Abstract
Purpose
The improvement of testicular volume, testosterone levels and sperm concentration was suggested to be significantly associated with the number of internal spermatic veins (ISVs) ligated during varicocelectomy. Herein, we investigated preoperative color Doppler ultrasonography (CDU) findings as potential preoperative predictors of the number of ISVs requiring ligation during microsurgical subinguinal varicocelectomy.
Materials and Methods
In a prospective evaluation of 40 patients, maximal vein size and maximal reflux velocity were measured, while the total cross-sectional area of the affected testicular veins during a Valsalva maneuver was calculated using CDU by a single uroradiologist. Microsurgical subinguinal varicocelectomies were performed by one urologist.
Results
Among the semen parameters, semen morphology showed significant improvement (p=0.033), which was much clearer in the patients with a higher number of ISVs ligated than a lower number of ISVs ligated. Among the various preoperative variables, maximal reflux velocity and total cross-sectional area on CDU were related to the number of ISVs ligated (r=-0.442, p=0.004; r=0.594, p=0.000, respectively). Furthermore, univariate and multivariate linear regression analyses showed that maximal reflux velocity and total cross-sectional area on CDU were independent predictive factors of the number of ISVs ligated.
Conclusion
Maximal reflux velocity and total cross-sectional area on CDU were related to the number of ISVs ligated. This means that the maximal reflux velocity and total cross-sectional area measured by preoperative CDU can predict the number of ISVs requiring ligation during microsurgical subinguinal varicocelectomy, which might be related to significant improvement of semen parameters after varicocelectomy.
Keywords: Internal spermatic vein, reflux velocity, varicocele
INTRODUCTION
Varicocele is defined as variceal dilatation and tortuosity of the testicular veins and pampiniform plexus, characterized by reflux flow in the affected veins.1 Varicocele is detected in approximately 15% of the normal male population, in 19% to 41% of males with primary infertility,2 and in 45% to 81% of males with secondary infertility.3,4 In a study of 9,034 infertile men sponsored by the World Health Organization, the incidence of varicocele in men with abnormal semen parameters was two times higher than that in men with normal semen (25.4% vs. 11.7%).5
The diagnostic method of varicocele is based on physical examination performed by specialists. However, this method is affected by low sensitivity and specificity, especially in cases of low grade varicocele. Gat, et al.6,7 found that using palpation only, left varicocele is missed in 10% and right varicocele is missed in 90% of cases. Thankfully, the diagnosis of varicoceles can also be achieved radiographically. Radiographic tests such as color Doppler ultrasonography (CDU) have been used to diagnose varicoceles.
CDU is the preferred method of diagnosing varicoceles. CDU defines the anatomic and physiologic aspects of varicoceles by measuring the size of the pampiniform plexus and blood flow parameters of the spermatic veins.8-11 Furthermore, reflux detected by CDU plays a valuable role in the diagnosis of varicoceles.12,13 Also, there have been many studies using CDU, which demonstrated significant correlation between varicocele diagnostic parameters and repair outcome, as well as successfully defining normative values and implications in the diagnosis of varicoceles.9,10,14,15
The success of a varicocele repair depends in part on the preoperative grade of the varicocele. Larger varicoceles are more likely to be associated with impairment of semen quality. Repair of larger varicoceles results in significantly greater improvement in semen quality than repair of smaller varicoceles does.16,17
In addition, it was recently suggested that the improvement of testicular volume, testosterone levels and sperm concentration was significantly associated with the number of internal spermatic veins (ISVs) ligated during varicocelectomy and that patients with more than 10 ligated veins have better chances to improve sperm concentration.18 Also, the number of veins ligated correlated positively with an increase in total motility (p=0.017).19 Furthermore, patients who showed significant improvement in their sperm density, motility and morphology 6 months after varicocelectomy had higher numbers of ligated veins (9.3±0.8 vs. 7.9±0.7) than patients who showed no improvement 6 months after surgery.20
However, to date, the relationship between preoperative findings, including CDU findings (vein size, maximal reflux velocity, and total cross-sectional area of the affected testicular veins showing venous reflux flow during a Valsalva maneuver), and intraoperative findings (number of veins ligated) during varicocelectomy has not yet been reported.
