Abstract
Retention of misfolded proteins by the endoplasmic reticulum (ER) is a quality control mechanism involving the participation of endogenous chaperones such as calnexin (CANX) which interact and restrict plasma membrane expression of gonadotropin releasing hormone receptor (GnRHR), a G protein coupled receptor. CANX also interacts with ERP-57, a thiol oxidoreductase chaperone present in the ER. CANX along with ERP-57, promotes the formation of disulfide bond bridges in nascent proteins. The human GnRH receptor (hGnRHR) is stabilized by two disulfide bond bridges (Cys14-Cys200 and Cys114-Cys196), that, when broken, its expression at plasma membrane decreases. To determine if the presence of chaperones CANX and ERP-57 exert an influence over membrane routing and second messenger activation, we assessed the effect of various mutants including those with broken bridges (Cys→Ala) along with the wild type hGnRHR. The effect of chaperones on mutants was insignificant, whereas the overexpression of ERP-57 led to a wild type hGnRHR retention which was further enhanced by cotransfection with CANX cDNA disclosing receptor retention by ERP-57 augmented by CANX, suggesting a quality control mechanism.
Keywords: hormone action, GPRC, protein folding, GnRH, receptor, protein retention, chaperone
INTRODUCTION
The human gonadotropin releasing hormone receptor (hGnRHR) is a G protein coupled receptor (GPCR) [1–3], stabilized by the presence of two disulfide bond bridges located between residues Cys114-Cys196 and Cys14-Cys200. These bridges provide the structural characteristics of the receptor that appear to be essential for its proper membrane expression [3, 4]. The GnRHR shows a pattern of convergent evolution that has lead to restriction of the plasma membrane expression (PME); as in the case of primate GnRHRs, where only about 50% is expressed in plasma membranes, in comparison to the total amount synthesized. Restriction of expression seems to be dependent of the presence of amino acid Lys191, absent in murine and rat sequences, and is usually replaced by Glu191 in non-rodent and non-primate mammals. When residue 191 is deleted from the amino acid sequence of hGnRHR, the PME is notoriously increased [5, 6]. Another interesting feature of GnRHR is the presence of a carboxyl terminal extension in reptiles, birds and fishes, but absent in mammals. When this extension is added to the hGnRHR, PME is also increased [7]. In clinical settings where mutations in the hGnRH receptor cause hypogonadotropic hypogonadism, there is a diminished expression due to hGnRHR misfolding that results in protein misrouting and inappropriate receptor trafficking. The observation that these mutations can be rescued with pharmacological chaperones, refolded and routed to the membrane may provide a therapeutic approach [8–11] for mutant receptor rescue within the endoplasmic reticulum (ER) [8–13]. The ER contains molecular chaperone proteins that retain misfolded or unfolded proteins [14] and targets them for degradation [14–16], albeit a dominant-negative effect, due to retention of wild type (WT) GnRHR forms contained in ER when mutant forms are present. This condition enables these proteins to be processed as oligomers while the chaperone system assesses the overall quality of the mutant-WT complex, often recognizing hetero-oligomers as defective [17]. GnRHR contains two disulfide bond bridges that tend to oxidize in the ER due to its high oxidative redox potential, hence promoting a disulfide bond formation, folding and oligomerization [14], of a determined array of chaperones.
Endoplasmic reticulum protein 57 (ERP-57) is a (54 kDa protein of the of the thiol oxidoreductase family with disulfide oxidoreductase and isomerase activity, particularly for N-glycosylated proteins and glycoproteins with no cysteine residues (also known as PDIA3), [18–25]. ERP-57 interacts with calnexin (CANX) a chaperone protein, and a lectin that directly binds glycoproteins through a transient oligosaccharide intermediate [26–28], thought to prevent a rapid degradation, as well as ER retention, of misfolded proteins [29, 30]. The presence of ERP-57 and CANX is apparently essential for binding the glycoprotein. Subsequently, ERP-57 is recruited by CANX and Calreticulin (CALR) to oxidize disulfide bonds [31] to modulate the folding process [20, 32, 33]. The actual interaction of CANX and ERP-57, seems to go through the tip of the arm domains of the lectin [34–36]. CANX interaction with glycoproteins may depend on the phosphorylation state of CANX cytoplasmic carboxyl terminus [37] and a decrease of PME of GnRHR retaining misfolded receptors and correct routing of folded ones to the plasma membrane [38].
The present study assesses the effect of GnRHR on PME when cotransfected with ERP-57, as the effect of this PME when cotransfecting GnRHR with a group of mutant receptors that were selected due to their differential expression levels in comparison to the wild type receptor (WT); of particular interest were receptors with broken disulfide bond bridges: Cys114Ala, Cys196Ala and Cys14Ala, Cys200Ala. The integrity of both cysteine bridges (Cys114-Cys195/196 and Cys14-Cys199/200) is required by the human receptor. The presence of these mutations in the hGnRHR is reflected by a decrease in PME, while the rat and mouse receptors are not affected by mutations in the Cys14-Cys199/200 [39]. Other mutations used were the construct of a rat extra cellular loop 2 (ECL2), based on differences of this particular loop between rats and humans (mutations at Ser186Gly, Gln189Pro and Ser203Pro). Due to its proximity with the disulfide bond bridge, we expected it to exert an effect upon the bridge formation [39]. Another group of mutants, used in this study, were selected because they also alter WT GnRHR PME. These include, a non-contiguous 4 amino acid sequence that cause a loss in the requirement for the Cys14-Cys/200 bridge: hWT+M (Leu112Phe/Gln208Glu/Leu300Val/Asp302Glu, these replace the human residues with orthologous rat residues called “M” as a shorthand [39]) and an extension found in the catfish GnRHR C-terminal (hWTGnRHR+C-tail); a feature lost in mammals. Both of this mutations, independently and added as a chimera to the human sequence, increase PME [7].
MATERIALS AND METHODS
Materials
The GnRH agonist, Buserelin(D-tert-butyl-Ser6, des-Gly10, Pro9, ethylamide-GnRH) was obtained through Hoechst-Roussel Pharmaceuticals (Somerville, NJ). DMEM, OPTI-MEM, Lipofectamine and PCR reagents were purchased from Invitrogen (Carlsbad, CA). Restriction enzymes, modified enzymes and competent cells for subcloning were purchased from Promega Corp. (Madison, WI). The Endofree Maxi-prep kit was purchased from QIAGEN (Valencia, CA). ERP-57, ERP-72 and Calnexin (CANX) cDNA was obtained from Open Biosystems (Huntsville, Al. MHS1011-9199116, MHS1011-75436 and MHS1011-60083, respectively). IN3, (2S)-2-[5-[2-(2-azabicyclo[2.2.2]oct-2-yl)-1,1-dimethyl-2-oxo-ethyl]-2-(3,5-dimethylphenyl)-1H-indol-3-yl]-N-(2-pyridin-4-ylethyl)-propan-1-amine, was obtained from Merck Co., Inc. (Rahway, NJ).
