Abstract
Interleukin 17 (IL-17) plays a critical role in the pathogenesis of inflammatory and autoimmune diseases. Here we report that Act1, the key adaptor for IL-17R, forms a complex with IKKi upon IL-17 stimulation. Using IKKi-deficient mice, we show that IKKi was required for IL-17-induced inflammatory gene expression in primary airway epithelial cells, neutrophilia and pulmonary inflammation. IKKi deficiency abolished IL-17-induced Act1-TRAF2/5 complex formation, MAPK activation and mRNA stability, whereas the Act1-TRAF6-NFκB axis was retained. IKKi was required for IL-17-induced Act1 phosphorylation on serine 311, adjacent to a putative TRAF binding motif. S311A mutation impaired IL-17-mediated Act1-TRAF2/5 interaction and gene expression. Thus, IKKi is a novel kinase modulating IL-17 signaling through its impact on Act1 phosphorylation and consequent function.
Introduction
The inflammatory T helper cell population (TH17), distinct from the classical TH1 and TH2 subsets, has provided important understanding about T cell-mediated immunity. Interleukin (IL)-17 (IL-17, IL-17A), a key proinflammatory cytokine mainly produced by the TH17 cell lineage, is required for host defense against extracellular microorganisms and contributes to the development and pathogenesis of inflammatory and autoimmune diseases1–8. IL-17 concentrations are elevated in many inflammatory conditions such as multiple sclerosis, rheumatoid arthritis and psoriasis. Elevated IL-17 concentrations were also found in the lung and blood of allergic asthma patients and linked to severity of asthma9–11. The main function of IL-17A is to coordinate local tissue inflammation via the upregulation of proinflammatory and neutrophil-mobilizing cytokines and chemokines, which include IL-6, GM-CSF, TNF, IL-1, CXCL1(KC), CCL2(MCP-1), CXCL2(MIP-2), CCL7(MCP-3), and CCL20(MIP-3A). IL-17 deficiency leads to diminished antigen-specific T cell-mediated immune responses, including allergen-induced pulmonary inflammation and airway hyper-responsiveness5,12. Although recent studies have begun to unravel some aspects of IL-17-inititated signal transduction13–19, precise molecular definition of the mechanism(s) through which IL-17 couples with multiple endpoints remains unclear. Identification of intermediate signaling components and elucidation of their mechanisms of action are crucial for the development of new therapeutic strategies to attenuate this major pro-inflammatory pathway.
IL-17 signals through a heteromeric receptor complex composed of two receptor chains IL-17RA and IL-17RC20,21. The adaptor protein Act1 has been identified as an essential component in the IL-17 signalling pathway and is required for IL-17-dependent immune responses13,18,20. IL-17RA, IL-17RC, and Act1 are all members of a protein family, defined by a conserved SEFIR domain, that is responsible for the homotypical interaction between the receptor complex and Act1 (ref. 22). Using a mouse model of allergic pulmonary inflammation, we observed that TH2 responses and lung inflammation in Act1-deficient mice were markedly reduced when compared to littermate control wild-type mice. Importantly, Act1 deficiency in epithelial cells reduced IL-17-induced pulmonary neutrophilia in the airway, indicating the essential role of epithelial-derived Act1 in IL-17-mediated pulmonary inflammation23.
Act1 contains two tumor necrosis factor receptor-associated factor (TRAF) binding sites; a helix-loop-helix domain at the N-terminus and a coiled-coil domain, which contains the SEFIR domain, at the C-terminus24. Upon IL-17 stimulation, Act1 is recruited to IL-17R through SEFIR-SEFIR domain interaction, which is followed by recruitment of the TGF-β Activated Kinase 1 (TAK1) and E3 ubiquitin ligase TRAF6 that mediate ‘downstream’ signalling events18,25. Importantly, we previously reported that Act1 is a novel U-box E3 ubiquitin ligase, whose activity is essential for IL-17-mediated signaling pathways (including NF-κB (nuclear factor κB), Jnk (c-Jun N-terminal kinase), p38 MAPK (mitogen-activated protein kinase) and Erk (extracellular signal regulated kinase) activation) and for inflammatory gene expression (encoding Cxcl1, Csf2 and Il6) in mammalian cells26. By using Ubc13/Uev1A E2 complex, we demonstrated that Act1 mediates Lys 63-linked ubiquitination of TRAF6, an important signaling component of IL-17-mediated NF-κB activation. However, it is important to note that TRAF6 is not required for IL-17-induced Erk and p38 activation, indicating the existence of an IL-17-induced, Act1-mediated but TRAF6-independent pathway. In addition to induction of gene transcription, IL-17 stimulation also prolongs the half-life of chemokine mRNAs, which is also mediated by an Act1-dependent but TRAF6-independent pathway14.
One important question is how Act1 is activated and regulated upon IL-17 stimulation to mediate the various downstream signaling pathways. In this study, we found that, upon IL-17 stimulation, the kinase IKKi (also known as IKKε)27,28 forms a complex with Act1 and mediates IL-17-induced Act1 phosphorylation, which is critical for the modulation of Act1-dependent signaling. Furthermore, using IKKi-deficient mice, we show that IKKi was required for IL-17-induced inflammatory gene expression (Cxcl1, Cxcl2, Tnf, Il6 and Csf3) in primary airway epithelial cells, neutrophilia and pulmonary inflammation, indicating that IKKi is a key component of the inflammatory response to IL-17. The identification of IKKi as a critical upstream kinase of Act1 in the IL-17 pathway will facilitate the development of new anti-inflammatory therapies to attenuate IL-17-dependent autoimmune inflammatory diseases.
