past, current <5 years, current ≥5 years, missing), case status (case, control).
Body mass index at blood collection (continuous).
Body mass index: weight (kg)/height (m)2.
Table 4.
Estimated Least-Squares Mean Telomere Length (z-score) and 95% Confidence Interval by Moderate or Vigorous Physical Activity Reported in 1988, Nurses’ Health Study, United States
| Moderate- or Vigorous-Intensity Activity, hours/weeka |
Ptrendb | ||||||||||
| <1 (n = 3,775) |
1–<2 (n = 1,161) |
2–<4 (n = 1,269) |
4–<7 (n = 823) |
≥7 (n = 660) |
|||||||
| LTL | 95% CI | LTL | 95% CI | LTL | 95% CI | LTL | 95% CI | LTL | 95% CI | ||
| Age adjusted | −0.02 | −0.05, 0.009 | −0.007 | −0.06, 0.05 | 0.08 | 0.03, 0.13 | 0.05 | −0.02, 0.12 | 0.007 | −0.07, 0.08 | 0.02 |
| Multivariatec | −0.005 | −0.06, 0.05 | 0.01 | −0.06, 0.09 | 0.10 | 0.03, 0.18 | 0.07 | −0.01, 0.16 | 0.03 | −0.06, 0.13 | 0.02 |
| Multivariate + body mass indexd,e | −0.004 | −0.06, 0.06 | 0.01 | −0.06, 0.09 | 0.10 | 0.02, 0.17 | 0.07 | −0.02, 0.16 | 0.03 | −0.07, 0.12 | 0.05 |
Abbreviations: CI, confidence interval; LTL, leukocyte telomere length.
Includes brisk walking, jogging, running, biking, swimming, tennis, and calisthenics or aerobics.
P values are 2 sided.
Adjusted for age in years (continuous), pack-years of smoking (0, >0−<20, 20−<40, ≥40, missing), menopausal status (pre-, post-, dubious), postmenopausal hormone use (never, past, current <5 years, current ≥5 years, missing), case status (case, control), easy walking (0, >0–<1, 1–<2.5, ≥2.5 hours/week).
Body mass index at blood collection (continuous).
Body mass index: weight (kg)/height (m)2.
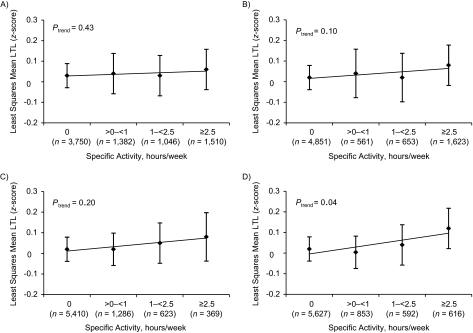
We evaluated walking at an easy (<3 miles/hour) or brisk (≥3 miles/hour) pace, biking, and calisthenics or aerobics, the 4 most commonly reported activities among participants (Figure 1) (1 mile = 1.6 km). After adjustment for age and other variables, calisthenics or aerobics was associated with a 0.10-SD increase in LTL when comparing the most active women with the least active (Ptrend = 0.04). Results were slightly attenuated, but remained significant, after adjustment for body mass index. Although women with more brisk walking and biking appeared to have longer telomeres, this was nonsignificant.
Figure 1.
Estimated least-squares mean leukocyte telomere length (LTL) (z-score) and 95% confidence interval by hours/week of easy walking (A), brisk walking (B), biking (C), and calisthenics or aerobics (D) reported in 1988 in the Nurses’ Health Study, United States. Adjusted for age in years (continuous), pack-years of smoking (0, >0–<20, 20–<40, ≥40, missing), menopausal status (pre-, post-, dubious), postmenopausal hormone use (never, past, current <5 years, current ≥5 years, missing), case status (case, control), and other specific activities (0–<1, 1–<2, 2–<4, 4–<7, ≥7 hours/week). All P values are 2 sided.