Wild and Mutant Receptors
The wild-type (WT) hGnRHR cDNA was subcloned intopcDNA3.1 at KpnI and XbaI restriction enzymes sites. Mutant receptor cDNAs for transfection were constructed by overlap extension PCR (38, 39); the purity and identity of plasmid DNA was verified by dye terminator cycle sequencing, Applied Biosystems (Foster City, CA).
Chaperone Proteins
Chaperone proteins CANX, ERP-57 and ERP-72 were subcloned into pcDNA3.1 using standard PCR conditions. Engineered restriction sites were incorporated at the 5′ and 3′ ends of the protein nucleotide sequence to allow subcloning into pcDNA3.1 at KpnI and Xba1 restriction sites. Kozak’s consensus sequence was additionally included in the primer design, and the sequences are as follows: 5′gtacggtaccaccgccaccatgxxxxxxx3′ and 5′gtactctagattaxxxxxxx3′, where “x” denotes the first and last 5–7 codons of chaperone nucleotide sequences.
Mutant Chaperone ERP-57 (Phe299Trp)
This mutant was constructed with the ERP-57 sequence, changing Phe299 to Trp299 through overlap extension PCR. Precision and identity of plasmid DNA was verified by dye terminator cycle sequencing, Applied Biosystems (Foster City, CA).
Transient transfection and Co-transfection
Cos 7 cells were cultured and plated in growth medium (DMEM, 10% fetal calf serum (FCS), 20μg/mL gentamicin) at 37°C and 5% CO2 in a humidified atmosphere incubator. Medium was previously warmed to 37°C in water bath and then added to cells. For cotransfection of mutant or WT receptors as well as chaperone proteins CANX, ERP-57, ERP-72 and mutant ERP-57 Phe299Trp, 5×104 cells were plated in 0.25mL of growth medium in 48-well Costar cell culture plates. After a 24 hour period, cells were washed with 0.25mL/well OPTI-MEM, then transfected with WT or mutant GnRHR (25ng/well), accordingly CANX, ERP-57, ERP-72 and ERP-57 Phe299Trp was also transfected, empty vector (pcDNA 3.1 without insert “empty vector” was used to bring the total cDNA to 100 ng/well). Lipofectamine was used according to manufacturer’s instructions. Five hours after transfection, 0.125mL DMEM with 20% FCS and 20μg/mL and gentamicin was added to the wells [40].
Inositol Phosphate (IP) Assays
Twenty-three hours after transfection the medium was removed and replaced with 0.25mL of fresh growth medium, IN3 was added, where indicated, in 1% final DMSO (vehicle) in respective media to cells for 4 hours (50). Twenty-seven hours after transfection the cells were washed twice with 0.5mL DMEM containing 0.1% BSA/20μg/mL gentamicin then “pre-loaded” for 18 hours with 0.25mL of 4μCi/mL myo-[2-3 H (N)]-inositol, in inositol free DMEM, then washed twice with 0.30mL DMEM (inositol free) containing 5mM LiCl and treated for 2 hours with 0.25mL of a saturating concentration of Buserelin (10−7 M) in the same medium. Total IP was determined by Dowex Anion exchange chromatography and liquid scintillation spectroscopy [41]. In the range of 0–100 ng receptor DNA, IP production is a good measure of plasma membrane expression of the hGnRHR [17, 39].
Statistics
Experiments were repeated at least 3 times with triplicate determinations in each showing the means ± SEM of replicates by experiment; significance was determined by ANOVA and verified with individual Student’s t test. P < 0.05 for a degree of significance (SigmaStat 3.0, Jandel Scientific Software, Chicago IL).
RESULTS
Cotransfection of ERP-57 chaperone with broken disulfide bond bridge mutant hGnRH receptors
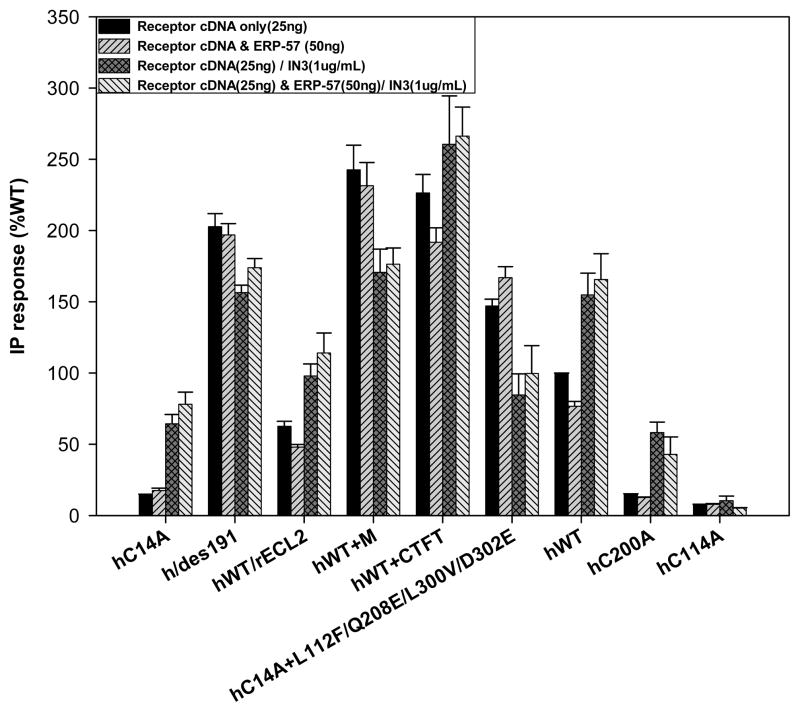
The ER chaperone ERP-57, with its thiol oxide reductase activity, was cotransfected with the hGnRHR mutants that had broken disulfide bond bridges due to mutation (Cys114Ala, Cys196Ala, Cys14Ala and Cys200Ala). We observed no significant difference in the Inositol Phosphate (IP) response to 10−7 Buserelin comparing those receptors cotransfected with or without ERP-57 (50ng). This experiment was performed since the Cys14-Cys200 bridge is essential for proper receptor trafficking in mammalian species other than rat and human [39]. Consistent with this observation, the Cys14-Cys200 bridge appeared to impact expression of hGnRHR, but not the rat counterpart [39] When rescue was attempted with pharmacoperone IN3, we obtained an increase in the PME, still, the difference between both groups (i.e. with and without over-expressed ERP-57 cDNA) was not significant (Figure 1). An interesting observation was the significant decrease in total IP production when ERP-57 was cotransfected with WT hGnRHR, an effect that was completely lost when cells were treated with pharmacoperone IN3 (Figure 1).