Results
IKKi forms a complex with Act1 upon IL-17 stimulation
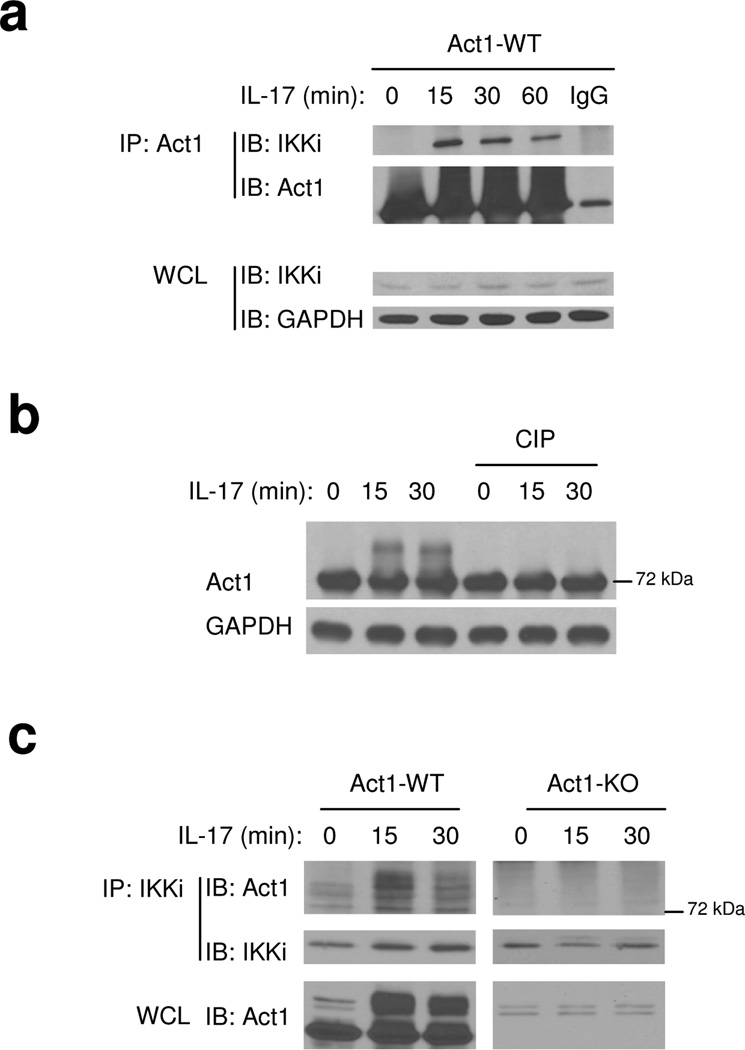
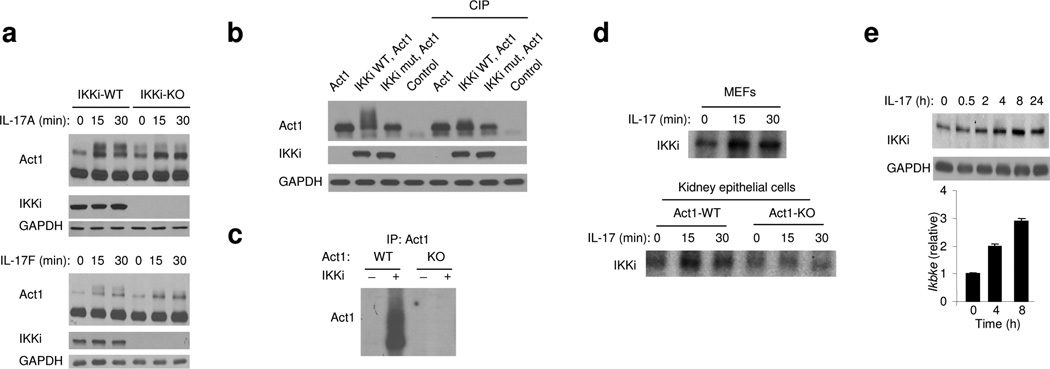
To understand how Act1 is activated and linked to downstream signaling factors upon IL-17 stimulation, we searched for unique proteins that co-immunoprecipitate with tagged-Act1 reconstituted into Act1-deficient primary mouse embryonic fibroblasts (MEFs). Since IL-17R and TLR–IL-1R mediate many similar downstream signaling events, a set of candidate signaling components that have been implicated in TLR–IL-1R pathways were tested for their interaction with Act1. Through this search, we found that Act1 (~72 kDa) forms a complex with the IKK-related kinase IKKi (also known as IKK epsilon) in response to IL-17 stimulation (Fig. 1a). IKKi was previously shown to play an important role in the innate immune response by activating IRF3 (interferon regulatory factor 3) and IRF7 during viral infection29–32. IL-17 induced a form of Act1 higher apparent molecule weight, which shifted down to its normal size upon phosphatase treatment (Fig. 1b), suggesting that Act1 is phosphorylated upon IL-17 stimulation. Interestingly, when immunoprecipitation was performed with anti-IKKi on cell lysates from untreated and IL-17-treated MEFs, only the modified form of Act1 was detected in the immune complex of IKKi (Fig. 1c), implying that IKKi interacts primarily with this form of Act1.
Figure 1. IKKi forms a complex with Act1 upon IL-17 stimulation.
A. Cell lysates from Act1-deficient MEFs infected with retroviral WT Act1 (Act1-WT) untreated or treated with IL-17 (50 ng/ml) for 0, 15, 30 and 60 min were immunoprecipitated with anti-Act1 or IgG, followed by immunoblot analysis with anti-IKKi, anti-Act1 and anti-GAPDH. WCL: whole cell lysates.
B. Lysates from Act1 WT reconstituted MEFs treated with IL-17 (50 ng/ml) for 0, 15 and 30 min were untreated or treated with phosphatase (CIP, 1 h, 37°C)], followed by immunoblot analysis with anti-Act1 and anti-GAPDH.
C. Cell lysates from wild-type and Act1-deficient MEFs untreated or treated with IL-17 (50 ng/ml) for 0, 15 and 30 min were immunoprecipitated with anti-IKKi, followed by immunoblot analysis with anti-Act1 and anti-IKKi. WCL: whole cell lysates.
The data shown in this figure are representation of three independent experiments.
