Abstract
Cardiovascular disease remains the leading cause of death in the Western world and myocardial infarction is one of the primary facets of this disease. The limited natural self-renewal of cardiac muscle following injury and restricted supply of heart transplants has encouraged researchers to investigate other means to stimulate regeneration of damaged myocardium. The plasticity of stem cells toward multiple lineages offers the potential to repair the heart following injury. Embryonic stem cells have been extensively studied for their ability to differentiate into early cardiomyocytes, however the pathway has only been partially defined and inadequate efficiency limits their clinical applicability. Some studies have shown cardiomyogenesis from adult mesenchymal stem cells, from both bone marrow and adipose tissue, but their differentiation pathway remains poorly detailed and these results remain controversial. Despite promising results using stem cells in animal models of cardiac injury, the driving mechanisms behind their differentiation down a cardiomyogenic pathway have yet to be determined. Currently there is a paucity of information regarding cardiomyogenesis on the systems level. Stem cell differentiation results from multiple signaling parameters operating in a tightly regulated spatiotemporal pattern. Investigating this phenomenon from a systems biology perspective could unveil the abstruse mechanisms controlling cardiomyogenesis that would otherwise require extensive in vitro testing.
Introduction
Each year over 8 million Americans suffer from myocardial infarction and there is roughly one death every minute in the US as a result of some coronary event [1]. Myocardial infarction (MI), resulting from a blockage of a coronary artery, typically leads to cardiomyocyte ischemia and death and eventual ventricular hypertrophy and heart failure [2]. The heart has traditionally been viewed as one of the few organs lacking the capacity for self-renewal. Despite recent studies showing limited long term turnover of cardiomyocytes, the mammalian heart remains incapable of adequately replacing damaged myocardium [3]. Cellular cardiomyoplasty, or cell transplantation, offers an exciting approach to regenerate myocardium post-MI by replacing the necrotic cardiomyocytes. Scientists have recently turned to stem cells as a potential avenue for rebuilding the heart after injury. Stem cells are characterized by their ability to differentiate into multiple lineages, including cardiomyocytes. While animal studies have shown some success in regenerating heart muscle in vivo, and several critical molecular chaperones have been identified in vitro, the overall pathway of cardiomyogenic differentiation remains largely unknown [4–7]. This review will discuss the major types of stem cells presently available to scientists and the current evidence for their subsequent differentiation into cardiomyocytes, with the hope that systems biologists can synthesize the combined information to diagram the overarching mechanisms driving cardiomyogenesis.
This review will focus mainly on in vitro cardiomyogenesis of human stem cells. While several in vivo studies have seen varying degrees of myocardial regeneration from stem cell delivery post-MI, the in vivo environment presents innumerable confounding variables. These studies are useful in demonstrating the feasibility of stem cell cardiac regeneration, but offer little help in elucidating the mechanisms that control cardiac differentiation and thus will only be briefly highlighted here.
Cardiogenesis and Cardiomyocyte Characterization
Before attempting to manipulate stem cell differentiation to a cardiac phenotype, it is imperative to gain a thorough understanding of the developmental biology of the heart. The heart arises from the lateral plate mesoderm and develops from the primary and secondary heart fields that segregate from a common progenitor [8–10]. The primary heart field gives rise to the left ventricle and atria whereas the secondary heart field gives rise to most of the right ventricle, ventricular septum and the outflow tracks [11, 12]. The primary heart field adapts a crescent shape, and then fuses at the midline to create a heart tube, which ultimately contributes to the left ventricle and atria. Next, several developmental steps are required to create a mature four chambered heart. First, cells are recruited from the lateral plate mesoderm to form the secondary heart field, and the tube loops around to form a C-shape. During cardiac looping, the compartments of the heart become visible as the loop begins ballooning out, and the outflow tract, embryonic right ventricle, embryonic left ventricle, atria, and sinus venosus are defined [13]. The right atrium becomes connected to the right ventricle as the left ventricle subsequently joins the outflow tract.
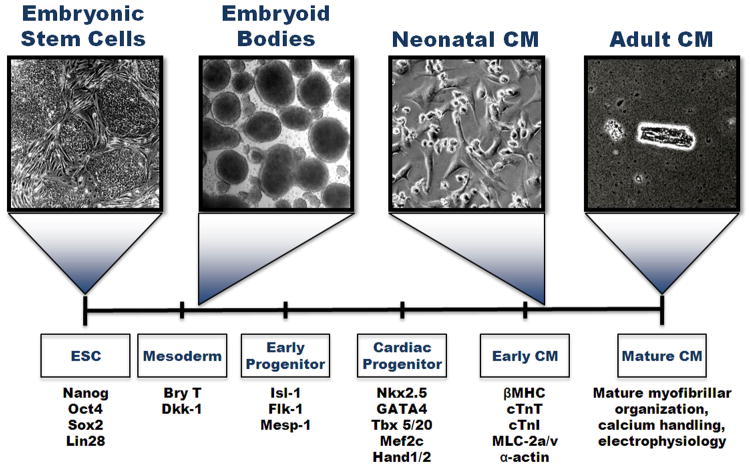
Several unique transcription factors and proteins have been identified to characterize the stages of cardiac development. First, differentiation into mesoderm, one of the three germ layers, can be identified by the transcription factor Brachyury (T). Pre-cardiac mesoderm expresses Mesp-1, Is1-1, and Flk-1. However, Flk-1 is also a marker for hematopoietic progenitor cells, and is found in the endothelium. Isl-1 may prove to be a difficult marker to use as well since its expression is transient and not seen in the adult heart. To identify cardiac progenitors, Nkx2.5 is one of the earliest cardiac transcription factors seen in the developing heart field, but in the mouse it is also associated with many other tissues [14]. Other markers include Tbx5/20, GATA4, Mef2c and Hand1/2 [15]. Cardiomyocytes are distinguished by their characteristic protein expression, including Troponin T (cTnT) and I (cTnI), myosin light chain atrial/ventricle (MLC-2a and MLC-2v, respectively), connexin-43, and myosin heavy chains (αMHC and βMHC). In addition, cardiac α-actinin will appear as striated, indicating the formation of sarcomeres. However, a fully differentiated adult cardiomyocyte will display an organized myofibrillar and sarcomeric pattern throughout the cell [16–18]. Further maturation is indicated by increased calcium transients compared to primordial cardiomyocytes, as well as the formation of several ion channels including the L-type Ca2+ channel (ICa), the sodium channel (INa), and the hyperpolarization-activated pacemaker channel (If) [19]. Action potentials also change from pacemaker-like to more ventricular-like and atrial-like with higher upstroke velocity [20]. Figure 1 displays these stages of cardiac development in relation to differentiating embryonic stem cells.
Figure 1.
Through a series of elegant lineage-tracing studies in both the chick and mouse, it was seen that both heart fields are marked by expression of Flk-1 and the transcription factor Nkx2.5 while solely the second heart field is marked by Isl-1, a transcription factor that is transiently expressed [10, 21–23]. When Nkx2.5+ cells are isolated in vitro, the majority of the cells differentiated to form cardiomyocytes and conduction system cells, but some were able to differentiate into smooth muscle cells [23]. The isolation of cells with a capacity for bipotential development in vitro was a significant achievement [24], but later isolation and differentiation of cells from cardiac progenitors proved to be tripotential, forming endothelium, smooth muscle and myocardium [24–26]. The study of heart development has provided insights into the natural progression of stem cell maturation, which can further be used as potential cues to direct differentiation towards the cardiac lineage.
Cardiomyogenesis of Human Embryonic Stem Cells
Human embryonic stem cells (hESCs) are derived from the pluripotent inner cell mass of the blastocyst, and are characterized by their ability to self-renew indefinitely while maintaining an undifferentiated phenotype, and to form derivatives of all three germ layers. While this plasticity broadens the regenerative potential of these cells, it also makes their differentiation difficult to control. Additionally, their origin from pre-implantation fertilized blastocysts have led to issues for their use and study, and many ethical guidelines and regulations have been set into place to assure derivation and use of hESCs is done according to ethical standards [27, 28]. However, the pluripotency of hESCs offers potential for myocardial regeneration post-MI [29, 30]. The first report of hESCs spontaneously differentiating into cardiomycytes was described by Kehat et al in 2001, however considerable effort has since been made to control this cardiomyogenesis [31]. These hESC-derived cardiomyocytes exhibit many similar behaviors of adult cardiomyocytes, including characteristic electrophysiology expression and response to chronotropic drugs; however, these profiles more closely resemble a fetal phenotype [18, 32–35]. Cardiomyocytes are generally difficult to differentiate, with generation efficiencies less than 10% with most protocols [36]. Many of the early protocols had a poorly defined combination of factors and high variability between serum lots, thereby making them difficult to reproduce. In addition, the differentiation potential among hESC lines is highly variable, as some will differentiate more readily into cardiomyocytes than others. For example, one hESC line derived at the University of Wisconsin, the H9 line, is considered to have a higher propensity to differentiate toward mesoderm than other commonly used hESC lines [18]. Thus, much of the progress over the past ten years has focused on better means for isolating, enriching, and characterizing the portion of hESCs that spontaneously differentiate into cardiomyocytes.