Therefore, we investigated the relationship between the preoperative findings and the number of ISVs ligated as well as preoperative CDU findings as preoperative predictors of the number of ISVs requiring ligation during microsurgical subinguinal varicocelectomy, which might be related to significant improvement of semen parameters after varicocelectomy.
MATERIALS AND METHODS
This study was approved by the Institutional Review Board of the Gachon University Gil Hospital. A total of 40 men with clinical varicocele underwent prospectively microsurgical subinguinal varicocelectomy for treatment of testicular pain, discomfort, scrotal mass, or infertility. The exclusion criteria for this study were previous history of genitourinary trauma, scrotal surgery including varicocelectomy, or groin surgery including herniorrhaphy, which might have a strong influence on CDU findings.
Preoperative evaluation included a complete history and physical examination, semen analysis, and CDU examination. Varicoceles were diagnosed and graded by physical examination with the patient in a supine position, standing at rest and during a Valsalva maneuver.
CDU examination was performed by single uroradiologist at the level of the subinguinal area just over the superior-lateral edge of the testis, with patients in supine position. Using CDU, right and left testis sizes, maximal vein size and maximal reflux velocity of the affected testicular veins during the Valsalva maneuver were measured. Also, on CDU images (cross-sectional view), which showed venous reflux flow during the Valsalva maneuver, total cross-sectional area of the affected testicular veins was calculated using the ellipse formula, i.e., π/4×major×minor axis.
All patients underwent microsurgical subinguinal varicocelectomy performed by one urologist. The testicular artery, cremasteric artery, lymphatics, and vas deferens and its vessels were preserved. All spermatic, cremasteric and gubernacular veins greater than 2 mm in diameter were doubly ligated with 4-Zero silk ties and divided. The numbers of lymphatics and arteries preserved as well as veins that should be ligated during operation were recorded.
The results are expressed as mean±standard deviation or numbers of patients. Statistical analysis was performed using Chi-square tests, Students' t-tests, and paired t-tests with SPSS software, version 12.0. The relationships between the study variables were analyzed using Pearson's linear correlation. To identify the factors influencing the number of ISVs ligated, univariate and multivariate analyses were performed using a linear regression model. Statistical significance was reached when p<0.05.
RESULTS
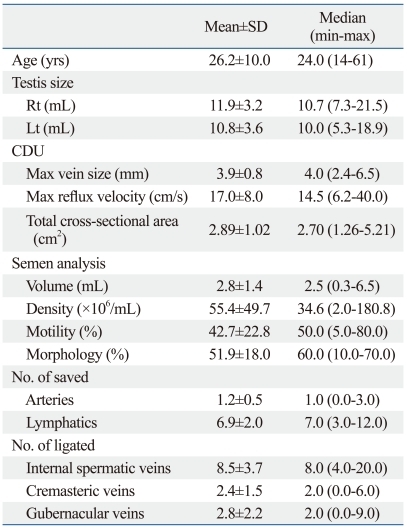
The patients' characteristics are summarized in Table 1. The mean patient age was 26.2±10.0 (14-61) years. Physical examinations revealed that 6 (15.0%) and 34 (85.0%) patients had grade 2 or 3 varicoceles, respectively. All patients had varicocele on the left side. During microsurgical subinguinal varicocelectomy, we encountered and saved 1.2±0.5 arteries and 6.9±2.0 lymphatics. We ligated 8.5±3.7 ISVs, 2.4±1.5 cremasteric veins and 2.8±2.2 gubernacular veins.
Table 1.
Characteristics of the Study Population
CDU, color Doppler ultrasonography.
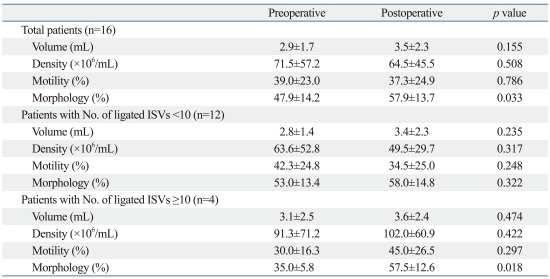
Of 40 patients, a total of 16 patients underwent both preoperative and postoperative semen analyses. Among the semen parameters, semen morphology showed improvement with statistical significance (p=0.033) (Table 2). Furthermore, in 12 patients with less than 10 ISVs, there was improvement in semen volume and morphology, but it was not statistically significant. In 4 patients with 10 ISVs or more, there was improvement in all semen parameters, but only semen morphology showed statistically significant change (Table 2).