IKKi in IL-17-induced inflammatory gene expression
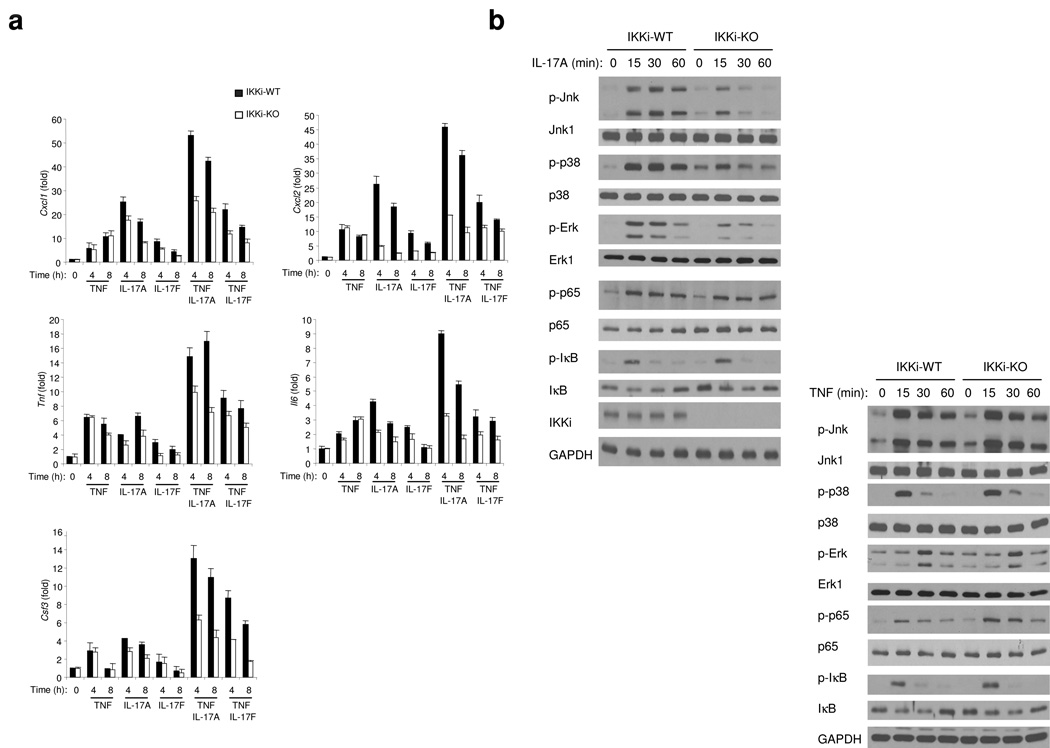
The fact that IKKi forms a complex with Act1 in response to IL-17 stimulation suggests that IKKi may play a role in IL-17-mediated signaling pathways. We first examined whether IKKi is required for IL-17-induced inflammatory gene expression. This study was done with differentiated murine primary airway epithelial cells cultured at an air-liquid interface, since we previously reported that Act1 deficiency in epithelial cells reduced IL-17-induced inflammatory gene expression and neutrophilia23. It is well documented that IL-17A and IL-17F can induce the expression of genes encoding proinflammatory molecules alone and acting in synergy with TNF. To determine if IKKi is required for the IL-17 signaling pathway, we analyzed the expression of several proinflammatory genes (Cxcl1, Cxcl2, Tnf, Il6 and Csf3) by real-time PCR after treatment of IKKi-deficient and wild-type (control) airway epithelial cells with TNF, IL-17A, IL-17F, IL-17A plus TNF or IL-17F plus TNF (Fig. 2a). TNF-stimulated expression of CXCL1, CXCL2, TNF, IL-6 and CSF3 was not different for IKKi-deficient and wild-type cells, indicating that IKKi is not involved in mediating signaling pathways via TNF. In contrast, expression of CXCL1, CXCL2, TNF, IL-6 and CSF3 induced by IL-17A and IL-17F alone or in synergy with TNF was significantly attenuated in IKKi-deficient airway epithelial cells, demonstrating that IKKi is important for the response to IL-17 (Fig. 2a).
Figure 2. IKKi is required for IL-17-mediated pro-inflammatory gene expression.
A. Real-time PCR analysis of CXCL1, TNF, CXCL2, IL-6 and CSF3 in wild-type and IKKi-deficient airway epithelial cells untreated or stimulated with TNF (10ng/ml), IL-17A (50 ng/ml), IL-17F (50 ng/ml), TNF+IL-17A or TNF+IL-17F at the basal surface of the epithelial cells for 0, 4 and 8 hours. Expression of mRNA is presented as fold of induction.
B. Immunoblot analysis of p-Jnk, Jnk1, p-p38, p38, p-Erk, Erk1, p-p65, p65, p-IκB, IκB, IKKi and GAPDH of lysates from wild-type and IKKi-deficient airway epithelial cells untreated or treated with IL-17A (50 ng/ml) or with TNF (10ng/ml) for indicated times.
The data shown in this figure are representation of three independent experiments.
To study the direct impact of IKKi on IL-17-induced signaling events, airway epithelial cells prepared from wild-type and IKKi-deficient mice were examined for capacity of IL-17 to stimulate MAPK activation. IL-17-induced p38 activation was abolished in airway epithelial cells from IKKi-deficient mice compared with that in wild-type control cells while Erk signaling was greatly reduced and Jnk signaling was somewhat reduced (Fig. 2b). In contrast, IL-17 induced similar extent of IκB phosphorylation and NF-κB activation (p65 phosphorylation) in wild-type and IKKi-deficient cells. Taken together, while IL-17-mediated cytokine and chemokine production was abolished in IKKi-deficient cells, IKKi deficiency substantially reduced IL-17-induced MAPK activation. As a control, we showed that IKKi deficiency did not have any effect on TNF-induced signaling events, indicating the specificity of IKKi in IL-17 signaling (Fig. 2b).
IKKi is required for IL-17-mediated neutrophilia
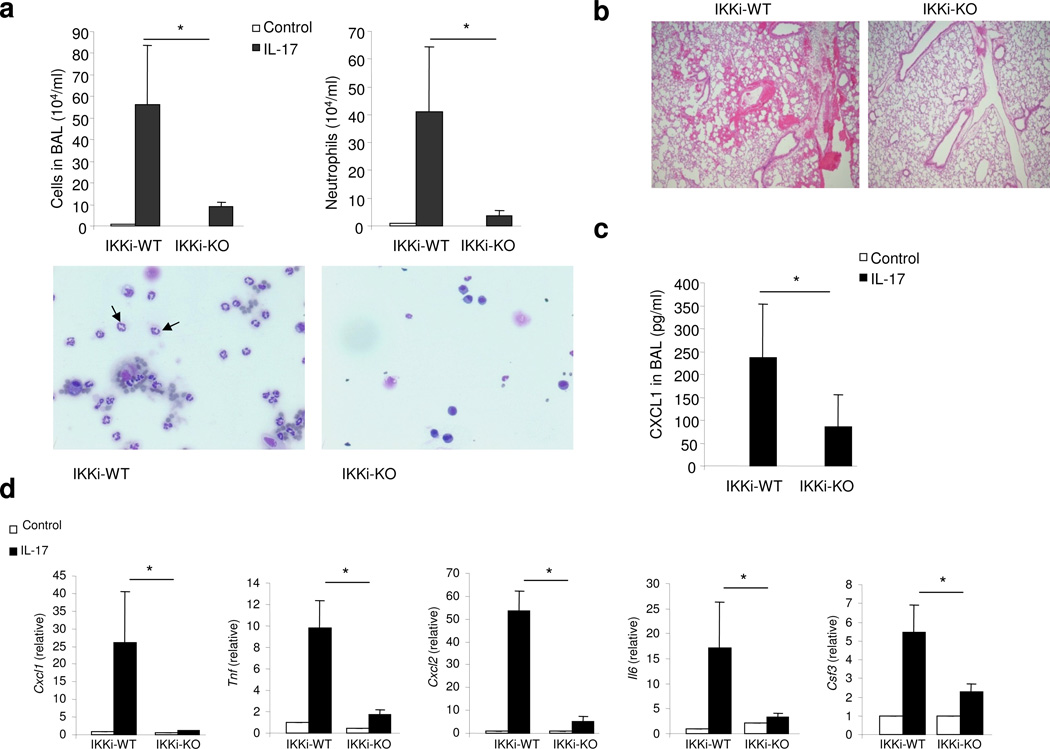
The main function of IL-17 is to coordinate local tissue inflammation via the up-regulation of proinflammatory and neutrophil-mobilizing cytokines and chemokines. We have recently reported that epithelial-derived Act1 is required for IL-17-mediated neutrophilia and allergic pulmonary inflammation23. Since IKKi forms a complex with Act1 upon IL-17 stimulation and plays an important role in IL-17-mediated inflammatory gene expression (Fig. 2), we next examined the in vivo impact of IKKi deficiency on IL-17-induced pulmonary inflammation. Wild-type and IKKi-deficient mice on a C57BL/6 background were treated with rIL-17 through intranasal injection. Twenty-four hours after challenge, the mice were sacrificed and analyzed for bronchalveolar lavage (BAL) cells and lung inflammation. The number of infiltrating cells, especially neutrophils, was significantly reduced in IKKi-deficient mice compared with that observed in wild-type mice (Fig. 3a). Histological analysis of lung tissue showed decreased lung inflammation in IKKi-deficient mice (Fig. 3b). The decreased inflammatory phenotype correlates with the decreased abundance of CXCL1 (KC) (a potent neutrophil chemokine) in the BAL and lung (Fig. 3c,d). Furthermore, IL-17-induced expression of CXCL1, CXCL2, TNF, IL-6 and CSF3 was abolished in the lung tissue of IKKi-deficient mice compared with that in wild-type mice (Fig. 3d). Taken together, these results indicate that IKKi is required for IL-17-mediated neutrophilia and pulmonary inflammation.