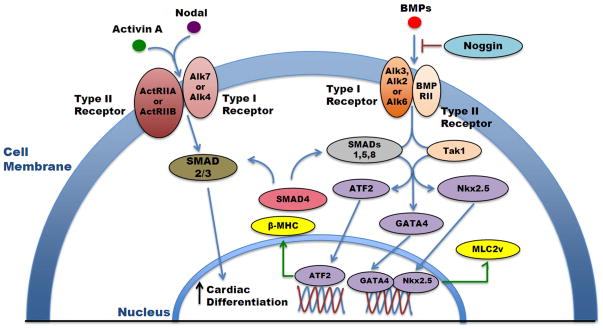
The recapitulation of heart development can provide useful insight for optimizing differentiation protocols. There have been three major families of growth factors that have been identified as being important regulators of cardiac development: the transforming growth factor beta-family (TGFβ) such as BMPs and Activin (Figure 2), the Wnt family, and the fibroblast growth factor (FGF) family [37–39]. While these pathways are able to be distinguished from each other, there may be a cooperative interplay between these developmental pathways [40]. It is becoming evident that there is a temporal and spatial segregation of molecular signals that are required to create the correct environment for cardiac differentiation. The TGFβ, Wnt and FGF families all play an important role on differentiation to any lineage, but are especially important for mesoderm formation. These signals must be delivered at appropriate combinations and timing, and subsequently turned off at the appropriate time to allow for specific differentiation. Addition of BMP4 during embryoid body differentiation was found to induce the development of mesoderm, or Brachyury (T) positive tissue. However, the Wnt/β-catenin signals play a biphasic role in mouse ES differentiation, as these signals must then be inhibited to promote precardiac progenitor formation [41]. If the signaling is not removed, the cells will instead form Flk-1+ mesoderm that moves toward a hemangioblast lineage [42]. Using Nodal, or adding Activin A to act as a surrogate for Nodal, enhanced mesendoderm formation, but a careful balance must be achieved, as higher concentrations of Activin A promoted formation of endoderm over mesoderm [43]. The subsequent addition of BMP 4, in addition to Activin A, is thought to further commit the cells down a cardiac lineage, and generate cardiomoycytes [44, 45]. However, BMPs have also exhibited biphasic activity and must be expressed and inhibited at appropriate intervals [46]. For example, the endogenous BMP inhibitor Noggin, when in use with mESCs, has been seen to increase cardiomyogenesis. Similarly, when a BMP small molecule inhibitor was used within the first 24 hours of culture, it robustly increased cardiomyogenesis and reduced differentiation into endothelial and smooth muscle lineages [47]. Conversely, this BMP inhibition must be removed in later differentiation steps as BMPs have also been shown to upregulate SMAD signaling and MAP3K7 signaling for proper heart development [48, 49]. The role of FGF, however, is less defined. While studies have suggested a synergistic partnership between members of the FGF and BMP families to promote heart induction during development, the precise pathway and interaction remains unclear [39, 48].
Figure 2.
Currently, there are three main methods for differentiating hESCs to the cardiac lineage: embryoid body formation, co-culture with other cell types, and monolayer differentiation. The first, and most widely used approach, is the formation of embryoid bodies (EBs), where hESCs are clustered into multicellular three-dimensional aggregates that will differentiate to comprise all three germ layers [50]. Over time, the EBs may spontaneously form contracting regions, that when dissociated are positive for cardiac markers [18]. The aggregates generally range in size, however methods are currently being developed to control EB size and uniformity [15, 51, 52]. EB size may play an important role in differentiation, as it has been shown that EBs with different initial aggregate size will affect the efficiency of creating contracting EBs [51]. Enhancing differentiation during EB formation using growth factors has also been explored, primarily using activin A and BMP4 [53]. After initial embryoid body formation, the cells are dissociated and plated onto feeder-free coated tissue culture plates for further differentiation and analysis, and spontaneously beating cells can clearly be seen under a light microscope.
A separate method of culture employs a supportive stromal layer that will enhance differentiation toward a cardiac lineage. For example, a co-culture approach using undifferentiated hESCs with a mouse visceral endoderm-like cell line (END-2) has shown increased cardiac differentiation [16]. Approximately 15–20% of the cells were seen to be contracting between 12–21 days of co-culture, and the cells stained positive for α-actinin; however, the sarcomeric proteins were not well defined. This approach may enhance differentiation as a result of cell-cell interaction or possibly paracrine effects from the END-2 cells, as culture of hESCs in END-2 conditioned media also enhanced cardiomyogenesis [54]. To avoid the confounding variables associated with a separate cell-line feeder layer, several groups have encouraged cardiomyogenesis by using monolayers of high density undifferentiated stem cells plated on some sort of extracellular matrix coating, representing a feeder-free system. These cells are then treated under various conditions to induce differentiation. Laflamme et al used serum-free media supplemented with BMP4 and Activin A to generate beating cardiomyocytes [44]. Additionally, Yao et al demonstrated Activin A and BMP4 could push hESC in monolayer culture toward the cardiac lineage [45]. However, other groups have shown that cell-cell interactions within the EB stimulate markers for mesendoderm formation, which may argue in favor for EB differentiation [55].
To assess the maturity of the hESC-derived cardiomyocytes, gene expression profiles can be used. Cao et al used microarray analysis at different time points during EB formation and compared the expression profiles to human fetal heart cells [32]. The hESC cardiomyocytes did contain a bias toward mesodermal and cardiac lineages, but there were still characteristics of all three cell layers. They found that the gene expression of structural and force generating proteins were similar between the hESC cardiomyocytes and fetal hearts, but metabolic process genes were upregulated in the fetal heart. These pathways are necessary for mature cardiomyocytes to contract forcefully, indicating that the cardiomyocytes from stem cells were still fairly immature [32].
In addition to monitoring gene and protein expression to determine cardiomyogenesis, several groups have examined the functionality of the differentiated cardiomyocytes. Mature cardiomyocytes have a characteristic response to electrical stimuli and pharmacological agents that alter heart rate. Examination of the electrophysiology of hESC-derived cardiomyocytes using microelectrodes showed distinct action potential (AP) formation, with characteristics of atria, ventricles and pacemaker cells [18]. However, these action potentials prove to be more similar to embryonic cardiac cells than adult with regard to AP amplitude, duration and depolarization [18]. Cao et al measured calcium handling in addition to AP formation, and found that hESC-derived cardiomyocytes were similar to fetal cardiomyocytes, and that the AP profiles showed no change from three to six weeks post differentiation. When examining cellular response to pharmacological agents, Xu et al demonstrated that their differentiated cells, when incubated with dilatiazem, an ion blocker, decreased the beating rate in a dose-dependent fashion while isoprenaline and phenylephrine increased the rate of contraction, as seen in adult cardiomyocytes. In particular, cells at an earlier stage of differentiation were not responsive to clenbuterol, while cultures that were allowed to differentiate for a longer period of time (greater than 60 days) increased beating frequency. These results indicate that longer culture times increase the maturation of the cells [56]. Despite the mature protein expression of these differentiated hESCs, there remains difficulty in producing cells that appropriately respond to external stimuli in a mature manner.
While many protocols attempt to generate fully mature cardiomyocytes, the efficiency is generally low. Several newer protocols aim at generating a cardiovascular progenitor that possesses tripotentiality, forming endothelium, smooth muscle cells, and cardiomyocytes. These cardiac precursors generally display Flk-1, c-kit, and Isl-1, and may be attractive to study since mature cardiomyocytes are non-proliferative and committed to cardiac muscle. Although several groups report that cardiomyocytes generated from hESCs are still able to maintain proliferation after differentiation, the cardiac progenitors would be proliferative and retain the ability to differentiate [17, 57, 58]. Yang et al in 2008 identified tripotential, contractile progenitor cells with Flk-1+ and c-kit negative expression in half of the hESC population, where in addition to cardiomyocyte formation, the cell population also contained endothelial and vascular smooth muscle potential [45]. This protocol combined activin A, BMP4, Dikkopf-1 (DKK1), VEGF, and bFGF in serum-free media using embryoid body formation, with the rationale that activin A and BMP4 promote hESC cardiac development, DKK1 acts as a Wnt inhibitor, and VEGF promotes the viability of the progenitor population. bFGF was added at a later time point to continue the development of the cardiovascular lineages. Also in 2008, Leschik et al published a separate protocol to generate cardiac committed, but not fully differentiated cardiomyocytes [59].This protocol aimed at inexpensively reproducing cardiac progenitor cells, as only one growth factor (BMP2) was used for only four days. These cells expressed various cardiac progenitor markers such as Tbx6, Mef2c, and Mesp2, and were able to differentiate into functional contracting cardiomyocyte-like cells. This protocol suggests that a subpopulation of beating hESCs can be produced that is comprised of up to 95% cardiomyocyte progenitors, potentially improving the overall efficiency of hESC cardiomyogenesis.
The need for a controlled differentiation environment has led scientists to utilize high throughput screening techniques to search for novel low molecular weight compounds that can enhance differentiation to a cardiac lineage [36, 60, 61]. By screening chemical libraries for “hits”, small molecules can be identified that improve the efficiency of hESC cardiomyogenesis, although the pathway of differentiation is not yet well defined using these synthetic molecules. Multiple developmental pathways affect the early stages of differentiation, as evidenced by the wide array of factors implicated for in vitro cardiomyogenesis. It is evident that the sequence and concentration of induction cues introduced in culture play a critical role in determining which differentiation pathway the hESCs follow. The mechanism of these pathways to form cardiac progenitors or cardiomyocytes has yet to be elucidated and will need to be mastered before use of hESCs for clinical therapies can continue.
The main focus of differentiation has traditionally been to use soluble cues to direct cardiomyogenesis, but other external cues may also enhance differentiation. The extracellular matrix (ECM) has been shown to play a pivotal role in controlling cell maturation and differentiation. Conventional protocols have cultured hESCs on gelatin-coated surfaces, however, recent studies have begun to examine the potential for matrix-directed renewal [62, 63] or differentiation [22, 63–69] of stem cells. In particular, one study showed that hESCs cultured on ECM produced by cardiac fibroblasts significantly improved the speed and extent of cardiomyogenesis [70]. Additionally, a rotary orbital suspension culture increased the proportion of contracting EBs and increased sarcomeric muscle protein expression when compared to static culture systems [71]. Electrical stimulation of hESC for increased cardiac differentiation has also been explored with moderate success [72]. Despite the rudimentary understanding of the controlling parameters for hESC cardiomyogenesis, embryonic stem cells remain the most thoroughly defined cell type. However, the cardiomyogenic potential of other cell types, including induced pluripotent stem cells and adult mesenchymal stem cells, is rapidly gaining popularity due to their autologous nature and ease of isolation and culture with the latter.
