Abstract
To estimate the association of antiretroviral therapy initiation with incident acquired immunodeficiency syndrome (AIDS) or death while accounting for time-varying confounding in a cost-efficient manner, the authors combined a case-cohort study design with inverse probability-weighted estimation of a marginal structural Cox proportional hazards model. A total of 950 adults who were positive for human immunodeficiency virus type 1 were followed in 2 US cohort studies between 1995 and 2007. In the full cohort, 211 AIDS cases or deaths occurred during 4,456 person-years. In an illustrative 20% random subcohort of 190 participants, 41 AIDS cases or deaths occurred during 861 person-years. Accounting for measured confounders and determinants of dropout by inverse probability weighting, the full cohort hazard ratio was 0.41 (95% confidence interval: 0.26, 0.65) and the case-cohort hazard ratio was 0.47 (95% confidence interval: 0.26, 0.83). Standard multivariable-adjusted hazard ratios were closer to the null, regardless of study design. The precision lost with the case-cohort design was modest given the cost savings. Results from Monte Carlo simulations demonstrated that the proposed approach yields approximately unbiased estimates of the hazard ratio with appropriate confidence interval coverage. Marginal structural model analysis of case-cohort study designs provides a cost-efficient design coupled with an accurate analytic method for research settings in which there is time-varying confounding.
Keywords: acquired immunodeficiency syndrome, case-cohort studies, cohort studies, confounding bias, HIV, pharmacoepidemiology, selection bias
Marginal structural models (1) are used increasingly to estimate the total, joint (2), or direct/indirect (3) associations of exposures with an outcome of interest. Using inverse probability weighting, such structural models can be employed to consistently estimate exposure associations when 1 or more time-varying confounders are affected by a prior time-varying exposure. Marginal structural Cox proportional hazards regression models (4) are commonly used when the outcome is time-to-event (5–8).
The case-cohort design is an efficient approach for estimating the hazard ratio accurately when obtaining exposure or covariate information is costly (9–12). In this design, exposure or covariate information is collected only for a random subcohort, as well as for all participants who have experienced the event of interest (and henceforth are cases). Cost savings are obtained by not collecting exposure and covariate information on noncases outside the subcohort. A case-cohort analysis typically proceeds with a standard Cox regression model for the subcohort, augmented with the cases outside the subcohort to improve precision (13, 14). Prior methodological work suggests that case-cohort designs may be analyzed using methods for causal inference based on potential outcomes (15, 16). However, to date, there has not been an example analysis of a case-cohort design using marginal structural models. Here we illustrate this approach with an analysis of the association between highly active antiretroviral therapy (HAART) initiation and incident acquired immunodeficiency syndrome (AIDS) or death (7).
MATERIALS AND METHODS
Study cohort
This analysis used data from the Multicenter AIDS Cohort Study (17) and the Women’s Interagency HIV Study (18). Beginning in 1984, the Multicenter AIDS Cohort Study enrolled 6,972 homosexual and bisexual men in Baltimore, Maryland; Chicago, Illinois; Pittsburgh, Pennsylvania; and Los Angeles, California. Beginning in 1994, the Women’s Interagency HIV Study enrolled 3,772 women in New York, Chicago, Los Angeles, San Francisco, and Washington, DC. Approximately every 6 months, participants in both studies underwent a physical examination, completed an extensive interviewer-administered questionnaire that obtained information on use of antiretroviral therapy, and provided a blood sample. Positive enzyme-linked immunosorbent assays with confirmatory Western blots were used to determine seropositivity for human immunodeficiency virus type 1 (HIV-1). Institutional review boards approved all protocols and informed consent forms, which were completed by participants in both cohorts.
The cost of these semiannual study visits varied by cohort, by site within each cohort, and over time, but the cost in 2007 was approximately $1,560 per participant per visit. Approximately $600 of the $1,560 was required for determination of covariate information based on biomarkers (e.g., CD4 cell count, HIV-1 RNA viral load), and this $600 may be saved for visits where covariate information is not required. However, the balance of the $1,560 is required in order to conduct the visit and obtain and store biologic specimens, regardless of whether the covariate information is needed presently. These costs will be used to quantify savings for the case-cohort design.
Analyses presented here included the 950 men and women who were alive, HIV-1-seropositive, and not using antiretroviral therapies in April 1995, just before effective therapy became available (the first highly active regimen was approved by the US Food and Drug Administration on December 6, 1995). Each participant contributed a maximum of 24 study visits, beginning with the first semiannual visit after April 1995 (the baseline visit). For the present report, participants were followed until: 1) incident AIDS or death, 2) initiation of non-HAART treatment, 3) dropout (i.e., 2 consecutive missed visits), or 4) study completion in September 2007. For participants who were missing baseline data on any time-varying covariate but remained eligible, baseline was redefined as the first visit with complete data. This approach is analogous to the inclusion of late entries in survival analysis (19) and assumes that such late entries are noninformative (20).
An illustrative subcohort was selected as a 20% simple random sample without replacement from the 950 participants. Among the 190 participants chosen for the subcohort, 41 (22%) were cases. The case cohort was defined as the subcohort of 190 participants plus the 170 cases (211 − 41 = 170) outside the subcohort.
Measurements
The endpoint of interest was first diagnosis of clinical AIDS or death from any cause, as recently described (21). The exposure of interest was initiation of HAART, versus no antiretroviral therapy. Data on a number of fixed and time-varying covariates were recorded.
Levels of T-lymphocyte subsets were determined by immunofluorescence using flow cytometry in laboratories participating in the National Institute of Allergy and Infectious Diseases Quality Assurance Program. T-lymphocyte subsets were measured in purified peripheral blood mononuclear cells or ethylenediaminetetraacetic acid-anticoagulated whole blood by staining with fluorescent dye-conjugated monoclonal antibodies that were specific for CD4 lymphocytes (Becton Dickinson, Mountain View, California) (22).
Plasma HIV-1 RNA viral load levels were measured in laboratories participating in the proficiency testing program of the National Institute of Allergy and Infectious Diseases Virology Quality Assurance Laboratory. To harmonize viral load detection limits, which varied from 50 copies/mL to 400 copies/mL across cohorts and over time, we set values below 400 copies/mL to a value of 200 copies/mL. Missing time-varying covariate information after the baseline visit (9%) was carried forward from the most recent prior observed value.