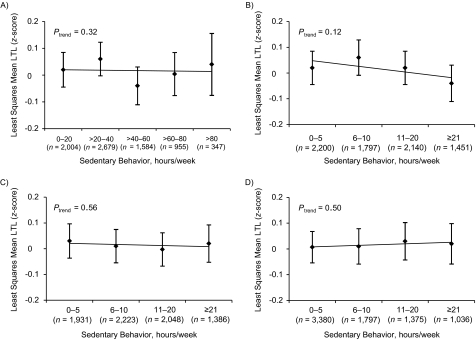
Sedentary behaviors, including total time spent sitting and time spent on specific types of sitting, were unassociated with LTL after adjustment for physical activity and other variables (Figure 2). Findings remained similar after additional adjustment for body mass index. After jointly classifying total activity (<9, ≥9 MET-hours/week) and total hours spent sitting (<34.5, ≥34.5 hours/week), we found that activity was similarly associated with longer LTL regardless of whether individuals were more sedentary or less sedentary; hours spent sitting remained unassociated (data not shown). Compared with women who were less active and more sedentary, those who were more active and less sedentary had the largest increase in LTL (SD = 0.11; P = 0.0007).
Figure 2.
Estimated least-squares mean leukocyte telomere length (LTL) (z-score) and 95% confidence interval by hours/week of total sitting (A), sitting while watching television (B), other sitting at home (C), and sitting at work or while away from home (D) reported in 1992 in the Nurses’ Health Study, United States. Adjusted for age in years (continuous), pack-years of smoking (0, >0–<20, 20–<40, ≥40, missing), menopausal status (pre-, post-, dubious), postmenopausal hormone use (never, past, current <5 years, current ≥5 years, missing), case status (case, control), and total activity in 1992 (<3, 3–<9, 9–<18, 18–<27, ≥27, missing metabolic equivalent (MET)-hours/week). All P values are 2 sided.
Results were consistent across age group, smoking status, and weight change between 1988 and blood collection. Compared with lean women (body mass index, <25 kg/m2), overweight women (body mass index, ≥25 kg/m2) had a stronger association between activity and LTL. This difference was statistically significant for moderate- or vigorous-intensity activity (Pinteraction = 0.02) but not for total activity (Pinteraction = 0.06).
The nested case-control studies observed null or modest associations between telomere length and disease development, and the LTLs of cases and controls were similar in the present analysis, reducing the possibility that preclinical disease at the time of blood collection influenced the observed associations. To address this, we repeated the analyses after restricting to controls (n = 4,562) and observed results similar to those from the main analyses. For instance, total activity remained positively associated with LTL (0.07-SD increase between the lowest and highest category; Ptrend = 0.009), while total sitting remained unassociated.
DISCUSSION
In this large cross-sectional analysis, women who were moderately or highly active had longer LTLs than less active women after adjustment for age and other potential confounders. This relation remained after additional adjustment for body mass index. Although the association was modest, the difference in telomere length corresponded on average to 4.4 years of aging, comparable to the difference observed when comparing smokers with nonsmokers (4.6 years) (20). Similarly, greater moderate- or vigorous-intensity activity was associated with longer telomeres independently of body mass index. We observed the longest telomeres among women who engaged in moderate or vigorous activities 2–4 hours per week, an amount corresponding to current US guidelines (2.5 hours/week) (13). There was no additional increase in LTL for the most active women compared with those who were moderately active, suggesting that even moderate amounts of activity may influence LTL. These associations were more pronounced in overweight women, although this observation requires confirmation. Among individual activities, calisthenics or aerobics was positively associated with LTL, possibly because of the less variable intensity with which it may be performed compared with other activities. Few women engaged in regular vigorous activities, which limited statistical power to examine other vigorous activities, such as jogging, running, swimming, and tennis.
More sedentary participants had LTL similar to that of those less sedentary. Conceivably, measurement error in self-reported sedentary behavior may have attenuated associations, limiting the power to detect a true association. However, sedentary behaviors have been associated with increased risk of type 2 diabetes and obesity in the NHS (21), providing further evidence for the validity of our findings. The role of sedentary behavior in LTL has not been examined previously and therefore requires further study.
Our group previously examined diet and other lifestyle factors in relation to LTL in a subset of 2,284 NHS participants (15) and observed no association between total activity and LTL. As the primary focus of this previous analysis was on dietary factors, it did not evaluate activity intensity and type or sedentary behaviors. In addition, previously observed associations between lifestyle factors and LTL have been modest in magnitude (17, 20), potentially limiting statistical power. We therefore conducted the present analysis to evaluate activity and sedentary behavior in detail in the largest study population to date.
