Abstract
In animal and clinical trials low-level laser therapy (LLLT) using red, infrared and mixed wavelengths has been shown to delay the development of skeletal muscle fatigue. However, the parameters employed in these studies do not allow a conclusion as to which wavelength range is better in delaying the development of skeletal muscle fatigue. With this perspective in mind, we compared the effects of red and infrared LLLT on skeletal muscle fatigue. A randomized double-blind placebo-controlled crossover trial was performed in ten healthy male volunteers. They were treated with active red LLLT, active infrared LLLT (660 or 830 nm, 50 mW, 17.85 W/cm2, 100 s irradiation per point, 5 J, 1,785 J/cm2 at each point irradiated, total 20 J irradiated per muscle) or an identical placebo LLLT at four points of the biceps brachii muscle for 3 min before exercise (voluntary isometric elbow flexion for 60 s). The mean peak force was significantly greater (p < 0.05) following red (12.14%) and infrared LLLT (14.49%) than following placebo LLLT, and the mean average force was also significantly greater (p < 0.05) following red (13.09%) and infrared LLLT (13.24%) than following placebo LLLT. There were no significant differences in mean average force or mean peak force between red and infrared LLLT. We conclude that both red than infrared LLLT are effective in delaying the development skeletal muscle fatigue and in enhancement of skeletal muscle performance. Further studies are needed to identify the specific mechanisms through which each wavelength acts.
Keywords: Muscle performance, Peak force, Phototherapy
Introduction
In strenuous physical activity, muscles typically show a progressive decline in performance which largely recovers after a period of rest. This reversible phenomenon is usually described as skeletal muscle fatigue. Muscle fatigue has central and peripheral components, and studies of the role of these components in the development of fatigue have been conducted across the years [1]. Several factors including type and intensity of exercise, muscle groups involved and the biochemical environment affect fatigue development [2]. Although skeletal muscle fatigue has been exhaustively investigated, the mechanisms involved in its development are still not fully understood.
The first clinical trial testing low-level laser therapy (LLLT) in musculoskeletal pain, published in 1980, investigated the effects of LLLT on rheumatoid arthritis [3]. Since this first clinical trial was published, several positive effects of LLLT have been identified in pathologies including osteoarthritis [4], tendinopathies [5, 6], wounds [7, 8], back pain [9], neck pain [10–12], peripheral nerve injuries [13] and stroke [14]. Some of the main physiological effects attributed to LLLT are related to soft tissue metabolism. Across different pathologies, increased microcirculation [15], enhanced ATP synthesis and stimulation of the mitochondrial respiratory chain [16] and mitochondrial function [17] have been observed after LLLT. Reductions in the release of reactive oxygen species (ROS) and in creatine phosphokinase activity, and increased production of antioxidants and heat shock proteins have also been found after LLLT [18, 19].
Several animal and human trials have shown that LLLT with red and infrared wavelengths has modulatory effects on inflammatory markers (PGE2, TNF-α, IL-1β, plasminogen activator), reduces the inflammatory process itself (edema, hemorrhage, necrosis, neutrophil cell influx) and modulates leukocyte activity (macrophages, lymphocytes, neutrophils) [5, 20–25].
Skeletal muscle fatigue is a novel area of research in phototherapy. In animal experiments, phototherapy with 655 nm red [26] and 904 nm infrared [27] wavelengths, and clinical trials employing red [28], infrared [29, 30] and mixed [31] wavelengths has been shown to delay the development of skeletal muscle fatigue. However, the parameters of application (such as power output, time of irradiation, doses, etc.) employed in these studies do not allow a conclusion as to whether red or infrared wavelengths produce better results in delaying the development of skeletal muscle fatigue.
With this perspective in mind, we decided to compare the effects of red and infrared LLLT (with the same parameters of application for both wavelengths) on skeletal muscle fatigue.
Materials and methods
The study was designed as a crossover randomized double-blinded placebo-controlled trial. All subjects signed a written declaration of informed consent and their rights were protected. The volunteers were recruited from among untrained healthy male physiotherapy students (n = 10). The protocol for this study was approved by the research ethics committee.