Figure 3. IKKi is required for IL-17-mediated pulmonary inflammation.
A. Total BAL and differential cell count were analyzed in samples from control or IL-17 challenged (1 µg through intranasal injection for 24 h) wild-type and IKKi-deficient mice (n=6) P<0.05. Cytospins prepared from the BAL of control or IL-17 challenged wild-type and IKKi-deficient mice were stained with Hema3, and differential cell counting was performed using standard morphological criteria. Magnification x400.
B. The lung sections from control or IL-17 challenged wild-type and IKKi-deficient mice (1 µg through intranasal injection for 24 h) were stained with H&E. Magnification x100.
C. ELISA of CXCL1 chemokine in BAL fluid from control or IL-17 challenged wild-type and IKKi-deficient mice.
D. Real-time PCR analysis of CXCL1, TNF, CXCL2, IL-6 and CSF3 in the lung tissues from control or IL-17 treated wild-type and IKKi-deficient mice. Expression of mRNA is presented as arbitrary units (mean ± s.d.) relative to the expression of mRNA encoding β-Actin. P<0.05.
The data shown in this figure are representation of two independent experiments.
IKKi mediates chemokine mRNA stabilization
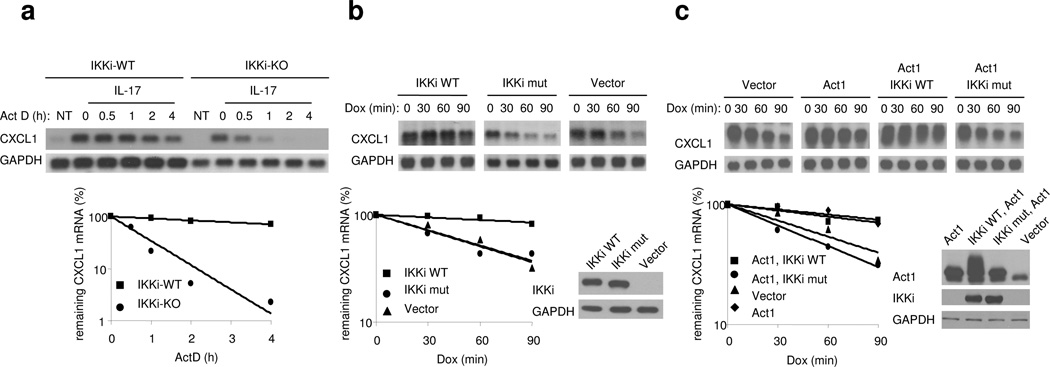
IL-17 has been shown to modulate gene expression at both transcriptional and post-transcriptional levels14,18. Because NF-κB activation was not affected in IKKi-deficient cells, we reasoned that IKKi might be necessary for the ability of IL-17 to promote the stabilization of chemokine mRNA, as we have previously reported14. Wild-type and IKK-deficient MEFs were first treated with TNF for 0.5 h to promote transcription of the Cxcl1 gene followed by treatment with actinomycin D (to block transcription) along with IL-17 (for mRNA stabilization) for 0.5–4 h. Although CXCL1 mRNAs were induced to similar extent in wild-type and IKKi-deficient MEFs after the initial treatment with TNF, the mRNA decayed more rapidly and reached a lower plateau in IKKi-deficient MEFs compared with wild-type cells (Fig. 4a). These results indicate that IKKi is necessary for IL-17-mediated CXCL1 mRNA stabilization.
Figure 4. The kinase activity of IKKi is required for chemokine mRNA stabilization.
A. Wild-type and IKKi-deficient MEFs were pretreated with TNF (10 ng/ml) for 1 h, followed by the treatment with IL-17 and ActD (5 µg/ml) for the indicated times. Total RNAs were prepared from these cells, followed by analysis of CXCL1 and GAPDH mRNA abundance by RNA hybridization blot analysis.
B. HeLa tet-off cells were transfected with 1 µg of pTRE2 CXCL1Δ4 and 1 µg of pcDNA3, IKKi WT or K38A IKKi mutant. The transfected cells were treated with Dox (1 µg/ml) and incubated for the indicated times, followed by analysis of CXCL1 and GAPDH mRNA levels by RNA hybridization blot analysis.
C. HeLa tet-off cells were transfected with 1 µg of pTRE2 CXCL1Δ4 and 1 µg of pcDNA3, Act1+ IKKi WT, or Act1+K38A IKKi mutant. The transfected cells were treated with Dox (1 µg/ml) and incubated for the indicated times, followed by analysis of CXCL1 and GAPDH mRNA levels by RNA hybridization blot analysis.
The data shown in this figure are representation of three independent experiments.