Table 2.
Comparison between Preoperative and Postoperative Semen Parameters
ISVs, internal spermatic veins.
Paired t-test was used.
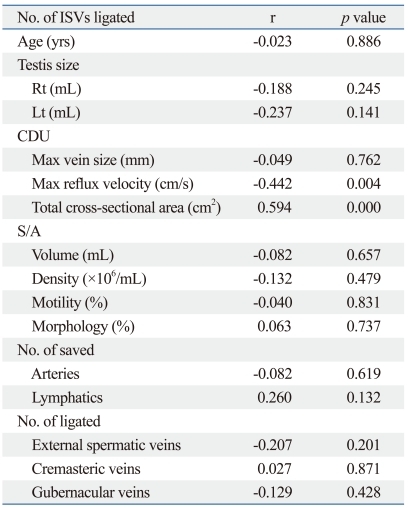
Table 3 summarizes the relationships found between the number of ISVs ligated and other preoperative and intraoperative variables. Maximal reflux velocity and total cross-sectional area on CDU were related to the number of ISVs ligated (r=-0.442, p=0.004; r=0.594, p=0.000, respectively)(Table 3).
Table 3.
Correlation Study between the Number of ISVs Ligated and Other Preoperative and Intraoperative Variables
CDU, color Doppler ultrasonography; S/A, semen analysis; ISVs, internal spermatic veins.
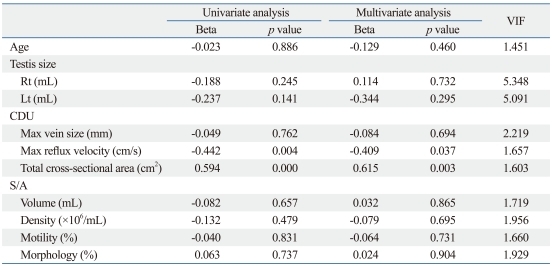
Furthermore, the univariate and multivariate linear regression analyses showed that among various variables, maximal reflux velocity and total cross-sectional area on CDU were independent predictive factors for the number of ISVs ligated (Table 4).
Table 4.
Univariate and Multivariate Linear Regression Analyses of the Various Variables for the Number of ISVs Ligated
CDU, color Doppler ultrasonography; S/A, semen analysis; ISVs, internal spermatic veins; Beta, standardized beta coefficient; VIF, variance inflation factor.
DISCUSSION
The pathogenesis of varicocele can be explained using fluid mechanics. Varicocele is caused by increased hydrostatic pressure in the impaired testicular venous drainage system.6,7,21-24 Gat, et al.6,7,21-24 found that due to the destruction of one-way valves, pathologic hydrostatic pressure is produced in the testicular venous microcirculatory system about 5 times higher than normal, exceeding arteriolar pressure. The pressure gradient between the arterioles and venules in testicular tissue is thereby reversed, leading to persistent hypoxia in the seminiferous tubules, as well as in Sertoli and Leydig cells. Hypoxia due to varicocele may cause progressive deterioration of sperm production, and may further lead to azoospermia and then finally to maturation arrest.7,22,24
These relationships between varicocele and infertility have raised the possibility that varicocele is a bilateral disease.6,7,21-23 Actually, according to urologic studies in infertile men, the rate of bilateral varicocele is about 10%.25,26 Furthermore, Gat, et al.21 found that varicocele was confirmed by venography in 255 of 286 (89.2%) infertile men: 45 (17.6%) men had left-sided varicocele, 4 (1.5%) right-sided varicocele, and 206 (80.8%) bilateral varicocele.21 Another study showed that the right side was affected in 86% (725/840) of infertile men with varicocele.7
Several studies support the importance of the number of veins ligated. Belani, et al.27 showed that grade 3 varicoceles have a greater number of large veins compared with grade 1 varicoceles. Hopps, et al.28 reported that the varicocele grade was not related with the maximum diameter of ISVs but with the number of ISVs found during varicocelectomy. Also, they recorded the numbers of internal and external spermatic arteries, veins and lymphatics within the subinguinal portion during microscopic varicocelectomy and compared these findings to the microanatomy observed with the inguinal approach. In the subinguinal approach compared with the inguinal approach, there were a smaller number of large ISVs per cord (0.4 vs. 1.9, respectively) and a greater number of small ISVs per cord (7.9 vs. 4.7, respectively). Therein, they discussed their results using a schematic diagram of spermatic cord microanatomy and showed that ISVs become substantially less numerous as they course from the subinguinal region through the inguinal canal to the internal inguinal ring. They also reported that in the subinguinal approach, ISVs were more complex than in the inguinal approach.
