Abstract
Gb3 and iGb3 are physiologically important trihexosylceramides with a terminal α-d-Galp-(1→4)-β-d-Galp- and α-d-Galp-(1→3)-β-d-Galp sequence, respectively. In particular iGb3 is attracting considerable attention as it is believed to serve as a ligand for natural killer T cells. Whether or not iGb3 is present in humans and which enzyme might be responsible for its synthesis is at present a matter of lively debate. In the current investigation we evaluated human blood group B galactosyltransferase (GTB) for its ability to catalyze the formation of iGb3 from lactosylceramide and UDP-Gal. GTB is a retaining glycosyltransferase that in vivo catalyzes the transfer of galactose from UDP-Gal donors to OH-3 of Gal on the H-antigen (α-l-Fucp-(1→2)-β-d-Galp) acceptor forming the blood group B antigen. GTB tolerates modifications in donor and acceptor substrates and its ability to accept lactosides as acceptors makes it a possible candidate for iGb3 production in humans. For comparison iGb3 and Gb3 were also synthesized from the same acceptor using an α-(1→3)- and α-(1→4)- specific galactosyltransferase, respectively. All of the enzymes tested catalyzed the desired reactions. Product characterization by NMR analysis clearly differentiated between the Gal-α-(1→3)-Gal and Gal-α-(1→4)-Gal product, with the GTB product being identical to that of the α-(1→3)-GalT-catalyzed reaction. The rate of transfer by GTB however was very low, only 0.001% of the rate obtained with a good substrate, H antigen disaccharide (octyl α-l-Fucp-(1→2)-β-d-Galp). This is too low to account for the possible formation of the iGb3 structure in humans in vivo.
Keywords: NKT cells, iGb3, Gb3, Human blood group B galactosyltransferase, α-(1→4)-Galactosyltransferase, Bovine α-(1→3)-galactosyltransferase
1. Introduction
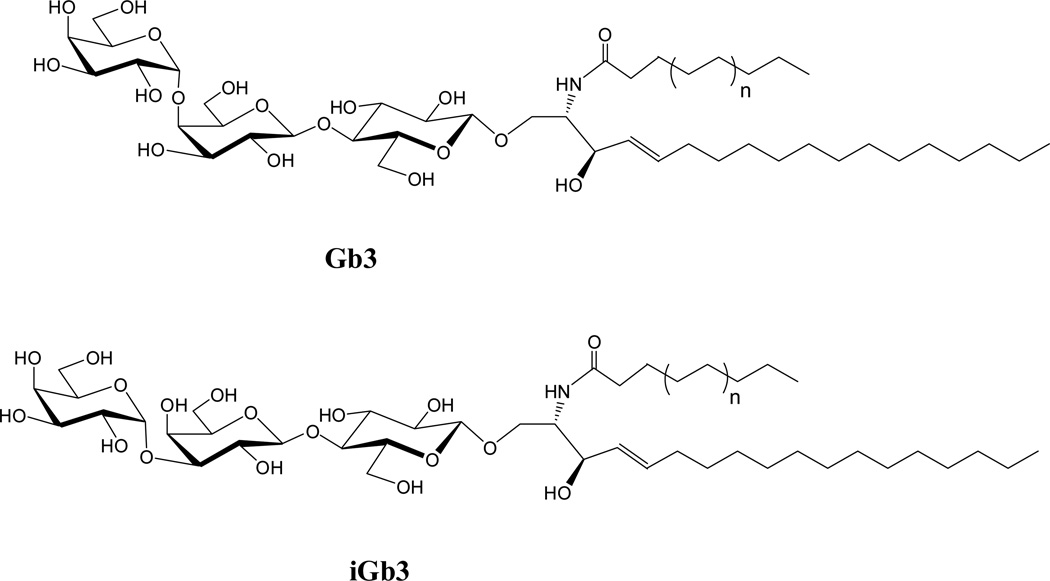
Gb3 and iGb3 are trihexosylceramides, which initiate the globo (Gb) and isoglobo (iGb) series, respectively. They are very similar in molecular structure and differ only with respect to the terminal glycosidic linkage, which is α-d-Gal-(1→4)-β-d-Gal in Gb3 and α-d-Gal-(1→3)-β-d-Gal in iGb3 (Fig. 1). Like other ceramides, Gb3 and iGb3 are involved in important physiologic events and they provide a good example for how subtle differences in molecular structure result in distinct physiological functions.