Randomization and blinding procedures
The randomization procedure consisted of the simple drawing of a card (A, B or C), which determined whether active red LLLT, active infrared LLLT or placebo LLLT should be given before the first exercise session. For the second session, participants were crossed over to receive one of the other treatments. For the third session, participants were again crossed over to receive the remaining treatment. The order of treatments is detailed in Table 1.
Table 1.
Order of LLLT treatments
| Card | Session | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| A | Red | Infrared | Placebo |
| B | Infrared | Placebo | Red |
| C | Placebo | Red | Infrared |
The code from the drawing was delivered to a technician who preset the control unit accordingly to either an active LLLT or placebo LLLT mode. The technician was also instructed not to communicate the type of treatment given to the participant, the therapist applying the laser treatment, or the observers. Thus, the treatment allocation was concealed from the participants, the therapist, and the observers. Blinding of the participants and the therapist was further maintained by the use of opaque goggles during the LLLT procedures. The goggles also served to protect the eyes from the LLLT radiation.
Inclusion/exclusion criteria
Healthy untrained male Caucasian physiotherapy students aged between 19 and 27 years were included in the study. Exclusion criteria consisted of any previous musculoskeletal injury to the shoulder, elbow or wrist region, regular strength training (more than once per week) for the previous 2 months, and the use of any kind of nutritional supplement or pharmacological agent. Ten subjects who met the inclusion and exclusion criteria were included in the trial.
Procedures
To provide a standard testing condition for the elbow, we used a Scott exercise bench with an inclination angle of 45°.
Period of evaluation
Care was taken to standardize the exercise protocols and testing sessions. Exercises were performed in a standardized sitting position in three separate sessions 7 days apart. Therefore, each subject performed all exercise sessions on the same day of the week and at the same time of day. Subjects were instructed not to change their daily activities during the 48 hours before the exercise tests.
Fatigue protocol
At the beginning of each exercise session the subjects performed a series of muscle stretching exercises involving all the major muscles of the nondominant arm (two repetitions of 60 s for each muscle group), finishing with the flexor muscles of the nondominant elbow. Then, the subjects were seated on the Scott bench, with their knees and hips flexed at 90°, and the nondominant elbow positioned in flexion of 90°. Using a cable linked to a force transducer (connected to a computer), subjects were instructed to perform an isometric elbow flexion (with the nondominant elbow flexed at 90°) for 60 s (Fig. 1). During execution of the exercise protocol subjects received verbal encouragement provided by one of the observers.
Fig. 1.

Exercise protocol being performed
LLLT procedure
The laser device was calibrated before and after data acquisition, and the equipment showed the same power output in both calibrations. The optical power was measured using a Newport multifunction optical meter model 1835C. The stability of the laser during irradiation was measured by collecting 4% of the light as a partial reflection. During each exercise session (7 days apart) the subjects received a single treatment with active red LLLT, active infrared LLLT or placebo LLLT (all using a the same laser diode device: Thera Lase; DMC, São Carlos, SP, Brazil). The treatment sequence was in accordance with the randomization procedure. Red, infrared or placebo LLLT was administered immediately after the stretching exercises (exactly 3 min before the exercise fatigue test). The biceps muscle belly of the nondominant arm was divided into four parts to provide four irradiation points evenly distributed along the ventral side of the muscle belly so that LLLT radiation was delivered to most of the muscle belly.
LLLT irradiation was performed with the probe in direct contact with the skin applying slight pressure, and held stationary perpendicular to the skin. The dose and other parameters for the LLLT (red, infrared and placebo) were chosen based in previous studies developed by our research group [28–30], and are summarized in Table 2. Immediately after LLLT the subjects were repositioned and started the exercise protocol. The time between LLLT and starting the testing was 180 s.
Table 2.
LLLT parameters
| Parameter | Value |
|---|---|
| Wavelength (nm) | |
| Red | 660 ± 2 |
| Infrared | 830 ± 2 |
| Laser output frequency | Continuous |
| Power output (mW) | 50 |
| Spot diameter (cm) | 0.06 |
| Spot size (cm2) | 0.0028 |
| Power density (W/cm2) | 17.85 |
| Energy per point (J) | 5 |
| Energy density (J/cm2) | 1,785 |
| Treatment time per point (s) | 100 |
| Number of points | 4 |
| Total energy delivered (J) | 20 |
| Application mode | Probe held stationary in skin contact at 90° with slight pressure |
Outcomes and statistical analysis
The outcomes analyzed were the mean peak force and the mean average force achieved during the fatigue protocol in three exercise sessions. Data are expressed as means and their respective standard deviations. ANOVA with the Tukey-Kramer post test was used to determine if there were significant differences in peak force and average force following treatment with the red, infrared and placebo LLLT. All statistical analyses were performed using GraphPad InStat version 3.00 for Windows (GraphPad Software, San Diego CA). The significance level was set at p < 0.05.