Furthermore, recently, some studies have revealed the relationship between the number of veins ligated during varicocelectomy and semen analysis outcomes. Pasqualotto, et al.18 suggested that, compared to the patients with less than 10 ligated ISVs, the patients with more than 10 ligated ISVs have better chances to improve testicular volume, testosterone levels and sperm concentration. Shindel, et al.19 found that the number of veins ligated correlated positively with an increase in total motility (p=0.017). Chen, et al.20 found that patients who showed significant improvement in their sperm density, motility and morphology 6 months after varicocelectomy had higher numbers of ligated veins (9.3±0.8 vs. 7.9±0.7) than patients who showed no improvement 6 months after surgery.20 These imply that for a higher number of ligated ISVs, there is greater improvement in semen parameters. In the present study, in spite of the insufficient data concerning pre- and post-operative semen parameters, semen morphology showed significant improvement (p=0.033), which was much clearer in the patients with a higher number of ISVs ligated than a lower number of ISVs ligated during operation (Table 2).
We assumed total cross-sectional area to be the sum of the cross-sectional area of each individual vein. Also, we considered that on CDU images (cross-sectional view) which showed venous reflux flow during a Valsalva maneuver, total cross-sectional area of the affected testicular veins could be calculated using the ellipse formula, i.e., π/4×major×minor axis. Correlation study (Table 3) showed that total cross-sectional area on CDU was positively related to the number of ISVs ligated (r=0.594, p=0.000) (Table 3). This positive correlation between total cross-sectional area on CDU and the number of ISVs ligated substantiates that it is reasonable to assume that total cross sectional area is the sum of the cross-sectional area of each individual vein. Therefore, we suggest that different numbers of veins mainly contribute to the observation of such different cross-sectional areas.
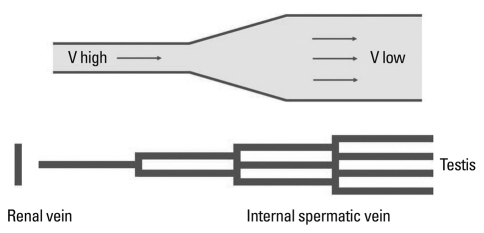
In the present study, maximal reflux velocity on CDU was negatively related to the number of ISVs ligated (r=-0.442, p=0.004)(Table 3). We applied hydrodynamics29,30 to analyze our results (Fig. 1). Distal ISVs have more branches than proximal ISVs, and the former have larger total cross-sectional area than the latter. When reflux occurs in the ISVs during a Valsalva maneuver, the flow rate (volume of blood per unit of time flowing through a given segment) of blood reflux is the same at all levels of the vein, irrespective of the degree of branching (division). However, the velocity (the linear speed, or distance per unit of time, with which blood flows forward through a given segment) at which blood flows through different segments of the vascular tree varies because flow velocity is inversely proportional to total cross-sectional area of all vessels, at any given level of the ISVs. It can be explained by the fact that flow (F) equals the product of mean velocity (V) times cross-sectional area (A).
Fig. 1.
Analyzation from the viewpoint of Hydrodynamics. V, velocity of blood flow during the Valsalva maneuver.
F=V×A
F: flow rate of blood flow
V: velocity of blood flow
A: cross-sectional area
As can be seen in this equation, total venous cross-sectional area increases as the number of ISV branches increases, and flow velocity decreases as the number of ISV branches increases (Fig. 1). As such, veins with more branches have larger total cross-sectional areas and lower reflux velocities.