Population-based studies of activity and telomere length have been limited and inconsistent. Our results agree with those of previous cross-sectional studies linking activity with longer telomeres (6, 14, 17, 19) but not with others reporting null associations (15, 16, 18). These inconsistencies may be due in part to differences in physical activity assessment, covariate adjustment, and study populations, as well as limitations in sample size. On average, participants were younger in the studies by Cherkas et al. (14) and Zhu et al. (17) compared with participants in studies that reported null findings. LTL has been reported to remain more stable among older individuals (36), and attenuated associations have been observed among older individuals (14), potentially limiting statistical power in studies of older participants. Although our study comprised middle-aged and older women, its large sample size enabled us to detect modest associations previously unobserved in the NHS (15).
Most studies broadly examined physical activity among many other lifestyle characteristics (6, 15, 17, 18); only one examined separately vigorous-intensity activity (17), and none addressed individual types of activity. Our findings for moderate or vigorous activity and calisthenics or aerobics were consistent with those of Zhu et al. (17), who reported a positive association between vigorous activity and LTL among adolescent girls. Our results extend the literature by showing in the largest study to date that even moderate amounts of activity, including that of moderate or vigorous intensity, may be associated with longer telomeres in middle-aged and older women.
Oxidative stress and inflammation accelerate telomere attrition (2, 37) and, therefore, potentially mediate the association between activity and LTL. Exercise helps to maintain energy balance and to reduce obesity (12), which may decrease levels of oxidative stress and inflammation (38, 39). Although slightly attenuated, associations remained significant after adjustment for body mass index, suggesting that the relation between activity and LTL may in part be mediated through factors other than body mass. One possibility is that moderate amounts of regular activity may generate low levels of reactive oxygen species that induce adaptive increases in endogenous antioxidant defenses, while high amounts of activity may generate excess reactive oxygen species that counteract these defenses (40–44). Although current evidence for this hypothesis is inconsistent, our results and those of others (19, 45, 46) may be consistent with this mechanism. Activity may also help to prevent insulin resistance (12), which has been associated with increased inflammation, oxidative stress, and telomere attrition (47–50). Additionally, activity may help to reduce the effects of chronic stress (51), which has been linked to telomere shortening (52). Telomerase, an enzyme counteracting telomere shortening, may also play a role. In mice, those randomized to short- and long-term exercise exhibited elevated telomerase activity compared with inactive controls (53, 54). In humans, telomerase activity was higher among trained athletes (54) and after a 3-month lifestyle intervention, which included the addition of moderate aerobic exercise (55); however, others observed no association (19).
The strengths of our study include the large study population and detailed assessment of activity, sedentary behavior, and lifestyle variables. However, the cross-sectional design precludes us from establishing a temporal relation between activity and LTL. Conceivably, individuals with shorter telomeres may possess existing chronic diseases that reduce their ability to exercise; this was unlikely as our results were similar after excluding individuals who were diagnosed with heart disease (n = 149), stroke (n = 34), diabetes (n = 277), or cancer (n = 450) prior to blood collection. We were unable to address physical activity at various times of life. Given that LTL is thought to reflect telomere shortening over time (2), the role of activity accumulated over a participant’s lifetime is likely important. To address this, future studies should compare the role of recent activity with that of activity accumulated over many years in LTL dynamics. Additionally, we primarily assessed recreational activity and were unable to address specifically household or occupational activity, although results remained similar after adjustment for employment status (e.g., homemaker, retired, full-time, part-time). We assessed LTL using a single measure, which prevented us from directly examining telomere attrition. Addressing this requires a prospective study with repeated LTL assessments. Unmeasured or residual confounding was also possible, although adjustment for a number of lifestyle and dietary factors and the evaluation of many others did not appreciably alter our results. Our study population predominantly comprised registered nurses of European ancestry. Because telomere dynamics may differ among African Americans and Hispanics (17, 56, 57), our results may not be generalizable to women of other ethnicities. However, the homogeneity among NHS participants strengthens the internal validity of our findings by maximizing the quality of self-reported activity and reducing residual confounding.
