Abstract
Purpose
Vascular endothelial growth factor (VEGF) and angiopoietin-2 (Ang-2) are major mediators of angiogenesis and are induced by tissue inflammation and hypoxia. The purpose of this study was to investigate whether serum VEGF and Ang-2 are associated with the presence of hemoptysis and the extent of systemic inflammation in patients with inflammatory lung diseases.
Materials and Methods
We prospectively enrolled 52 patients with inflammatory lung disease between June 2008 and October 2009.
Results
The median values of VEGF and Ang-2 were 436 pg/mL and 2383 pg/mL, respectively. There was a significant positive correlation between serum Ang-2 and VEGF levels. VEGF levels were not significantly different according to the presence of hemoptysis. C-reactive protein (CRP) and Ang-2 level were significantly higher in patients without hemoptysis (n=26) than in those with hemoptysis (n=26; p<0.001 and p<0.001, respectively). CRP and arterial oxygen tension (PaO2) were significantly correlated with both serum VEGF (p=0.032 and p=0.016, respectively) and Ang-2 levels (p<0.001 and p=0.041, respectively), after adjusting for other factors. Age and the absence of hemoptysis were factors correlated with serum Ang-2 levels
Conclusion
Our study suggests that serum VEGF and Ang-2 levels are associated with PaO2 and the severity of inflammation rather than the presence of hemoptysis in patients with inflammatory lung diseases. Thus, hemoptysis may not be mediated by increased serum levels of VEGF and Ang-2 in patients with inflammatory lung diseases, and further studies are required to determine the mechanisms of hemoptysis.
Keywords: Angiogenesis, angiopoietin-2, hemoptysis, inflammation, vascular endothelial growth factor
INTRODUCTION
Angiogenesis is characterized by the formation of new microvessels from preexisting vasculature and is associated with numerous inflammatory conditions, such as atherosclerosis, arthritis, retinopathy, and tumor growth.1 Previous studies provided evidence that inflammation exists in a mutually dependent association with angiogenesis.2,3
Among the numerous angiogenic factors, vascular endothelial growth factor (VEGF) is the most extensively studied and is significantly related to the severity of inflammatory lung diseases, such as active tuberculosis, chronic bronchitis, pulmonary aspergilloma, and pulmonary disease caused by cystic fibrosis.4-7 Overexpression of VEGF is also significantly correlated with lung cancer progression and metastasis. Antiangiogenic agents have received widespread attention as targets for lung cancer therapy.8,9 Recent studies reported that VEGF elevation correlates significantly with the presence of hemoptysis in patients with pulmonary aspergilloma.6 Another major factor involved in angiogenesis, angiopoietin-2 (Ang-2), is also induced by hypoxemia10 and plays an important role in initiating vessel sprouting in concert with VEGF.11,12 In contrast with angiopoietin-1, which stabilizes blood vessels, Ang-2 destabilizes blood vessels, initiating angiogenic changes instead of regression, and promotes the neovascularization of tumor cells.13-16
The purpose of this study was to investigate whether serum VEGF and Ang-2 were associated with the presence of hemoptysis and the level of systemic inflammation in patients with inflammatory lung diseases.
MATERIALS AND METHODS
Study population
The study was approved by the Institutional Review Board of Samsung Medical Center (a 2600-bed University affiliated hospital in Seoul, Korea) and was registered with www.clinicaltrials.gov (NCT01171768). Informed written consent was obtained from all participants.