The recent discovery of induced pluripotent stem cells (iPSCs) has sparked significant excitement for the generation of patient-specific pluripotent stem cells. These cells are reprogrammed somatic cells that have characteristics of embryonic stem cells without involving the destruction of an embryo. Takahashi et al first described the generation of human iPSCs from dermal fibroblasts using retroviruses containing the four transcription factors c-myc, Klf4, Oct3/4, and Sox2 [73]. The resulting iPSCs could easily form embryoid bodies (EBs) as discussed previously, express markers for all three germ layers, self-renew, and form teratomas – all of the signature characteristics of pluripotent embryonic stem cells. Several groups have explored the cardiomyogenic potential of these cells using existing protocols previously established for hESCs, as discussed earlier. Most commonly, EBs are formed from the iPSCs using either the hanging drop method or by aggregation during floating cultivation. Several EBs exhibited spontaneous beating within a few days and exhibited positive expression of the cardiomyogenic genes Tbx5, Nkx2.5, and MEF2c [74–76]. Several groups even showed typical cardiomyocyte-like responses to pharmacological agents that alter beating frequency [77, 78]. Together, these results demonstrate the ability of iPSCs, like hESCs, to differentiate into cardiomyocytes. However the genetic reconfiguration during viral reprogramming raises questions as to whether iPSCs follow the same molecular pathways to arrive at the desired cell lineage. While promising, these newest players in the stem cell cardiomyogenesis niche remain largely undefined.
Adult Mesenchymal Stem Cells
Bone Marrow-Derived Mesenchymal Stem Cells
The ethical and tumorigenicity concerns surrounding hESCs and iPSCs have sparked several researchers to investigate adult stem cell types for cardiomyogenic capabilities. One of the more extensively studied adult stem cell types, bone marrow-derived mesenchymal stem cells (MSCs), have expressed plasticity towards a variety of cell lineages including muscle [79, 80], bone [81, 82], cartilage [83, 84], and neurons [85]. Some studies have shown that these cells differentiate into cardiomyocyte-like cells under a variety of stimuli, although true cardiomyogenesis is controversial. 5-azacytidine (5-aza), a cytosine analog and DNA demethylating agent, has become a common media additive to induce differentiation. Makino et al were among the first to describe the in vitro cardiomyogenic potential of MSCs by incubating rat MSCs in 3 μM 5-azacytidine [86]. This treatment produced spontaneously beating cells within 30% of the culture that stained positively for myosin, actinin, and desmin. RT-PCR further showed positive expression of cardiac markers MHC, MLC-2v, GATA4, and Nkx 2.5. Xu et al expanded these findings to human MSCs using 10 μM 5-azacytidine and saw up to 80% of the isolated cells express similar positive cardiac markers after 3 weeks of culture [87]. However, bFGF and horse serum, commonly used in myogenic differentiation of mESCs, were also present in the media for the duration of this study. These additives had little effect on the control MSCs that did not receive 5-aza treatment, thus suggesting a synergistic role of several compounds in the enhanced cardiomyogenic differentiation of hMSCs compared to Makino’s findings. Several cells also positively stained for adipogenic markers, thus indicating that this method is not entirely cardiac specific. Furthermore, Liu et al were unable to repeat these studies, seeing no cardiac gene expression despite applying a range of 5-aza concentrations [88]. These studies raise questions as to whether these cells are undergoing true cardiomyogenesis as a result of 5-aza treatment, or if there are other latent factors contributing to these variable results.
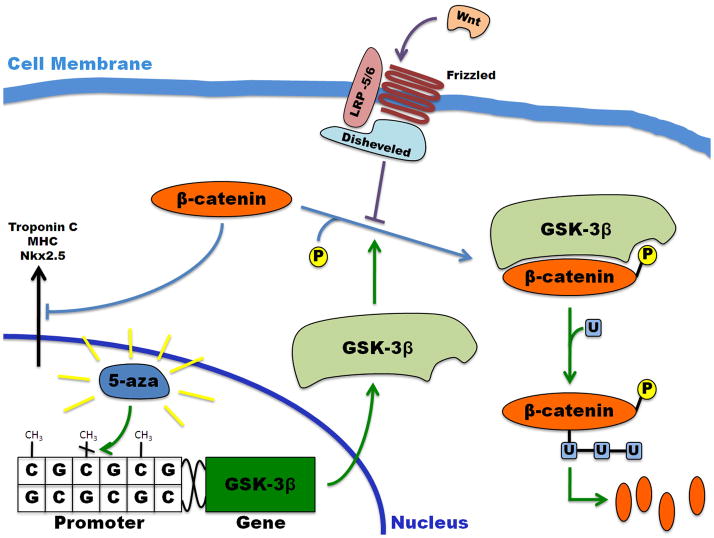
Until recently, the exact mechanism of 5-aza induced cardiomyogenesis was largely unknown and attributed to demethylation, and subsequent activation, of an unknown cardiac-related gene. However, a study by Cho et al in 2009 revealed that the upregulation in cardiomyocyte specific genes following 5-aza delivery resulted from the induced demethylation of CpG islands in the promoter of the glycogen synthase kinase (GSK)-3 gene, a major player in the Wnt signaling pathway [89]. Methylation of CpG sequences in a gene promoter has been postulated to play a role in silencing that gene. This study saw a time dependent increase in both GSK-3 isoforms, GSK-3α and GSK-3β , in response to 5-aza treatment. Genetically modified overexpression of GSK-3β resulted in a similar upregulation of cardiomyocyte specific genes in the absence of 5-aza. Furthermore, GSK-3β is known for phosphorylation of β-catenin, which leads to its eventual ubiquitinylated proteolytic degradation. This downregulation of β-catenin, either by 5-aza induced upregulation of GSK-3β or specific knockdown using silencing RNA, results in the apparent cardiomyogenesis of MSCs (Figure 3). Conversely, this study also saw chondrogenesis of the MSCs in response to β-catenin upregulation. These results suggest that the differentiation of MSCs could be induced by controlling elements of the Wnt signaling pathway further downstream from where 5-aza acts. However, the Wnt pathway has extensive applications within the body and the specific transcription factors bound to β-catenin as it localizes to the nucleus to inhibit cardiomyogenic gene expression remain unknown. Thus, further studies are still required to fully define and control the chemically-defined cardiomyogenic pathway.
Figure 3.
To better mimic the physical extracellular cues that these cells would naturally see in vivo, multiple laboratories have investigated the differentiation of MSCs co-cultured with differentiated cardiomyocytes. Research from Dr. Shinji Tomita’s group in Japan first showed that MSCs would beat synchronously with rat neonatal cardiomyocytes within a few days of co-culture [90]. Neonatal cardiomyocytes were used in this study due to the limited viability of adult cardiomyocytes ex vivo. These findings were later expanded to show that direct cell-cell contact between MSCs and cardiomyocytes was necessary for the induction of cardiomyocyte-like function in the stem cells. Only those MSCs cultured in direct contact with neonatal cardiomyocytes showed synchronous beating and positive staining for MHC, TnI, atrial natriuretic peptide, and connexin-43 [91]. MSCs cultured alone or in conditioned media showed no cardiomyocyte-like behavior, thus indicating that soluble factors alone were insufficient to cause beating or the presence of myogenic and gap junction proteins. Several studies have further confirmed this finding, suggesting that physical connections between MSCs and cardiomyocytes via gap junction proteins, like connexin-43, upregulate the expression of cardiomyogenic markers β MHC, cTnT, and α-actin [92–95]. Interestingly, the addition of 5-aza to these co-culture conditions had no increased effect on cardiomyogenic differentiation of the MSCs [96]. Thus, it is possible that the intracellular signaling between the MSCs and the mature cardiomyocytes is triggering a similar pathway as 5-aza when inducing cardiomyogenesis. In light of the conflicting results surrounding 5-aza induced differentiation, it may be more beneficial to focus on downstream regulators of cardiogenic genes, such as the Wnt pathway and other cardiac-related transcription factors.
Despite the moderate success of 5-aza and co-culture techniques, neither is an ideal solution for in vitro cardiomyogenesis. 5-aza has been shown to induce tumors in animals [97] and the cardiomyocyte co-culture system is too poorly defined to identify the controlling variables of differentiation. Shim et al developed a “cardiomyogenic medium” containing several additives such as insulin, transferrin, dexamethasone, ascorbate phosphate, linoleic acid, and sodium selenite [98]. This cocktail of soluble proteins and molecules was able to transdifferentiate nearly 90% of hMSCs in culture, which showed positive staining for cardiac markers TnI, connexin-43, and β MHC. These cells were also negative for myoD and skeletal MHC, indicating that the differentiation was cardiac specific and did not induce skeletal myogenesis. However, the role each element of the cardiomyogenic cocktail plays in differentiation remains unclear. Xaymardan et al also saw positive staining of MSCs for cardiac markers following treatment with VEGF and FGF-2 [99]. Both of these growth factors are commonly used in the cardiomyogenesis of embryonic stem cells, as discussed previously, and also for cardiac-directed differentiation of endothelial cells [100]. Using an elegant systems biology approach, Behfar and Terzic were able to identify specific cardiotrophic agents by analyzing the gene expression of cardiomyogenic embryonal mouse endodermal cells [101]. They then found that a specific combination of these factors, including TGFβ , BMP-2, FGF-4, VEGF, retinoic acid, and tumor necrosis factor α caused a significant upregulation and nuclear translocation of Nkx 2.5 and Mef2c. However, the total percentage of cardiomyogenic cells within the population was not reported, leaving doubts as to the efficiency of the established protocol.