To explore this potential regulatory mechanism in more detail we examined the impact of IKKi kinase activity on mRNA stabilization using the tet-off expression system33. The reporter plasmid (pTRE2 CXCL1Δ4) includes the 5’ untranslated region (5’ UTR) and coding region as well as the IL-17 sensitive portion of the 3’UTR of CXCL1 mRNA under control of a Tetracycline-response element (TRE). HeLa cells stably expressing the tet-controlled trans activator were transiently co-transfected with this reporter plasmid (pTRE2 CXCL1Δ4) along with wild-type or kinase-inactive IKKi (K38A Lys38→Ala38). In the absence of tet, or its analog doxycyline (dox), the tet trans activator drives strong transcription of the CXCL1 transgene. Following addition of dox to the culture medium transcription is abolished enabling the measurement of residual CXCL1 and GAPDH mRNA abundance over 0, 30, 60 and 90 min by RNA hybridization analysis. Wild-type IKKi, but not the kinase-inactive IKKi mutant, promoted CXCL1 mRNA stabilization (Fig. 4b). These results indicate that over-expression of IKKi is sufficient to mediate CXCL1 mRNA stabilization, and the kinase activity IKKi is required for this process.
Since it has been shown that overexpression of Act1 can mediate CXCL1 mRNA stabilization, we then examined the impact of IKKi kinase activity on Act1-mediated mRNA stabilization by using the tet-off expression system as described above33. HeLa tet-off cells were transiently co-transfected with pTRE2 CXCL1Δ4 along with Act1 and either wild-type or kinase inactive mutant IKKi. The transfected cells were treated with dox to allow reporter CXCL1 mRNA to decay for 0, 30, 60 and 90 min and remaining reporter CXCL1 mRNA and GAPDH mRNA were determined as above. The kinase-inactive IKKi mutant inhibited the ability of Act1 to mediate CXCL1 mRNA stabilization (Fig. 4c). These results suggest that the IKKi kinase-inactive mutant probably functions as a dominant negative mutant that inhbits signals downstream of Act1 that couple with CXCL1 mRNA stabilization.
IKKi mediates Act1 phosphorylation
Our results indicate that IKKi plays an essential role in IL-17-mediated gene expression and in vivo inflammatory response. We investigated the important question of how IKKi participates in IL-17 signaling at a molecular level. As shown in Figure 1, IL-17 induced phosphorylation of Act1, and only the modified form of Act1 was detected in immune complexes with IKKi, suggesting that IKKi may directly modulate Act1. To determine the role of IKKi in IL-17-induced Act1 phosphorylation, we examined Act1 modification in untreated and IL-17-treated airway epithelial cells from wild-type and IKKi-deficient mice. Importantly, IL-17 (IL-17A or IL-17F)-induced Act1 phosphorylation was greatly reduced in airway epithelial cells from IKKi-deficient mice compared to wild-type control cells (Fig. 5a). To determine whether the kinase activity of IKKi is important for Act1 phosphorylation, we overexpressed wild-type and kinase-inactive IKKi (IKKi-K38A) in human 293 cells, followed by immunoblot analysis of Act1. Wild-type IKKi but not IKKi-K38A induced a form of Act1 with slower migration, which shifted down to its normal size upon phosphatase treatment (Fig. 5b), indicating that the kinase activity of IKKi is required for Act1 phosphorylation. We then examined whether IKKi can directly phosphorylate Act1 in vitro. Act1 immunoprecipiated from wild-type MEFs was subjected to in vitro kinase assay with or without purified recombinant IKKi protein. Immunoprecipitates from Act1-deficient MEFs was used as a negative control. Importantly, we observed that recombinant IKKi was able to effectively phosphorylate Act1 in vitro (Fig. 5c), indicating the importance of IKKi in Act1 phosphorylation.
Figure 5. The kinase activity of IKKi is required for IL-17-induced Act1 phosphorylation.
A. Immunoblot analysis of Act1, IKKi and GAPDH in lysates from wild type and IKKi-deficient airway epithelial cells untreated or treated with IL-17A (50 ng/ml) or IL-17F (50ng/ml) for indicated times.
B. Lysates from 293HEK cells transfected with Act1+pcDNA3, Act1+ IKKi WT, Act1+K38A IKKi mutant or pcDNA3 were untreated or treated with phosphatase (CIP, 1 h at 37°C), followed by immunoblot analysis with antibodies against Act1, IKKi and GAPDH.
C. Lysates from wild-type or Act1-deficient MEFs untreated or treated with IL-17 (50 ng/ml, 20 min) were immunoprecipitated with anti-Act1 followed by in vitro kinase assay with or without recombinant IKKi-GST.
D. Lysates from wild-type MEFs or wild-type and Act1-deficient kidney epithelial cells untreated or treated with IL-17 (50 ng/ml, 0, 15 and 30 min) were immunoprecipitated with anti-IKKi followed by in vitro kinase assay.
E. Immunoblot and real-time PCR analysis of IKKi and GAPDH in lysates from wild type and IKKi-deficient airway epithelial cells untreated or treated with IL-17A (50 ng/ml) for indicated times.
The data shown in this figure are representation of three independent experiments.
While IKKi can mediate Act1 phosphorylation, it would also be important to examine IL-17-mediated IKKi activation. We immunoprecipitated IKKi from cell lysates of MEFs and primary kidney epithelial cells untreated and treated with IL-17, followed by in vitro kinase assay. Increased IKKi phosphorylation was detected in lysates from both cell types 15 min after IL-17 stimulation (Fig. 5d). Interestingly, IL-17-induced IKKi auto-phosphorylation was substantially reduced in Act1-deficient kidney epithelial cells. These results implicate that IL-17 stimulation probably leads to IKKi activation in an Act1-dependent manner. On the other hand, previous studies have shown the IKKi expression can be induced by extracellular stimuli, including lipopolysaccharide. Therefore, we examined whether IL-17 can also upregulate IKKi expression. IL-17 stimulation indeed induced moderate amounts of IKKi at both mRNA and protein levels (Fig. 5e). Taken together, our results show that IL-17 stimulation impacts on both IKKi expression and activation.