Statistical methods
In this article, capital letters represent variables and lowercase letters represent possible values of the variables or constants. Let T be the time from study entry to AIDS or death. Due to study completion, dropout, or inadequate therapy initiation, T may be right-censored. Let Ts be the potential time from study entry to AIDS or death under the regimen of HAART initiation at time s. Letting denote the hazard at time t for the random variable Ts, a marginal structural Cox proportional hazards model is , where h0(t) is the hazard for participants not exposed by time t (i.e., all Ts where s > t), and x(t) = 1 if t > s and x(t) = 0 otherwise. For this model, β is the log hazard ratio comparing the regimens previously exposed to treatment with those not exposed to treatment by time t. The specification of exposure to HAART is a simple initiation indicator and does not depend on time since initiation or cumulative exposure, which is in keeping with most (7, 8, 23) but not all (21) prior reports. We also consider structural models for cumulative average exposure to HAART.
Cohort and subcohort estimation.
In Appendix 1, we describe standard methods for drawing inference assuming the above model using data from the full cohort. Methods for the analysis of the subcohort follow directly from those for the full cohort. The only distinction is that the subcohort analysis is conducted using only the data from the 190 (out of 950) participants chosen at random for the subcohort. This restriction to the 190 subcohort participants also applies for calculation of the inverse probability weights described in Appendix 1.
Case cohort.
For case-cohort designs, the Cox partial likelihood must be adjusted to account for sampling of covariate information from only a subset of participants. Here we extend Prentice’s approach to estimation of the hazard ratio from a case-cohort study design (12). Assuming no tied failure times, for the case-cohort design the contribution to the weighted partial likelihood corresponding to participant i failing at time ti is
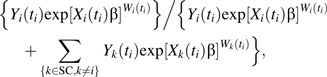 |
where Y, X, and W are as defined in Appendix 1 and SC denotes the 190 participants in the subcohort. The distinction between the contributions to the weighted partial likelihood for the full cohort and those shown here is that the sum in the denominator of the weighted partial likelihood is taken over the members of the subcohort and the case, rather than the full cohort membership.
For the case-cohort design, the inverse probability weights are estimated using the subcohort set SC as well as cases outside the subcohort. Contributions to the models for the inverse probability weights were themselves weighted inversely to the sampling probability for the case-cohort design. Specifically, cases (inside or outside the subcohort) were given a weight of 1, while noncases inside the subcohort were given a weight of 5 (1/0.2 = 5). Noncases outside the subcohort were not used to estimate the inverse probability weights.
Unadjusted and adjusted hazard ratio estimates were obtained for comparison from standard unweighted Cox models without and with adjustment for the same set of variables as used in the marginal structural model. A Monte Carlo simulation experiment is presented in Appendix 2. The simulation examines some finite-sample properties of the proposed method. All analyses were conducted with SAS, version 9.2 (SAS Institute, Inc., Cary, North Carolina), using robust variance estimates (24) to calculate 95% confidence intervals and P values for all case-cohort designs and marginal structural models (see Appendix in Cole et al. (25)).
RESULTS
At study entry, the 950 cohort participants had a mean age of 39 years (standard deviation (SD), 8), a CD4 cell count of 498 cells/mm3 (SD, 279), and a viral load of 4.5 log10 copies/mL (SD, 0.7) for the 74% participants with detectable values; 61% were women, and 59% were African-American. The 190 subcohort participants had similar characteristics at study entry (Table 1).
Table 1.
Characteristics of the Cohort (Men and Women Infected With HIV Type 1) and the 20% Subcohort at Baseline and During Follow-up, Multicenter AIDS Cohort Study and Women’s Interagency HIV Study, 1996–2007
| Characteristic | Cohort (n = 950) |
Subcohorta (n = 190) |
||||
| % | Mean (SD) | No. | % | Mean (SD) | No. | |
| At baseline in 1996 | ||||||
| Mean age, years | 39 (8) | 38 (8) | ||||
| Female sex | 61 | 578 | 57 | 108 | ||
| African-American race | 59 | 560 | 54 | 102 | ||
| Mean CD4 cell count, no. of cells/mm3 | 498 (279) | 489 (283) | ||||
| Mean log10 HIV-1 RNA level, no. of copies/mL | 4.5 (0.7)b | 4.5 (0.7)c | ||||
| Over follow-up between 1996 and 2007 | ||||||
| No. of person-years | 4,456 | 861 | ||||
| No. of exposed person-years | 2,974 | 590 | ||||
| No. with incident AIDS or death | 211 | 41 | ||||
Abbreviations: AIDS, acquired immunodeficiency syndrome; HIV, human immunodeficiency virus; SD, standard deviation.
The subcohort comprised 20% of 950 cohort participants chosen at random.
Among 705 detectable measurements.
Among 145 detectable measurements.
Over the course of follow-up, the 950 cohort participants contributed 4,456 person-years, of which 2,974 person-years were accrued after antiretroviral therapy initiation. During follow-up, 180 cases of incident AIDS and 31 AIDS-free deaths were observed, 259 participants were censored at inadequate therapy initiation, 173 participants were lost to follow-up, and 307 participants completed the study event-free.
The 190 subcohort participants contributed 861 person-years, of which 590 person-years were accrued after antiretroviral therapy initiation. During follow-up, 34 cases of incident AIDS and 7 AIDS-free deaths were observed, 94 participants were censored at inadequate therapy initiation or lost to follow-up, and 55 participants completed the study event-free (Table 1).
The inverse probability weights for the cohort had a mean of 0.95 (SD, 0.65) with a range of 0.14–9.60 and first and 99th percentiles of 0.22 and 4.12, respectively. The inverse probability weights for the subcohort had a mean of 0.98 (SD, 0.66), with a range of 0.09–4.91 and first and 99th percentiles of 0.15 and 3.77, respectively. The inverse probability weights for the case cohort had a mean of 0.98 (SD, 0.65), with a range of 0.10–7.29 and first and 99th percentiles of 0.16 and 3.26, respectively.