In summary, findings from this large cross-sectional study suggest that activity—even in moderate amounts—may be associated with longer LTL in middle-aged and older women, providing insight into the understanding of how regular exercise may benefit health on the cellular level. Although the associations were modest, our findings warrant further investigation in large prospective studies with detailed activity and sedentary behavior assessment and repeated measurements of telomere length over time.
Acknowledgments
Author affiliations: Department of Epidemiology, Harvard School of Public Health, Boston, Massachusetts (Mengmeng Du, Peter Kraft, Jiali Han, Edward Giovannucci, Susan E. Hankinson, Immaculata De Vivo); Program in Molecular and Genetic Epidemiology, Harvard School of Public Health, Boston, Massachusetts (Mengmeng Du, Jennifer Prescott, Peter Kraft, Jiali Han, Immaculata De Vivo); Channing Laboratory, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, Massachusetts (Mengmeng Du, Jennifer Prescott, Jiali Han, Susan E. Hankinson, Immaculata De Vivo); Clinical Research Program, Department of Dermatology, Brigham and Women’s Hospital and Harvard Medical School, Boston, Massachusetts (Jiali Han); and Department of Nutrition, Harvard School of Public Health, Boston, Massachusetts (Edward Giovannucci).
This study was supported by the National Institutes of Health (grants R01 CA082838, P01 CA87969, R01 CA49449, R01 HL034594, R01 HL088521, and T32 ES01664 to M. D. and T32 CA09001 to J. P.).
The authors thank Qun Guo for programming support and assistance with data preparation, as well as Patrice Soule, Jason Wong, and Robert Farquhar for assistance collecting telomere length data.
The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. The contents hereof are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Conflict of interest: none declared.
Glossary
Abbreviations
- LTL
leukocyte telomere length
- MET
metabolic equivalent
- MET-hours/week
metabolic equivalent hours of activity/week
- NHS
Nurses’ Health Study
- SD
standard deviation
- T/S
telomere repeat copy number/single-gene (36B4) copy number
References
- 1.Blasco MA. Telomeres and human disease: ageing, cancer and beyond. Nat Rev Genet. 2005;6(8):611–622. doi: 10.1038/nrg1656. [DOI] [PubMed] [Google Scholar]
- 2.Aviv A. Telomeres and human aging: facts and fibs. Sci Aging Knowledge Environ. 2004;2004(51):pe43. doi: 10.1126/sageke.2004.51.pe43. (doi:10.1126/sageke.2004.51.pe43) [DOI] [PubMed] [Google Scholar]
- 3.Calado RT, Young NS. Telomere diseases. N Engl J Med. 2009;361(24):2353–2365. doi: 10.1056/NEJMra0903373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wentzensen IM, Mirabello L, Pfeiffer RM, et al. The association of telomere length and cancer: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2011;20(6):1238–1250. doi: 10.1158/1055-9965.EPI-11-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu X, Amos CI, Zhu Y, et al. Telomere dysfunction: a potential cancer predisposition factor. J Natl Cancer Inst. 2003;95(16):1211–1218. doi: 10.1093/jnci/djg011. [DOI] [PubMed] [Google Scholar]
- 6.Willeit P, Willeit J, Mayr A, et al. Telomere length and risk of incident cancer and cancer mortality. JAMA. 2010;304(1):69–75. doi: 10.1001/jama.2010.897. [DOI] [PubMed] [Google Scholar]
- 7.Ma H, Zhou Z, Wei S, et al. Shortened telomere length is associated with increased risk of cancer: a meta-analysis. PLoS One. 2011;6(6):e20466. doi: 10.1371/journal.pone.0020466. (doi:10.1371/journal.pone.0020466) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sampson MJ, Winterbone MS, Hughes JC, et al. Monocyte telomere shortening and oxidative DNA damage in type 2 diabetes. Diabetes Care. 2006;29(2):283–289. doi: 10.2337/diacare.29.02.06.dc05-1715. [DOI] [PubMed] [Google Scholar]