IL-17 induces phosphorylation of Act1 at S311 site
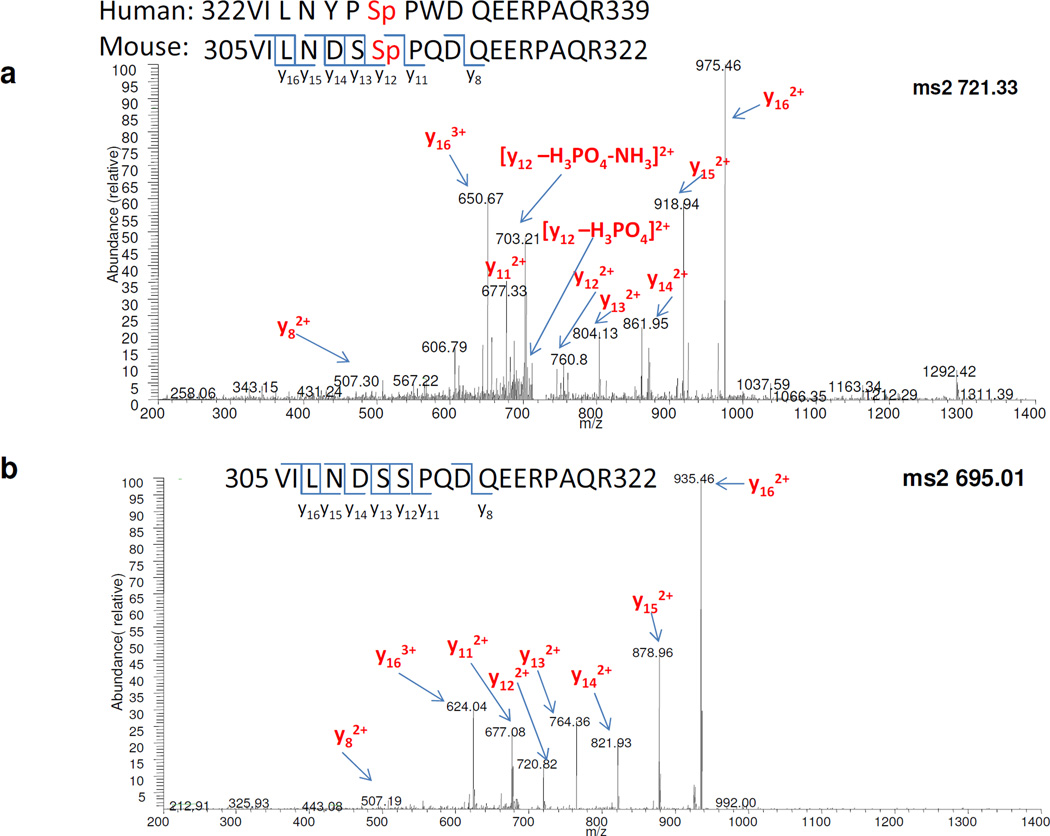
We next attempted to identify the IL-17-induced IKKi-mediated Act1 phosphorylation sites by MS/MS. Act1−/− MEFs were reconstituted with mouse Act1 and stimulated by IL-17 for 30 min, followed by immunoprecipitation of the mouse Act1. The Act1-immunocomplex was separated by SDS–polyacrylamide gel electophoresis. Bands corresponding to Act1, including regions covering modified forms of Act1 (~72 kDa) were excised and subjected to in-gel digestion by trypsin. The proteins in-gel digests were analyzed by ESI nano LC- FT MS. We confirmed that the major protein was Act1 with sequence coverage of 60%. The Act1 peptide “305VILNDSSPQDEERPAQR322” was determined to be phosphorylated on Ser 311; importantly, this peptide species was found in IL-17-treated samples but not in untreated samples. This result was repeated with biological triplicates using tandem MS. Mass spectra of this peptide in both modified [m/z (mass over charge ratio) of 721.3311], and unmodified (m/z of 694.6769) forms are shown in Fig. 6a,b. These peptides are triply charged; the mass difference of the two precursor ions is therefore (721.3311-694.6769) × 3 = 79.9626 Da, corresponding closely to the mass of a phosphate group (PO43−, Mw: 79.9663 Da). The consistent cleavage patterns of modified and unmodified peptides in MS further demonstrated the accurate characterization of the phosphopeptide with high confidence.
Figure 6. Identification of S311 phosphorylation site of Act1 by MS.
A. MS/MS spectrum of the Act1 phosphopeptide (305VILNDSSpPQDEERPAQR322) precursor ions at m/z of 721.33 Da.
B. MS/MS spectrum of unmodified peptide of the same sequence (305VILNDSSPQDEERPAQR322) precursor ions at m/z of 695.01 Da.
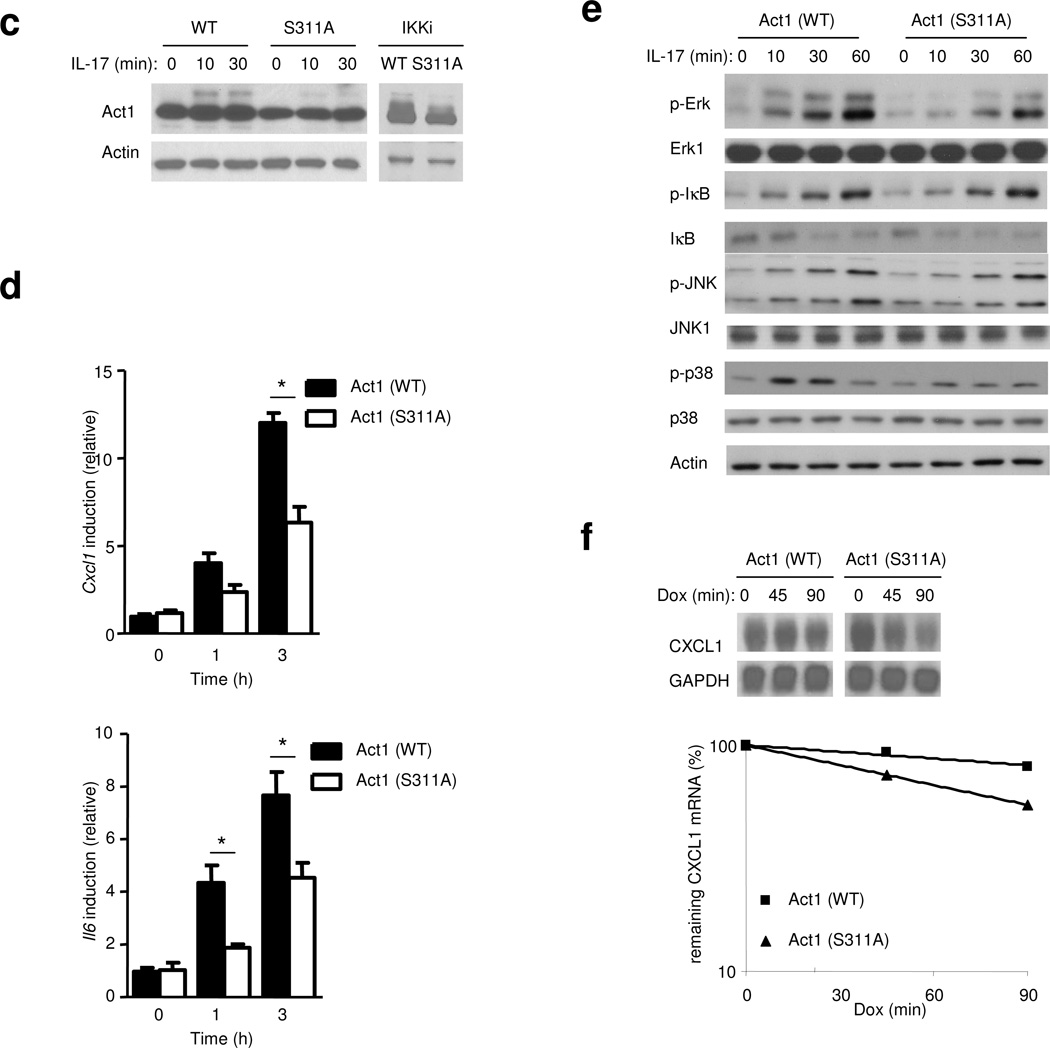
C. Act1-deficient MEFs infected with either retroviral WT-mAct1 or Act1 mutant S11A were untreated or treated with IL-17 (50 ng/ml) for 0, 15 or 30 min, followed by immunoblot analysis with anti-Act1 and anti-Actin.