Figure 1.
Cotransfection was performed with the hWT GnRHR as well as hGnRHR mutants in COS-7 cells due to their different IP production. Each one of these proteins was stimulated and their inositol phosphate (IP) production determined with and without chaperone ERP-57 (50ng). Further on, pharmacoperone rescue was attempted using IN3 (1μg/mL) where the IP production is increased in most of these receptors. As observed, hWT GnRHR IP production is decreased when cotransfected with ERP-57(50ng), rescue is evident with pharmacoperone IN3 (1μg/mL). Mutations such as hC14A, hC200A and hC114A prevent disulfide bond bridge formation in the hGnRHR, hence a lower IP production, other mutations such as h/des191, hWT+CTFT and hWT+M actually increase IP production (“M” is a shorthand notation for receptor with mutations in Leu112Phe/Gln208Glu/Leu300Val/Asp302Glu. The hWT/rECL2 mutant construct of a rat extracellular loop 2, based on the differences of this particular loop between rats and humans (mutations at Ser186Gly, Gln189Pro and Ser203Pro) has also been seen to have variations in IP production. “C-tail” refers to the carboxyl terminal extension found in catfish GnRHR, a feature lost in mammals that increases PME (7).
ERP-57 had no effect when K191 was deleted from sequence, which already had a greater expression when compared to the WT receptor. The same situation was observed in other mutants such as hWT+M (“M” is a shorthand notation for receptor with mutations Leu112Phe/Gln208Glu/Leu300Val/Asp302Glu) that replaces the human residues with orthologous rat residues. These mutations, which convert the hGnRHR to the rat ortholog of four residues, also conveys rat receptor characteristics (loss of dependence on the Cys14-Cys200 bridge and greater PME) upon the human receptor [39], as does the hWT+C-tail (catfish tail carboxyl terminal extension) [7]. The only mutant that presented a slight decrease in expression was hWT+rECL2 (mutations on the ECL2 makes it more rat like, Ser186Gly, Gln189Pro and Ser203Pro) with an average expression variation of 50.2%.
Cotransfection with ERP-57 and CANX
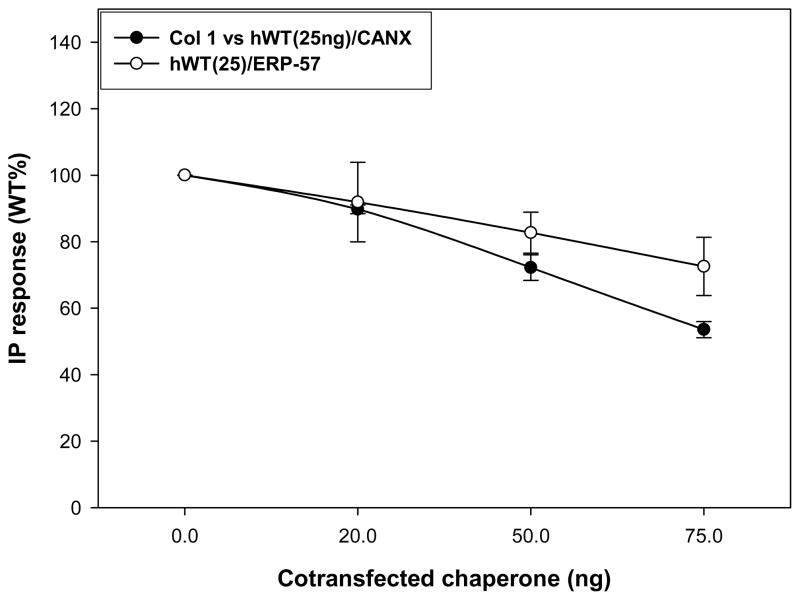
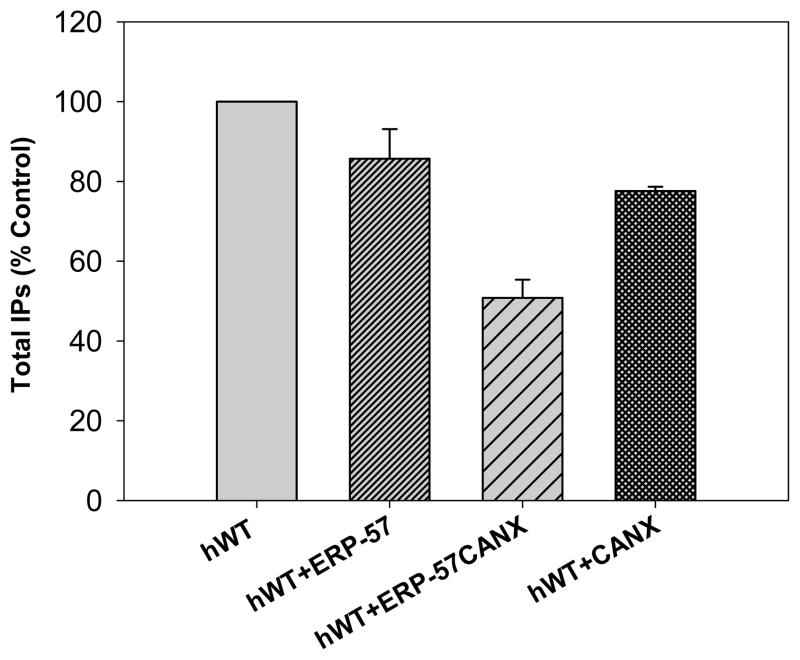
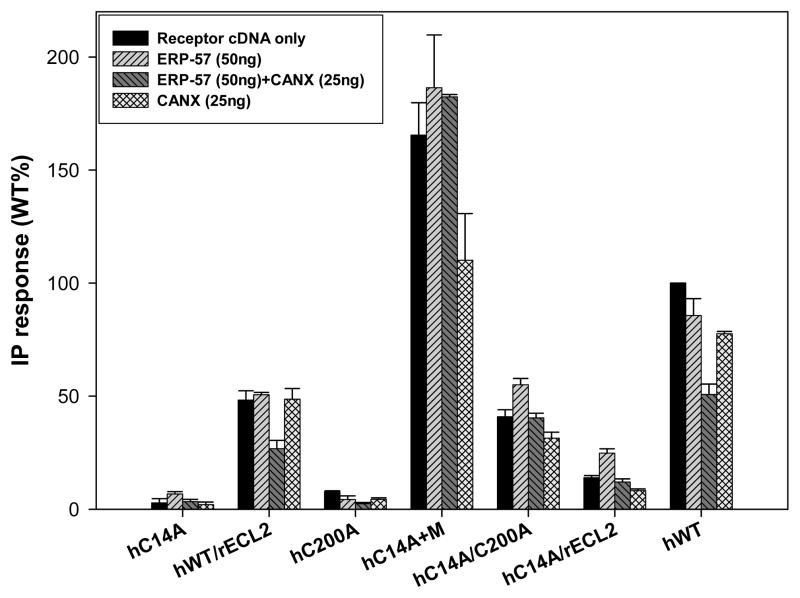
Cotransfecting CANX with hWT GnRH cDNA (or that from other proteins) resulted in a marked decrease in their PME [29, 30, 38]. This decrease in receptor PME observed with increasing amounts of cotransfected ERP-57 (50ng) and CANX (25ng) is shown in Figure 2; CANX being the one to present a lower PME and probably a higher retention efficiency of the protein. Since ERP-57 interacts with CANX to achieve thiol reductase function, we cotransfected both chaperones with mutants and WT GnRH receptors. There was no significant change in PME with mutants such as hCys14Ala, hCys200Ala and hCys14Ala/Cys200Ala. An impact was observed exclusively with hWT receptor, decreasing PME that increased when the two chaperones were cotransfected together (Figures 3, 4). The double mutant hCys14Ala/Cys200Ala presented an increased expression with ERP-57(50ng) only in comparison to chaperone free conditions, contrasting with mutants containing one Ala-substituted side of the disulfide bond bridge, as in hCys14Ala or hCys200Ala. Cotransfecting ERP-57 (50ng) and CANX (25ng) with several of these mutants revealed that only the PME of the hWT was altered when CANX and ERP-57 were cotransfected (Figures 3, 4).