- 9.Salpea KD, Talmud PJ, Cooper JA, et al. Association of telomere length with type 2 diabetes, oxidative stress and UCP2 gene variation. Atherosclerosis. 2010;209(1):42–50. doi: 10.1016/j.atherosclerosis.2009.09.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fitzpatrick AL, Kronmal RA, Gardner JP, et al. Leukocyte telomere length and cardiovascular disease in the Cardiovascular Health Study. Am J Epidemiol. 2007;165(1):14–21. doi: 10.1093/aje/kwj346. [DOI] [PubMed] [Google Scholar]
- 11.Ji LL, Gomez-Cabrera MC, Vina J. Exercise and hormesis: activation of cellular antioxidant signaling pathway. Ann N Y Acad Sci. 2006;1067(1):425–435. doi: 10.1196/annals.1354.061. [DOI] [PubMed] [Google Scholar]
- 12.McTiernan A. Mechanisms linking physical activity with cancer. Nat Rev Cancer. 2008;8(3):205–211. doi: 10.1038/nrc2325. [DOI] [PubMed] [Google Scholar]
- 13.US Department of Health and Human Services. 2008 Physical Activity Guidelines for Americans: Be Active, Healthy, and Happy! Washington, DC: US Department of Health and Human Services; 2008. [Google Scholar]
- 14.Cherkas LF, Hunkin JL, Kato BS, et al. The association between physical activity in leisure time and leukocyte telomere length. Arch Intern Med. 2008;168(2):154–158. doi: 10.1001/archinternmed.2007.39. [DOI] [PubMed] [Google Scholar]
- 15.Cassidy A, De Vivo I, Liu Y, et al. Associations between diet, lifestyle factors, and telomere length in women. Am J Clin Nutr. 2010;91(5):1273–1280. doi: 10.3945/ajcn.2009.28947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Woo J, Tang N, Leung J. No association between physical activity and telomere length in an elderly Chinese population 65 years and older. Arch Intern Med. 2008;168(19):2163–2164. doi: 10.1001/archinte.168.19.2163. [DOI] [PubMed] [Google Scholar]
- 17.Zhu H, Wang X, Gutin B, et al. Leukocyte telomere length in healthy Caucasian and African-American adolescents: relationships with race, sex, adiposity, adipokines, and physical activity. J Pediatr. 2011;158(2):215–220. doi: 10.1016/j.jpeds.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mirabello L, Huang WY, Wong JY, et al. The association between leukocyte telomere length and cigarette smoking, dietary and physical variables, and risk of prostate cancer. Aging Cell. 2009;8(4):405–413. doi: 10.1111/j.1474-9726.2009.00485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ludlow AT, Zimmerman JB, Witkowski S, et al. Relationship between physical activity level, telomere length, and telomerase activity. Med Sci Sports Exerc. 2008;40(10):1764–1771. doi: 10.1249/MSS.0b013e31817c92aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Valdes AM, Andrew T, Gardner JP, et al. Obesity, cigarette smoking, and telomere length in women. Lancet. 2005;366(9486):662–664. doi: 10.1016/S0140-6736(05)66630-5. [DOI] [PubMed] [Google Scholar]
- 21.Hu FB, Li TY, Colditz GA, et al. Television watching and other sedentary behaviors in relation to risk of obesity and type 2 diabetes mellitus in women. JAMA. 2003;289(14):1785–1791. doi: 10.1001/jama.289.14.1785. [DOI] [PubMed] [Google Scholar]
- 22.Hankinson SE, Willett WC, Manson JE, et al. Alcohol, height, and adiposity in relation to estrogen and prolactin levels in postmenopausal women. J Natl Cancer Inst. 1995;87(17):1297–1302. doi: 10.1093/jnci/87.17.1297. [DOI] [PubMed] [Google Scholar]
- 23.De Vivo I, Prescott J, Wong JY, et al. A prospective study of relative telomere length and postmenopausal breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2009;18(4):1152–1156. doi: 10.1158/1055-9965.EPI-08-0998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han J, Qureshi AA, Prescott J, et al. A prospective study of telomere length and the risk of skin cancer. J Invest Dermatol. 2009;129(2):415–421. doi: 10.1038/jid.2008.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prescott J, McGrath M, Lee IM, et al. Telomere length and genetic analyses in population-based studies of endometrial cancer risk. Cancer. 2010;116(18):4275–4282. doi: 10.1002/cncr.25328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ainsworth BE, Haskell WL, Leon AS, et al. Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc. 1993;25(1):71–80. doi: 10.1249/00005768-199301000-00011. [DOI] [PubMed] [Google Scholar]