Figure 1.
Structures of Gb3 and iGb3, with n=1 in the ceramides synthesized in the current investigation.
Gb3 belongs to those glycolipids predominantly expressed in cancerous tissues.1 Gb3 over-expression is best characterized in colorectal adenoma2 and Burkitt’s lymphoma3 but occurs also in other cancers such as breast cancer4 and testicular carcinoma.5 In colorectal adenoma a clear correlation between Gb3 over-expression and the metastatic potential of the cancer is seen.2 Moreover, Gb3 is a possible target in colon cancer therapy as it functions as receptor for shiga toxin 1, which can cause apoptosis selectively in the receptive cancer cells.6 Gb3 over expression occurs also in Fabry’s disease, an X-linked disorder in glycolipid metabolism.7
The physiological relevance of iGb3 on the other hand is less clear and has been a matter of lively debate for the past five years.8–11 The starting point of the debate is a report by Zhou et al. who found that iGb3 is able to stimulate NKT cells in both human and mouse and concluded that iGb3 might be the principal endogenous ligand responsible for NKT cell development and function.8
NKT cells are a T lymphoside sub lineage with potent immuno-regulatory properties.12 They have been associated with many disease conditions which include bacterial infections13 and cancer14 as well as autoimmune diseases, such as type 1 diabetes15 and atherosclerosis.16 During development in the thymus, NKT cells go through a complex maturation and selection process in which an endogenous ligand is believed to be involved.12 The chemical identity of this ligand has puzzled researches for almost two decades. Zhou’s report8 providing strong evidence for iGb3 being this elusive ligand has thus been received with great attention in the NKT field.
Subsequent studies further supported the finding that iGb3 serves as ligand for NKT cells.17–19 The theory that iGb3 is the endogenous ligand responsible for NKT cell development and function in vivo has been challenged, mainly because there was no evidence for the presence of isoglobo ceramides in humans. As a matter of fact HPLC analysis first failed to detect iGb3 in human tissues.20 Moreover, it was also not possible to detect the mRNA of iGb3 synthase, the enzyme responsible for iGb3 synthesis, despite extensive analysis.21 In the same study Christiansen et al. further suggest that human iGb3 synthase, even if it were expressed, would not be functional due to several mutations that differentiate the human enzyme from its functional counterpart in other mammals. Indeed, it has been shown that exchanging a single amino acid in the rat iGb3 synthase to the one found in the human enzyme at equivalent position resulted in total inactivation of the rat enzyme.21 Nevertheless it was finally possible to detect iGb4, the lysosomal derivative of iGb38 in human thymus with very sensitive mass spectrometric analysis.22 This suggests that there is an enzyme responsible for iGb3 production in humans. If it is not iGb3 synthase, there must be another related enzyme, such as the blood group B galactosyltransferase (GTB), performing this function. GTB is a retaining glycosyltransferase that in vivo catalyzes the transfer of galactose from UDP-Gal donors to OH-3 of Gal on the H-antigen (Fuc-α-(1→2)Gal) acceptor forming the blood group B antigen. GTB tolerates modifications in donor and acceptor substrates23 and its ability to accept lactosides24 as acceptors makes it a possible candidate for iGb3 production in humans. As GTB is only present in individuals having blood group B or AB, it is unlikely that GTB is the only enzyme responsible for iGb3 production in humans. Alternatively, iGb3 is not the sole endogenous ligand responsible for NKT cell development. However NKT cell number differs greatly between different individuals25 and it is possible that the GTB catalyzed synthesis of iGb3 accounts to some extent for that difference.