D. Act1-deficient MEFs infected with either retroviral WT-mAct1 or Act1 mutant S11A, followed by treatments with IL-17A (50 ng/ml) for 1 h and 3 h. CXCL1 and IL-6 mRNA was measured by real-time PCR.
E. Act1-MEFs infected either WT-mAct1 or Act1 mutant S11A were untreated or treated with IL-17 (50 ng/ml) for 0, 15, 30 or 60 min, followed by immunoblot analysis with antibodies against p-Erk, Erk1, p-IκB, IκB, p-Jnk, Jnk, p-p38, p38 and Actin.
F. HeLa tet-off cells were transfected with 1 µg of pTRE2 CXCL1Δ4 and 1 µg of Act1 or Act1 S311A mutant. The transfected cells were treated with dox (1 µg/ml) and incubated for the indicated times, followed by isolation of total RNA and RNA hybridization blot analysis for the determination of CXCL1 and GAPDH mRNA levels.
The data shown in this figure are representation of three independent experiments.
Moreover, Ser 311 (human Ser 328) is precisely located from the detection of product ions “y112+ and y122+ (doubly charged)”. In both modified and unmodified peptides, m/z of y112+ is 677.3 and 677.1 (1 Da mass tolerance for product ions); while m/z of y122+ is 760.8 in MS of modified peptide and 720.8 in MS of unmodified peptide. The mass difference of these two product ions is (760.8-720.8) × 2 = 80 Da. Other y ions after y12 in modified MS, y13, y14, y15, y16 all exhibit an 80 Da mass shift compared to their counterparts in unmodified MS. The evidence that a phosphate group is attached to y12 ions but not y11 ions proved that Ser 311, the only differing amino acid between the two species, is the phosphorylated site.
We then examined whether IKKi can phosphorylate Act1 on S311 in vitro. Act1 immunoprecipiated from wild-type MEFs was subjected to in vitro kinase assay with or without purified recombinant IKKi protein. The peptide “305VILNDSSPQDEERPAQR322” with the phosphorylated Ser 311 was found in IKKi-treated samples but not in untreated samples (data not shown). To evaluate the biological consequence of S311 phosphorylation event, we generated an Act1-S311A point mutant. The IL-17-induced Act1 modification (phosphorylation) was reduced in Act1−/− MEFs reconstituted with S311A mutant as compared to those reconstituted with wild-type Act1 (Fig. 6c). Furthermore, IKKi-mediated Act1 modification was also decreased in 293 cells transfected with IKKi+Act1-S311A, compared to those transfected with IKKi+WT Act1. Taken together, these results suggest that S311 is indeed a site that contributes to IL-17-induced, IKKi-mediated Act1 phosphorylation. Furthermore, IL-17-induced CXCL1 and IL-6 mRNA expression was reduced in Act1−/− MEFs reconstituted with Act1-S311A compared to MEFs reconstituted with wild-type Act1 (Fig. 6d). Consistent with the role of IKKi in IL-17-induced MAPK activation and mRNA stabilization, IL-17-induced Erk and p38 activation as well as CXCL1 mRNA stabilization were decreased in Act1−/− MEFs reconstituted with Act1-S311A (Fig. 6e,f), demonstrating that IKKi-mediated Act1 phosphorylation is important for IL-17 signaling. While S311A mutation did not affect IL-17-induced NF-κB activation (Fig. 6e and data not shown), it is interesting to note that IL-17-induced IκB phosphorylation in MEFs was prolonged as compared to that in epithelial cells (Fig. 2b)26.