Patients presented to the Samsung Medical Center for the treatment of benign inflammatory lung diseases, including bronchiectasis, aspergilloma, pneumonia, and post-tuberculosis destroyed lung, between June 2008 and December 2009. Diagnosis of post-tuberculosis destroyed lung (inactive tuberculosis) was considered when chest computed tomography (CT) scan showed the findings compatible with fibrotic sequelae of old tuberculosis and all mycobacterial examinations of sputum or bronchial washing fluid were negative. Patients with hemoptysis (hemoptysis group) were enrolled if they had hemoptysis within 2 weeks before study enrollment, and the patients without hemoptysis (no hemoptysis group) were enrolled if they did not have any hemoptysis more than 2 years before study enrollment. All study patients except one were admitted to general ward or emergency department for the management of symptomatic benign lung diseases. We excluded patients with neoplastic conditions, including lung cancer, vasculitis, and lung disease associated with collagen vascular disease. Serum samples for VEGF and Ang-2, clinical data, such as age, gender, smoking status, clinical symptoms, and laboratory data, including peripheral white blood cell (WBC) count, hemoglobin (Hb), prothrombin time (PT), C-reactive protein (CRP), and arterial oxygen tension (PaO2), were obtained at study enrollment.
Determination of sample size
The required sample size was calculated based on VEGF levels according to the presence of hemoptysis in patients with asperilloma.6 Assuming 437±164 pg/mL of VEGF in the hemoptysis group and 162±102 pg/mL in the no hemoptysis group, a sample size of 42 patients (21 per group) was required to detect a 120 pg/mL difference between the two groups with a power of 90% and α error of 0.05 using a two-tailed test.17 Anticipating a potential 20% drop-out rate, we planned to include 52 subjects (26 per group).
Measurement of VEGF and Ang-2
Serum samples from each individual were obtained at the time of study enrollment. Sera were stored at -80℃. Serum VEGF and Ang-2 concentrations were measured in triplicate for each sample using a commercial enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems, Minneapolis, MN, USA). The detection ranges of ELISA kits for VEGF and Ang-2 were 31.2-2000 pg/mL and 46.9-3000 pg/mL, respectively. Therefore, serum was diluted to measure serum Ang-2 levels.
Statistical analysis
Data presented are expressed as either the median and interquartile range (IQR, 25th and 75th percentiles) or the number (percentage) of patients. For univariate analysis or relation with hemoptysis, categorical variables were analyzed using a Pearson χ2 test or Fisher's exact test; continuous variables were analyzed using a Mann-Whitney U test. VEGF and Ang-2 levels did not fit normality assumptions; thus, log-transformed VEGF and Ang-2 (ln VEGF and ln Ang-2) values were used in analyses. As the VEGF and Ang-2 levels were measured in triplicate from single patient samples, a mixed model was used to analyze associations between parameters. Additionally, to take random effects between subjects into account, we used the 'random statement' function in PROC MIXED. All p values are two-sided with p<0.05 considered to indicate statistical significance. Statistical analyses were performed using the SAS software (ver. 9.1; SAS Institute, Cary, NC, USA).
RESULTS
Baseline clinical and laboratory features
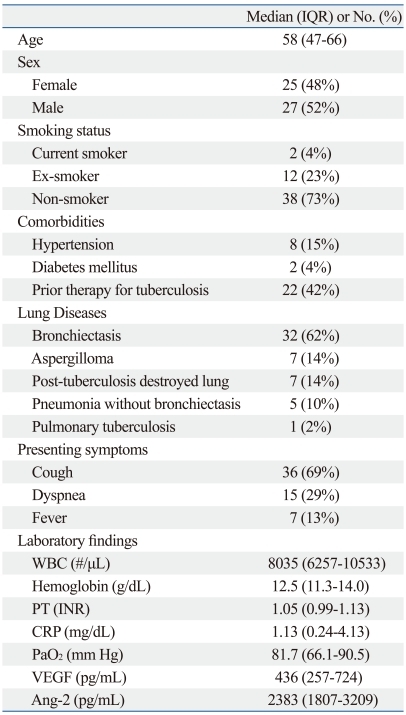
Characteristics of the enrolled patients are listed in Table 1. In this study, 52 study patients underwent extensive evaluations for underlying disease including chest CT scan (n=46), bronchoscopy (n=21), and sputum bacterial and mycobacterial examinations (n=44). There were 25 men and 27 women, with a median age of 58 years (range, 47-66). Of the 52 patients, 14 patients (27%) had a history of smoking, and 22 (42%) had a history of tuberculosis treatment. The most common disease in enrolled patients was bronchiectasis (62%); 14% had an aspergilloma, and 14% had post-tuberculosis destroyed lung. Median VEGF and Ang-2 levels were 436 pg/mL (257-724) and 2383 pg/mL (1807-3209), respectively. In total, 5 patients received oxygen therapy (1-2 L/min) on arterial blood gas analysis.