In the present study, we measured maximal reflux velocity and total cross-sectional areas during a Valsalva maneuver, which is not reflective of the patient's normal or resting state. Although the Valsalva maneuver is artificial, reflux on CDU during a Valsalva maneuver is significant for diagnosing varicocele and is more obviously and more coherently obtained than at a resting state.9,10,12,13,31
Furthermore, we feel that these CDU findings (maximum reflux velocity and total cross-sectional area) during a Valsalva maneuver can explain the circumstances of ISVs at resting state. As shown in Fig. 1 and the study by Hopps, et al.,28 the blood of ISVs at resting state flows from wide spaces to narrow spaces. Therefore, we think that the blood flow of ISVs at resting state is similar with the Bottleneck phenomenon (effect) in traffic (a bottleneck is a place where a road becomes narrow or where it meets another road so that the traffic slows down or stops, often causing traffic jams). If the flow rate of blood flow is constant, in the case of larger cross-sectional area, velocity of blood flow is decreased in comparison with smaller cross-sectional area.
According to Bernoulli's equation, on the condition that total pressure should be constant, as velocity of blood flow is decreased, static pressure is increased.
Total pressure=dynamic pressure+static pressure=constant
ρV12/2+P1=ρV22/2+P2=constant
ρ: density of the fluid
V: velocity of blood flow
P: static pressure
As such, in the resting state, veins with more branches have larger total cross-sectional areas, lower velocity of blood flow, and higher static pressure. This higher static pressure is similar with traffic jams in a bottleneck. Although Gat, et al.6,7,21-24 suggested that hydrostatic pressure depends only on the vertical height of the ISVs, we conjecture that hydrostatic pressure depends on not only the vertical height of the ISVs but also the division of the ISVs. Also, we feel that this static pressure, due to the division of the ISVs, might also have some influence on the affected testis.
In addition, there is another possible explanation using Poiseuille's law. According to Poiseuille's law, pressure is proportional to the length of a pipe.
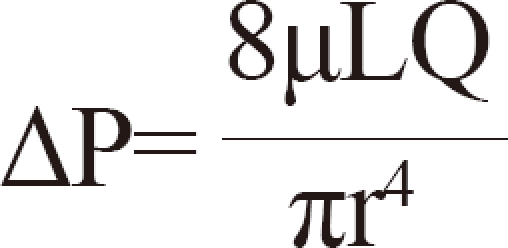
ΔP: pressure difference
L: length of pipe
µ: dynamic viscosity
Q: volumetric flow rate
r: radius
π: mathematical constant
Applying Poiseuille's law to the spermatic venous system, with greater numbers of nonfunctioning venous valves, the longer the combined length of the columns of the spermatic vein will be. This leads to increasing pressure of the pampiniform plexus on the venous drainage system. Therefore, we think that the defect of spermatic venous valves could cause not only the increasing hydrostatic pressure in an erect position at resting state but also the increasing venous drainage pressure in a supine position at resting state on the basis of Poiseuille's law.
Thus, theoretically, hydrodynamics supports our results that maximal reflux velocity and total cross-sectional area measured by preoperative CDU are able to predict the number of ISVs that require ligation during microsurgical subinguinal varicocelectomy.