Figure 2.
A dose response curve was elaborated in order to evidence the most dramatic and significant IP production restriction by the chaperones ERP-57 and Calnexin (CANX) when cotransfected with hWT GnRHR (25ng). Co-transfection of CANX or ERP-57 cDNAs restricts human WT GnRH receptor plasma membrane expression. Total inositol phosphate (IP) production, as a result of transfecting increasing amounts (25–75 ng) of chaperone (CANX) and disulfide oxidoreductase (ERP-57), with a constant amount of human WT GnRH receptor (25ng) in Cos 7 cells.
Figure 3.
The hWT receptor IP percentage production further evidences the attenuating effect of co-transfection of ERP-57 and CANX cDNAs when compared to the hWT GnRHR, the effect of each chaperone protein (decreased IP production) and the combination of the two (a probable synergistic effect). Total inositol phosphate (IP) production of human WT GnRH receptor cDNA (25ng) cotransfected with: empty vector (75ng), ERP-57 (75ng), ERP-57 + CANX (50ng, 25ng), and CANX alone (25ng).
Figure 4.
Buserelin (a GnRH receptor agonist) dose response curves for Cos 7 cells expressing: human WT GnRH receptor, ERP-57, CANX + ERP-57, and CANX alone illustrate PME decrease, inferring receptor retention at the ER. Cos 7 cells were transiently transfected with human WT GnRH receptor (25ng) and: ERP-57 (75 ng), CANX + ERP-57 (50ng, 25ng), and CANX alone (25ng) and total inositol phosphate (IP) production was measured. The final amount of DNA was 100 ng by adding a complementary amount of empty vector.
Observations with mutant ERP-57
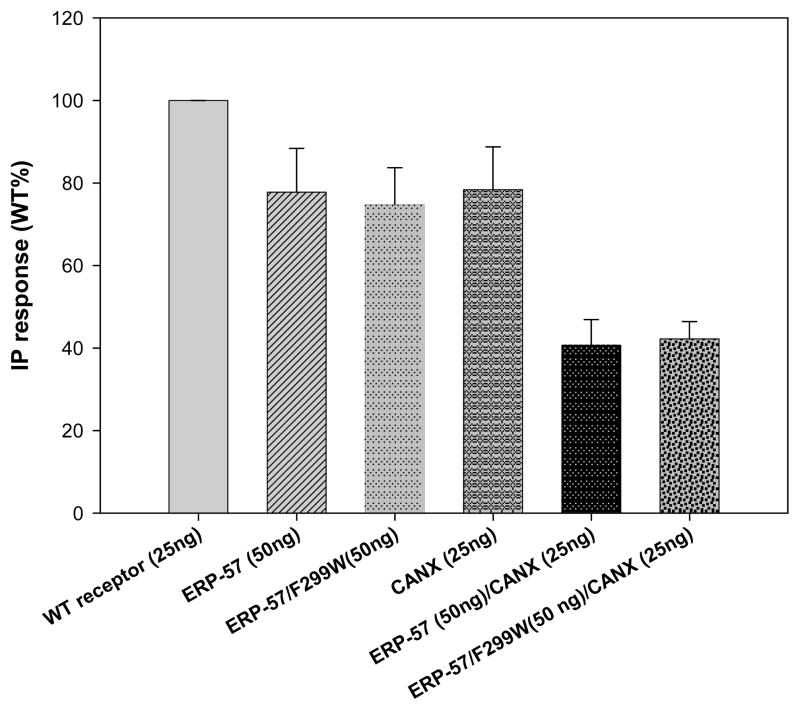
The interaction between ERP-57 and CANX has been suggested by a report [42] of a mutant with a point mutation at position 299 in the ERP-57 sequence (Phe→Trp), reducing the interaction between these two chaperones. We constructed the same mutant (ERP-57/Phe299Trp) and cotransfected it with our mutant and WT hGnRH receptors, comparing PME to the wild type ERP-57 chaperone. No significant differences were observed between these two, although both chaperones presented a decreased receptor PME. When cotransfected with CANX, the PME of the hGnRH receptors was even lower than seen previously, yet no difference between the wild type and mutant ERP-57 chaperone was registered (Figure 5) even when CANX was additionally transfected.
Figure 5.
Cotransfection was performed with the hWT GnRHR as well as hGnRHR mutants in COS-7 cells due to their different IP production. Each one of these proteins was stimulated and their inositol phosphate (IP) production determined without any cotransfected chaperone, with ERP-57 (50ng), CANX (25ng) and ERP-57(50ng)+CANX(25ng). The hWT GnRHR IP production is decreased when cotransfected with ERP-57(50ng) and with CANX (25ng), further restriction is evident when both of these proteins are cotransfected with the hWT GnRHR. Mutations such as hC14A, hC200A and hC14/C200A will prevent disulfide bond bridge formation in the hGnRHR, hence a lower IP production, other mutations such as hC14a+M will increase IP production (“M” is a shorthand notation for receptor with mutations in Leu112Phe/Gln208Glu/Leu300Val/Asp302Glu. (7)). Total inositol phosphate (IP) production of human WT GnRH receptor cDNA (25ng) cotransfected with: empty vector (75ng), ERP-57 (75 ng), ERP-57 + CANX (50ng, 25ng), and CANX alone (25ng). The final amount of DNA was 100ng by adding a complementary amount of empty vector.