Results
Ten untrained healthy male students met the inclusion criteria and were included in study. Their average age was 22.30 ± 2.26 years, their body weight was 75.40 ± 6.54 kg and their height was 176.90 ± 7.19 cm.
Mean peak forces achieved in the exercise test were 23.83 ± 4.51 kgf following red LLLT, 24.33 ± 4.88 kgf following infrared LLLT and 21.25 ± 4.93 kgf following placebo LLLT. The peak force achieved following red LLLT was significantly higher than that achieved following placebo LLLT (p < 0.05), and similarly following infrared LLLT was significantly higher than following placebo LLLT (p < 0.01). However, the peak force achieved following red LLLT was not significantly different from that achieved following infrared LLLT (p > 0.05). The results are summarized in Fig. 2.
Fig. 2.
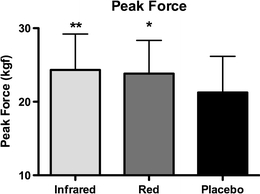
Mean peak forces achieved in the exercise protocol (error bars represents standard deviations). *p < 0.05, **p < 0.01, vs. placebo
Mean average forces achieved in the exercise test were 15.46 ± 1.98 kgf following red LLLT, 15.48 ± 2.84 kgf following infrared LLLT and 13.67 ± 2.05 kgf following placebo LLLT. The mean average force following red LLLT was significantly higher than that following placebo LLLT (p < 0.05), and similarly following infrared LLLT was significantly higher than following placebo LLLT (p < 0.05). As with peak force, there were also no significant differences in mean average force between red and infrared LLLT (p > 0.05). The results are summarized in Fig. 3.
Fig. 3.
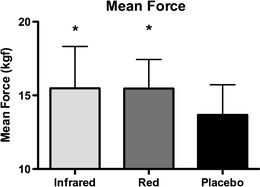
Mean average forces achieved in the exercise protocol (error bars represents standard deviations). *p < 0.05, vs. placebo
Discussion
In this trial we employed an isometric exercise protocol in contrast to the repeated elbow flexions exercise protocol employed in our previous human trials [28–31]. This is the first time that we have compared the effects of LLLT at two wavelengths on skeletal muscle fatigue in humans. Red LLLT (660 nm) significantly increased peak force by 12.14% (p < 0.05) and mean average force by 13.09% (p < 0.05) compared to placebo LLLT. We have previously reported positive effects of red LLLT (655 nm) in delaying the development of skeletal muscle fatigue during an elbow flexion exercise protocol performed by male professional volleyball players [28].
The peak force and the average force were significantly greater (14.49% and 13.24%, respectively) following infrared LLLT (830 nm) than following placebo LLLT (p < 0.01 and p < 0.05, respectively). We have previously also observed positive effects of infrared LLLT employing single [29] and cluster probes [30] with different wavelengths (830 nm and 810 nm, respectively) on skeletal muscle fatigue in male volleyball players. Animal studies performed by our research group have also demonstrated that red LLLT [26] and infrared LLLT [27] can significantly delay the development of skeletal muscle fatigue and increase muscle performance. However, differences in LLLT parameters used in previous studies do not allow an effective comparison between red and infrared LLLT in human and animal trials as mentioned above.
Surprisingly, no differences were observed in peak force and average force between red and infrared LLLT. Laser radiation at infrared wavelengths penetrates better through human skin than red wavelengths [32], and for this reason we expected that the results with infrared LLLT would be better than with red LLLT in this trial. With this perspective, further studies are warranted to investigate the specific mechanisms by which each wavelength acts in delaying skeletal muscle fatigue.