Table 1.
Baseline Characteristics of Enrolled Patients (n=52)
WBC, white blood cell; PT, prothrombin time; CRP, C-reactive protein; VEGF, vascular endothelial growth factor; Ang-2, angiopoietin-2; IQR, interquartile range; INR, international normalized ratio; PaO2, arterial oxygen tension.
Comparison of clinical laboratory features depending on the presence of hemoptysis
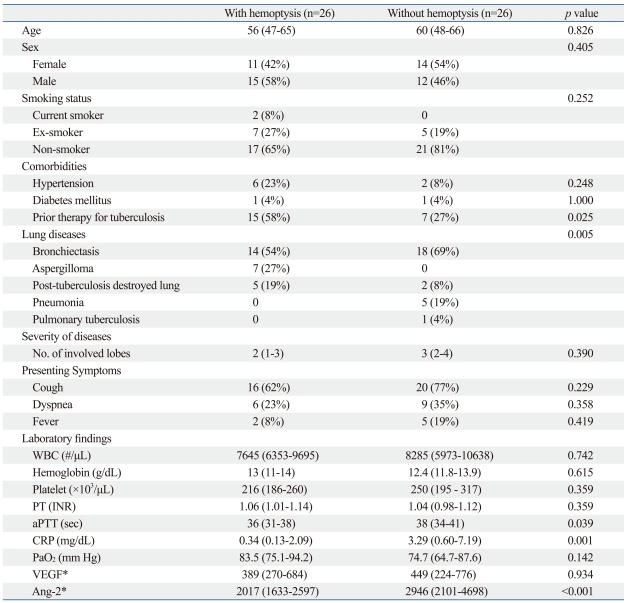
In patients with hemoptysis (n=26), bronchiectasis (54%), aspergilloma (27%), and post-tuberculosis destroyed lung (19%) were observed. In those without hemoptysis (n=26), bronchiectasis (69%), post-tuberculosis destroyed lung (8%), pneumonia (19%), and pulmonary tuberculosis (4%) were observed. Patients with hemoptysis had more significant history of tuberculosis treatment compared with those without hemoptysis. However, the median CRP and Ang-2 levels were significantly higher in patients without hemoptysis than in those with hemoptysis (CRP; 0.34 vs. 3.29 mg/dL; p<0.001, and Ang-2; 2017 vs. 2946 pg/mL; p<0.001). There was no significant difference in age, gender, smoking history, presenting symptoms, or laboratory findings (including WBC, Hb, PT, PaO2, serum VEGF levels) (Table 2).
Table 2.
Comparison of Clinical Laboratory Features according to the Presence of Hemoptysis
WBC, white blood cell; PT, prothrombin time; aPTT, activated partial thromboplastin time; CRP, C-reactive protein; VEGF, vascular endothelial growth factor; Ang-2, angiopoietin-2; INR, international normalized ratio; PaO2, arterial oxygen tension.
*The analysis used a mixed model, accounting for the random effect between subjects (logarithmic transformed VEGF and Ang-2 values were used).
Correlation between serum VEGF or angiopoietin-2 and other parameters
The median VEGF levels were 375 pg/mL in bronchiectasis, 472 pg/mL in aspergilloma, 554 pg/mL in post-tuberculosis destroyed lung, and 451 pg/mL in pneumonia. The median Ang-2 levels were 2444 pg/mL in bronchiectasis, 1689 pg/mL in aspergilloma, 3021 pg/mL in post-tuberculosis destroyed lung, and 4344 pg/mL in pneumonia.