Other mammalian glycosyltransferases related to iGb3 synthase, namely Forssman synthase26, α-(1→3)-galactosyltransferase (α-(1→3)-GalT), and blood group A N-acetylgalactosaminyltransferase (GTA)27 are less likely candidates for iGb3 production in humans. Forssman synthase, which is not functional in humans and GTA can be excluded because they are both GalNAc transferases. Furthermore, α-(1→3)-GalT, the enzyme that due to its substrate specificity is most suited to take on the role of iGb3 synthase, is not expressed in humans.28
In the current study we therefore focused on GTB and investigated whether GTB has the ability to synthesize iGb3 from lactocyl ceramide and UDP galactose. For comparison Gb3 and iGb3 were also synthesized from the same precursor using α-(1→4)-GalT from N. meningitidis and bovine α-(1→3)-GalT respectively. Both enzymes are known to add galactose to lactosides with the desired regiospecificity.29, 30
2. Results and Discussion
GTB, α-(1→3)-GalT and α-(1→4)-GalT were first tested for their ability to add galactose to lactosyl ceramide using a sensitive radiochemical assay.31 All experiments, with exception of those involving α-(1→4)-GalT were carried out in duplicate. The average error of determination was about 10%.
Due to the low solubility of naturally occurring lactosyl ceramides, a lactosyl ceramide species with a short acyl chain (C8) was used in the assay. This synthetic ceramide could readily be solubilised in the aqueous buffer by the addition of methyl-β-cyclodextrin. For comparison, the enzymes were also assayed using acceptors with hydrophobic aglycones, either lactose-gr (8-methoxycarbonyloctyl-β-D-Galp(1–4) β-D-Glcp) or H-antigen disaccharide (octyl-α-l-Fucp-(1→2)-β-d-Galp). Lactose-gr is routinely used in our laboratory to assay α-(1→3)-GalT and α-(1→4)-GalT activity and H antigen disaccharide is routinely used to assay the activity of GTB.
All the enzymes tested catalyzed the addition of galactose to lactosyl ceramide (Table 1). The highest specific activity with lactosyl ceramide as substrate was observed in the α-(1→4)-GalT-catalyzed synthesis of Gb3, namely 7.6 mU/mg protein. This is about 70 % of the activity observed with lactose-gr. An enzymatic route involving α-(1→4)-GalT thus provides a promising alternative to elaborative chemical methods for production of Gb3 and related compounds which are currently under investigation as vaccines for cancer therapy.32
Table 1.
Specific activities of α-(1→4)-GalT, α-(1→3)-GalT and GTB assayed with hydrophobic acceptors.
| Specific activity (mU/mg) |
|||
|---|---|---|---|
| Acceptors (300 µM) |
|||
| Enzyme | Lactosyl ceramide |
Lactose-gr. | H-antigen disaccharide-octyl |
| α-(1→4)-GalT | 7.6 | 10.9 | - |
| α-(1→3)-GalT | 0.065 | 1.13 | - |
| GTB | 0.0016 | 0.0090 | 212 |
The specific activity observed in the α-(1→3)-GalT assay with lactosyl ceramide was considerably lower, 0.065 mU/mg protein which is 5% of the activity with lactose-gr. The lower activity was expected due to the fact that this enzyme preferentially uses glycoproteins rather than glycolipids as substrates.30
The lowest specific activity was obtained in the GTB assay with lactosyl ceramide, 0.0016 mU/mg protein. This is only about 0.001% of the specific activity obtained with H-antigen disaccharide and about 18 % of the activity observed with lactose-gr. Changes at the sugar moiety thus affect the specific activity of the GTB enzyme more than the chemical nature of the aglycone.
To verify the product structure, the enzymatic reactions with lactosyl ceramide were carried out on 200 µg scale. The reaction was followed by TLC, monitoring the formation of a new spot at Rf 0.4. Unfortunately it was difficult to judge the degree of conversion, due to co-migration of methyl cyclodextrin and lactosyl ceramide. The reactions were thus terminated when TLC analysis indicated that the amount of product was sufficient for MS and NMR analyses.