Oxidative stress and production of ROS play important roles in the development of skeletal muscle fatigue. However, the mechanisms through which ROS play a role in the development of fatigue are not fully understood [1]. On the other hand, it is known that oxidative stress leads to an impairment in contractile muscle function resulting in muscle fatigue [33]. To counteract these effects, organisms present antioxidant defenses involving, for example, the action of such enzymes as superoxide dismutase (SOD) and catalase (CAT) which are responsible for the dismutation of the superoxide radical and hydrogen peroxide, respectively [34]. Some studies have sought to determine whether LLLT has effects on oxidative stress and ROS production in skeletal muscles. Avni et al. [18] investigated the effects of infrared LLLT (810 nm) in ischemic–reperfusion injury in the gastrocnemius muscles of rats. They found that LLLT protects skeletal muscles from degeneration following acute ischemic–reperfusion injury. This was evident by a significantly (p < 0.05) higher increase in creatine phosphokinase activity and a lower activity of acid phosphatase in the LLLT-treated muscles. The content of antioxidants and heat shock proteins were also higher in the LLLT-treated muscles than in injured nonirradiated muscles. In another study [19], infrared LLLT (904 nm) was tested in traumatic injury of the gastrocnemius muscle in rats. LLLT reduced the inflammatory response and blocked the effects of ROS release, which led the authors to conclude that LLLT can attenuate some of the negative consequences of inflammation and fibrosis and optimize muscle healing.
Disruption in mitochondrial function is one of mechanisms involved in the development of skeletal muscle fatigue [35]. Recently, Xu et al. [17] induced mitochondrial dysfunction by electrical stimulation in cultured C2C12 cells, and irradiated the cells with infrared LLLT (810 nm) at different doses. Mitochondrial function improved after electrical stimulation in muscle cells with LLLT doses between 0.33 to 8.22 J/cm2, and LLLT doses of 0.33 and 1.338 J/cm2 reversed the dysfunctional state induced by the electrical stimulation. A similar effect could possibly have contributed to the decrease in skeletal muscle fatigue observed in the current study.
An improvement in mitochondrial activity and in ATP synthesis could also be mechanisms by which LLLT affects skeletal muscle fatigue. Silveira et al. [16] evaluated the effects of 5 days treatment with infrared LLLT (904 nm) after traumatic injury of the gastrocnemius muscle in rats. The infrared LLLT significantly increased the activities of complexes I, II, III, and IV and also of succinate dehydrogenase. The authors concluded that treatment with LLLT induced an increase in ATP synthesis, and that this could have accelerated the muscle healing process. In another recent study, for first time an in vivo intact skeletal muscle was irradiated with the aim of testing the effects of phototherapy on cytochrome oxidase [36]. Light emitting diode therapy (LEDT) with a red wavelength (660 nm) was used to irradiate the temporalis muscle in rats. The LEDT significantly increased cytochrome oxidase activity in white, red and intermediate fibers, which indicates enhancement in the metabolic oxidative capacity of different types of muscle fibers. These findings support the use of phototherapy to enhance the aerobic potential of skeletal muscle.
In a recent study Joensen et al. [37] analyzed the thermal effects of 810 nm (200 mW) and 904 nm (60 mW) infrared LLLT at different doses (2, 6, 9 and 12 J) in subjects with different skin colors (light, medium and dark). The infrared LLLT did not significantly change skin temperature (less than 2.0°C) in subjects with light and medium skin colors. In the present study the subjects were Caucasian and we used infrared LLLT with parameters similar to those tested by Joensen et al. [37]. Thus we consider that the performance enhancement observed in our study could not have been due to thermal effects in the irradiated tissue.
The World Anti-Doping Code published in 2009 [38] by World Anti-Doping Agency (WADA) states that a substance or method can be considered as doping if two of the following three criteria are fulfilled: (1) medical or other scientific evidence, pharmacological effect or experience that the substance or method, alone or in combination with other substances or methods, has the potential to enhance or enhances sport performance; (2) medical or other scientific evidence, pharmacological effect or experience that the use of the substance or method represents an actual or potential health risk to the athlete; and (3) WADA's determination that the use of the substance or method violates the spirit of sport described in the introduction to the code. Therefore, as phototherapy does not have side effects and is not a potential health risk, and also does not violate the spirit of sport described in the introduction to the code, we do not think that phototherapy can be considered as doping when used before exercise.