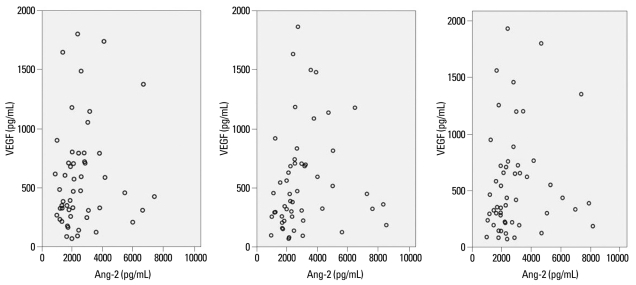
Serum Ang-2 levels were significantly correlated with serum VEGF levels (p=0.028) (Fig. 1). Serum VEGF levels demonstrated a significant positive correlation with WBC and a negative correlation with PaO2 levels (Table 3). Age, gender, smoking status, presence of hemoptysis, and CRP levels showed no significant correlation with VEGF levels (Table 3). Ang-2 levels showed significantly positive correlations with age, WBC, and CRP levels, while demonstrating a negative correlation with PaO2 levels (Table 3).
Fig. 1.
Correlation between serum VEGF and Ang-2 levels. VEGF, vascular endothelial growth factor; Ang-2, angiopoietin-2. p=0.028.
Table 3.
Univariate Analysis of Associations between Serum VEGF or Angiopoietin-2 levels and Other Measured Parameters
Ln, natural logarithm; WBC, white blood cell; PT, prothrombin time; CRP, C-reactive protein; VEGF, Vascular endothelial growth factor; Ang-2, angiopoietin-2; SE, standard error; PaO2, arterial oxygen tension.
Analysis using a mixed model to account for the random effect between subjects (logarithmic transformed VEGF and Ang-2 values were used).
*Reference category.
†Smoking status: smokers (current smoker or ex-smoker) and non-smoker.
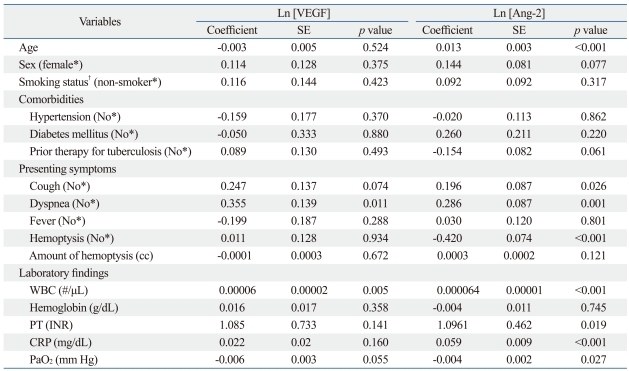
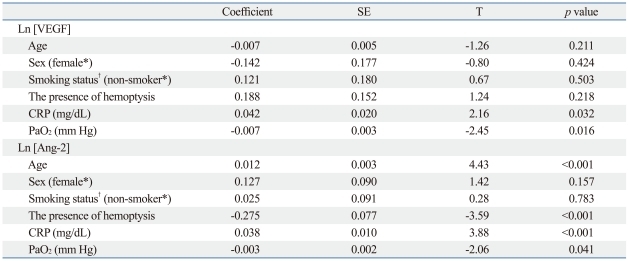
Multivariate analysis using PROC MIXED for repeated measures data was performed to identify factors significantly correlated with serum VEGF or Ang-2 levels. CRP levels and PaO2 were found to be significant correlated with both serum VEGF (p=0.032 and p=0.016, respectively) and Ang-2 levels (p<0.001 and p=0.041, respectively) after adjusting for other factors (age, gender, smoking history, and the presence of hemoptysis) (Table 4). Age and the absence of hemoptysis were significantly correlated with serum Ang-2 levels (Table 4).
Table 4.
Multivariate Correlations between Serum VEGF or Angiopoietin-2 Levels and Other Measured Parameters
Ln, natural logarithm; T, t statistic value; VEGF, vascular endothelial growth factor; Ang-2, angiopoietin-2; CRP, C-reactive protein; SE, standard error; PaO2, arterial oxygen tension.