The α-(1→4)-GalT reaction was stopped after one hour of reaction time. Prolonged incubation in the presence of excess UDP Gal resulted in the addition of another galactose to Gb3 (result not shown), and thus should be avoided. The α-(1→3)-GalT and GTB-catalyzed reactions were allowed to proceed 22 hrs and 44 hrs, respectively. Mass spectrometric analysis of the products isolated by preparative TLC showed single peaks corresponding to MNa+ ions (m/z ratio 934.4).
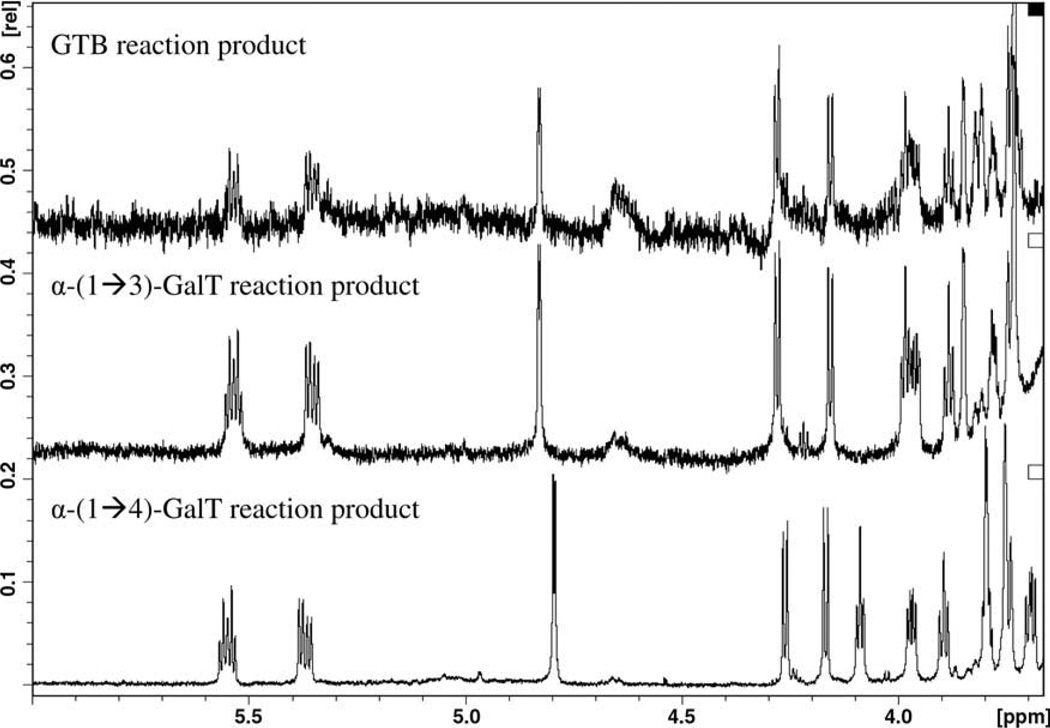
Comparison of the NMR spectra shows clear differences between the products obtained by α-(1→4)-GalT-catalyzed synthesis and those obtained by the action of α-(1→3)-GalT and GTB (Fig. 2). Due to the low amount of material, (with respect to NMR sensitivity) only the proton signals of the carbohydrate moiety were assigned. Nevertheless, the carbohydrate part was fully assigned (Table 2) and characteristic chemical shifts were observed for H1, H2, H3 and H5 of α-Gal, and for H2, H3 and H4 of the β-Gal when comparing the α-(1→3) with the α-(1→4) linkage. The α-(1→4) linkage was confirmed by an NOE from H1 of the α. galactose to H4 of the β galactose, and the α-(1→3) linkage was confirmed by NOE from H1 of α-galactose to H3 and H4 of the β-galactose.
Figure 2.
Partial NMR spectra of products obtained in the GTB, α-(1→3)-GalT and α-(1→4)-GalT reaction with lactocyl ceramide and UDP-Gal.
Table 2.