In summary, maximal reflux velocity and total cross-sectional area on CDU were related to the number of ISVs ligated. This suggests that the maximal reflux velocity and total cross-sectional area measured by preoperative CDU can predict the number of ISVs requiring ligation during microsurgical subinguinal varicocelectomy, which might be related to significant improvement of semen parameters after varicocelectomy.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Cornud F, Belin X, Amar E, Delafontaine D, Hélénon O, Moreau JF. Varicocele: strategies in diagnosis and treatment. Eur Radiol. 1999;9:536–545. doi: 10.1007/s003300050706. [DOI] [PubMed] [Google Scholar]
- 2.Nagler HM, Luntz RK, Martinis FG. Varicocele. In: Lipshultz LI, Howards SS, editors. Infertility in the Male. 3rd ed. St. Louis: Mosby-Year Book; 1997. pp. 336–359. [Google Scholar]
- 3.Greenberg SH. Varicocele and male fertility. Fertil Steril. 1977;28:699–706. doi: 10.1016/s0015-0282(16)42669-5. [DOI] [PubMed] [Google Scholar]
- 4.Jarow JP, Coburn M, Sigman M. Incidence of varicoceles in men with primary and secondary infertility. Urology. 1996;47:73–76. doi: 10.1016/s0090-4295(99)80385-9. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. The influence of varicocele on parameters of fertility in a large group of men presenting to infertility clinics. Fertil Steril. 1992;57:1289–1293. [PubMed] [Google Scholar]
- 6.Gat Y, Bachar GN, Zukerman Z, Belenky A, Gorenish M. Physical examination may miss the diagnosis of bilateral varicocele: a comparative study of 4 diagnostic modalities. J Urol. 2004;172:1414–1417. doi: 10.1097/01.ju.0000138540.57137.5f. [DOI] [PubMed] [Google Scholar]
- 7.Gat Y, Gornish M, Navon U, Chakraborty J, Bachar GN, Ben-Shlomo I. Right varicocele and hypoxia, crucial factors in male infertility: fluid mechanics analysis of the impaired testicular drainage system. Reprod Biomed Online. 2006;13:510–515. doi: 10.1016/s1472-6483(10)60638-4. [DOI] [PubMed] [Google Scholar]
- 8.Petros JA, Andriole GL, Middleton WD, Picus DA. Correlation of testicular color Doppler ultrasonography, physical examination and venography in the detection of left varicoceles in men with infertility. J Urol. 1991;145:785–788. doi: 10.1016/s0022-5347(17)38451-3. [DOI] [PubMed] [Google Scholar]
- 9.Chiou RK, Anderson JC, Wobig RK, Rosinsky DE, Matamoros A, Jr, Chen WS, et al. Color Doppler ultrasound criteria to diagnose varicoceles: correlation of a new scoring system with physical examination. Urology. 1997;50:953–956. doi: 10.1016/S0090-4295(97)00452-4. [DOI] [PubMed] [Google Scholar]
- 10.Kocakoc E, Serhatlioglu S, Kiris A, Bozgeyik Z, Ozdemir H, Bodakci MN. Color Doppler sonographic evaluation of inter-relations between diameter, reflux and flow volume of testicular veins in varicocele. Eur J Radiol. 2003;47:251–256. doi: 10.1016/s0720-048x(02)00182-1. [DOI] [PubMed] [Google Scholar]
- 11.Liguori G, Trombetta C, Garaffa G, Bucci S, Gattuccio I, Salamè L, et al. Color Doppler ultrasound investigation of varicocele. World J Urol. 2004;22:378–381. doi: 10.1007/s00345-004-0421-0. [DOI] [PubMed] [Google Scholar]
- 12.Dhabuwala CB, Kumar AB, Kerkar PD, Bhutawala A, Pierce J. Patterns of Doppler recordings and its relationship to varicocele in infertile men. Int J Androl. 1989;12:430–438. doi: 10.1111/j.1365-2605.1989.tb01333.x. [DOI] [PubMed] [Google Scholar]
- 13.Hoekstra T, Witt MA. The correlation of internal spermatic vein palpability with ultrasonographic diameter and reversal of venous flow. J Urol. 1995;153:82–84. doi: 10.1097/00005392-199501000-00029. [DOI] [PubMed] [Google Scholar]
- 14.Hussein AF. The role of color Doppler ultrasound in prediction of the outcome of microsurgical subinguinal varicocelectomy. J Urol. 2006;176:2141–2145. doi: 10.1016/j.juro.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 15.Cina A, Minnetti M, Pirronti T, Vittoria Spampinato M, Canadè A, Oliva G, et al. Sonographic quantitative evaluation of scrotal veins in healthy subjects: normative values and implications for the diagnosis of varicocele. Eur Urol. 2006;50:345–350. doi: 10.1016/j.eururo.2006.02.055. [DOI] [PubMed] [Google Scholar]