Analysis using a mixed model to account for the random effect between subjects (logarithmic transformed VEGF and Ang-2 values were used).
*Reference category.
†Smoking status: smokers (current smoker or ex-smoker) and non-smoker.
DISCUSSION
In the present study, serum VEGF levels did not differ according to the presence of hemoptysis. However, we demonstrated that serum VEGF and Ang-2 levels in patients with inflammatory lung diseases correlated significantly with elevations in serum CRP and reduction of PaO2.
Angiogenesis, the growth of new capillary blood vessels from pre-existing vasculature, is an important contributor to tissue inflammation and abnormal remodeling in inflammatory diseases. Consistent with previous data that levels of VEGF are significantly elevated in inflammatory disorders, including lung inflammatory disease,6,7,18,19 our present results also demonstrated that VEGF levels were increased in inflammatory lung diseases, such as bronchiectasis, aspergilloma, post-tuberculosis destroyed lung, and pneumonia, when compared with published normal values.20,21 Additionally, elevated VEGF levels correlated with severity of inflammation. Significant change in the serum VEGF levels with the treatment of inflammatory lung diseases have been reported.4,6 To our best knowledge, however, our present study is the first to demonstrate that serum VEGF levels are positively correlated with serum CRP levels and WBCs. In a previous study on patients undergoing major surgery, there was no significant correlation between increases in serum VEGF levels and serum IL-6 or CRP levels.22
Recently, several clinical studies have shown that circulating levels of Ang-2 are increased in septic shock patients, as well as non-septic patients with, or at risk of, acute lung injury/acute respiratory distress syndrome; this is related to vascular permeability and pulmonary dysfunction.23-26 In these studies, serum Ang-2 levels were positively correlated with serum CRP levels. In the present study, Ang-2 levels were positively correlated with CRP levels and WBCs, both representing markers of inflammation. Compared with the levels of Ang-2 in previous studies,23,25,27 the median level of Ang-2 in septic shock patients was greater than 4000 pg/mL; this is significantly higher than the levels seen in the current study (median Ang-2 level of 2383 pg/mL). However, the levels of Ang-2 in the current study are similar to data measured in cancer patients, including lung, breast, and prostate,15,28 and were greater than the levels of Ang-2 in the healthy volunteers without lung disease (1000-1500 pg/mL).28 Our findings suggest that high Ang-2 levels are associated with the severity of inflammatory lung disease in patients without sepsis, and that the Ang-2 levels in inflammatory diseases are similar to the levels reported in the setting of various cancers.15,28 To our best knowledge, this is the first reported study to evaluate Ang-2 levels in inflammatory lung diseases, including bronchiectasis, post-tuberculosis destroyed lung, and aspergilloma.
During development of inflammatory tissue, hypoxic conditions can promote angiogenesis, leading to induced VEGF and Ang-2 production. Expression of Ang-2 was elevated in cultured endothelial cells exposed to hypoxia,29-31 while VEGF mRNA expression was increased.32,33 In the present study, PaO2 levels were correlated inversely with VEGF and Ang-2 levels; suggesting that angiogenesis mediators may be released in response to low PaO2 in patients with inflammatory lung diseases.
Significant associations between VEGF and Ang-2 levels have been noted in earlier studies in patients with various tumors,28 diabetes mellitus,34 and asthma.35 The present study demonstrated that serum VEGF and Ang-2 levels had a significant positive correlation in inflammatory lung diseases.