1H NMR chemical shift assignments for iGb3 and Gb3.
| iGb3 | H-1 | H-2 | H-3 | H-4 | H-5 | H-6a | H-6b |
|---|---|---|---|---|---|---|---|
| α(1→3)-Gal | 4.84 | 3.58 | 3.63 | 3.74 | 3.99 | 3.52 | 3.41 |
| β(1→4)-Gal | 4.28 | 3.42 | 3.48 | 3.86 | 3.97 | 3.89 | 3.78 |
| β–Glc | 4.16 | 3.05 | 3.34 | 3.30 | 3.29 | 3.63 | 3.59 |
| Gb3 | H-1 | H-2 | H-3 | H-4 | H-5 | H-6a | H-6b |
| α(1→4)-Gal | 4.79 | 3.65 | 3.56 | 3.74 | 4.09 | 3.49 | 3.45 |
| β(1→4)-Gal | 4.25 | 3.29 | 3.39 | 3.79 | 3.97 | 3.89 | 3.79 |
| β–Glc | 4.16 | 3.04 | 3.33 | 3.30 | 3.28 | 3.65 | 3.59 |
This study thus clearly shows that GTB, the blood group B enzyme is able to catalyze the synthesis of iGb3 in vitro, however, the rate observed is probably too low in order to account for iGb3 synthesis in vivo. In addition, the synthetic utility of α-(1→3)- and α-(1→4)- specific galactosyltransferases for the production of iGb3 and Gb3 ceramides was demonstrated.
3. Material and Methods
3.1. Materials
D-lactocylceramide-β1-1´-N-octanoyl-D-erythro-sphingosine was from Avanti Polar Lipids (Alabaster AL, USA). Methyl-β-cyclodextrin was from Sigma-Aldrich (St. Louis MO, USA). Radioactive [6-3H]-labelled UDP-Gal, was from American Radiolabeled Chemicals (St. Louis, MO, USA). Sep-Pak C18® reverse-phase cartridges were obtained from Waters (Milford, MA, USA). Ecolite® liquid scintillation cocktail was from MP Biomedicals (Santa Ana CA, USA). UDP-galactose was from Pharma-Waldhof GmbH (Dusseldorf), octyl α-l-Fucp-(1→2)-β-d-Galp (H-antigen disaccharide)33 and 8-methoxycarbonyloctyl-β-D-Galp(1–4) β-D-Glcp23 (lactose-gr) were prepared following literature procedures.
3.2 Enzyme production
Recombinant human GTB (E.C. 2.4.1.37)34 and calf thymus α-(1→3)-GalT (E.C. 2.4.1.87)30 were expressed in E. coli and purified according to previous published methods. His tagged α-(1→4)- GalT from N. meningitidis29 was expressed in E. coli and purified by chromatography on a HiTrapTM column from GE Healthcare Bio Science AB (Uppsala, Sweden). The activity of the enzymes were determined by the radiochemical assay.31 The reaction mixtures (10 µl) contained 0.1 M MOPS buffer pH 7.0, 20 mM MnCl2, 0.1 % BSA and varying amounts of substrates as follows: 5 nmol H-antigen disaccharide and 1.3 nmol UDP-Gal (5×104 dpm) for GTB, 5 nmol lactose-gr and 5 nmol UDP-Gal (5×104 dpm) for α1,3-GalT and 20 nmol lactose-gr and 1.3 nmol UDP-Gal (5×104 dpm) for α1,4-GalT. The enzymatic reactions were started by the addition of enzyme and incubated at 37°C for 20 min. Control reactions were carried out without the additions of acceptor. After reaction the mixtures were diluted with 500 µL water and applied to C18 Sep Pack cartridges. The cartridges were first washed with water (100 mL) to remove unreacted donor and then eluted with 3.5 mL methanol to which 10 mL Ecolite® liquid scintillation cocktail were added. Radioactivity was then measured on a Beckman LS 6500 scintillation counter from Beckman Coulter, Fullerton (CA, USA). One mU of enzyme activity is defined as the amount producing one nmol of product/min. The enzyme amounts referred to in section 3.5 are determined under the assay conditions described above.