- 16.Steckel J, Dicker AP, Goldstein M. Relationship between varicocele size and response to varicocelectomy. J Urol. 1993;149:769–771. doi: 10.1016/s0022-5347(17)36203-1. [DOI] [PubMed] [Google Scholar]
- 17.Jarow JP, Ogle SR, Eskew LA. Seminal improvement following repair of ultrasound detected subclinical varicoceles. J Urol. 1996;155:1287–1290. [PubMed] [Google Scholar]
- 18.Pasqualotto FF, Lucon AM, de Góes PM, Sobreiro BP, Hallak J, Pasqualotto EB, et al. Relationship between the number of veins ligated in a varicocelectomy with testicular volume, hormonal levels and semen parameters outcome. J Assist Reprod Genet. 2005;22:245–249. doi: 10.1007/s10815-005-5147-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shindel AW, Yan Y, Naughton CK. Does the number and size of veins ligated at left-sided microsurgical subinguinal varicocelectomy affect semen analysis outcomes? Urology. 2007;69:1176–1180. doi: 10.1016/j.urology.2007.01.086. [DOI] [PubMed] [Google Scholar]
- 20.Chen SS, Chen LK. Predictive factors of successful varicocelectomy in infertile patients. Urol Int. 2011;86:320–324. doi: 10.1159/000322825. [DOI] [PubMed] [Google Scholar]
- 21.Gat Y, Bachar GN, Zukerman Z, Belenky A, Gornish M. Varicocele: a bilateral disease. Fertil Steril. 2004;81:424–429. doi: 10.1016/j.fertnstert.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 22.Gat Y, Zukerman Z, Chakraborty J, Gornish M. Varicocele, hypoxia and male infertility. Fluid Mechanics analysis of the impaired testicular venous drainage system. Hum Reprod. 2005;20:2614–2619. doi: 10.1093/humrep/dei089. [DOI] [PubMed] [Google Scholar]
- 23.Gat Y, Gornish M, Heiblum M, Joshua S. Reversal of benign prostate hyperplasia by selective occlusion of impaired venous drainage in the male reproductive system: novel mechanism, new treatment. Andrologia. 2008;40:273–281. doi: 10.1111/j.1439-0272.2008.00883.x. [DOI] [PubMed] [Google Scholar]
- 24.Gat Y, Gornish M, Chakraborty J, Perlow A, Levinger U, Pasqualotto F. Azoospermia and maturation arrest: malfunction of valves in erect poster of humans leads to hypoxia in sperm production site. Andrologia. 2010;42:389–394. doi: 10.1111/j.1439-0272.2010.01083.x. [DOI] [PubMed] [Google Scholar]
- 25.Dubin L, Amelar RD. Etiologic factors in 1294 consecutive cases of male infertility. Fertil Steril. 1971;22:469–474. doi: 10.1016/s0015-0282(16)38400-x. [DOI] [PubMed] [Google Scholar]
- 26.Kursh ED. What is the incidence of varicocele in a fertile population? Fertil Steril. 1987;48:510–511. doi: 10.1016/s0015-0282(16)59432-1. [DOI] [PubMed] [Google Scholar]
- 27.Belani JS, Yan Y, Naughton CK. Does varicocele grade predict vein number and size at microsurgical subinguinal repair? Urology. 2004;64:137–139. doi: 10.1016/j.urology.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 28.Hopps CV, Lemer ML, Schlegel PN, Goldstein M. Intraoperative varicocele anatomy: a microscopic study of the inguinal versus subinguinal approach. J Urol. 2003;170:2366–2370. doi: 10.1097/01.ju.0000097400.67715.f8. [DOI] [PubMed] [Google Scholar]
- 29.Sherwood L. Human Physiology: From Cells to Systems. 7th ed. Belmont: Brooks/Cole; 2010. pp. 362–363. [Google Scholar]
- 30.Guyton AC, Hall JE. Text book of medical physiology. 10th ed. Philadelphia: Saunders Elsevier; 2000. Overview of the circulation; Medical physics of pressure, flow, and resistance; pp. 144–151. [Google Scholar]
- 31.Hirsh AV, Cameron KM, Tyler JP, Simpson J, Pryor JP. The Doppler assessment of varicoceles and internal spermatic vein reflux in infertile men. Br J Urol. 1980;52:50–56. doi: 10.1111/j.1464-410x.1980.tb02919.x. [DOI] [PubMed] [Google Scholar]