Consistent with prior research (7), in the full cohort the unadjusted hazard ratio for AIDS or death was 1.02 (95% confidence interval (CI): 0.72, 1.43), and the adjusted hazard ratio was 0.96 (95% CI: 0.68, 1.36). The inverse probability-weighted hazard ratio for AIDS or death was 0.41 (95% CI: 0.26, 0.65) (Table 2). For the inverse probability-weighted hazard ratio, the ratio of the upper confidence limit to the lower confidence limit, a measure of precision, was 2.5. As expected (26), when we truncated the inverse probability weights at the first and 99th percentiles, the hazard ratio was somewhat muted yet slightly more precise (hazard ratio = 0.48, 95% CI: 0.31, 0.74); untruncated inverse probability weights were used henceforth. The treatment initiation association appeared to increase over follow-up time, with a hazard ratio of 0.61 (95% CI: 0.22, 1.66) before 2 years and a hazard ratio of 0.38 (95% CI: 0.23, 0.61) after 2 years from study entry (P for homogeneity = 0.39). Two years was chosen as the point at which approximately half of the events had occurred; henceforth, this possible departure from proportional hazards was disregarded.
Table 2.
Cohort, 20% Subcohort, and Case-Cohort Analyses of the Association Between Highly Active Antiretroviral Therapy and Incident AIDS or Death Among 950 Men and Women Infected With HIV Type 1, Multicenter AIDS Cohort Study and Women’s Interagency HIV Study, 1996–2007
| Analysis and Model | Hazard Ratio | 95% Confidence Interval | Standard Errora |
| Cohort | |||
| Unadjusted | 1.02 | 0.72, 1.43 | 0.176 |
| Adjustedb | 0.96 | 0.68, 1.36 | 0.178 |
| Weightedb | 0.41 | 0.26, 0.65 | 0.231 |
| Subcohort | |||
| Unadjusted | 1.27 | 0.57, 2.86 | 0.412 |
| Adjusted | 1.37 | 0.60, 3.17 | 0.426 |
| Weighted | 0.53 | 0.23, 1.24 | 0.436 |
| Case-cohort | |||
| Unadjusted | 0.97 | 0.61, 1.54 | 0.238 |
| Adjusted | 1.09 | 0.69, 1.73 | 0.235 |
| Weighted | 0.47 | 0.26, 0.83 | 0.293 |
Abbreviations: AIDS, acquired immunodeficiency syndrome; HIV, human immunodeficiency virus.
Standard error for log hazard ratio; robust standard error for case-cohort and weighted models.
Adjusted and weighted models both controlled for time-varying prior CD4 cell count and HIV-1 RNA level, specified as restricted cubic splines.
As expected, results for the subcohort were less precise than those for the full cohort. The unadjusted hazard ratio for AIDS or death was 1.27 (95% CI: 0.57, 2.86), and the adjusted hazard ratio was 1.37 (95% CI: 0.60, 3.17). The inverse probability-weighted hazard ratio for AIDS or death was 0.53 (95% CI: 0.23, 1.24) (Table 2). For the inverse probability-weighted hazard ratio, the ratio of upper to lower confidence limits was 5.39, or 2.16 times that of the full cohort precision.
Finally, results for the case-cohort design recovered much of the precision lost in the subcohort design. The unadjusted hazard ratio for AIDS or death was 0.97 (95% CI: 0.61, 1.54), and the adjusted hazard ratio was 1.09 (95% CI: 0.69, 1.73). The inverse probability-weighted hazard ratio for AIDS or death was 0.47 (95% CI: 0.26, 0.83) (Table 2). For the inverse probability-weighted hazard ratio, the ratio of upper to lower confidence limits was 3.19, or 1.28 times that of the full cohort precision.
Table 3 summarizes dollar and precision costs relative to the full cohort. While the costs for the full cohort design would be about $14.5 million, the costs for the subcohort and case-cohort designs would be about $10 million and $10.5 million, respectively. For the subcohort design, the dollar savings are more than offset by the decreased precision, as seen by the proportional precision costs’ being larger than unity. However, for the case-cohort design, the dollar savings are offset by the decreased precision, such that the proportional precision costs remain below unity and therefore in favor of the case-cohort study design under a fixed budget.
Table 3.
Costs and Precision Costs for Cohort, 20% Subcohort, and Case-Cohort Marginal Structural Analyses of Highly Active Antiretroviral Therapy and Incident AIDS or Death Among 950 Men and Women Infected With HIV Type 1, Multicenter AIDS Cohort Study and Women’s Interagency HIV Study, 1996–2007
| Analysis and Visit Type | No. of Visits | Cost, dollarsa | Proportional Costs | Precision Costsb | Proportional Precision Costs |
| Cohort | |||||
| Full | 9,172 | 14,308,320 | |||
| Partial | 0 | 0 | |||
| Total | 14,308,320 | 1c | 3.31 | 1c | |
| Subcohort | |||||
| Full | 1,766 | 2,754,960 | |||
| Partial | 7,406 | 7,109,760 | |||
| Total | 9,864,720 | 0.69 | 4.30 | 1.30 | |
| Case-cohort | |||||
| Full | 2,891 | 4,509,960 | |||
| Partial | 6,281 | 6,029,760 | |||
| Total | 10,539,720 | 0.74 | 3.09 | 0.93 |
Abbreviations: AIDS, acquired immunodeficiency syndrome; HIV, human immunodeficiency virus.
Costs of a full study visit were approximated at $1,560 and costs of a partial study visit at $960 (excluding biomarker determinations).
Precision costs were defined as costs (in millions of dollars) × standard error.
Proportional costs and precision costs were taken with respect to the full cohort.
Similar results were obtained when modeling the cumulative average exposure. For instance, using the full cohort, the unadjusted, adjusted, and marginal structural model estimates of the hazard ratio for cumulative average exposure to HAART were 0.73 (95% CI: 0.44, 1.21), 0.84 (95% CI: 0.50, 1.42), and 0.24 (95% CI: 0.12, 0.47), respectively. In the case-cohort marginal structural model analysis, the hazard ratio estimate was 0.30 (95% CI: 0.12, 0.73).
Monte Carlo simulation results are provided in Appendix 2. Briefly, the simulations demonstrated that the proposed case-cohort approach yields approximately unbiased estimates with appropriate confidence interval coverage and recovers a large portion of the precision present in the full cohort design (Appendix Table 1).