3.3 Protein determination
The Bradford method using a kit from Bio-Rad was utilized for protein determinations with bovine gamma globulin as reference protein standard
3.4 Enzyme activity assay
The rate of enzyme-catalyzed Gb3 and iGB3 formation was measured by a radiochemical assay.31 To increase the sensitivity of the assay a lower concentration of unlabeled donor was used than in the standard assay (see section 3.2). The reaction mixture (20 µl) contained 6.0 nmol acceptor and 0.2 nmol UDP-Gal (1.5×106 dpm) in 0.1 M MOPS buffer, pH 7.0, containing 40 mM MnCl2, 0.2 % BSA and 0.6 % methyl-β-cyclodextrin. For comparison the reaction was also carried out using lactose-gr and H-antigen disaccharide as substrates under otherwise identical conditions. The reactions were initiated by addition of enzyme and were allowed to proceed for 20 minutes at 37°C. Control reactions were carried out without the additions of acceptor. As enzyme activities differed by several orders of magnitude, depending on which acceptor substrate was used, it was necessary to adjust the amount of enzyme added accordingly. In case of GTB the very concentrated stock solution (73 U/ml, according to the assay described in section 3.2) was diluted 10 times to measure enzyme activity with lactosyl ceramide and 100 000 times to measure the activity with the H antigen disaccharide. Samples were then treated as described above with the exception that 250 mL water were used to remove unreacted radiolabelled donor.
3.5 Enzymatic production of Gb3 and iGb3 for mass spectrometry and NMR analyses
Gb3 and iGB3 was synthesized from lactosyl ceramide and UDP-galactose. Reaction was initiated by the addition of 75 µL buffered enzyme solution to 150 µL substrate solution containing 625 nmol UDP-Gal, 66.5 nmol lactosyl ceramide and 2 mg methyl-β-cyclodextrin. The quantities of enzyme used were 2.4 U α-(1→4)-GalT, 0.1 U α-(1→3)-GalT and 5.25 U GTB with units measured as described in section 3.2. The reactions were allowed to proceed at ambient temperature and were stopped after 1, 22 and 44 hr respectively.
3.5 TLC analysis and product isolation
Formation of Gb3 and iGb3 was followed by TLC using CHCl3:MeOH:H2O (65:35:5) as eluent. Gb3 and iGb3 migrated with an Rf value of 0.4. Orcinol-sulfuric acid reagent was used for visualization of sugar containing compounds. The same TLC system was used for product isolation. 50 µL of the reaction mixture were applied as band to a TLC plate measuring 5×5cm. After developing a minor stripe was cut off at the edge of the plate for orcinol staining. A band was then scraped from the remaining plate, where orcinol staining indicated the presence of product. The silica powder was then extracted twice with 0.5 mL of the chloroform mixture to obtain the product. The solvent was thereafter removed in a rotary evaporator, and the sample was lyophilized before NMR and mass spectrometric analyses.
3.6 Product characterization
Mass spectra were recorded on an electro-spray ionization ion trap mass spectrometer from Bruker Daltonics (Bremen, Germany), operated in positive ion mode. All NMR spectra were recorded at 25°C on a Bruker Avance 800 instrument at 799.96 MHz for proton and 201.12 MHz for carbon using a 5 mm cryo probe. The sample contained freeze dried material dissolved in 600 µL DMSO:D2O (98:2) in a 5 mm NMR tube. The ppm scales were calibrated to DMSO for proton (2.5 ppm). The assignment was based on Bruker standard experiments as 2D DQFCOSY, NOESY and TOCSY recorded with 4K data points and 512 increments and an acquisition time off 0.23s, 0.6s NOESY mixing time and 0.08s spinlock in the TOCSY experiment. In addition a 1D proton spectra was recorded.