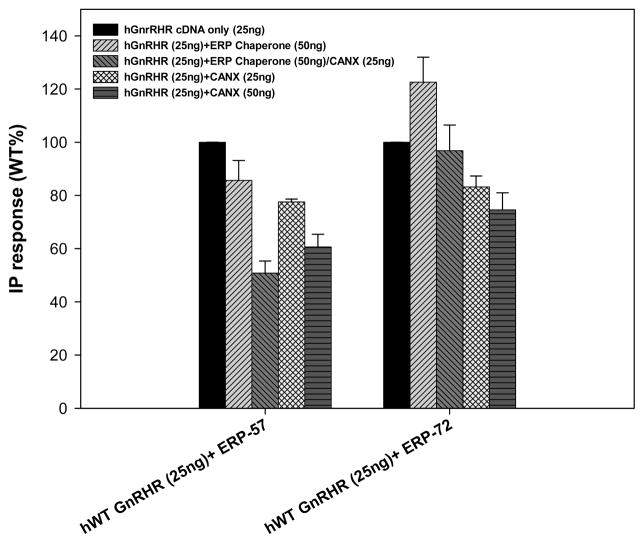
Observations with mutant ERP-72 chaperone
ERP-72 is another member of the Protein Disulfide Isomerase family, sharing sequence identity as well as isomerase activity [45–47] with PDI. Transfecting this chaperone with hWT and mutant GnRH receptors yielded no PME modification; hWT GnRHR presented greater expression with ERP-72+CANX than the receptor cotransfected with ERP-57. This could be attributed to the ERP-57+CANX interaction mentioned previously (Figures 6 and 7). The ERP-72 chaperone actually increased PME in comparison with chaperone free hWT GnRHR. Receptor rescue experiments performed with pharmacoperone IN3 showed rescue of the protein expression, yet failed to be significant enough to reveal any effect generated by the cotransfection of ERP-57 and CANX.
Figure 6.
Total IP production response was further determined for the ERP-57 mutant receptor ERP-57 (Phe299Trp), although IP production is restricted, compared to the chaperone free production, no difference was observed in the effect of this mutant compared to the non-mutant ERP-57; such an observation is also made when this mutant was co-transfected with calnexin. All Cos 7 cells were transiently transfected with hWT GnRH cDNA (25 ng) and different chaperones were added: ERP-57 (75 ng), ERP-57/Phe299Trp mutant (75ng), Calnexin (75ng), ERP-57 (50ng) and CANX (25ng), ERP-57/Phe299Trp mutant (50 ng) and CANX (25ng).
Figure 7.
ERP-57 and ERP-72 action, diminishing PME of WT GnRH receptors, as measured by total inositol phosphate (IP) production in Cos 7 cells transiently cotransfected with human WT GnRH receptor (25ng) and chaperones: ERP-57(50ng), ERP-57 (50ng) and CANX (25ng), CANX (25ng) and CANX (50ng); same combinations for ERP-72. ERP72 exhibited a non-significant IP production increase, yet no restriction was observed when co-transfected with Calnexin.
DISCUSSION
Human GnRHR mutants with broken disulfide bond bridges (Cys14Ala, Cys200Ala, Cys114Ala, Cys196Ala) do not assume the conformation needed to pass the cellular quality control system (QCS), due to misfolding, resulting in ER retention and inability to reach the plasma membrane. These mutants exhibit a low PME compared with the hWT hGnRHR, hence, integrity of both disulfide bond bridges is key for the human GnRHR (Cys14-Cys200 and Cys114-Cys196), yet this is not true for other species [39].
Cotransfecting these mutants with ERP-57 (with its thiol oxide reductase activity) did not have any significant difference in the IP response with receptors cotransfected without this chaperone. Rescue with pharmacoperone IN3 resulted in a threefold increase in the PME of both groups.
Previous observations have revealed a diminished PME when the disulfide bond bridges are broken by mutating the residues on either side of the bridge (Cys14Ala, Cys200Ala, Cys114Ala and Cys196) [39]. Cotransfecting ERP-57 (50 ng), a chaperone that is part of the QCS within the ER, one would expect an interaction where ERP-57 could increase PME due to its thiol oxide reductase activity or diminish the expression by identifying the mutant as improperly folded. The receptor, with the double mutation Cys14Ala/Cys200Ala, had an increased expression when cotransfected with ERP-57 as opposed to the chaperone free mutant (Figure 5); one might speculate that the lack of the disulfide bond is compensated by other modification due to ERP-57, where cysteine residues could be involved, forming disulfide bonds as described by Farmery et al. [43].
We examined the effect of ERP-57 on other GnRHR mutants, such as those lacking K191. It has been observed that cow, sheep and humans include this particular amino acid in the GnRHR sequence; its deletion significantly increased PME [6], as observed with WT hGnRH containing the C-tail. When cotransfecting these particular mutants with chaperone protein ERP-57 (50ng), no significant difference was found between receptor PME, with or without the chaperone protein. This could be due to the fact that this particular mutant does not interact with the chaperone. Both these mutants (hGnRHR des K191 and WT hGnRH+C-tail) have a greater PME than the hWT receptor [8]. The K191 seems to be characteristic of primates and its deletion increases the efficiency in receptor routing to PM, likely by modifying receptor conformation and increasing chances of forming the Cys14-Cys200 bridge.
Addition of the catfish tail sequence is also known to increase the hGnRHR PME [7], since it is quite probable that the lack of the catfish tail is accompanied by structural rearrangements in other parts of the receptor, resulting in lower PME. Chaperone interaction may also be important in the recognition of these conformational differences which may be identified by other chaperones, or a set of several interacting chaperone proteins. Due to the significantly increased expression of these mutants, ERP-57 chaperone activity may be unperceivable in these particular cases.
Other mutants tested were WT hGnRH + rECL2. Point mutations in the ECL2 render the receptor more rat-like (Gly186/Pro189/Pro202) [39], hence diminishing PME; also the WT hGnRHR + M (“M” is a shorthand notation for receptor with mutations in Leu112Phe/Gln208Glu/Leu300Val/Asp302Glu), a series of mutations that were all steric (no major change in their charge or hydrophobicity) [39], had dramatic increases in the PME of the receptor. Still none of these had significant modifications in their levels of IP production when cotransfected with chaperone ERP-57 (50ng).