Although controversial, based on the results of these studies, it suggests that a population of cells may exist within the bone marrow that possesses the potential to differentiate into cardiomyocytes or cardiomyocyte-like cells. However, several factors have been implicated in this process and the exact controlling mechanisms have yet to be clearly defined. In fact, one study has even shown the presence of certain muscle markers in freshly isolated bone marrow stromal cells under basal conditions [102]. This suggests the possibility of a rare pre-existing myocardial progenitor present within the bone marrow. Thus, the wide discrepancy in cardiomyocyte differentiation cues for MSCs could be the result of amplification of these cardiac-specific progenitors, as opposed to true transdifferentiation of multipotent stem cells toward the cardiomyocyte lineage.
Adipose-Derived Mesenchymal Stem Cells
In addition to bone marrow, adipose tissue has been discovered as a depot of mesenchymal stem cells. These adipose-derived mesenchymal stem cells (ASCs) can be isolated in relatively high numbers from either excised adipose tissue or from lipoaspirate [103]. Several groups have investigated the multipotency of these stem cells and identified differentiation towards multiple cell lineages, including osteocytes [104, 105], adipocytes [106, 107], chondrocytes [83, 108], myoblasts [109, 110], neurons [111], and hepatocytes [112]. As this review focuses on the cardiomyogenic potential of these cells, readers are referred to more in-depth reviews for further detail [113, 114]. In 2003, Rangappa et al first showed the conversion of rabbit ASCs to a cardiomyocyte lineage by incubating the cells in 9 μM 5-azacytidine for 24 hours [115]. However, despite the ability of 5-aza to encourage the apparent cardiomyogenesis of MSCs, there is not yet enough evidence to support its equivalent function in ASCs. Additionally, the specific targets of this demethylating agent remain unknown and thus preclude its classification as a cardiogenic factor.
Separately, several other groups have shown spontaneous beating of small populations of ASCs in culture. Shortly following the Rangappa study, Planat-Bénard et al published a study showing a miniscule subpopulation of cells (0.02–0.07%) that expressed contractile activity within a heterogeneous population of adipose stromal vascular fraction cells [117]. Despite the presence of surrounding adipocytes and fibroblasts, these beating cells tested positive for Nkx2.5, GATA4, MLC-2v, and MLC-2a. They also positively stained for Mef2C, β MHC, and connexin-43, and negatively for the skeletal marker myoD. Song et al further confirmed these results, reporting a range of 0.005% to 0.07% spontaneously beating cells within a population of human ASCs [118]. Additionally, this study reported a significant level of VEGF in the media surrounding these beating hASCs, and a complete absence of contractile activity following the blocking of VEGF receptor Flk-1. These results suggest that the presence of VEGF could be a crucial factor in the cardiomyogenesis of hASCs. However, no supporting evidence has since been published for this conclusion. Other studies have investigated the ability of nitric oxide donors and cellular uptake of homogenized mature cardiomyocyte extracts as in vitro differentiation cues [119, 120]. While these techniques have resulted in cardiomyocyte-like behavior of hASCs, a defined molecular pathway for cardiomyocyte differentiation remains to be outlined. Gene expression analyses, such as those used by Behfar and Terzic, could provide insight into the appropriate factors to promote hASC cardiogenesis.
Paucity of Systems Biology Analysis in Cardiomyogenesis Research
Current research investigating the cardiomyogenesis of stem cells has identified several key players, but a systematic approach to the process has not yet been defined. Looking across all stem cell types, it appears that the Wnt signaling pathway, β-catenin, and specific growth factors such as VEGF, FGF, and BMP-2 each significantly contribute to the process. However, their roles and interactions have only been partially defined to date. Biological development is never controlled by the static presence of one single molecule. Growth and maturation of any tissue result from the appropriate spatiotemporal delivery of multiple factors at defined intervals. Control over the specific timing and concentration of each stimulus is crucial for proper development of any organ. To date, there has been limited investigation of human stem cell differentiation using a systems biology approach. Fathi et al combined proteome and transcriptome analyses to examine hESCs at various stages of EB differentiation. Accordingly, they identified several proteins associated with self-renewal (Oct4 and Nanog) that were downregulated in differentiating EBs [121]. Furthermore, Aranda et al discovered specific microRNA patterns at different levels of differentiation when analyzing the epigenetic profile of hESCs [122]. However, systemic analysis of cardiogenic factors has primarily been concentrated on mouse ESCs. Faustino et al analyzed the gene expression of mouse ESCs at various timepoints in cardiomyogenesis and were able to identify distinct patterns of upregulation and downregulation of specific genes at the undifferentiated, cardiopoietic, and cardiomyocyte stages [123]. Ventura and Branzi have also published a detailed review of autocrine and intracrine signaling pathways that have been identified for mouse ESC cardiogenesis. Similar analyses of human adult stem cells could help label the “stemness” of these multipotent cells and verify the extent of their cardiomyogenic ability. Metabolomics has recently emerged as a growing field within systems biology to analyze the products of intracellular biochemical reactions, and thus generate connections between a cell’s gene expression and its functional metabolic activity. Post-transcriptional modification can prevent protein activity despite an upregulation in mRNA expression, and thus analysis of the metabolites provides insight into which biochemical pathways are actually active. Additional studies that analyze the metabolome of hESC-derived cardiomyocytes could aid in characterization of the phenotypic changes seen in these differentiating cells. The propensity of embryonic stem cells to form cardiomyocytes also varies greatly between cell lines [124]. Proteomic and metabolomic analyses of these cell lines could potentially identify trends among highly cardiomyogenic cell lines and serve as an initial screening method for differentiation capacity of newly derived lines.
Furthermore, high throughput screening analysis could hasten the discovery of latent contributors to differentiation. This type of non-hypothesis driven research could facilitate faster identification of novel cardiogenic factors than traditional experimentation. Willems et al used high throughput screening and high content screening to identify several small molecule candidates that potentially improve cardiomyogenesis of hESCs [36]. This strategy could be further utilized to discover other compounds that improve ESC viability and maturation as well, and also expanded for use with adult stem cell types. In addition to chemical cues, many studies have implicated external physical factors, such as matrix stiffness and biochemical composition, as contributors to enhanced stem cell differentiation and maturation [125]. While there are limited studies investigating the influence of this feature specifically for cardiomyogenesis, it adds yet another parameter to consider when investigating this type of lineage specificity [126, 127]. Matrix stiffness has also been linked to enhanced maturation and force development of neonatal cardiomyocytes. Gene expression and metabolomics analysis potentially offer a means for identifying signal transduction pathways that develop during cardiomyocyte maturation. This information would aid in quantifying the efficiency of newly developed differentiation protocols.
As empirical data supporting the cardiomyogenesis of various stem cells continues to surface, there emerges a growing need to synthesize this information into a coherent process. In silico experiments offer the possibility of monitoring and managing a host of parameters, but have only begun to investigate the field of cardiomyogenesis. The use of a systems biology approach holds potential for elucidating the controlling mechanisms behind cardiomyocyte differentiation, and thus permit the controlled generation of renewed myocardial tissue.
Conclusions
Regenerating healthy cardiac muscle following myocardial infarction continues to be a significant challenge for tissue engineering. Stem cells offer an exciting potential solution, and have even seen some degree of success in improving heart function in animal models of MI. However, there are several hurdles that must be overcome before this hypothesis can become a reality in the clinic. Most current differentiation protocols lack the efficiency to produce clinically-relevant numbers of committed cardiomyocytes in a reasonable timeframe. Additionally, several approaches use hazardous compounds or molecules that may pose challenges for clinical translation. Undifferentiated stem cells also impose the risk of tumorigenicity upon implantation. Despite these setbacks, progress in improving the efficiency of cardiomyogenesis in vitro and defining the controlling mechanisms brings the generation of therapeutic myocardial tissue closer to realization; a goal that can be rapidly advanced through the use of a systems biology approach.