Acknowledgements
The authors wish to thank Durita Djuurhus for skilful technical assistance and Warren Wakarchuk for the α-(1→3)- and α-(1→4)- specific galactosyltransferase clones. This study was funded by a grant from the National Institute of Health (R01NS061767). The spectra at 800 MHz were obtained on the Bruker Avance 800 spectrometer of the Danish Instrument Center for NMR Spectroscopy of Biological Macromolecules.
References
- 1.Hakomori S. Adv. Exp. Med. Biol. 2001;491:369–402. doi: 10.1007/978-1-4615-1267-7_24. [DOI] [PubMed] [Google Scholar]
- 2.Kovbasnjuk O, Mourtazina R, Baibakov B, Wang T, Elowsky C, Choti MA, Kane A, Donowitz M. PNAS. 2005;102:19087–19092. doi: 10.1073/pnas.0506474102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mangeney M, Lingwood CA, Taga S, Caillou B, Tursz T, Wiels J. Cancer Research. 1993;53:5314–5319. [PubMed] [Google Scholar]
- 4.Johansson D, Kosovac E, Moharer J, Ljuslinder I, Brännström T, Johansson A, Behnam-Motlagh P. BMC Cancer. 2009;9:67. doi: 10.1186/1471-2407-9-67. Epub 2009 Feb 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kang J-L, Rajpert-De Meyts E, Wiels J, Skakkebæk NE. Virchows Arch. 1995;426:369–374. doi: 10.1007/BF00191346. [DOI] [PubMed] [Google Scholar]
- 6.Falguières T, Maak M, von Weyhern C, Sarr M, Sastre X, Poupon M-F, Robine S, Johannes L, Janssen K-P. Mol. Cancer Ther. 2008;7:2498–2508. doi: 10.1158/1535-7163.MCT-08-0430. [DOI] [PubMed] [Google Scholar]
- 7.Schiffmann R. Pharmacol. Ther. 2009;122:65–77. doi: 10.1016/j.pharmthera.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Zhou D, Mattner J, Cantu C, 3rd, Schrantz N, Yin N, Gao Y, Sagiv Y, Hudspeth K, Wu Y-P, Yamashita T, Teneberg S, Wang D, Proia RL, Levery SB, Savage PB, Teyton L, Bendelac A. Science. 2004;306:1786–1789. doi: 10.1126/science.1103440. [DOI] [PubMed] [Google Scholar]
- 9.Ahmad S. Nature Reviews Immunology. 2007;7:325. [Google Scholar]
- 10.Kronenberg M, Gapin L. PNAS. 2007;104:5713–5714. doi: 10.1073/pnas.0701493104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sedwick C. PloS Biology. 2008;6:1358. doi: 10.1371/journal.pbio.0060281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bendelac A, Savage PB, Teyton L. Annu. Rev. Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 13.Mattner J, DeBord KL, Nahed I, Goff RD, Cantu C, 3rd, Zhou D, Saint-Mezard P, Wang V, Gao Y, Yin N, Hoebe K, Schneewind O, Walker D, Beutler B, Teyton L, Savage PB, Bendelac A. Nature. 2005;434:525–529. doi: 10.1038/nature03408. [DOI] [PubMed] [Google Scholar]
- 14.Terabe M, Berzofsky JA. Adv. Cancer Res. 2008;101:277–348. doi: 10.1016/S0065-230X(08)00408-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Novak J, Griseri T, Beaudoin L, Lehuen A. Int Rev Immunol. 2007;26:49–72. doi: 10.1080/08830180601070229. [DOI] [PubMed] [Google Scholar]
- 16.van Puijvelde GH, van Wanrooij EJ, Hauer AD, de Vos P, van Berkel TJ, Kuiper J. Thromb Haemost. 2009;102:223–230. doi: 10.1160/TH09-01-0020. [DOI] [PubMed] [Google Scholar]