DISCUSSION
Using a combination of a case-cohort study design and a marginal structural Cox model analysis, we estimated that relative to no antiretroviral therapy, initiation of HAART decreased the hazard of AIDS or death by 53%. A similar result was found using the combination of a full cohort design and a marginal structural Cox model analysis, but a dramatically attenuated result was found using standard analytic methods for both the case-cohort and full cohort designs. The combination of a case-cohort design with marginal structural model analysis was found to be favorable in terms of a combination of monetary cost and precision for the present example. In settings where there is a larger cost savings associated with not collecting exposure or covariate information from persons outside the subcohort or where the outcome is less frequent, the case-cohort study design will be even more advantageous.
While we are unaware of any prior work on marginal structural models for case-cohort designs, Joffe and Rosenbaum (15) and Mansson et al. (16) describe case-cohort studies using propensity scores, which have features in common with the present work but are restricted to time-fixed exposures.
There are several competing approaches to estimating the hazard ratio from a case-cohort design. Early work by Kupper et al. (10) and Miettinen (11) dealt with estimation of the incidence density ratio under the dual assumptions of the hazards’ being proportional across exposure groups and constant over time, both assumptions being within levels of measured variables. Modern approaches, since the work of Prentice (12), allow the hazards for exposure groups to be nonconstant over time but continue to assume proportionality across exposure groups. Borgan and Samuelsen (27) reported Monte Carlo simulations showing that Prentice’s approach (12) and Barlow’s approach (28), the latter using inverse probability of sampling weights, provide similar inferences when the subcohort is of reasonable size relative to the full cohort (i.e., ≥15% of the full cohort), as is the case in our setting. Further details on differences between these approaches are discussed by Barlow et al. (13), Borgan and Samuelsen (27), Langholz and Jiao (29), and Kulathinal et al. (30).
The present results should be interpreted with consideration of the following limitations. First, like all observational analyses, the estimates have a causal interpretation only under the assumption of no unmeasured confounding. This assumption probably holds approximately here, because the most important clinical and laboratory information used by physicians as indications for therapy initiation was collected and used in the models for the estimation of the weights (31). As described previously (7, 26), numerous additional functional forms for the weight models were explored (e.g., longer covariate histories, more flexible splines), as well as a broader set of covariates (e.g., age, race, body mass index, HIV-related symptoms, use of Pneumocystis jiroveci pneumonia prophylaxis, and red blood, platelet, CD3, and CD8 cell counts), but such alternative model specifications did not appreciably alter the results. If the assumption of no unmeasured confounders is correct and the model used to create the treatment weights is correctly specified, then weighting creates a pseudopopulation in which the probability of therapy initiation is not a function of the time-varying covariates (i.e., no confounding exists), but the association of therapy initiation with AIDS or death is the same as in the actual study population.
Second, and as in all prospective analyses with right-censoring, the results are based on the assumption that right-censoring is ignorable, conditional on measured covariates. Neither the present analyses nor past analyses (5, 7, 25, 32) suggested that there was notable selection bias due to measured variables in these data.
Third, the results rely on the assumptions that therapy initiation and time to AIDS or death are measured without error. There are 3 pertinent subpoints. Firstly, we did not account for therapy cessation in these analyses. The inverse probability-weighted analysis was therefore estimating an “intention-to-treat effect” (32) of therapy initiation versus no antiretroviral therapy in a hypothetical randomized clinical trial in which 1) participants were randomly assigned to begin continuous therapy at different visits, 2) all participants initially complied and began therapy at their assigned visit, and 3) a portion later discontinued therapy. Secondly, the timing of therapy initiation may have been misreported. Cole et al. (21) have described methods with which to account for such measurement error. Thirdly, these results may have been sensitive to the relative infrequency of data collection (i.e., 6-month intervals). Misclassification due to this coarse measurement (with respect to time) could have reintroduced some confounding, which could have biased the estimated hazard ratios in either direction (26). Indeed, therapy is initiated during the interval between study visits by physicians who have access to information beyond that collected for the study. Therefore, a sensitivity analysis to deviations from the no-unmeasured-confounding assumption is appropriate but was beyond the scope of this article.
Fourth, the inferences rely on correct model specification. There are 2 pertinent subpoints. Firstly, we assume that the most recent prior observed value is a reasonable substitute for the 9% missing time-varying covariate information that was carried forward. Given the relatively small amount of missing time-varying covariate data, one would expect results to be robust to violations of this assumption. Secondly, inferences rely on correct specification of the final structural model relating HAART exposure to incident AIDS or death. We fitted a simple initiation indicator, as well as cumulative average exposure, and found similar results.
Fifth, our approach to implementing a marginal structural model for the case-cohort design is not fully efficient. One way to improve the efficiency would be to use information from the full cohort that is currently ignored. Recently, Breslow et al. (33, 34) discussed stratified case-cohort studies in the context of 2-stage study designs (35, 36). They demonstrated that use of the full cohort in the analysis of case-cohort studies may improve precision for estimation of covariate associations in the full cohort, as well as product terms between these covariates and the exposure of interest. In our setting, a modification of the association of therapy with incident AIDS or death by baseline CD4 cell count has been reported (7). This may be a topic of future work.
Finally, we assume both consistency (37, 38) and positivity (37, 39), which would be granted by design in a randomized trial but are subject to possible violations in observational studies. For causal contrasts to be interpretable, either there must be a single route to the exposure or the multiple routes must yield similar effects. This consistency assumption is likely to hold approximately in the present setting, where the exposure is a treatment (40, 41). Valid use of inverse probability weights requires that there not be a probability of 0 or 1 that participants are exposed at any level of the confounders (26). This positivity assumption was met in theory in our study, as some participants with high CD4 cell counts and low viral loads initiated therapy while others with low CD4 cell counts and high viral loads did not, and this assumption was not violated in practice, as previously discussed (26).
In conclusion, the association of HAART initiation with incident AIDS or death using a marginal structural model analysis of a full cohort design was approximated well using an analogous analysis of a case-cohort design. The combination of the cost-efficient case-cohort design with accurate analytic methods based on potential outcomes provides the epidemiologist with a useful tool for etiologic inference.