References
- 1.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Stafford R, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J Subcommittee AHASCaSS. Heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 2.Mann DL. Mechanisms and models in heart failure: A combinatorial approach. Circulation. 1999;100:999–1008. doi: 10.1161/01.cir.100.9.999. [DOI] [PubMed] [Google Scholar]
- 3.Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabé-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H, Jovinge S, Frisén J. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valina C, Pinkernell K, Song Y-H, Bai X, Sadat S, Campeau RJ, Le Jemtel TH, Alt E. Intracoronary administration of autologous adipose tissue-derived stem cells improves left ventricular function, perfusion, and remodelling after acute myocardial infarction. Eur Heart J. 2007;28:2667–77. doi: 10.1093/eurheartj/ehm426. [DOI] [PubMed] [Google Scholar]
- 5.Laflamme M, Chen K, Naumova A, Muskheli V, Fugate J, Dupras S, Reinecke H, Xu C, Hassanipour M, Police S. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25:1015–24. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 6.Hu X, Wang J, Chen J, Luo R, He A, Xie X, Li J. Optimal temporal delivery of bone marrow mesenchymal stem cells in rats with myocardial infarction. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2007;31:438–43. doi: 10.1016/j.ejcts.2006.11.057. [DOI] [PubMed] [Google Scholar]
- 7.Fraser JK, Schreiber RE, Zuk PA, Hedrick MH. Adult stem cell therapy for the heart. Int J Biochem Cell Biol. 2004;36:658–66. doi: 10.1016/j.biocel.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 8.Kelly RG, Buckingham ME. The anterior heart-forming field: voyage to the arterial pole of the heart. Trends Genet. 2002:210–6. doi: 10.1016/s0168-9525(02)02642-2. [DOI] [PubMed] [Google Scholar]
- 9.Abu-Issa R, Kirby M. Heart field: from mesoderm to heart tube. Annual Reviews. 2007 doi: 10.1146/annurev.cellbio.23.090506.123331. [DOI] [PubMed] [Google Scholar]
- 10.Cai C, Liang X, Shi Y, Chu P, Pfaff S, Chen J. Isl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart. Developmental cell. 2003 doi: 10.1016/s1534-5807(03)00363-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verzi MP, McCulley DJ, De Val S, Dodou E, Black BL. The right ventricle, outflow tract, and ventricular septum comprise a restricted expression domain within the secondary/anterior heart field. Dev Biol. 2005;287:134–45. doi: 10.1016/j.ydbio.2005.08.041. [DOI] [PubMed] [Google Scholar]
- 12.Waldo K, Hutson M, Ward C, Zdanowicz M. Secondary heart field contributes myocardium and smooth muscle to the arterial pole of the developing heart. Developmental. 2005 doi: 10.1016/j.ydbio.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 13.Christoffels V, Habets P, Franco D. Chamber formation and morphogenesis in the developing mammalian heart. Developmental. 2000 doi: 10.1006/dbio.2000.9753. [DOI] [PubMed] [Google Scholar]
- 14.Moses KA, DeMayo F, Braun RM, Reecy JL, Schwartz RJ. Embryonic expression of an Nkx2-5/Cre gene using ROSA26 reporter mice. Genesis. 2001;31:176–80. doi: 10.1002/gene.10022. [DOI] [PubMed] [Google Scholar]
- 15.Yoon BS, Yoo SJ, Lee JE, You S, Lee HT, Yoon HS. Enhanced differentiation of human embryonic stem cells into cardiomyocytes by combining hanging drop culture and 5-azacytidine treatment. Differentiation. 2006;74:149–59. doi: 10.1111/j.1432-0436.2006.00063.x. [DOI] [PubMed] [Google Scholar]
- 16.Mummery C, Ward-van Oostwaard D, Doevendans P, Spijker R, van den Brink S, Hassink R, van der Heyden M, Opthof T, Pera M, de la Riviere AB, Passier R, Tertoolen L. Differentiation of human embryonic stem cells to cardiomyocytes: role of coculture with visceral endoderm-like cells. Circulation. 2003;107:2733–40. doi: 10.1161/01.CIR.0000068356.38592.68. [DOI] [PubMed] [Google Scholar]
- 17.Snir M, Kehat I, Gepstein A, Coleman R, Itskovitz-Eldor J, Livne E, Gepstein L. Assessment of the ultrastructural and proliferative properties of human embryonic stem cell-derived cardiomyocytes. Am J Physiol Heart Circ Physiol. 2003;285:H2355–63. doi: 10.1152/ajpheart.00020.2003. [DOI] [PubMed] [Google Scholar]
- 18.He JQ, Ma Y, Lee Y, Thomson JA, Kamp TJ. Human embryonic stem cells develop into multiple types of cardiac myocytes: action potential characterization. Circ Res. 2003;93:32–9. doi: 10.1161/01.RES.0000080317.92718.99. [DOI] [PubMed] [Google Scholar]
- 19.Wei H, Juhasz O, Li J, Tarasova YS, Boheler KR. Embryonic stem cells and cardiomyocyte differentiation: phenotypic and molecular analyses. J Cell Mol Med. 2005;9:804–17. doi: 10.1111/j.1582-4934.2005.tb00381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wobus AM, Rohwedel J, Maltsev V, Hescheler J. Development of cardiomyocytes expressing cardiac-specific genes, action potentials, and ionic channels during embryonic stem cell-derived cardiogenesis. Ann N Y Acad Sci. 1995;752:460–9. doi: 10.1111/j.1749-6632.1995.tb17456.x. [DOI] [PubMed] [Google Scholar]
- 21.Ema M, Takahashi S, Rossant J. Deletion of the selection cassette, but not cis-acting elements, in targeted Flk1–lacZ allele reveals Flk1 expression in multipotent mesodermal progenitors. Blood. 2006;107:111–7. doi: 10.1182/blood-2005-05-1970. [DOI] [PubMed] [Google Scholar]
- 22.Moretti A, Caron L, Nakano A, Lam JT, Bernshausen A, Chen Y, Qyang Y, Bu L, Sasaki M, Martin-Puig S, Sun Y, Evans SM, Laugwitz KL, Chien KR. Multipotent embryonic isl1+ progenitor cells lead to cardiac, smooth muscle, and endothelial cell diversification. Cell. 2006;127:1151–65. doi: 10.1016/j.cell.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 23.Wu SM, Fujiwara Y, Cibulsky SM, Clapham DE, Lien CL, Schultheiss TM, Orkin SH. Developmental origin of a bipotential myocardial and smooth muscle cell precursor in the mammalian heart. Cell. 2006;127:1137–50. doi: 10.1016/j.cell.2006.10.028. [DOI] [PubMed] [Google Scholar]
- 24.Wu S, Fujiwara Y, Cibulsky S, Clapham D, Lien C. Developmental origin of a bipotential myocardial and smooth muscle cell precursor in the mammalian heart. Cell. 2006 doi: 10.1016/j.cell.2006.10.028. [DOI] [PubMed] [Google Scholar]
- 25.Kattman SJ, Huber TL, Keller GM. Multipotent flk-1+ cardiovascular progenitor cells give rise to the cardiomyocyte, endothelial, and vascular smooth muscle lineages. Developmental cell. 2006:723–32. doi: 10.1016/j.devcel.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 26.Moretti A, Caron L, Nakano A, Lam J, Bernshausen A. Multipotent embryonic isl1+ progenitor cells lead to cardiac, smooth muscle, and endothelial cell diversification. Cell. 2006 doi: 10.1016/j.cell.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 27.Dickens BM. International Society for Stem Cell Research (ISSCR) Guidelines for the Conduct of Human Embryonic Stem Cell Research (December 2006) Med Law. 2008;27:179–90. [PubMed] [Google Scholar]
- 28.Daley GQ, Ahrlund Richter L, Auerbach JM, Benvenisty N, Charo RA, Chen G, Deng HK, Goldstein LS, Hudson KL, Hyun I, Junn SC, Love J, Lee EH, McLaren A, Mummery CL, Nakatsuji N, Racowsky C, Rooke H, Rossant J, Scholer HR, Solbakk JH, Taylor P, Trounson AO, Weissman IL, Wilmut I, Yu J, Zoloth L. Ethics. The ISSCR guidelines for human embryonic stem cell research. Science. 2007;315:603–4. doi: 10.1126/science.1139337. [DOI] [PubMed] [Google Scholar]
- 29.Klug MG, Soonpaa MH, Koh GY, Field LJ. Genetically selected cardiomyocytes from differentiating embronic stem cells form stable intracardiac grafts. J Clin Invest. 1996;98:216–24. doi: 10.1172/JCI118769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johkura K, Cui L, Suzuki A, Teng R, Kamiyoshi A, Okamura S, Kubota S, Zhao X, Asanuma K, Okouchi Y, Ogiwara N, Tagawa Y, Sasaki K. Survival and function of mouse embryonic stem cell-derived cardiomyocytes in ectopic transplants. Cardiovasc Res. 2003;58:435–43. doi: 10.1016/s0008-6363(02)00730-7. [DOI] [PubMed] [Google Scholar]
- 31.Kehat I, Kenyagin-Karsenti D, Snir M, Segev H, Amit M, Gepstein A, Livne E, Binah O, Itskovitz-Eldor J, Gepstein L. Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyocytes. J Clin Invest. 2001;108:407–14. doi: 10.1172/JCI12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cao F, Wagner RA, Wilson KD, Xie X, Fu JD, Drukker M, Lee A, Li RA, Gambhir SS, Weissman IL, Robbins RC, Wu JC. Transcriptional and functional profiling of human embryonic stem cell-derived cardiomyocytes. PLoS ONE. 2008;3:e3474. doi: 10.1371/journal.pone.0003474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu J, Fu JD, Siu CW, Li RA. Functional sarcoplasmic reticulum for calcium handling of human embryonic stem cell-derived cardiomyocytes: insights for driven maturation. Stem cells (Dayton, Ohio) 2007;25:3038–44. doi: 10.1634/stemcells.2007-0549. [DOI] [PubMed] [Google Scholar]