Chaperone protein ERP-57 may not recognize some point mutations, or simply not interact with the hGnRHR when certain point mutations are present in the ECL2 as well as the disulfide bond bridges. Still, a significant finding was a 14% decrease in IP production when ERP-57 was cotransfected with the human WT receptor; a finding that was further enhanced (49%) when this chaperone was cotransfected in combination with CANX (25ng), another chaperone of the QCS within the ER, whose PME restriction qualities have been described on this receptor [38].
Cotransfection with ERP-57 and CANX
Cotransfecting the hWT GnRHR with ERP-57 and CANX yielded a 49% decreased expression in comparison to the WT receptor free of any chaperone interaction. The PME was decreased by 22% when hWT GnRHR was cotransfected with CANX only. ERP-57 recruited by CANX to oxidize disulfide bonds, being that both chaperones are part of the QCS in the ER and together they seem to recognize hWT GnRHR as an improperly folded protein and were being retained in the ER [29,30,38], hence decreasing IP production CANX and hGnRHR interactions have shown a diminished IP production when cotransfected together [38]. Therefore, interaction between these chaperones enhanced receptor retention within the ER. ERP-57 has been observed to provide initial protection to glycoprotein degradation rather than CANX. Later on, roles seem to be exchanged and ERP-57 moved on to its thiol oxidoreductase function [44]; this might also explain the reduced expression of the receptor when GnRHR is cotransfected with ERP-57 only.
Mutant ERP-57 Chaperone Protein
Once we had observed a high PME restriction with CANX and ERP-57 combined, we tested the actual interactions between these two chaperones by constructing an ERP-57 mutant described by Russel SJ et al [42]. This mutant (ERP-57 Phe299Trp) is reported to have less than a 17% interaction with CANX and CALR. We cotransfected this mutant chaperone with hWT GnRHR and found no significant difference in PME compared to normal ERP-57, in combination with hWT GnRHR. Adding CANX to these chaperones did not alter the difference between the mutant chaperone and the normal one (no significant difference, p ≥ 0.05); still both presented a mean 58.57% less IP production than hWT GnRHR. Our observations indicate no interaction between these two chaperones in the presence of hGnRHR, still both exerted a PME restriction. One might also conclude that these two chaperones act in different segments of the receptor structure, and instead of this being a synergistic effect between the two chaperones, it is the net result of individual proteins exerting their own effect. Further studies are needed to establish the actual effect and interaction of these two chaperone proteins and their effect on the hWT GnRHR.
Observations with mutant ERlP-72 Chaperone Protein
Another important member of the Protein Disulfide Isomerase family is ERP-72, which shares sequence identity with PDI and ERP-57; and, as such, it is recognized to function both as a disulfide oxidase and reductase, catalyzing the formation of disulfide bonds in nascent proteins [45–47], although interactions with ERP-57 and CANX have been reported [34–36], the same cannot be said for ERP-72. This chaperone was cotransfected with hGnRHR to see how it affected the IP production (PME), using the same amount of chaperone protein as in the ERP-57 experiments (50ng). An increased IP production was observed compared to the expression of the hWT GnRHR without any chaperone interaction; although this increase by ERP-72 on hWT GnRHR is not statistically significant (p ≥ 0.05). ERP-72 and CANX have been reported to interact, yet their relation remains unclear [48]. Further experiments are needed to establish the actual interaction between ERP-72 and hWT GnRHR.
These experiments show hWT GnRHR is a protein whose expression is modified by the QCS chaperones. Their interaction may be complicated by the fact that many of these proteins interact among themselves before exerting any effect on the nascent protein. Other chaperones, such as HSP-90 and CALX, may be tested to see their own specific effect on the hGnRHR as well as further detail in the interaction of ERP-57 and ERP-72 with this specific receptor. Thiol oxidase/reductase chaperones are an important component of the ER QCS that is still being studied. Since these chaperones interact with cysteine residues, they probably also interact with the hWT GnRHR at the ECL-2 (a site that contains the cysteine residues), the disulfide bond bridges and the Lys191 residue—when deleted, it dramatically increases PME.
Our research failed to identify a specific site where ERP-57, with or without CANX, may interact with the human GnRHR, but it shows that the WT receptor IP production and therefore PME is affected by these two chaperone proteins. Different interactions may take place with ERP-72, but these do not seem to be as important as the ones with ERP-57.
Acknowledgments
This research was supported by NIH grants HD-19899, RR-00163, HD18185, TW/HD-00668.
References
- 1.Palczewski K, Kumasaka T, Hori T, Behnke CA, Motoshima H, Fox BA, Le Trong I, Teller DC, Okada T, Stenkamp RE, Yamamoto M, Miyano M. Crystal structure of rhodopsin: A G protein-coupled receptor. Science. 2000;289:739–745. doi: 10.1126/science.289.5480.739. [DOI] [PubMed] [Google Scholar]
- 2.Söderhall JA, Polymeropoulos EE, Paulini K, Gunther E, Kuhne R. Antagonist and agonist binding models of the human gonadotropin-releasing hormone receptor. Biochem Biophys Res Commun. 2005;333:568–582. doi: 10.1016/j.bbrc.2005.05.142. [DOI] [PubMed] [Google Scholar]
- 3.Millar RP, Lu ZL, Pawson AJ, Flanagan CA, Morgan K, Maudsley SR. Gonadotropin-releasing hormone receptors. Endocr Rev. 2004;25:235–275. doi: 10.1210/er.2003-0002. [DOI] [PubMed] [Google Scholar]