Acknowledgments
Author affiliations: Department of Epidemiology, Gillings School of Global Public Health, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina (Stephen R. Cole); Department of Biostatistics, Gillings School of Global Public Health, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina (Michael G. Hudgens); Department of Medicine, School of Medicine, University of California, San Francisco, San Francisco, California (Phyllis C. Tien); San Francisco Veterans Affairs Medical Center, San Francisco, California (Phyllis C. Tien); Montefoire Medical Center, Bronx, New York (Kathryn Anastos); Albert Einstein College of Medicine, Bronx, New York (Kathryn Anastos); Department of Infectious Diseases and Microbiology, Graduate School of Public Health, University of Pittsburgh, Pittsburgh, Pennsylvania (Lawrence Kingsley); Department of Preventive Medicine, Feinberg School of Medicine, Northwestern University, Chicago, Illinois (Joan S. Chmiel); and Department of Epidemiology, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, Maryland (Lisa P. Jacobson).
Dr. Stephen Cole was partially supported by National Institutes of Health grant R01-AA-017594. Dr. Michael Hudgens was partially supported by National Institutes of Health grant R01-AI-085073. The Multicenter AIDS Cohort Study is funded by the National Institute of Allergy and Infectious Diseases, with additional supplemental funding from the National Cancer Institute (grants UO1-AI-35042, UL1-RR025005 (General Clinical Research Center), UO1-AI-35043, UO1-AI-37984, UO1-AI-35039, UO1-AI-35040, UO1-AI-37613, and UO1-AI-35041). The Women’s Interagency HIV Study is funded by the National Institute of Allergy and Infectious Diseases (grants UO1-AI-35004, UO1-AI-31834, UO1-AI-34994, UO1-AI-34989, UO1-AI-34993, and UO1-AI-42590) and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (grant UO1-HD-32632). The study is cofunded by the National Cancer Institute, the National Institute on Drug Abuse, and the National Institute on Deafness and Other Communication Disorders. Funding is also provided by the National Center for Research Resources (UCSF-CTSI grant UL1 RR024131).
The authors thank Hana Lee and Drs. James M. Robins and Miguel A. Hernán for expert advice.
The Multicenter AIDS Cohort Study (http://www.statepi.jhsph.edu/macs/macs.html) includes the following study centers: Baltimore, Maryland—Bloomberg School of Public Health, Johns Hopkins University: Joseph B. Margolick (Principal Investigator (PI)), Haroutune Armenian, Barbara Crain, Adrian Dobs, Homayoon Farzadegan, Joel Gallant, John Hylton, Lisette Johnson, Shenghan Lai, Ned Sacktor, Ola Selnes, James Shepard, Chloe Thio; Chicago, Illinois—Howard Brown Health Center, Feinberg School of Medicine, Northwestern University, and Cook County Bureau of Health Services: John P. Phair (PI), Joan S. Chmiel (Co-PI), Sheila Badri, Bruce Cohen, Craig Conover, Maurice O’Gorman, David Ostrow, Frank Palella, Daina Variakojis, Steven M. Wolinsky; Los Angeles, California—School of Public Health and School of Medicine, University of California, Los Angeles: Roger Detels (PI), Barbara R. Visscher (Co-PI), Aaron Aronow, Robert Bolan, Elizabeth Breen, Anthony Butch, Thomas Coates, Rita Effros, John Fahey, Beth Jamieson, Otoniel Martínez-Maza, Eric N. Miller, John Oishi, Paul Satz, Harry Vinters, Dorothy Wiley, Mallory Witt, Otto Yang, Stephen Young, Zuo Feng Zhang; Pittsburgh, Pennsylvania—Graduate School of Public Health, University of Pittsburgh: Charles R. Rinaldo (PI), Lawrence Kingsley (Co-PI), James T. Becker, Robert W. Evans, John Mellors, Sharon Riddler, Anthony Silvestre; Data Coordinating Center—Bloomberg School of Public Health, Johns Hopkins University: Lisa P. Jacobson (PI), Alvaro Muñoz (Co-PI), Keri Althoff, Christopher Cox, Gypsyamber D’Souza, Stephen J. Gange, Elizabeth Golub, Janet Schollenberger, Eric C. Seaberg, Sol Su; National Institutes of Health—National Institute of Allergy and Infectious Diseases: Robin E. Huebner; National Cancer Institute: Geraldina Dominguez; National Heart, Lung, and Blood Institute: Cheryl McDonald.
Data from the Women’s Interagency HIV Study (WIHS) were collected by the WIHS Collaborative Study Group at the following centers: New York City/Bronx Consortium (PI, Kathryn Anastos); Brooklyn, New York (PI, Howard Minkoff); Washington, DC, Metropolitan Consortium (PI, Mary Young); Connie Wofsy Study Consortium of Northern California (PI, Ruth Greenblatt); Los Angeles County/Southern California Consortium (PI, Alexandra Levine); Chicago Consortium (PI, Mardge Cohen); and Data Coordinating Center (PI, Stephen Gange).
The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Conflict of interest: none declared.
Glossary
Abbreviations
- AIDS
acquired immunodeficiency syndrome
- CI
confidence interval
- HAART
highly active antiretroviral therapy
- HIV-1
human immunodeficiency virus type 1
- SD
standard deviation
APPENDIX 1.
Standard Analysis of a Marginal Structural Cox Model
The hazard ratio, exp(β), may be estimated consistently in the full cohort under the dual assumptions of no unmeasured confounders and no unmeasured informative censoring (42) by maximizing a weighted partial likelihood as described below. Let S be the time from study entry to highly active antiretroviral therapy (HAART) initiation for persons who initiate HAART before T, and S = T otherwise. Note that S is interval-censored because therapy initiation is known only to occur between semiannual study visits: We take the right side of the interval as S.
Assuming no tied failure times, for the full cohort design the contribution to the weighted partial likelihood corresponding to participant i failing at time ti is
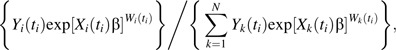 |
where Yi(t) is an indicator of participant i’s being in the risk set at time t, Xi(t) = 1 if t > Si and Xi(t) = 0 otherwise, and the inverse probability weights are , defined below.