However, in contrast to a previous study,6 our study did not find a significant association between the presence of hemoptysis and VEGF levels. This lack of an association may be explained by differences in the distribution of disease between the hemoptysis and the no hemoptysis groups. As shown in Table 2, the prevalence of bronchiectasis (54%), aspergilloma (27%), and post-tuberculosis destroyed lung (19%) in the hemoptysis group differed from the no hemoptysis group where bronchiectasis (69%), pneumonia (19%), and post-tuberculosis destroyed lung (8%) were prevalent. When the VEGF levels were compared between patients with aspergilloma, which were present only in the hemoptysis group, and those with pneumonia, which were present only in the non-hemoptysis group, no significant difference in VEGF levels was observed. Additionally, when comparison was restricted to patients with bronchiectasis from both groups, VEGF levels were not significantly correlated with the presence of hemoptysis. The lack of a significant difference in VEGF levels between the hemoptysis and no hemoptysis groups can be partially explained by the different disease distribution in the two groups. In previous reports of associations between VEGF levels and hemoptysis in 21 pulmonary aspergilloma patients,6 the mean serum VEGF levels in 6 patients with hemoptysis and 15 patients without hemoptysis were 437 pg/mL and 162 pg/mL, respectively. The reported serum VEGF levels were similar to the aspergilloma patients with hemoptysis in the current study.
The present study has several other limitations. The measurements of VEGF and Ang-2 levels were made with serum, not lung tissue; it is unclear whether serum VEGF or Ang-2 levels reflect the levels in lung tissue. Also, because the present study was conducted with multiple inflammatory lung diseases, the sample size might have influenced the statistical power; this limits the interpretation of the study despite the fact that a formal sample size calculation was performed. Finally, the timing of measurement of serum angiogenesis markers in this study was not uniform in relation to stages of lung injury. Since the level of serum angiogenesis markers could vary in different stages of lung injury, our data should be interpreted conservatively. A larger-scale study in patients with bronchiectasis, aspergilloma, and pneumonia is needed to further clarify the association between hemoptysis and VEGF levels.
In conclusion, serum VEGF and Ang-2 levels were associated with the PaO2 and the severity of inflammation, rather than the presence of hemoptysis in patients with inflammatory lung diseases. Hemoptysis in patients with inflammatory lung diseases may not be mediated by increased serum levels of VEGF and Ang-2. Further studies are required to determine the mechanism(s) of hemoptysis in patients with inflammatory lung disease.
ACKNOWLEDGEMENTS
This research was supported by the IN-SUNG Foundation for Medical Research (CA98761).
This study was supported by the Samsung Medical Center Clinical Research Development Program grant, #CRS-109-10-1.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 2.Jackson JR, Seed MP, Kircher CH, Willoughby DA, Winkler JD. The codependence of angiogenesis and chronic inflammation. FASEB J. 1997;11:457–465. [PubMed] [Google Scholar]
- 3.Dvorak HF, Brown LF, Detmar M, Dvorak AM. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol. 1995;146:1029–1039. [PMC free article] [PubMed] [Google Scholar]
- 4.Matsuyama W, Hashiguchi T, Matsumuro K, Iwami F, Hirotsu Y, Kawabata M, et al. Increased serum level of vascular endothelial growth factor in pulmonary tuberculosis. Am J Respir Crit Care Med. 2000;162:1120–1122. doi: 10.1164/ajrccm.162.3.9911010. [DOI] [PubMed] [Google Scholar]