Conclusion
We conclude that both red and infrared LLLT are effective in delaying the development of skeletal muscle fatigue and in enhancing skeletal muscle performance. The optimal parameters of application, as well as dose–response patterns for several wavelengths still need to be identified in further studies. Further studies are also needed to identify the specific mechanisms by which each wavelength acts.
Acknowledgement
The authors would like to thank Fundo de Apoio a Pesquisa (FAP/UNINOVE) for financial support.
Ethical standards
This research was carried out in accordance with current Brazilian laws relating to experiments in humans.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Allen DG, Lamb GD, Westerblad H. Skeletal muscle fatigue: cellular mechanisms. Physiol Rev. 2008;88:287–332. doi: 10.1152/physrev.00015.2007. [DOI] [PubMed] [Google Scholar]
- 2.Weir JP, Beck TW, Cramer JT, Housh TJ. Is fatigue all in your head? A critical review of the central governor model. Br J Sports Med. 2006;40:573–586. doi: 10.1136/bjsm.2005.023028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldman JA, Chiapella J, Casey H, et al. Laser therapy of rheumatoid arthritis. Lasers Surg Med. 1980;1:93–101. doi: 10.1002/lsm.1900010110. [DOI] [PubMed] [Google Scholar]
- 4.Hegedus B, Viharos L, Gervain M, Gálfi M. The effect of low-level laser in knee osteoarthritis: a double-blind, randomized, placebo-controlled trial. Photomed Laser Surg. 2009;27:577–584. doi: 10.1089/pho.2008.2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bjordal JM, Lopes-Martins RA, Iversen VV. A randomised, placebo controlled trial of low level laser therapy for activated achilles tendinitis with microdialysis measurement of peritendinous prostaglandin E2 concentrations. Br J Sports Med. 2006;40:76–80. doi: 10.1136/bjsm.2005.020842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stergioulas A, Stergioula M, Aarskog R, Lopes-Martins RA, Bjordal JM. Effects of low-level laser therapy and eccentric exercises in the treatment of recreational athletes with chronic achilles tendinopathy. Am J Sports Med. 2008;36:881–887. doi: 10.1177/0363546507312165. [DOI] [PubMed] [Google Scholar]
- 7.Ozcelik O, Cenk Haytac M, Kunin A, Seydaoglu G. Improved wound healing by low-level laser irradiation after gingivectomy operations: a controlled clinical pilot study. J Clin Periodontol. 2008;35:250–254. doi: 10.1111/j.1600-051X.2007.01194.x. [DOI] [PubMed] [Google Scholar]
- 8.Schubert MM, Eduardo FP, Guthrie KA, et al. A phase III randomized double-blind placebo-controlled clinical trial to determine the efficacy of low level laser therapy for the prevention of oral mucositis in patients undergoing hematopoietic cell transplantation. Support Care Cancer. 2007;15:1145–1154. doi: 10.1007/s00520-007-0238-7. [DOI] [PubMed] [Google Scholar]
- 9.Basford JR, Sheffield CG, Harmsen WS. Laser therapy: a randomized, controlled trial of the effects of low-intensity Nd:YAG laser irradiation on musculoskeletal back pain. Arch Phys Med Rehabil. 1999;80:647–652. doi: 10.1016/S0003-9993(99)90167-3. [DOI] [PubMed] [Google Scholar]
- 10.Gur A, Sarac AJ, Cevik R, Altindag O, Sarac S. Efficacy of 904 nm gallium arsenide low level laser therapy in the management of chronic myofascial pain in the neck: a double-blind and randomize-controlled trial. Lasers Surg Med. 2004;35:229–235. doi: 10.1002/lsm.20082. [DOI] [PubMed] [Google Scholar]