Let J denote the number of follow-up study visits for an individual, let vj equal the time from baseline to follow-up visit j = 1 to J, and let v0 = 0. In the case where acquired immunodeficiency syndrome (AIDS) occurs during the interval (vj−1, vj], the time of AIDS replaces the visit time vj. In the case where death occurs after visit J, the time of death replaces the visit time vJ. Let X(vj) = 1 indicate exposure initiation in the interval (vj−1, vj]; by design, X(v0) = 0. Let L(vj) be the vector of covariates measured at visit j, with baseline covariates denoted as L(v0). Let C(vj) = 1 indicate that the participant was censored because of dropout or initiation of non-HAART therapy during the interval (vj−1, vj]; by design, C(v0) = 0. Overbars are used to denote histories, such that ; and are defined analogously. The inverse probability weights are defined as
and
where P[ | ] is the conditional probability function. The denominator for each term in WX(t) is the probability that a participant received her own observed treatment at time vj given her past exposure and covariate history and that she remained uncensored.
The estimate of β was obtained in 2 steps: First, the inverse probability weights were estimated for each individual at each failure time, and then the weighted partial likelihood was maximized conditional on the estimated weights. We estimated the components of the inverse probability weights using pooled logistic regression models (43), as previously described (25). In the pooled logistic regression model, baseline covariates included age, sex, CD4 cell count, and log10 viral load. Time-varying covariate histories were modeled as restricted cubic splines with 4 knots located at the 5th, 33rd, 67th, and 95th percentiles for CD4 cell count and log10 viral load, both taken at visit j − 1. Using SAS, the time-varying inverse probability weights (and exposure) were accommodated with a counting process style of data input, with entry and exit times given as vj−1 and vj for j = 1 to J. Inverse probability weights were calculated in the same manner for cumulative average HAART, but the marginal structural Cox model replaced X(t) with . This cumulative average could be generalized to allow for unequal visit intervals.
APPENDIX 2.
Monte Carlo Simulation Experiment
Simulation design
We conducted 5,000 simulations each of 1,000 participants for 2 scenarios: β = 0 and β = log(1/2). We first describe a method for simulating data such that the marginal structural Cox model given in the main text holds when there are 2 possible exposure times, at times t = 0 and t = t1 > 0. We assume here that β ≤ 0, such that exposure is not harmful. Let T0, , and denote the potential survival times corresponding to regimens where the first exposure is at t = 0, the first exposure is at t = t1, and there is never exposure, respectively. Note that T∞ has the corresponding hazard and that we do not assume . The steps needed to generate the potential survival times are:
Draw T∞ from an exponential distribution with hazard h0(t) = λ and survival function S0(t) ≡ Pr(T∞ > t) = exp(−λt).
Let T0 = T∞exp(−β).
Let,where and I(.) is the indicator function, equal to 1 when the condition (.) is true and 0 otherwise.
A proof that this method yields potential outcomes satisfying the marginal structural Cox model is available from the authors.
We set λ = 1 and t1 = 0.1. For each participant, we set the baseline covariates to constant values 0, so they may be ignored without consequence. We generated a baseline exposure as Bernoulli with a probability of 1/3. Next, we generated a first time-varying confounder as Bernoulli with a marginal probability of 0.5 dependent on baseline exposure by an odds ratio of 8 and the potential survival time under no exposure (T∞) by an odds ratio of 5 per unit of time. Then, we generated a second time-varying confounder as a standard normal dependent on baseline exposure (with a mean difference of 2 for exposed) and the potential survival time under no exposure (with a mean difference of 0.5 per unit of time). Finally, for the approximately two-thirds of subjects who were unexposed at baseline, we generated a time-varying exposure at t1 = 0.1 as Bernoulli with a marginal probability of 0.5 dependent on the first time-varying confounder by an odds ratio of 0.1 and on the second time-varying confounder by an odds ratio of 0.75 per unit. The observed survival time was assigned as the potential survival time that coincided with the observed exposure history. We set survival times longer than the 25th percentile as administratively censored, such that there were 25% events. For subcohort analysis, we selected a 20% subsample at random without replacement.
Simulation analysis
For each scenario and each of the 5,000 simulations, we conducted 5 analyses with the following models: 1) an unadjusted Cox proportional hazards model using the full cohort; 2) a Cox model using the full cohort, adjusting for the 2 time-varying confounders in the linear predictor; 3) a marginal structural Cox model using the full cohort; 4) a marginal structural Cox model using only data from the subcohort; and 5) a marginal structural Cox model using the cases and the cohort, as described in the main text.
For each of the 5 analyses, we calculated bias, 95% confidence interval coverage, statistical power, and root mean squared error. Bias was computed as the average difference between an estimator and the true log hazard ratio. Confidence interval coverage was computed as the proportion of times the confidence interval contained the true hazard ratio. Statistical power was computed as the proportion of simulations in which the 95% confidence interval excluded the null value of unity. The empirical variance was defined as the square of the empirical standard error (i.e., the standard deviation of the 5,000 estimates). The root mean squared error was calculated by taking the square root of the sum of the square of the bias and the empirical variance for the estimator.
Simulation results
Neither unadjusted Cox models nor adjusted Cox models provided unbiased estimates of the hazard ratio. The mean stabilized inverse probability weights for the cohort and case-cohort designs were 1.00 and 1.02, respectively. For all 3 designs (i.e., cohort, subcohort, and case-cohort), the marginal structural Cox model provided an approximately unbiased estimate of the hazard ratio, approximately valid type 1 error when testing the null hypothesis of a hazard ratio equal to 1, and appropriate 95% confidence interval coverage (Appendix Table 1).
The empirical standard errors differed notably across the designs. In scenario 2 (alternative hypothesis), the empirical standard error for the inverse probability-weighted cohort was 0.144, while for the 20% random subcohort, precision was lost, such that the empirical standard error was 0.332. This difference accords with theory in that, with sample sizes of 1,000 and 200, the ratio of the standard errors should be approximately 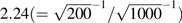 and was observed to be 2.31 (0.332/0.144 = 2.31). In the case-cohort design, the empirical standard error was improved over the subcohort design to 0.225.
and was observed to be 2.31 (0.332/0.144 = 2.31). In the case-cohort design, the empirical standard error was improved over the subcohort design to 0.225.