- 34.Binah O, Dolnikov K, Sadan O, Shilkrut M, Zeevi-Levin N, Amit M, Danon A, Itskovitz-Eldor J. Functional and developmental properties of human embryonic stem cells-derived cardiomyocytes. Journal of electrocardiology. 2007;40:S192–6. doi: 10.1016/j.jelectrocard.2007.05.035. [DOI] [PubMed] [Google Scholar]
- 35.Laflamme MA, Gold J, Xu C, Hassanipour M, Rosler E, Police S, Muskheli V, Murry CE. Formation of human myocardium in the rat heart from human embryonic stem cells. The American journal of pathology. 2005;167:663–71. doi: 10.1016/S0002-9440(10)62041-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Willems E, Bushway PJ, Mercola M. Natural and synthetic regulators of embryonic stem cell cardiogenesis. Pediatr Cardiol. 2009;30:635–42. doi: 10.1007/s00246-009-9409-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marvin MJ, Di Rocco G, Gardiner A, Bush SM, Lassar AB. Inhibition of Wnt activity induces heart formation from posterior mesoderm. Genes Dev. 2001;15:316–27. doi: 10.1101/gad.855501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Klaus A, Birchmeier W. Developmental signaling in myocardial progenitor cells: a comprehensive view of Bmp- and Wnt/beta-catenin signaling. Pediatr Cardiol. 2009;30:609–16. doi: 10.1007/s00246-008-9352-7. [DOI] [PubMed] [Google Scholar]
- 39.Filipczyk AA, Passier R, Rochat A, Mummery CL. Regulation of cardiomyocyte differentiation of embryonic stem cells by extracellular signalling. Cell Mol Life Sci. 2007;64:704–18. doi: 10.1007/s00018-007-6523-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sumi T, Tsuneyoshi N, Nakatsuji N, Suemori H. Defining early lineage specification of human embryonic stem cells by the orchestrated balance of canonical Wnt/{beta}-catenin, Activin/Nodal and BMP. Development. 2008 doi: 10.1242/dev.021121. [DOI] [PubMed] [Google Scholar]
- 41.Naito AT, Shiojima I, Akazawa H, Hidaka K, Morisaki T, Kikuchi A, Komuro I. Developmental stage-specific biphasic roles of Wnt/beta-catenin signaling in cardiomyogenesis and hematopoiesis. Proc Natl Acad Sci U S A. 2006;103:19812–7. doi: 10.1073/pnas.0605768103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gadue P, Huber TL, Paddison PJ, Keller GM. Wnt and TGF-beta signaling are required for the induction of an in vitro model of primitive streak formation using embryonic stem cells. Proc Natl Acad Sci U S A. 2006;103:16806–11. doi: 10.1073/pnas.0603916103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ueno S, Weidinger G, Osugi T, Kohn AD, Golob JL, Pabon L, Reinecke H, Moon RT, Murry CE. Biphasic role for Wnt/beta-catenin signaling in cardiac specification in zebrafish and embryonic stem cells. Proc Natl Acad Sci U S A. 2007;104:9685–90. doi: 10.1073/pnas.0702859104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, Reinecke H, Xu C, Hassanipour M, Police S, O'Sullivan C, Collins L, Chen Y, Minami E, Gill EA, Ueno S, Yuan C, Gold J, Murry CE. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25:1015–24. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 45.Yao S, Chen S, Clark J, Hao E, Beattie GM, Hayek A, Ding S. Long-term self-renewal and directed differentiation of human embryonic stem cells in chemically defined conditions. Proc Natl Acad Sci U S A. 2006;103:6907–12. doi: 10.1073/pnas.0602280103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marques SR, Yelon D. Differential requirement for BMP signaling in atrial and ventricular lineages establishes cardiac chamber proportionality. Dev Biol. 2009;328:472–82. doi: 10.1016/j.ydbio.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hao J, Daleo MA, Murphy CK, Yu PB, Ho JN, Hu J, Peterson RT, Hatzopoulos AK, Hong CC. Dorsomorphin, a selective small molecule inhibitor of BMP signaling, promotes cardiomyogenesis in embryonic stem cells. PLoS ONE. 2008;3:e2904. doi: 10.1371/journal.pone.0002904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barron M, Gao M, Lough J. Requirement for BMP and FGF signaling during cardiogenic induction in non-precardiac mesoderm is specific, transient, and cooperative. Dev Dyn. 2000;218:383–93. doi: 10.1002/(SICI)1097-0177(200006)218:2<383::AID-DVDY11>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 49.Faure S, de Santa Barbara P, Roberts DJ, Whitman M. Endogenous patterns of BMP signaling during early chick development. Dev Biol. 2002;244:44–65. doi: 10.1006/dbio.2002.0579. [DOI] [PubMed] [Google Scholar]
- 50.Itskovitz-Eldor J, Schuldiner M, Karsenti D, Eden A, Yanuka O, Amit M, Soreq H, Benvenisty N. Differentiation of human embryonic stem cells into embryoid bodies compromising the three embryonic germ layers. Mol Med. 2000;6:88–95. [PMC free article] [PubMed] [Google Scholar]
- 51.Mohr JC, Zhang J, Azarin SM, Soerens AG, de Pablo JJ, Thomson JA, Lyons GE, Palecek SP, Kamp TJ. The microwell control of embryoid body size in order to regulate cardiac differentiation of human embryonic stem cells. Biomaterials. 31:1885–93. doi: 10.1016/j.biomaterials.2009.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Burridge PW, Anderson D, Priddle H, Barbadillo Munoz MD, Chamberlain S, Allegrucci C, Young LE, Denning C. Improved human embryonic stem cell embryoid body homogeneity and cardiomyocyte differentiation from a novel V-96 plate aggregation system highlights interline variability. Stem Cells. 2007;25:929–38. doi: 10.1634/stemcells.2006-0598. [DOI] [PubMed] [Google Scholar]
- 53.Yang L, Soonpaa MH, Adler ED, Roepke TK, Kattman SJ, Kennedy M, Henckaerts E, Bonham K, Abbott GW, Linden RM, Field LJ, Keller GM. Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. Nature. 2008;453:524–8. doi: 10.1038/nature06894. [DOI] [PubMed] [Google Scholar]
- 54.Graichen R, Xu X, Braam SR, Balakrishnan T, Norfiza S, Sieh S, Soo SY, Tham SC, Mummery C, Colman A, Zweigerdt R, Davidson BP. Enhanced cardiomyogenesis of human embryonic stem cells by a small molecular inhibitor of p38 MAPK. Differentiation. 2008;76:357–70. doi: 10.1111/j.1432-0436.2007.00236.x. [DOI] [PubMed] [Google Scholar]
- 55.Tran TH, Wang X, Browne C, Zhang Y, Schinke M, Izumo S, Burcin M. Wnt3a-induced mesoderm formation and cardiomyogenesis in human embryonic stem cells. Stem Cells. 2009;27:1869–78. doi: 10.1002/stem.95. [DOI] [PubMed] [Google Scholar]
- 56.Xu C, Police S, Rao N, Carpenter M. Characterization and enrichment of cardiomyocytes derived from human embryonic stem cells. Circulation research. 2002 doi: 10.1161/01.res.0000035254.80718.91. [DOI] [PubMed] [Google Scholar]
- 57.Xu C, Police S, Rao N, Carpenter MK. Characterization and enrichment of cardiomyocytes derived from human embryonic stem cells. Circ Res. 2002;91:501–8. doi: 10.1161/01.res.0000035254.80718.91. [DOI] [PubMed] [Google Scholar]
- 58.McDevitt TC, Laflamme MA, Murry CE. Proliferation of cardiomyocytes derived from human embryonic stem cells is mediated via the IGF/PI 3-kinase/Akt signaling pathway. J Mol Cell Cardiol. 2005;39:865–73. doi: 10.1016/j.yjmcc.2005.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Leschik J, Stefanovic S, Brinon B, Puceat M. Cardiac commitment of primate embryonic stem cells. Nat Protoc. 2008;3:1381–7. doi: 10.1038/nprot.2008.116. [DOI] [PubMed] [Google Scholar]
- 60.Wu X, Ding S, Ding Q, Gray NS, Schultz PG. Small molecules that induce cardiomyogenesis in embryonic stem cells. J Am Chem Soc. 2004;126:1590–1. doi: 10.1021/ja038950i. [DOI] [PubMed] [Google Scholar]
- 61.Takahashi T, Lord B, Schulze PC, Fryer RM, Sarang SS, Gullans SR, Lee RT. Ascorbic acid enhances differentiation of embryonic stem cells into cardiac myocytes. Circulation. 2003;107:1912–6. doi: 10.1161/01.CIR.0000064899.53876.A3. [DOI] [PubMed] [Google Scholar]
- 62.Nur EKA, Ahmed I, Kamal J, Schindler M, Meiners S. Three-dimensional nanofibrillar surfaces promote self–renewal in mouse embryonic stem cells. Stem Cells. 2006;24:426–33. doi: 10.1634/stemcells.2005-0170. [DOI] [PubMed] [Google Scholar]
- 63.Gerecht S, Burdick JA, Ferreira LS, Townsend SA, Langer R, Vunjak-Novakovic G. Hyaluronic acid hydrogel for controlled self-renewal and differentiation of human embryonic stem cells. Proc Natl Acad Sci U S A. 2007;104:11298–303. doi: 10.1073/pnas.0703723104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gerecht-Nir S, Cohen S, Ziskind A, Itskovitz-Eldor J. Three-dimensional porous alginate scaffolds provide a conducive environment for generation of well-vascularized embryoid bodies from human embryonic stem cells. Biotechnol Bioeng. 2004;88:313–20. doi: 10.1002/bit.20248. [DOI] [PubMed] [Google Scholar]
- 65.Flaim CJ, Chien S, Bhatia SN. An extracellular matrix microarray for probing cellular differentiation. Nat Methods. 2005;2:119–25. doi: 10.1038/nmeth736. [DOI] [PubMed] [Google Scholar]