- 11.Chow RT, Heller GZ, Barnsley L. The effect of 300 mW, 830 nm laser on chronic neck pain: a double-blind, randomized, placebo-controlled study. Pain. 2006;124:201–210. doi: 10.1016/j.pain.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 12.Chow RT, Johnson MI, Lopes-Martins RA, Bjordal JM. Efficacy of low-level laser therapy in the management of neck pain: a systematic review and meta-analysis of randomised placebo or active-treatment controlled trials. Lancet. 2009;374:1897–1908. doi: 10.1016/S0140-6736(09)61522-1. [DOI] [PubMed] [Google Scholar]
- 13.Rochkind S, Leider-Trejo L, Nissan M, Shamir MH, Kharenko O, Alon M. Efficacy of 780-nm laser phototherapy on peripheral nerve regeneration after neurotube reconstruction procedure (double-blind randomized study) Photomed Laser Surg. 2007;25:137–143. doi: 10.1089/pho.2007.2076. [DOI] [PubMed] [Google Scholar]
- 14.Lampl Y, Zivin JA, Fisher M, et al. Infrared laser therapy for ischemic stroke: a new treatment strategy: results of the NeuroThera Effectiveness and Safety Trial-1 (NEST-1) Stroke. 2007;38:1843–1849. doi: 10.1161/STROKEAHA.106.478230. [DOI] [PubMed] [Google Scholar]
- 15.Tullberg M, Alstergren PJ, Ernberg MM. Effects of low-power laser exposure on masseter muscle pain and microcirculation. Pain. 2003;105:89–96. doi: 10.1016/S0304-3959(03)00166-0. [DOI] [PubMed] [Google Scholar]
- 16.Silveira PC, Silva LA, Fraga DB, Freitas TP, Streck EL, Pinho R. Evaluation of mitochondrial respiratory chain activity in muscle healing by low-level laser therapy. J Photochem Photobiol B. 2009;95:89–92. doi: 10.1016/j.jphotobiol.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 17.Xu X, Zhao X, Liu TC, Pan H. Low-intensity laser irradiation improves the mitochondrial dysfunction of C2C12 induced by electrical stimulation. Photomed Laser Surg. 2008;26:197–202. doi: 10.1089/pho.2007.2125. [DOI] [PubMed] [Google Scholar]
- 18.Avni D, Levkovitz S, Maltz L, Oron U. Protection of skeletal muscles from ischemic injury: low-level laser therapy increases antioxidant activity. Photomed Laser Surg. 2005;23:273–277. doi: 10.1089/pho.2005.23.273. [DOI] [PubMed] [Google Scholar]
- 19.Rizzi CF, Mauriz JL, Freitas Correa DS, et al. Effects of low-level laser therapy (LLLT) on the nuclear factor (NF)-kappaB signaling pathway in traumatized muscle. Lasers Surg Med. 2006;38:704–713. doi: 10.1002/lsm.20371. [DOI] [PubMed] [Google Scholar]
- 20.Albertini R, Aimbire FS, Correa FI, et al. Effects of different protocol doses of low power gallium-aluminum-arsenate (Ga-Al-As) laser radiation (650 nm) on carrageenan induced rat paw oedema. J Photochem Photobiol B. 2004;74:101–107. doi: 10.1016/j.jphotobiol.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Albertini R, Villaverde AB, Aimbire F, et al. Anti-inflammatory effects of low-level laser therapy (LLLT) with two different red wavelengths (660 nm and 684 nm) in carrageenan-induced rat paw edema. J Photochem Photobiol B. 2007;89:50–55. doi: 10.1016/j.jphotobiol.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 22.Aimbire F, Albertini R, Pacheco MT, et al. Low-level laser therapy induces dose-dependent reduction of TNFalpha levels in acute inflammation. Photomed Laser Surg. 2006;24:33–37. doi: 10.1089/pho.2006.24.33. [DOI] [PubMed] [Google Scholar]
- 23.Aimbire F, Lopes-Martins RA, Albertini R, et al. Effect of low-level laser therapy on hemorrhagic lesions induced by immune complex in rat lungs. Photomed Laser Surg. 2007;25:112–117. doi: 10.1089/pho.2006.1041. [DOI] [PubMed] [Google Scholar]
- 24.Correa F, Lopes Martins RA, Correa JC, Iversen VV, Joensen J, Bjordal JM. Low-level laser therapy (GaAs lambda = 904 nm) reduces inflammatory cell migration in mice with lipopolysaccharide-induced peritonitis. Photomed Laser Surg. 2007;25:245–249. doi: 10.1089/pho.2007.2079. [DOI] [PubMed] [Google Scholar]