From another perspective, also in the alternative scenario, the statistical power for the inverse probability-weighted results across the 3 designs is notable, because this difference illustrates the advantage of the case-cohort design over the subcohort design. The cohort design had approximately 99% statistical power, while the subcohort design had only 52% statistical power. The case-cohort design had statistical power of approximately 90% and therefore recovered much of the information available in the full cohort.
Appendix Table 1.
Results From 5,000 Cohort, Subcohort, and Case-Cohort Marginal Structural Analysesa for Null and Alternative Scenarios
| Cohort Analysis |
Subcohort Analysis (IP-Weighted) | Case-Cohort Analysis (IP-Weighted) | |||
| Unadjusted | Adjusted | IP-Weighted | |||
| Scenario 1: null hypothesis | |||||
| Bias | 0.336 | 0.113 | 0.001 | 0.006 | −0.015 |
| Average standard error | 0.143 | 0.156 | 0.162 | 0.363 | 0.212 |
| Empirical standard errorb | 0.138 | 0.150 | 0.157 | 0.363 | 0.228 |
| 95% CI coverage, % | 33.4 | 90.1 | 95.8 | 94.7 | 93.4 |
| Type 1 error, % | 66.6 | 9.9 | 4.2 | 5.3 | 6.6 |
| Root MSE | 0.363 | 0.188 | 0.157 | 0.363 | 0.228 |
| Relative root MSE | 1 | 2.31 | 1.45 | ||
| Scenario 2: alternative hypothesis | |||||
| Bias | 0.671 | 0.146 | 0.001 | 0.004 | −0.004 |
| Average standard error | 0.139 | 0.156 | 0.156 | 0.351 | 0.217 |
| Empirical standard errorb | 0.131 | 0.138 | 0.144 | 0.332 | 0.225 |
| 95% CI coverage, % | <0.1 | 87.3 | 96.7 | 96.2 | 94.0 |
| Statistical power, % | 4.3 | 95.9 | 99.4 | 51.5 | 89.6 |
| Root MSE | 0.684 | 0.201 | 0.144 | 0.332 | 0.225 |
| Relative root MSE | 1 | 2.31 | 1.56 | ||
Abbreviations: CI, confidence interval; IP, inverse probability; MSE, mean squared error.
1,000 participants per cohort with 25% events; 200 participants per subcohort.
Standard deviation of 5,000 log hazard ratio estimates.
References
- 1.Robins JM, Hernán MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11(5):550–560. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 2.Hernán MA, Brumback B, Robins JM. Marginal structural models to estimate the joint causal effect of non-randomized treatments. J Am Stat Assoc. 2001;96:440–448. [Google Scholar]
- 3.VanderWeele TJ. Marginal structural models for the estimation of direct and indirect effects. Epidemiology. 2009;20(1):18–26. doi: 10.1097/EDE.0b013e31818f69ce. [DOI] [PubMed] [Google Scholar]
- 4.Cox DR. Regression models and life-tables [with discussion] J R Stat Soc (B) 1972;34(2):187–220. [Google Scholar]
- 5.Hernán MA, Brumback B, Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology. 2000;11(5):561–570. doi: 10.1097/00001648-200009000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Cook NR, Cole SR, Hennekens CH. Use of a marginal structural model to determine the effect of aspirin on cardiovascular mortality in the Physicians’ Health Study. Am J Epidemiol. 2002;155(11):1045–1053. doi: 10.1093/aje/155.11.1045. [DOI] [PubMed] [Google Scholar]
- 7.Cole SR, Hernán MA, Robins JM, et al. Effect of highly active antiretroviral therapy on time to acquired immunodeficiency syndrome or death using marginal structural models. Am J Epidemiol. 2003;158(7):687–694. doi: 10.1093/aje/kwg206. [DOI] [PubMed] [Google Scholar]
- 8.Sterne JA, Hernán MA, Ledergerber B, et al. Long-term effectiveness of potent antiretroviral therapy in preventing AIDS and death: a prospective cohort study. Swiss HIV Cohort Study. Lancet. 2005;366(9483):378–384. doi: 10.1016/S0140-6736(05)67022-5. [DOI] [PubMed] [Google Scholar]
- 9.Mantel N. Synthetic retrospective studies and related topics. Biometrics. 1973;29(3):479–486. [PubMed] [Google Scholar]
- 10.Kupper LL, McMichael AJ, Spirtas R. Hybrid epidemiologic study design useful in estimating relative risk. J Am Stat Assoc. 1975;70(351):524–528. [Google Scholar]
- 11.Miettinen O. Estimability and estimation in case-referent studies. Am J Epidemiol. 1976;103(2):226–235. doi: 10.1093/oxfordjournals.aje.a112220. [DOI] [PubMed] [Google Scholar]
- 12.Prentice RL. A case-cohort design for epidemiologic cohort studies and disease prevention trials. Biometrika. 1986;73(1):1–11. [Google Scholar]
- 13.Barlow WE, Ichikawa L, Rosner D, et al. Analysis of case-cohort designs. J Clin Epidemiol. 1999;52(12):1165–1172. doi: 10.1016/s0895-4356(99)00102-x. [DOI] [PubMed] [Google Scholar]
- 14.Therneau TM, Li H. Computing the Cox model for case cohort designs. Lifetime Data Anal. 1999;5(2):99–112. doi: 10.1023/a:1009691327335. [DOI] [PubMed] [Google Scholar]
- 15.Joffe MM, Rosenbaum PR. Invited commentary: propensity scores. Am J Epidemiol. 1999;150(4):327–333. doi: 10.1093/oxfordjournals.aje.a010011. [DOI] [PubMed] [Google Scholar]
- 16.Månsson R, Joffe MM, Sun W, et al. On the estimation and use of propensity scores in case-control and case-cohort studies. Am J Epidemiol. 2007;166(3):332–339. doi: 10.1093/aje/kwm069. [DOI] [PubMed] [Google Scholar]
- 17.Kaslow RA, Ostrow DG, Detels R, et al. The Multicenter AIDS Cohort Study: rationale, organization, and selected characteristics of the participants. Am J Epidemiol. 1987;126(2):310–318. doi: 10.1093/aje/126.2.310. [DOI] [PubMed] [Google Scholar]
- 18.Barkan SE, Melnick SL, Preston-Martin S, et al. The Women’s Interagency HIV Study. WIHS Collaborative Study Group. Epidemiology. 1998;9(2):117–125. [PubMed] [Google Scholar]