- 5.Kanazawa H. Role of vascular endothelial growth factor in the pathogenesis of chronic obstructive pulmonary disease. Med Sci Monit. 2007;13:RA189–RA195. [PubMed] [Google Scholar]
- 6.Inoue K, Matsuyama W, Hashiguchi T, Wakimoto J, Hirotsu Y, Kawabata M, et al. Expression of vascular endothelial growth factor in pulmonary aspergilloma. Intern Med. 2001;40:1195–1199. doi: 10.2169/internalmedicine.40.1195. [DOI] [PubMed] [Google Scholar]
- 7.McColley SA, Stellmach V, Boas SR, Jain M, Crawford SE. Serum vascular endothelial growth factor is elevated in cystic fibrosis and decreases with treatment of acute pulmonary exacerbation. Am J Respir Crit Care Med. 2000;161:1877–1880. doi: 10.1164/ajrccm.161.6.9905022. [DOI] [PubMed] [Google Scholar]
- 8.Donovan EA, Kummar S. Targeting VEGF in cancer therapy. Curr Probl Cancer. 2006;30:7–32. doi: 10.1016/j.currproblcancer.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 9.Mattern J, Koomägi R, Volm M. Association of vascular endothelial growth factor expression with intratumoral microvessel density and tumour cell proliferation in human epidermoid lung carcinoma. Br J Cancer. 1996;73:931–934. doi: 10.1038/bjc.1996.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nilsson I, Shibuya M, Wennström S. Differential activation of vascular genes by hypoxia in primary endothelial cells. Exp Cell Res. 2004;299:476–485. doi: 10.1016/j.yexcr.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Tsigkos S, Koutsilieris M, Papapetropoulos A. Angiopoietins in angiogenesis and beyond. Expert Opin Investig Drugs. 2003;12:933–941. doi: 10.1517/13543784.12.6.933. [DOI] [PubMed] [Google Scholar]
- 12.Hashizume H, Falcón BL, Kuroda T, Baluk P, Coxon A, Yu D, et al. Complementary actions of inhibitors of angiopoietin-2 and VEGF on tumor angiogenesis and growth. Cancer Res. 2010;70:2213–2223. doi: 10.1158/0008-5472.CAN-09-1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pouysségur J, Dayan F, Mazure NM. Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature. 2006;441:437–443. doi: 10.1038/nature04871. [DOI] [PubMed] [Google Scholar]
- 14.Boussat S, Eddahibi S, Coste A, Fataccioli V, Gouge M, Housset B, et al. Expression and regulation of vascular endothelial growth factor in human pulmonary epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2000;279:L371–L378. doi: 10.1152/ajplung.2000.279.2.L371. [DOI] [PubMed] [Google Scholar]
- 15.Park JH, Park KJ, Kim YS, Sheen SS, Lee KS, Lee HN, et al. Serum angiopoietin-2 as a clinical marker for lung cancer. Chest. 2007;132:200–206. doi: 10.1378/chest.06-2915. [DOI] [PubMed] [Google Scholar]
- 16.Jain RK. Molecular regulation of vessel maturation. Nat Med. 2003;9:685–693. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- 17.Hayes RJ, Bennett S. Simple sample size calculation for cluster-randomized trials. Int J Epidemiol. 1999;28:319–326. doi: 10.1093/ije/28.2.319. [DOI] [PubMed] [Google Scholar]
- 18.Kraft A, Weindel K, Ochs A, Marth C, Zmija J, Schumacher P, et al. Vascular endothelial growth factor in the sera and effusions of patients with malignant and nonmalignant disease. Cancer. 1999;85:178–187. [PubMed] [Google Scholar]
- 19.Alatas F, Alatas O, Metintas M, Ozarslan A, Erginel S, Yildirim H. Vascular endothelial growth factor levels in active pulmonary tuberculosis. Chest. 2004;125:2156–2159. doi: 10.1378/chest.125.6.2156. [DOI] [PubMed] [Google Scholar]
- 20.Hyodo I, Doi T, Endo H, Hosokawa Y, Nishikawa Y, Tanimizu M, et al. Clinical significance of plasma vascular endothelial growth factor in gastrointestinal cancer. Eur J Cancer. 1998;34:2041–2045. doi: 10.1016/s0959-8049(98)00282-2. [DOI] [PubMed] [Google Scholar]