- 25.Hemvani N, Chitnis DS, George M, Chammania S. In vitro effect of nitrogen and He-Ne laser on the apoptosis of human polymorphonuclear cells from burn cases and healthy volunteers. Photomed Laser Surg. 2005;23:476–479. doi: 10.1089/pho.2005.23.476. [DOI] [PubMed] [Google Scholar]
- 26.Lopes-Martins RA, Marcos RL, Leonardo PS, et al. Effect of low-level laser (Ga-Al-As 655 nm) on skeletal muscle fatigue induced by electrical stimulation in rats. J Appl Physiol. 2006;101:283–288. doi: 10.1152/japplphysiol.01318.2005. [DOI] [PubMed] [Google Scholar]
- 27.Leal Junior EC, Lopes-Martins RA, de Almeida P, Ramos L, Iversen VV, Bjordal JM. Effect of low-level laser therapy (GaAs 904 nm) in skeletal muscle fatigue and biochemical markers of muscle damage in rats. Eur J Appl Physiol. 2010;108:1083–1088. doi: 10.1007/s00421-009-1321-1. [DOI] [PubMed] [Google Scholar]
- 28.Leal Junior EC, Lopes-Martins RA, Dalan F, et al. Effect of 655-nm low-level laser therapy on exercise-induced skeletal muscle fatigue in humans. Photomed Laser Surg. 2008;26:419–424. doi: 10.1089/pho.2007.2160. [DOI] [PubMed] [Google Scholar]
- 29.Leal Junior EC, Lopes-Martins RA, Vanin AA, et al. Effect of 830 nm low-level laser therapy in exercise-induced skeletal muscle fatigue in humans. Lasers Med Sci. 2009;24:425–431. doi: 10.1007/s10103-008-0592-9. [DOI] [PubMed] [Google Scholar]
- 30.Leal Junior EC, Lopes-Martins RA, Frigo L, et al. Effects of low-level laser therapy (LLLT) in the development of exercise-induced skeletal muscle fatigue and changes in biochemical markers related to post-exercise recovery. J Orthop Sports Phys Ther. 2010;40:524–532. doi: 10.2519/jospt.2010.3294. [DOI] [PubMed] [Google Scholar]
- 31.Leal Junior EC, Lopes-Martins RA, Rossi RP, et al. Effect of cluster multi-diode light emitting diode therapy (LEDT) on exercise-induced skeletal muscle fatigue and skeletal muscle recovery in humans. Lasers Surg Med. 2009;41:572–577. doi: 10.1002/lsm.20810. [DOI] [PubMed] [Google Scholar]
- 32.Enwemeka CS. Intricacies of dose in laser phototherapy for tissue repair and pain relief. Photomed Laser Surg. 2009;27:387–393. doi: 10.1089/pho.2009.2503. [DOI] [PubMed] [Google Scholar]
- 33.Reid MB, Haack KE, Franchek KM, Valberg PA, Kobzik L, West MS. Reactive oxygen in skeletal muscle. I. Intracellular oxidant kinetics and fatigue in vitro. J Appl Physiol. 1992;73:1797–1804. doi: 10.1152/jappl.1992.73.5.1797. [DOI] [PubMed] [Google Scholar]
- 34.Halliwell B, Gutteridge JC. Free radicals in biology and medicine. Oxford: Oxford University Press; 2000. [Google Scholar]
- 35.Pedersen TH, Nielsen OB, Lamb GD, Stephenson DG. Intracellular acidosis enhances the excitability of working muscle. Science. 2004;305:1144–1147. doi: 10.1126/science.1101141. [DOI] [PubMed] [Google Scholar]
- 36.Hayworth CR, Rojas JC, Padilla E, Holmes GM, Sheridan EC, Gonzalez-Lima F. In vivo low-level light therapy increases cytochrome oxidase in skeletal muscle. Photochem Photobiol. 2010;86:673–680. doi: 10.1111/j.1751-1097.2010.00732.x. [DOI] [PubMed] [Google Scholar]
- 37.Joensen J, Demmink JH, Johnson MI, Iversen VV, Lopes-Martins RA, Bjordal JM. The thermal effects of therapeutic lasers with 810 and 904 nm wavelengths on human skin. Photomed Laser Surg. 2011;29:145–153. doi: 10.1089/pho.2010.2793. [DOI] [PubMed] [Google Scholar]
- 38.WADA (2009) World anti-doping code. World Anti-Doping Agency, Montreal. http://www.wada-ama.org/rtecontent/document/code_v2009_En.pdf. Accessed 1 Jul 2011