- 66.Hwang NS, Varghese S, Zhang Z, Elisseeff J. Chondrogenic differentiation of human embryonic stem cell-derived cells in arginine-glycine-aspartate-modified hydrogels. Tissue Eng. 2006;12:2695–706. doi: 10.1089/ten.2006.12.2695. [DOI] [PubMed] [Google Scholar]
- 67.Goetz AK, Scheffler B, Chen HX, Wang S, Suslov O, Xiang H, Brustle O, Roper SN, Steindler DA. Temporally restricted substrate interactions direct fate and specification of neural precursors derived from embryonic stem cells. Proc Natl Acad Sci U S A. 2006;103:11063–8. doi: 10.1073/pnas.0510926103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Garreta E, Genove E, Borros S, Semino CE. Osteogenic differentiation of mouse embryonic stem cells and mouse embryonic fibroblasts in a three-dimensional self–assembling peptide scaffold. Tissue Eng. 2006;12:2215–27. doi: 10.1089/ten.2006.12.2215. [DOI] [PubMed] [Google Scholar]
- 69.Chen SS, Fitzgerald W, Zimmerberg J, Kleinman HK, Margolis L. Cell-cell and cell-extracellular matrix interactions regulate embryonic stem cell differentiation. Stem Cells. 2007;25:553–61. doi: 10.1634/stemcells.2006-0419. [DOI] [PubMed] [Google Scholar]
- 70.Battista S, Guarnieri D, Borselli C, Zeppetelli S. The effect of matrix composition of 3D constructs on embryonic stem cell differentiation. Biomaterials. 2005 doi: 10.1016/j.biomaterials.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 71.Sargent, Berguig G, McDevitt T. Cardiomyogenic differentiation of embryoid bodies is promoted by rotary orbital suspension culture. Tissue Engineering Part A. 2009 doi: 10.1089/ten.tea.2008.0145. [DOI] [PubMed] [Google Scholar]
- 72.Serena E, Figallo E, Tandon N, Cannizzaro C. Electrical stimulation of human embryonic stem cells: Cardiac differentiation and the generation of reactive oxygen species. Experimental cell. 2009 doi: 10.1016/j.yexcr.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–72. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 74.Martinez-Fernandez A, Nelson TJ, Yamada S, Reyes S, Alekseev AE, Perez-Terzic C, Ikeda Y, Terzic A. iPS programmed without c-MYC yield proficient cardiogenesis for functional heart chimerism. Circulation Research. 2009;105:648–56. doi: 10.1161/CIRCRESAHA.109.203109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Martinez-Fernandez A, Nelson TJ, Ikeda Y, Terzic A. c-MYC independent nuclear reprogramming favors cardiogenic potential of induced pluripotent stem cells. Journal of cardiovascular translational research. 2010;3:13–23. doi: 10.1007/s12265-009-9150-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Germanguz I, Sedan O, Zeevi-Levin N, Shtreichman R, Barak E, Ziskind A, Eliyahu S, Meiry G, Amit M, Itskovitz-Eldor J, Binah O. Molecular characterization and functional properties of cardiomyocytes derived from human inducible pluripotent stem cells. J Cell Mol Med. 2009 doi: 10.1111/j.1582-4934.2009.00996.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zwi L, Caspi O, Arbel G, Huber I, Gepstein A, Park I-H, Gepstein L. Cardiomyocyte differentiation of human induced pluripotent stem cells. Circulation. 2009;120:1513–23. doi: 10.1161/CIRCULATIONAHA.109.868885. [DOI] [PubMed] [Google Scholar]
- 78.Zhang J, Wilson GF, Soerens AG, Koonce CH, Yu J, Palecek SP, Thomson JA, Kamp TJ. Functional cardiomyocytes derived from human induced pluripotent stem cells. Circulation Research. 2009;104:e30–41. doi: 10.1161/CIRCRESAHA.108.192237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kurpinski K, Lam H, Chu J, Wang A, Kim A, Tsay E, Agrawal S, Schaffer DV, Li S. Transforming growth factor-beta and notch signaling mediate stem cell differentiation into smooth muscle cells. Stem Cells. 2010;28:734–42. doi: 10.1002/stem.319. [DOI] [PubMed] [Google Scholar]
- 80.Lee J-H, Kosinski PA, Kemp DM. Contribution of human bone marrow stem cells to individual skeletal myotubes followed by myogenic gene activation. Experimental Cell Research. 2005;307:174–82. doi: 10.1016/j.yexcr.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 81.Hosogane N, Huang Z, Rawlins BA, Liu X, Boachie-Adjei O, Boskey AL, Zhu W. Stromal derived factor-1 regulates bone morphogenetic protein 2-induced osteogenic differentiation of primary mesenchymal stem cells. Int J Biochem Cell Biol. 2010 doi: 10.1016/j.biocel.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yoon DS, Yoo JH, Kim YH, Paik S, Han CD, Lee JW. The effects of COX-2 inhibitor during osteogenic differentiation of bone marrow-derived human mesenchymal stem cells. Stem Cells Dev. 2010 doi: 10.1089/scd.2009.0393. [DOI] [PubMed] [Google Scholar]
- 83.Puetzer JL, Petitte JN, Loboa EG. Comparative Review of Growth Factors for Induction of Three-Dimensional In Vitro Chondrogenesis in Human Mesenchymal Stem Cells Isolated from Bone Marrow and Adipose Tissue. Tissue engineering Part B, Reviews. 2010 doi: 10.1089/ten.TEB.2009.0705. [DOI] [PubMed] [Google Scholar]
- 84.Weiss S, Hennig T, Bock R, Steck E, Richter W. Impact of growth factors and PTHrP on early and late chondrogenic differentiation of human mesenchymal stem cells. J Cell Physiol. 2010;223:84–93. doi: 10.1002/jcp.22013. [DOI] [PubMed] [Google Scholar]
- 85.Tondreau T, Dejeneffe M, Meuleman N, Stamatopoulos B, Delforge A, Martiat P, Bron D, Lagneaux L. Gene expression pattern of functional neuronal cells derived from human bone marrow mesenchymal stromal cells. BMC Genomics. 2008;9:166. doi: 10.1186/1471-2164-9-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Makino S, Fukuda K, Miyoshi S, Konishi F, Kodama H, Pan J, Sano M, Takahashi T, Hori S, Abe H, Hata J, Umezawa A, Ogawa S. Cardiomyocytes can be generated from marrow stromal cells in vitro. J Clin Invest. 1999;103:697–705. doi: 10.1172/JCI5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Xu W, Zhang X, Qian H, Zhu W, Sun X, Hu J, Zhou H, Chen Y. Mesenchymal stem cells from adult human bone marrow differentiate into a cardiomyocyte phenotype in vitro. Exp Biol Med (Maywood) 2004;229:623–31. doi: 10.1177/153537020422900706. [DOI] [PubMed] [Google Scholar]
- 88.Liu Y, Song J, Liu W, Wan Y, Chen X, Hu C. Growth and differentiation of rat bone marrow stromal cells: does 5-azacytidine trigger their cardiomyogenic differentiation? Cardiovasc Res. 2003;58:460–8. doi: 10.1016/s0008-6363(03)00265-7. [DOI] [PubMed] [Google Scholar]
- 89.Yang M-C, Wang S-S, Chou N-K, Chi N-H, Huang Y-Y, Chang Y-L, Shieh M-J, Chung T-W. The cardiomyogenic differentiation of rat mesenchymal stem cells on silk fibroin-polysaccharide cardiac patches in vitro. Biomaterials. 2009;30:3757–65. doi: 10.1016/j.biomaterials.2009.03.057. [DOI] [PubMed] [Google Scholar]
- 90.Tomita S, Nakatani T, Fukuhara S, Morisaki T, Yutani C, Kitamura S. Bone marrow stromal cells contract synchronously with cardiomyocytes in a coculture system. Jpn J Thorac Cardiovasc Surg. 2002;50:321–4. doi: 10.1007/BF03032624. [DOI] [PubMed] [Google Scholar]
- 91.Fukuhara S, Tomita S, Yamashiro S, Morisaki T, Yutani C, Kitamura S, Nakatani T. Direct cell-cell interaction of cardiomyocytes is key for bone marrow stromal cells to go into cardiac lineage in vitro. J Thorac Cardiovasc Surg. 2003;125:1470–80. doi: 10.1016/s0022-5223(02)73610-6. [DOI] [PubMed] [Google Scholar]
- 92.Wang T, Xu Z, Jiang W, Ma A. Cell-to-cell contact induces mesenchymal stem cell to differentiate into cardiomyocyte and smooth muscle cell. Int J Cardiol. 2006;109:74–81. doi: 10.1016/j.ijcard.2005.05.072. [DOI] [PubMed] [Google Scholar]
- 93.Rose RA, Jiang H, Wang X, Helke S, Tsoporis JN, Gong N, Keating SCJ, Parker TG, Backx PH, Keating A. Bone marrow-derived mesenchymal stromal cells express cardiac-specific markers, retain the stromal phenotype, and do not become functional cardiomyocytes in vitro. Stem Cells. 2008;26:2884–92. doi: 10.1634/stemcells.2008-0329. [DOI] [PubMed] [Google Scholar]
- 94.Muscari C, Bonafé F, Carboni M, Govoni M, Stanic I, Gamberini C, Ricci F, Tazzari PL, Caldarera CM, Guarnieri C. Difluoromethylornithine stimulates early cardiac commitment of mesenchymal stem cells in a model of mixed culture with cardiomyocytes. J Cell Biochem. 2008;103:1046–52. doi: 10.1002/jcb.21683. [DOI] [PubMed] [Google Scholar]
- 95.Armiñán A, Gandía C, Bartual M, García-Verdugo JM, Lledó E, Mirabet V, Llop M, Barea J, Montero JA, Sepúlveda P. Cardiac differentiation is driven by NKX2.5 and GATA4 nuclear translocation in tissue-specific mesenchymal stem cells. Stem Cells Dev. 2009;18:907–18. doi: 10.1089/scd.2008.0292. [DOI] [PubMed] [Google Scholar]
- 96.Koninckx R, Hensen K, Daniëls A, Moreels M, Lambrichts I, Jongen H, Clijsters C, Mees U, Steels P, Hendrikx M, Rummens J-L. Human bone marrow stem cells co-cultured with neonatal rat cardiomyocytes display limited cardiomyogenic plasticity. Cytotherapy. 2009;11:778–92. doi: 10.3109/14653240902988818. [DOI] [PubMed] [Google Scholar]