- 27.Wolf AM, Hunter DJ, Colditz GA, et al. Reproducibility and validity of a self-administered physical activity questionnaire. Int J Epidemiol. 1994;23(5):991–999. doi: 10.1093/ije/23.5.991. [DOI] [PubMed] [Google Scholar]
- 28.Eliassen AH, Hankinson SE, Rosner B, et al. Physical activity and risk of breast cancer among postmenopausal women. Arch Intern Med. 2010;170(19):1758–1764. doi: 10.1001/archinternmed.2010.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giovannucci E, Colditz GA, Stampfer MJ, et al. Physical activity, obesity, and risk of colorectal adenoma in women (United States) Cancer Causes Control. 1996;7(2):253–263. doi: 10.1007/BF00051301. [DOI] [PubMed] [Google Scholar]
- 30.Manson JE, Hu FB, Rich-Edwards JW, et al. A prospective study of walking as compared with vigorous exercise in the prevention of coronary heart disease in women. N Engl J Med. 1999;341(9):650–658. doi: 10.1056/NEJM199908263410904. [DOI] [PubMed] [Google Scholar]
- 31.Hu FB, Sigal RJ, Rich-Edwards JW, et al. Walking compared with vigorous physical activity and risk of type 2 diabetes in women: a prospective study. JAMA. 1999;282(15):1433–1439. doi: 10.1001/jama.282.15.1433. [DOI] [PubMed] [Google Scholar]
- 32.Willett WC, Reynolds RD, Cottrell-Hoehner S, et al. Validation of a semi-quantitative food frequency questionnaire: comparison with a 1-year diet record. J Am Diet Assoc. 1987;87(1):43–47. [PubMed] [Google Scholar]
- 33.McGrath M, Wong JY, Michaud D, et al. Telomere length, cigarette smoking, and bladder cancer risk in men and women. Cancer Epidemiol Biomarkers Prev. 2007;16(4):815–819. doi: 10.1158/1055-9965.EPI-06-0961. [DOI] [PubMed] [Google Scholar]
- 34.Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30(10):e47. doi: 10.1093/nar/30.10.e47. (doi:10.1093/nar/30.10.e47) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosner B. Percentage points for a generalized ESD many-outlier procedure. Technometrics. 1983;25(2):165–172. [Google Scholar]
- 36.Frenck RW, Jr, Blackburn EH, Shannon KM. The rate of telomere sequence loss in human leukocytes varies with age. Proc Natl Acad Sci U S A. 1998;95(10):5607–5610. doi: 10.1073/pnas.95.10.5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.von Zglinicki T. Oxidative stress shortens telomeres. Trends Biochem Sci. 2002;27(7):339–344. doi: 10.1016/s0968-0004(02)02110-2. [DOI] [PubMed] [Google Scholar]
- 38.Pou KM, Massaro JM, Hoffmann U, et al. Visceral and subcutaneous adipose tissue volumes are cross-sectionally related to markers of inflammation and oxidative stress: the Framingham Heart Study. Circulation. 2007;116(11):1234–1241. doi: 10.1161/CIRCULATIONAHA.107.710509. [DOI] [PubMed] [Google Scholar]
- 39.Keaney JF, Jr, Larson MG, Vasan RS, et al. Obesity and systemic oxidative stress: clinical correlates of oxidative stress in the Framingham Study. Arterioscler Thromb Vasc Biol. 2003;23(3):434–439. doi: 10.1161/01.ATV.0000058402.34138.11. [DOI] [PubMed] [Google Scholar]
- 40.Radak Z, Chung HY, Goto S. Systemic adaptation to oxidative challenge induced by regular exercise. Free Radic Biol Med. 2008;44(2):153–159. doi: 10.1016/j.freeradbiomed.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 41.Covas MI, Elosua R, Fitó M, et al. Relationship between physical activity and oxidative stress biomarkers in women. Med Sci Sports Exerc. 2002;34(5):814–819. doi: 10.1097/00005768-200205000-00014. [DOI] [PubMed] [Google Scholar]