- 4.Davidson JS, Assefa D, Pawson A, Davies P, Hapgood J, Becker I, Flanagan C, Roeske R, Millar R. Irreversible activation of the gonadotropin-releasing hormone receptor by photoaffinity cross-linking: localization of attachment site to Cys residue in N-terminal segment. Biochemistry. 1997;36:12881–12889. doi: 10.1021/bi971377t. [DOI] [PubMed] [Google Scholar]
- 5.Arora KK, Chung HO, Catt KJ. Influence of a species-specific extracelular aminoacid on expression and function of the human gonadotropin releasing hormone receptor. Mol Endocrinol. 1999;13:890–896. doi: 10.1210/me.13.6.890. [DOI] [PubMed] [Google Scholar]
- 6.Maya-Nuñez G, Janovick JA, Conn P. Combined modification of intracellular and extracellular loci on human gonadotropin-releasing hormone receptor provides a mechanism for enhanced expression. Endocrine. 2000;13:401–407. doi: 10.1385/ENDO:13:3:401. [DOI] [PubMed] [Google Scholar]
- 7.Lin X, Janovick JA, Brothers S, Blomenrohr M, Bogerd J, Conn PM. Addition of catfish gonadotropin-releasing hormone (GnRH) receptor intracelular carboxyl-terminal tail to rat GnRH receptor alters receptor expression and regulation. Mol Endocrinol. 1998;12:161–171. doi: 10.1210/me.12.2.161. [DOI] [PubMed] [Google Scholar]
- 8.Conn PM, Leaños Miranda A, Janovick JA. Protein origami: therapeutic rescue of misfolded gene products. Mol Interv. 2002;2:308–316. doi: 10.1124/mi.2.5.308. [DOI] [PubMed] [Google Scholar]
- 9.Leaños-Miranda A, Ulloa-Aguirre A, Janovick JA, Conn PM. In vitro coexpression and pharmacological rescue of mutant gonadotropin-releasing hormona receptors causing hypogonadotrophic hypogonadism in humans expressing compound heterozygous alleles. J Clin Endocrinol Metab. 2005;90:3001–3008. doi: 10.1210/jc.2004-2071. [DOI] [PubMed] [Google Scholar]
- 10.Ulloa-Aguirre A, Janovick JA, Leaños Miranda A, Conn PM. Misrouted cell surface GnRH receptors as a disease aetiology for congenital isolated hypogonadotrophic hypogonadism. Hum Reprod Update. 2004;10:177–192. doi: 10.1093/humupd/dmh015. [DOI] [PubMed] [Google Scholar]
- 11.Ulloa-Aguirre A, Janovick JA, Brothers SP, Conn PM. Pharmacologic rescue of conformationally-defective proteins: implications for the treatment of human disease. Traffic. 2004;5:821–837. doi: 10.1111/j.1600-0854.2004.00232.x. [DOI] [PubMed] [Google Scholar]
- 12.Janovick JA, Maya Nuñez G, Conn PM. Rescue of hypogonadotrophic hypogonadism causing and manufactured GnRH receptor mutants by a specific protein-folding template: misrouted proteins as a novel disease etiology and therapeutic target. J Clin Endocrinol Metab. 2002;87:3255–3262. doi: 10.1210/jc.87.7.3255. [DOI] [PubMed] [Google Scholar]
- 13.Brothers SP, Janovick JA, Conn PM. Unexpected effects of epitope and chimeric tags on Gonadotropin-releasing hormone receptors: implications for understanding the molecular etiology of hypogonadotrophic hypogonadism. J Clin Endocrinol Metab. 2003;88:6107–6112. doi: 10.1210/jc.2003-031047. [DOI] [PubMed] [Google Scholar]
- 14.Dobson CM. Protein folding and misfolding. Nature. 2003;426:884–890. doi: 10.1038/nature02261. [DOI] [PubMed] [Google Scholar]
- 15.Stevens FJ, Argon Y. Protein folding in the ER. Semin Cell Dev Biol. 1999;10:443–454. doi: 10.1006/scdb.1999.0315. [DOI] [PubMed] [Google Scholar]
- 16.Ellgaard L. Catalysis of disulphide bond formation in the endoplasmic reticulum. Biochem Soc Trans. 2004;32:663–667. doi: 10.1042/BST0320663. [DOI] [PubMed] [Google Scholar]
- 17.Brothers SP, Cornea A, Janovick JA, Conn PM. Human loss of function gonadotropin-releasing hormone receptor mutants retain wild-type receptors in the endoplasmic reticulum: molecular basis of the dominant-negative effect. Mol Endocrinol. 2004;18:1787–1797. doi: 10.1210/me.2004-0091. [DOI] [PubMed] [Google Scholar]
- 18.Creighton TE, Hillson DA, Freedman RB. Catalysis by protein-disulfide isomerase of the unfolding and refolding of proteins with disulfide bonds. J Mol Biol. 1980;142:43–62. doi: 10.1016/0022-2836(80)90205-3. DOI:0022-2836(80)90205-3. [DOI] [PubMed] [Google Scholar]
- 19.Lyles MM, Gilbert HF. Catalysis of the oxidative folding of ribonuclease A by protein disulfide isomerase: pre-steady-state kinetics and the utilization of the utilization of the oxidizing equivalents of the isomerase. Biochemistry. 1991;30:619–625. doi: 10.1021/bi00217a005. [DOI] [PubMed] [Google Scholar]
- 20.Olive JD, van der Wal FJ, Bulleid NJ, High S. Interaction of the thiol-dependent reductase ERP-57 with nascent glycoproteins. Science. 1997;275:86–88. doi: 10.1126/science.275.5296.86. [DOI] [PubMed] [Google Scholar]
- 21.Koivunen P, Helaakoski T, Annunen P, Veijola J, Raisanen S, Pihlajaniemi T, Kivirikko KI. ERP-60 does not substitute for protein disulphide isomerase as the beta-subunit of prolyl 4 hydroxylase. Biochem J. 1996;316:599–605. doi: 10.1042/bj3160599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferrari DM, Soling HD. The protein disulphide-isomerase family: unraveling a string of folds. Biochem J. 1999;339:1–10. [PMC free article] [PubMed] [Google Scholar]
- 23.Frickel EM, Frei P, Bouvier M, Stafford WF, Helenius A, Glocksuber R, Ellgarrd L. ERP-57 is a multifunctional thioldisulfide oxidoreductase. J Biol Chem. 2004;279:18277–18287. doi: 10.1074/jbc.M314089200. [DOI] [PubMed] [Google Scholar]
- 24.Srivastava SP, Fuchs JA, Holtzman JL. The reported cDNA sequence for phospholipase C alpha encodes protein disulphide isomerase, isoenzyme Q-2 and not phospholipase-C. Biochem Biophys Res Commun. 1993;193:971–978. doi: 10.1006/bbrc.1993.1720. [DOI] [PubMed] [Google Scholar]
- 25.Antoniou AN, Ford S, Alphey M, Osborne A, Elliott T, Powis SJ. The oxidoreductase ERP-57 efficiently reduces partially folded in preference to fully folded MHC class I molecules. EMBO J. 2002;21:2655–2663. doi: 10.1093/emboj/21.11.2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hammond C, Braakman I, Helenius A. Role of N-linked oligosaccharide recognition, glucose trimming, and calnexin in glycoprotein folding and quality control. Proc Natl Acad Sci USA. 1994;91:913–917. doi: 10.1073/pnas.91.3.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ware FE, Vassilakos A, Peterson PA, Jackson MR, Lehrman MA, Williams DB. The molecular chaperone calnexin binds GlcMan9GlcNAc2 oligosaccharide as an initial step in recognizing unfolded glycoproteins. J Biol Chem. 1995;270:4697–4704. doi: 10.1074/jbc.270.9.4697. [DOI] [PubMed] [Google Scholar]