- 97.Carr BI, Reilly JG, Smith SS, Winberg C, Riggs A. The tumorigenicity of 5-azacytidine in the male Fischer rat. Carcinogenesis. 1984;5:1583–90. doi: 10.1093/carcin/5.12.1583. [DOI] [PubMed] [Google Scholar]
- 98.Shim WSN, Jiang S, Wong P, Tan J, Chua YL, Tan YS, Sin YK, Lim CH, Chua T, Teh M, Liu TC, Sim E. Ex vivo differentiation of human adult bone marrow stem cells into cardiomyocyte-like cells. Biochemical and biophysical research communications. 2004;324:481–8. doi: 10.1016/j.bbrc.2004.09.087. [DOI] [PubMed] [Google Scholar]
- 99.Xaymardan M, Tang L, Zagreda L, Pallante B, Zheng J, Chazen JL, Chin A, Duignan I, Nahirney P, Rafii S, Mikawa T, Edelberg JM. Platelet-derived growth factor-AB promotes the generation of adult bone marrow-derived cardiac myocytes. Circulation Research. 2004;94:E39–45. doi: 10.1161/01.RES.0000122042.51161.B6. [DOI] [PubMed] [Google Scholar]
- 100.Edelberg JM, Tang L, Hattori K, Lyden D, Rafii S. Young adult bone marrow-derived endothelial precursor cells restore aging-impaired cardiac angiogenic function. Circulation Research. 2002;90:E89–93. doi: 10.1161/01.res.0000020861.20064.7e. [DOI] [PubMed] [Google Scholar]
- 101.Behfar A, Terzic A. Derivation of a cardiopoietic population from human mesenchymal stem cells yields cardiac progeny. Nat Clin Pract Cardiovasc Med. 2006;3 (Suppl 1):S78–82. doi: 10.1038/ncpcardio0429. [DOI] [PubMed] [Google Scholar]
- 102.Corti S, Strazzer S, Del Bo R, Salani S, Bossolasco P, Fortunato F, Locatelli F, Soligo D, Moggio M, Ciscato P, Prelle A, Borsotti C, Bresolin N, Scarlato G, Comi GP. A subpopulation of murine bone marrow cells fully differentiates along the myogenic pathway and participates in muscle repair in the mdx dystrophic mouse. Experimental Cell Research. 2002;277:74–85. doi: 10.1006/excr.2002.5543. [DOI] [PubMed] [Google Scholar]
- 103.Bernacki S, Wall M, Loboa E. Isolation of human mesenchymal stem cells from bone and adipose tissue. Methods in cell biology. 2008;86:257. doi: 10.1016/S0091-679X(08)00011-3. [DOI] [PubMed] [Google Scholar]
- 104.Cho HH, Shin KK, Kim YJ, Song JS, Kim JM, Bae YC, Kim CD, Jung JS. NF-kappaB activation stimulates osteogenic differentiation of mesenchymal stem cells derived from human adipose tissue by increasing TAZ expression. J Cell Physiol. 2010;223:168–77. doi: 10.1002/jcp.22024. [DOI] [PubMed] [Google Scholar]
- 105.Giusta MS, Andrade H, Santos AV, Castanheira P, Lamana L, Pimenta AMC, Goes AM. Proteomic analysis of human mesenchymal stromal cells derived from adipose tissue undergoing osteoblast differentiation. Cytotherapy. 2010 doi: 10.3109/14653240903580270. [DOI] [PubMed] [Google Scholar]
- 106.Stacey D, Hanson S, Lahvis G, Gutowski K, Masters K. In vitro Adipogenic Differentiation of Preadipocytes Varies with Differentiation Stimulus, Culture Dimensionality, and Scaffold Composition. Tissue Engineering Part A. 2009 doi: 10.1089/ten.TEA.2008.0293. [DOI] [PubMed] [Google Scholar]
- 107.Hemmrich K, von Heimburg D, Cierpka K, Haydarlioglu S, Pallua N. Optimization of the differentiation of human preadipocytes in vitro. Differentiation. 2005;73:28–35. doi: 10.1111/j.1432-0436.2005.07301003.x. [DOI] [PubMed] [Google Scholar]
- 108.Jiang T, Liu W, Lv X, Sun H, Zhang L, Liu Y, Zhang WJ, Cao Y, Zhou G. Potent in vitro chondrogenesis of CD105 enriched human adipose-derived stem cells. Biomaterials. 2010;31:3564–71. doi: 10.1016/j.biomaterials.2010.01.050. [DOI] [PubMed] [Google Scholar]
- 109.Mizuno H, Zuk P, Zhu M, Lorenz H, Benhaim P, Hedrick M. Myogenic Differentiation by Human Processed Lipoaspirate Cells. Plastic and reconstructive surgery. 2002;109:199. doi: 10.1097/00006534-200201000-00030. [DOI] [PubMed] [Google Scholar]
- 110.Kim MH, Hong HN, Hong JP, Park CJ, Kwon SW, Kim SH, Kang G, Kim M. The effect of VEGF on the myogenic differentiation of adipose tissue derived stem cells within thermosensitive hydrogel matrices. Biomaterials. 2009:1–6. doi: 10.1016/j.biomaterials.2009.10.057. [DOI] [PubMed] [Google Scholar]
- 111.Safford KM, Hicok KC, Safford SD, Halvorsen Y-DC, Wilkison WO, Gimble JM, Rice HE. Neurogenic differentiation of murine and human adipose-derived stromal cells. Biochemical and biophysical research communications. 2002;294:371–9. doi: 10.1016/S0006-291X(02)00469-2. [DOI] [PubMed] [Google Scholar]
- 112.Banas A, Teratani T, Yamamoto Y, Tokuhara M, Takeshita F, Osaki M, Kato T, Okochi H, Ochiya T. Rapid hepatic fate specification of adipose-derived stem cells and their therapeutic potential for liver failure. J Gastroenterol Hepatol. 2009;24:70–7. doi: 10.1111/j.1440-1746.2008.05496.x. [DOI] [PubMed] [Google Scholar]
- 113.Zuk P, Zhu M, Ashjian P, De Ugarte D, Huang J, Mizuno H, Alfonso Z, Fraser J, Benhaim P, Hedrick M. Human adipose tissue is a source of multipotent stem cells. Molecular biology of the cell. 2002;13:4279. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gimble J, Guilak F. Adipose-derived adult stem cells: isolation, characterization, and differentiation potential. Cytotherapy. 2003;5:362–9. doi: 10.1080/14653240310003026. [DOI] [PubMed] [Google Scholar]
- 115.Rangappa S, Fen C, Lee EH, Bongso A, Sim EKW, Wei EKS. Transformation of adult mesenchymal stem cells isolated from the fatty tissue into cardiomyocytes. The Annals of thoracic surgery. 2003;75:775–9. doi: 10.1016/s0003-4975(02)04568-x. [DOI] [PubMed] [Google Scholar]
- 116.Lee W-CC, Sepulveda JL, Rubin JP, Marra KG. Cardiomyogenic differentiation potential of human adipose precursor cells. Int J Cardiol. 2009;133:399–401. doi: 10.1016/j.ijcard.2007.11.068. [DOI] [PubMed] [Google Scholar]
- 117.Planat-Bénard V, Menard C, André M, Puceat M, Perez A, Garcia-Verdugo J-M, Pénicaud L, Casteilla L. Spontaneous cardiomyocyte differentiation from adipose tissue stroma cells. Circulation Research. 2004;94:223–9. doi: 10.1161/01.RES.0000109792.43271.47. [DOI] [PubMed] [Google Scholar]
- 118.Song Y, Gehmert S, Sadat S, Pinkernell K, Bai X, Matthias N, Alt E. VEGF is critical for spontaneous differentiation of stem cells into cardiomyocytes. Biochemical and biophysical research communications. 2007;354:999–1003. doi: 10.1016/j.bbrc.2007.01.095. [DOI] [PubMed] [Google Scholar]
- 119.Rebelatto CK, Aguiar AM, Senegaglia AC, Aita CM, Hansen P, Barchiki F, Kuligovski C, Olandoski M, Moutinho JA, Dallagiovanna B, Goldenberg S, Brofman PS, Nakao LS, Correa A. Expression of cardiac function genes in adult stem cells is increased by treatment with nitric oxide agents. Biochemical and biophysical research communications. 2009;378:456–61. doi: 10.1016/j.bbrc.2008.11.061. [DOI] [PubMed] [Google Scholar]
- 120.Gaustad K, Boquest A, Anderson B, Gerdes A, Collas P. Differentiation of human adipose tissue stem cells using extracts of rat cardiomyocytes. Biochemical and biophysical research communications. 2004;314:420–7. doi: 10.1016/j.bbrc.2003.12.109. [DOI] [PubMed] [Google Scholar]
- 121.Fathi A, Pakzad M, Taei A, Brink TC, Pirhaji L, Ruiz G, Sharif Tabe Bordbar M, Gourabi H, Adjaye J, Baharvand H, Salekdeh GH. Comparative proteome and transcriptome analyses of embryonic stem cells during embryoid body-based differentiation. Proteomics. 2009;9:4859–70. doi: 10.1002/pmic.200900003. [DOI] [PubMed] [Google Scholar]
- 122.Aranda P, Agirre X, Ballestar E, Andreu EJ, Román-Gómez J, Prieto I, Martín-Subero JI, Cigudosa JC, Siebert R, Esteller M, Prosper F. Epigenetic signatures associated with different levels of differentiation potential in human stem cells. PLoS ONE. 2009;4:e7809. doi: 10.1371/journal.pone.0007809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Faustino RS, Behfar A, Perez-Terzic C, Terzic A. Genomic chart guiding embryonic stem cell cardiopoiesis. Genome Biol. 2008;9:R6. doi: 10.1186/gb-2008-9-1-r6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Osafune K, Caron L, Borowiak M, Martinez RJ, Fitz–Gerald CS, Sato Y, Cowan CA, Chien KR, Melton DA. Marked differences in differentiation propensity among human embryonic stem cell lines. Nat Biotechnol. 2008;26:313–5. doi: 10.1038/nbt1383. [DOI] [PubMed] [Google Scholar]
- 125.Engler AJ, Sweeney HL, Discher DE, Schwarzbauer JE. Extracellular matrix elasticity directs stem cell differentiation. Journal of musculoskeletal & neuronal interactions. 2007;7:335. [PubMed] [Google Scholar]
- 126.Engler AJ, Carag–Krieger C, Johnson CP, Raab M, Tang H-Y, Speicher DW, Sanger JW, Sanger JM, Discher DE. Embryonic cardiomyocytes beat best on a matrix with heart-like elasticity: scar-like rigidity inhibits beating. J Cell Sci. 2008;121:3794–802. doi: 10.1242/jcs.029678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Baharvand H, Azarnia M, Parivar K, Ashtiani SK. The effect of extracellular matrix on embryonic stem cell-derived cardiomyocytes. Journal of molecular and cellular cardiology. 2005;38:495–503. doi: 10.1016/j.yjmcc.2004.12.011. [DOI] [PubMed] [Google Scholar]