- 19.Lamarca R, Alonso J, Gómez G, et al. Left-truncated data with age as time scale: an alternative for survival analysis in the elderly population. J Gerontol A Biol Sci Med Sci. 1998;53(5):M337–M343. doi: 10.1093/gerona/53a.5.m337. [DOI] [PubMed] [Google Scholar]
- 20.Wang MC, Brookmeyer R, Jewell NP. Statistical models for prevalent cohort data. Biometrics. 1993;49(1):1–11. [PubMed] [Google Scholar]
- 21.Cole SR, Jacobson LP, Tien PC, et al. Using marginal structural measurement-error models to estimate the long-term effect of antiretroviral therapy on incident AIDS or death. Am J Epidemiol. 2010;171(1):113–122. doi: 10.1093/aje/kwp329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mellors JW, Muñoz A, Giorgi JV, et al. Plasma viral load and CD4+ lymphocytes as prognostic markers of HIV-1 infection. Ann Intern Med. 1997;126(12):946–954. doi: 10.7326/0003-4819-126-12-199706150-00003. [DOI] [PubMed] [Google Scholar]
- 23.Ray M, Logan R, Sterne JA, et al. The effect of combined antiretroviral therapy on the overall mortality of HIV-infected individuals. HIV-CAUSAL Collaboration. AIDS. 2010;24(1):123–137. doi: 10.1097/QAD.0b013e3283324283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.White HA. A heteroskedasticity-consistent covariance matrix estimator and a direct test of heteroskedasticity. Econometrica. 1980;48(4):817–838. [Google Scholar]
- 25.Cole SR, Hernán MA, Anastos K, et al. Determining the effect of highly active antiretroviral therapy on changes in human immunodeficiency virus type 1 RNA viral load using a marginal structural left-censored mean model. Am J Epidemiol. 2007;166(2):219–227. doi: 10.1093/aje/kwm047. [DOI] [PubMed] [Google Scholar]
- 26.Cole SR, Hernán MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol. 2008;168(6):656–664. doi: 10.1093/aje/kwn164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Borgan O, Samuelsen SO. A review of cohort sampling designs for Cox’s regression model: potentials in epidemiology. Nor Epidemiol. 2003;13(2):239–248. [Google Scholar]
- 28.Barlow WE. Robust variance estimation for the case-cohort design. Biometrics. 1994;50(4):1064–1072. [PubMed] [Google Scholar]
- 29.Langholz B, Jiao J. Computational methods for case-cohort studies. Comput Stat Data Anal. 2007;51(8):3737–3748. [Google Scholar]
- 30.Kulathinal S, Karvanen J, Saarela O, et al. Case-cohort design in practice—experiences from the MORGAM Project. Epidemiol Perspect Innov. 2007;4:15. doi: 10.1186/1742-5573-4-15. (doi:10.1186/1742-5573-4-15) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ahdieh L, Gange SJ, Greenblatt R, et al. Selection by indication of potent antiretroviral therapy use in a large cohort of women infected with human immunodeficiency virus. Am J Epidemiol. 2000;152(10):923–933. doi: 10.1093/aje/152.10.923. [DOI] [PubMed] [Google Scholar]
- 32.Cole SR, Hernán MA, Margolick JB, et al. Marginal structural models for estimating the effect of highly active antiretroviral therapy initiation on CD4 cell count. Am J Epidemiol. 2005;162(5):471–478. doi: 10.1093/aje/kwi216. [DOI] [PubMed] [Google Scholar]
- 33.Breslow NE, Lumley T, Ballantyne CM, et al. Improved Horvitz-Thompson estimation of model parameters from two-phase stratified samples: applications in epidemiology. Stat Biosci. 2009;1(1):32. doi: 10.1007/s12561-009-9001-6. (doi:10.1007/s12561-009-9001-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Breslow NE, Lumley T, Ballantyne CM, et al. Using the whole cohort in the analysis of case-cohort data. Am J Epidemiol. 2009;169(11):1398–1405. doi: 10.1093/aje/kwp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.White JE. A two stage design for the study of the relationship between a rare exposure and a rare disease. Am J Epidemiol. 1982;115(1):119–128. doi: 10.1093/oxfordjournals.aje.a113266. [DOI] [PubMed] [Google Scholar]
- 36.Walker AM. Anamorphic analysis: sampling and estimation for covariate effects when both exposure and disease are known. Biometrics. 1982;38(4):1025–1032. [PubMed] [Google Scholar]
- 37.Hernán MA, Robins JM. Estimating causal effects from epidemiological data. J Epidemiol Community Health. 2006;60(7):578–586. doi: 10.1136/jech.2004.029496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cole SR, Frangakis CE. The consistency statement in causal inference: a definition or an assumption? Epidemiology. 2009;20(1):3–5. doi: 10.1097/EDE.0b013e31818ef366. [DOI] [PubMed] [Google Scholar]
- 39.Westreich D, Cole SR. Invited commentary: positivity in practice. Am J Epidemiol. 2010;171(6):674–677. doi: 10.1093/aje/kwp436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hernán MA. Invited commentary: hypothetical interventions to define causal effects—afterthought or prerequisite? Am J Epidemiol. 2005;162(7):618–620. doi: 10.1093/aje/kwi255. [DOI] [PubMed] [Google Scholar]
- 41.Hernán MA, Taubman SL. Does obesity shorten life? The importance of well-defined interventions to answer causal questions. Int J Obes (Lond) 2008;32(suppl 3):S8–S14. doi: 10.1038/ijo.2008.82. [DOI] [PubMed] [Google Scholar]
- 42.Robins JM. Marginal structural models versus structural nested models as tools for causal inference. In: Halloran ME, Berry DA, editors. Statistical Models in Epidemiology, the Environment, and Clinical Trials. New York, NY: Springer Publishing Company; 2000. pp. 95–133. [Google Scholar]
- 43.Abbott RD. Logistic regression in survival analysis. Am J Epidemiol. 1985;121(3):465–471. doi: 10.1093/oxfordjournals.aje.a114019. [DOI] [PubMed] [Google Scholar]