- 21.Kikuchi K, Kubo M, Kadono T, Yazawa N, IHN H, Tamaki K. Serum concentrations of vascular endothelial growth factor in collagen diseases. Br J Dermatol. 1998;139:1049–1051. doi: 10.1046/j.1365-2133.1998.02563.x. [DOI] [PubMed] [Google Scholar]
- 22.Futami R, Miyashita M, Nomura T, Makino H, Matsutani T, Sasajima K, et al. Increased serum vascular endothelial growth factor following major surgical injury. J Nihon Med Sch. 2007;74:223–229. doi: 10.1272/jnms.74.223. [DOI] [PubMed] [Google Scholar]
- 23.Siner JM, Bhandari V, Engle KM, Elias JA, Siegel MD. Elevated serum angiopoietin 2 levels are associated with increased mortality in sepsis. Shock. 2009;31:348–353. doi: 10.1097/SHK.0b013e318188bd06. [DOI] [PubMed] [Google Scholar]
- 24.van der Heijden M, Pickkers P, van Nieuw Amerongen GP, van Hinsbergh VW, Bouw MP, van der Hoeven JG, et al. Circulating angiopoietin-2 levels in the course of septic shock: relation with fluid balance, pulmonary dysfunction and mortality. Intensive Care Med. 2009;35:1567–1574. doi: 10.1007/s00134-009-1560-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van der Heijden M, van Nieuw Amerongen GP, Koolwijk P, van Hinsbergh VW, Groeneveld AB. Angiopoietin-2, permeability oedema, occurrence and severity of ALI/ARDS in septic and non-septic critically ill patients. Thorax. 2008;63:903–909. doi: 10.1136/thx.2007.087387. [DOI] [PubMed] [Google Scholar]
- 26.Ricciuto DR, dos Santos CC, Hawkes M, Toltl LJ, Conroy AL, Rajwans N, et al. Angiopoietin-1 and angiopoietin-2 as clinically informative prognostic biomarkers of morbidity and mortality in severe sepsis. Crit Care Med. 2011;39:702–710. doi: 10.1097/CCM.0b013e318206d285. [DOI] [PubMed] [Google Scholar]
- 27.Orfanos SE, Kotanidou A, Glynos C, Athanasiou C, Tsigkos S, Dimopoulou I, et al. Angiopoietin-2 is increased in severe sepsis: correlation with inflammatory mediators. Crit Care Med. 2007;35:199–206. doi: 10.1097/01.CCM.0000251640.77679.D7. [DOI] [PubMed] [Google Scholar]
- 28.Caine GJ, Blann AD, Stonelake PS, Ryan P, Lip GY. Plasma angiopoietin-1, angiopoietin-2 and Tie-2 in breast and prostate cancer: a comparison with VEGF and Flt-1. Eur J Clin Invest. 2003;33:883–890. doi: 10.1046/j.1365-2362.2003.01243.x. [DOI] [PubMed] [Google Scholar]
- 29.Pugh CW, Ratcliffe PJ. Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med. 2003;9:677–684. doi: 10.1038/nm0603-677. [DOI] [PubMed] [Google Scholar]
- 30.Mandriota SJ, Pyke C, Di Sanza C, Quinodoz P, Pittet B, Pepper MS. Hypoxia-inducible angiopoietin-2 expression is mimicked by iodonium compounds and occurs in the rat brain and skin in response to systemic hypoxia and tissue ischemia. Am J Pathol. 2000;156:2077–2089. doi: 10.1016/S0002-9440(10)65079-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pichiule P, Chavez JC, LaManna JC. Hypoxic regulation of angiopoietin-2 expression in endothelial cells. J Biol Chem. 2004;279:12171–12180. doi: 10.1074/jbc.M305146200. [DOI] [PubMed] [Google Scholar]
- 32.Bruick RK, McKnight SL. Building better vasculature. Genes Dev. 2001;15:2497–2502. doi: 10.1101/gad.931601. [DOI] [PubMed] [Google Scholar]
- 33.Semenza GL. Regulation of hypoxia-induced angiogenesis: a chaperone escorts VEGF to the dance. J Clin Invest. 2001;108:39–40. doi: 10.1172/JCI13374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lim HS, Lip GY, Blann AD. Angiopoietin-1 and angiopoietin-2 in diabetes mellitus: relationship to VEGF, glycaemic control, endothelial damage/dysfunction and atherosclerosis. Atherosclerosis. 2005;180:113–118. doi: 10.1016/j.atherosclerosis.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 35.Kanazawa H, Nomura S, Asai K. Roles of angiopoietin-1 and angiopoietin-2 on airway microvascular permeability in asthmatic patients. Chest. 2007;131:1035–1041. doi: 10.1378/chest.06-2758. [DOI] [PubMed] [Google Scholar]