- 28.Williams DB. Beyond lectins: the calnexin/Calreticulin chaperone system of the endoplasmic reticulum. J Cell Sci. 2006;119:615–623. doi: 10.1242/jcs.02856. [DOI] [PubMed] [Google Scholar]
- 29.Liu Y, Choudhury P, Cabral CM, Sifers RN. Oligosaccharide modification in the early secretory pathway directs the selection of a misfolded glycoprotein for degradation by the proteasome. J Biol Chem. 1999;274:5861–5867. doi: 10.1074/jbc.274.9.5861. [DOI] [PubMed] [Google Scholar]
- 30.Bedard K, Szabo E, Michalak M, Opas M. Cellular functions of endoplasmic reticulum chaperones calreticulin, calnexin and ERP-57. Int Rev Cytol. 2005;245:91–121. doi: 10.1016/S0074-7696(05)45004-4. [DOI] [PubMed] [Google Scholar]
- 31.Zapun A, Darby NJ, Tessier DC, Michalak M, Bergeron JJ, Thomas DY. Enhanced catalysis of ribonuclease B folding by the interaction of calnexin or calreticulin with ERP-57. J Biol Chem. 1998;273:6009–6012. doi: 10.1074/jbc.273.11. [DOI] [PubMed] [Google Scholar]
- 32.Leach MR, Williams DB. Lectin-deficient calnexin is capable of binding class I histocompatibility molecules in vivo and preventing their degradation. J Biol Chem. 2004;279:9072–9079. doi: 10.1074/jbc.M310788200. [DOI] [PubMed] [Google Scholar]
- 33.Oliver JD, Roderick HL, Llewllyn DH, High S. ERP-57 functions as a subunit of specific complexes formed with the ER lectins calreticulin and calnexin. Mol Biol Cell. 1999;10:2573–2582. doi: 10.1091/mbc.10.8.2573. DOI:10:2573-2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frickel EM, Riek R, Jelesarov I, Helenius A, Withrich K, Ellgaard L. TROSY-NMR reveals interaction between ERP-57 and the tip of the calreticulin P-domain. Proc Natl Acad Sci USA. 2002;99:1954–1959. doi: 10.1073/pnas.042699099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leach MR, Cohen-Doyle MF, Thomas DY, Williams DB. Localization of the lectin ERP-57 binding, and polypeptide binding sites of calnexin and calreticulin. J Biol Chem. 2002;277:29686–29697. doi: 10.1074/jbc.M202405200. DOI:0.1074/jbc.M202405200. [DOI] [PubMed] [Google Scholar]
- 36.Pollock S, Kozlov G, Pelletier MF, Trempe JF, Jansen G, Sitnikov D, Bergeron JJ, Gerhing K, Ekiel I, Thomas DY. Specific interaction of ERP-57 and calnexin determined by NMR spectroscopy and an ER two-hybrid system. EMBO J. 2005;23:1020–1029. doi: 10.1038/sj.emboj.7600119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roderick HL, Lechleiter JD, Camacho P. Cytosolic phosphorylation of calnexin controls intracellular oscillations via an interaction with SERCA2b. J Cell Biol. 2000;149:1235–1248. doi: 10.1083/jcb.149.6.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brothers SP, Janovick JA, Conn PM. Calnexin regulated gonadotropin-releasing hormone receptor plasma membrane expression. J Mol Endocrinol. 2006;37:479–488. doi: 10.1677/jme.1.02142. [DOI] [PubMed] [Google Scholar]
- 39.Janovick JA, Knollman PE, Brothers SP, Ayala-Yañez R, Aziz AS, Conn PM. Regulation of G protein-coupled receptor trafficking by inefficient plasma membrane expression. J Biol Chem. 2006;281:8417–8425. doi: 10.1074/jbc.M510601200. [DOI] [PubMed] [Google Scholar]
- 40.Leaños-Miranda A, Ulloa-Aguirre A, Ji TH, Janovick JA, Conn PM. Dominant-negative action of disease-causing gonadotropin-releasing hormone receptor (GnRHR) mutants: a trait that potentially coevolved with decreased plasma membrane expression of GnRHR in humans. J Clin Endocrinol Metab. 2003;88:3360–3367. doi: 10.1210/jc.2003-030084. [DOI] [PubMed] [Google Scholar]
- 41.Huckle WR, Conn PM. Use of lithium ion in measurement of stimulated pituitary insositol phospholipid turnover. Methods Enzymol. 1987;141:149–155. doi: 10.1016/0076-6879(87)41063-X. [DOI] [PubMed] [Google Scholar]
- 42.Russell SJ, Ruddock LW, Salo KE, Oliver JD, Roebuck QP, Llewellyn DH, Roderick HL, Koivunen P, Myllyharju J, High S. The primary substrate binding site in the b′ domain of ERP-57 is adapted for endoplasmic reticulum lectin association. J Biol Chem. 2004;279:18861–18869. doi: 10.1074/jbc.M400575200. [DOI] [PubMed] [Google Scholar]
- 43.Farmery MR, Allen S, Allen AJ, Bulleid NJ. The role of ERP-57 in disulfide bond formation during the assembly of major histocompatibility complex class I in a synchronized semipermeabilized cell translation system. J Biol Chem. 2000;275:14933–14938. doi: 10.1074/jbc.275.20.14933. [DOI] [PubMed] [Google Scholar]
- 44.Frenkel Z, Shenkman M, Kondratyev M, Lederkremer GZ. Separate roles and different routing of calnexin and ERP-57 in endoplasmic reticulum quality control revealed by interactions with asialoglycoprotein receptor chains. Mol Biol Cell. 2004;15:2133–2142. doi: 10.1091/mbc.E03-12-0899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mazzarella RA, Srinivasan M, Haugejorden SM, Green M. ERP-72, an abundant luminar endoplasmic reticulum protein, contains three copies of the active site sequences of protein disulfide isomerase. J Biol Chem. 1990;265:1094–1101. doi: 10.1091/mbc.E03-12-0899. [DOI] [PubMed] [Google Scholar]
- 46.Van PN, Rupp K, Lampen A, Soling HD. CaBP2 is a rat homolog of ERP-72 with proteindisulfide isomerase activity. Eur J Biochem. 1993;213:789–795. doi: 10.1111/j.1432-1033.1993.tb17821.x. [DOI] [PubMed] [Google Scholar]
- 47.Satoh M, Shimada A, Keino H, Kashiwai A, Nagai N, Saga S, Hosokawa M. Functional characterization of 3 thioredoxin homology domains of ERP-72. Cell Stress Chaperones. 2005;10:278–284. doi: 10.1379/CSC-116R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sorensen S, Ranheim T, Bakken KS, Leren TP, Kulseth MA. Retention of mutant low density lipoprotein receptor in endoplasmic reticulum (ER) leads to ER stress. J Biol Chem. 2006;281:468–476. doi: 10.1074/jbc.M507071200. [DOI] [PubMed] [Google Scholar]