- 42.Pialoux V, Brown AD, Leigh R, et al. Effect of cardiorespiratory fitness on vascular regulation and oxidative stress in postmenopausal women. Hypertension. 2009;54(5):1014–1020. doi: 10.1161/HYPERTENSIONAHA.109.138917. [DOI] [PubMed] [Google Scholar]
- 43.Powers SK, Jackson MJ. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev. 2008;88(4):1243–1276. doi: 10.1152/physrev.00031.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Poulsen HE, Loft S, Vistisen K. Extreme exercise and oxidative DNA modification. J Sports Sci. 1996;14(4):343–346. doi: 10.1080/02640419608727720. [DOI] [PubMed] [Google Scholar]
- 45.Collins M, Renault V, Grobler LA, et al. Athletes with exercise-associated fatigue have abnormally short muscle DNA telomeres. Med Sci Sports Exerc. 2003;35(9):1524–1528. doi: 10.1249/01.MSS.0000084522.14168.49. [DOI] [PubMed] [Google Scholar]
- 46.Rae DE, Vignaud A, Butler-Browne GS, et al. Skeletal muscle telomere length in healthy, experienced, endurance runners. Eur J Appl Physiol. 2010;109(2):323–330. doi: 10.1007/s00421-010-1353-6. [DOI] [PubMed] [Google Scholar]
- 47.Ceriello A, Motz E. Is oxidative stress the pathogenic mechanism underlying insulin resistance, diabetes, and cardiovascular disease? The common soil hypothesis revisited. Arterioscler Thromb Vasc Biol. 2004;24(5):816–823. doi: 10.1161/01.ATV.0000122852.22604.78. [DOI] [PubMed] [Google Scholar]
- 48.Gardner JP, Li S, Srinivasan SR, et al. Rise in insulin resistance is associated with escalated telomere attrition. Circulation. 2005;111(17):2171–2177. doi: 10.1161/01.CIR.0000163550.70487.0B. [DOI] [PubMed] [Google Scholar]
- 49.Demissie S, Levy D, Benjamin EJ, et al. Insulin resistance, oxidative stress, hypertension, and leukocyte telomere length in men from the Framingham Heart Study. Aging Cell. 2006;5(4):325–330. doi: 10.1111/j.1474-9726.2006.00224.x. [DOI] [PubMed] [Google Scholar]
- 50.Al-Attas OS, Al-Daghri NM, Alokail MS, et al. Adiposity and insulin resistance correlate with telomere length in middle-aged Arabs: the influence of circulating adiponectin. Eur J Endocrinol. 2010;163(4):601–607. doi: 10.1530/EJE-10-0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Puterman E, Lin J, Blackburn E, et al. The power of exercise: buffering the effect of chronic stress on telomere length. PLoS One. 2010;5(5):e10837. doi: 10.1371/journal.pone.0010837. (doi:10.1371/journal.pone.0010837) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Epel ES, Blackburn EH, Lin J, et al. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci U S A. 2004;101(49):17312–17315. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Werner C, Hanhoun M, Widmann T, et al. Effects of physical exercise on myocardial telomere-regulating proteins, survival pathways, and apoptosis. J Am Coll Cardiol. 2008;52(6):470–482. doi: 10.1016/j.jacc.2008.04.034. [DOI] [PubMed] [Google Scholar]
- 54.Werner C, Fürster T, Widmann T, et al. Physical exercise prevents cellular senescence in circulating leukocytes and in the vessel wall. Circulation. 2009;120(24):2438–2447. doi: 10.1161/CIRCULATIONAHA.109.861005. [DOI] [PubMed] [Google Scholar]
- 55.Ornish D, Lin J, Daubenmier J, et al. Increased telomerase activity and comprehensive lifestyle changes: a pilot study. Lancet Oncol. 2008;9(11):1048–1057. doi: 10.1016/S1470-2045(08)70234-1. [DOI] [PubMed] [Google Scholar]
- 56.Hunt SC, Chen W, Gardner JP, et al. Leukocyte telomeres are longer in African Americans than in whites: the National Heart, Lung, and Blood Institute Family Heart Study and the Bogalusa Heart Study. Aging Cell. 2008;7(4):451–458. doi: 10.1111/j.1474-9726.2008.00397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Roux AV, Ranjit N, Jenny NS, et al. Race/ethnicity and telomere length in the Multi-Ethnic Study of Atherosclerosis. Aging Cell. 2009;8(3):251–257. doi: 10.1111/j.1474-9726.2009.00470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]