- 17.Schümann J, Mycko MP, Dellabona P, Casorati G, MacDonald HR. J Immunol. 2006;176:2064–2068. doi: 10.4049/jimmunol.176.4.2064. [DOI] [PubMed] [Google Scholar]
- 18.Xia C, Yao Q, Schümann J, Rossy E, Chen W, Zhu L, Zhang W, De Libero G, Wang PG. Bioorg. Med. Chem. Lett. . 2006;16:2195–2199. doi: 10.1016/j.bmcl.2006.01.040. [DOI] [PubMed] [Google Scholar]
- 19.Scott-Browne JP, Matsuda JL, Mallevaey T, White J, Borg NA, McCluskey J, Rossjohn J, Kappler J, Marrack P, Gapin L. Nat. Immunol. . 2007;8:1105–1113. doi: 10.1038/ni1510. [DOI] [PubMed] [Google Scholar]
- 20.Speak AO, Salio M, Neville DCA, Fontaine J, Priestman DA, Platt N, Heare T, Butters TD, Dwek RA, Trottein F, Exley MA, Cerundolo V, Platt FM. PNAS. 2007;104:5971–5976. doi: 10.1073/pnas.0607285104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Christiansen D, Milland J, Mouhtouris E, Vaughan H, Pellicci DG, McConville MJ, Godfrey DI, Sandrin MS. PLoS Biol. 2008;6:1527–1538. doi: 10.1371/journal.pbio.0060172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Y, Teneberg S, Thapa P, Bendelac A, Lervery SB, Zhou D. Glycobiology. 2008;18:158–165. doi: 10.1093/glycob/cwm129. [DOI] [PubMed] [Google Scholar]
- 23.Lowary TL, Hindsgaul O. Carbohydr. Res. 1993;249:163–195. doi: 10.1016/0008-6215(93)84068-h. [DOI] [PubMed] [Google Scholar]
- 24.Letts JA, Rose NL, Fang YR, Barry CH, Borisova SN, Seto NO, Palcic MM, Evans SV. J. Biol. Chem. 2006;281:3625–3632. doi: 10.1074/jbc.M507620200. [DOI] [PubMed] [Google Scholar]
- 25.Godfrey DI, Berzins SP. Nat Rev Immunol. 2007;7:505–518. doi: 10.1038/nri2116. [DOI] [PubMed] [Google Scholar]
- 26.Xu H, Storch T, Yu M, Elliott SP, Haslam DB. J. Biol. Chem. . 1999;274:29390–29398. doi: 10.1074/jbc.274.41.29390. [DOI] [PubMed] [Google Scholar]
- 27.Soya N, Shoemaker GK, Palcic MM, Klassen JS. Glycobiology. 2009;19:1224–1234. doi: 10.1093/glycob/cwp114. [DOI] [PubMed] [Google Scholar]
- 28.Joziasse DH, Sharper JH, Jabs EW, Sharper NL. J. Biol. Chem. 1991;266:6991–6998. [PubMed] [Google Scholar]
- 29.Wakarchuk WW, Cunningham A, Watson DC, Young NM. Protein Eng. . 1998;11:295–302. doi: 10.1093/protein/11.4.295. [DOI] [PubMed] [Google Scholar]
- 30.Blanken WM, Van den Eijnden DH. J Biol. Chem. 1985;260:12927–12934. [PubMed] [Google Scholar]
- 31.Palcic MM, Heerze LD, Pierce M, Hindsgaul O. Glycoconjugate J. 1988;5:49–63. [Google Scholar]
- 32.Werz DB, Castagner B, Seeberger PH. J. Am. Chem. Soc. 2007;129:2770–2771. doi: 10.1021/ja069218x. [DOI] [PubMed] [Google Scholar]
- 33.Kamath VP, Yeske RE, Gregson JM, Ratcliffe RM, Fang YR, Palcic MM. Carbhydr. Res. 2004;339:1141–1146. doi: 10.1016/j.carres.2003.12.027. [DOI] [PubMed] [Google Scholar]
- 34.Seto NOL, Palcic MM, Hindsgaul O, Bundle DR, Narang SA. Eur. J. Biochem. 1995;234:323–328. doi: 10.1111/j.1432-1033.1995.323_c.x. [DOI] [PubMed] [Google Scholar]