IKKi regulates the interaction of Act1 with TRAFs
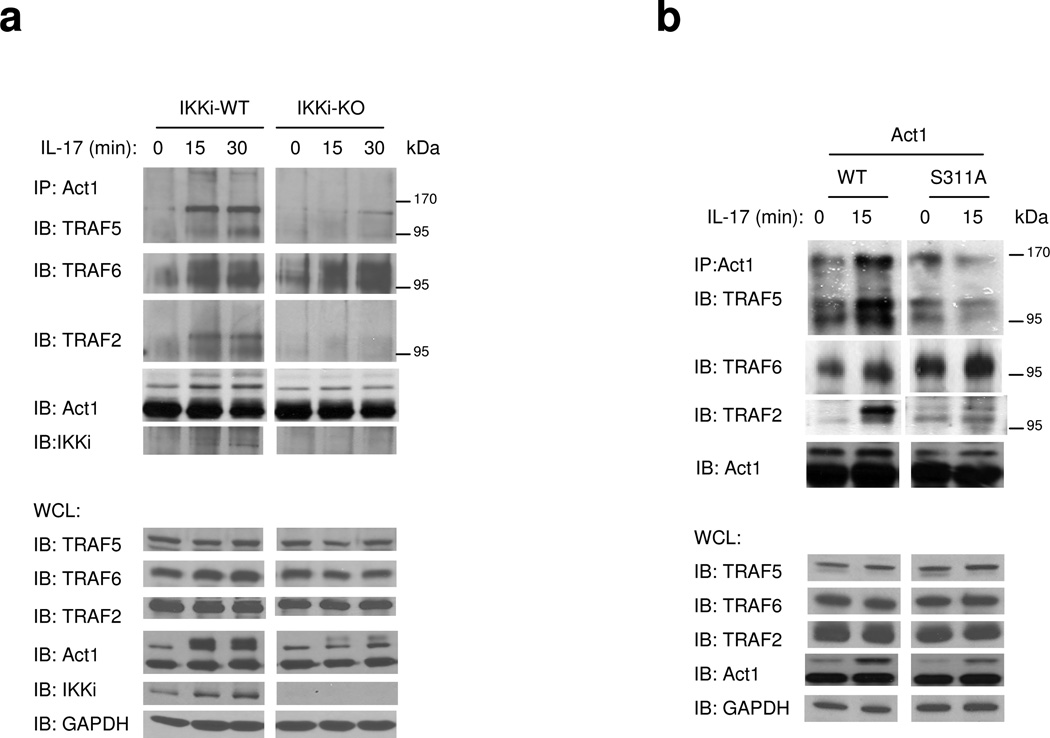
We have previously shown that Act1 forms a complex with TRAF6 upon IL-17 stimulation14,22, which is required for IL-17-mediated NF-κB activation, but not for IL-17-induced Act1-mediated Erk and p38 activation or mRNA stabilization14, demonstrating the existence of IL-17–Act1-mediated but TRAF6-independent pathways. The fact that IKKi deficiency abolished IL-17-induced MAPK activation and mRNA stabilization, but not NF-κB activation, suggests that IKKi-mediated signaling is TRAF6 independent. Importantly, we recently found that TRAF2 and TRAF5 are required for IL-17-induced mRNA stabilization, suggesting that TRAF2/5 may function downstream of IKKi to mediate IL-17–Act1-induced mRNA stabilization. The serine targeted by IKKi is located adjacent to a putative TRAF-interacting consensus motif (P/S/T/A-X-Q/E-acidic/polar) on Act1 (murine Act1 residues 310–317 and human Act1 residues 327–334). Therefore, we hypothesize that phosphorylation of this serine by IKKi may modulate the interaction between Act1 and different TRAFs. Upon IL-17 stimulation, Act1 formed complexes with the modified forms of TRAF2, TRAF5 and TRAF6 (Fig. 7a)18,26. We have previously shown that Act1 mediates TRAF6 ubiquitination upon IL-17 stimulation, which is required for IL-17-induced NF-κB activation26. The observed modification (Fig. 7b) of TRAF2, 5 and 6 is probably due to ubiquitination. Future studies are required to further define the IL-17-induced TRAF2 and TRAF5 modification. Interestingly, we found that IKKi deficiency abolished IL-17-induced Act1 interaction with TRAF2/5, but retained the Act1-TRAF6 interaction (Fig. 7a). Furthermore, IL-17-induced Act1-TRAF2/5 interaction, but not Act1-TRAF6 interaction was substantially reduced in Act1−/− MEFs reconstituted with Act1-S311A (Fig. 7b). Taken together, these results indicate that IKKi and phosphorylation on Ser 311 of Act1 influence the specificity of interactions between Act1 and different TRAFs.
Figure 7. The impact of phosphorylation of Act1-S311 on Act1’s interaction with TRAFs.
A. Cell lysates from wild type and IKKi-deficient kidney epithelial cells untreated or treated with IL-17 (50 ng/ml) for 0, 15 and 30 min were immunoprecipitated with anti-Act1, followed by immunoblot analysis with antibodies against TRAF2, TRAF5, TRAF6, IKKi and Act1. WCL (whole cell lysates). The data shown is a representation of three independent experiments.
B. Act1-deficient MEFs infected with either retroviral WT-mAct1 or Act1 mutant S11A were untreated or treated with IL-17A (50 ng/ml) for 0 or 15 min. Cell lysates were then immunoprecipitated with anti-Act1, followed by immunoblot analysis with antibodies against TRAF2, TRAF5, TRAF6 and Act1. WCL (whole cell lysates). The data shown is a representation of three independent experiments.
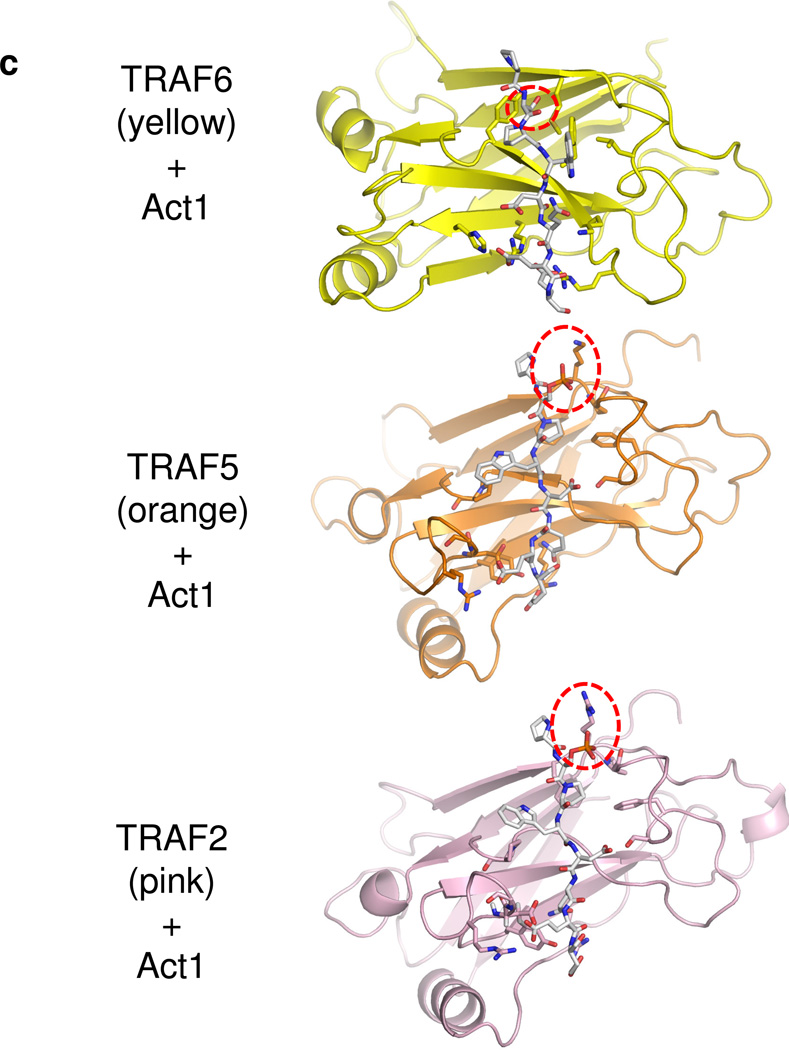
C. Computationally modeled interaction of the Act1 TRAF-binding motif (human Act1327–334) to the structures of TRAF domains of TRAF6 (yellow) and TRAF2 (pink) and a homology model of the TRAF domain of TRAF5 (orange). The Act1 target Serine/phosphoserine is marked with a dashed circle in each panel.
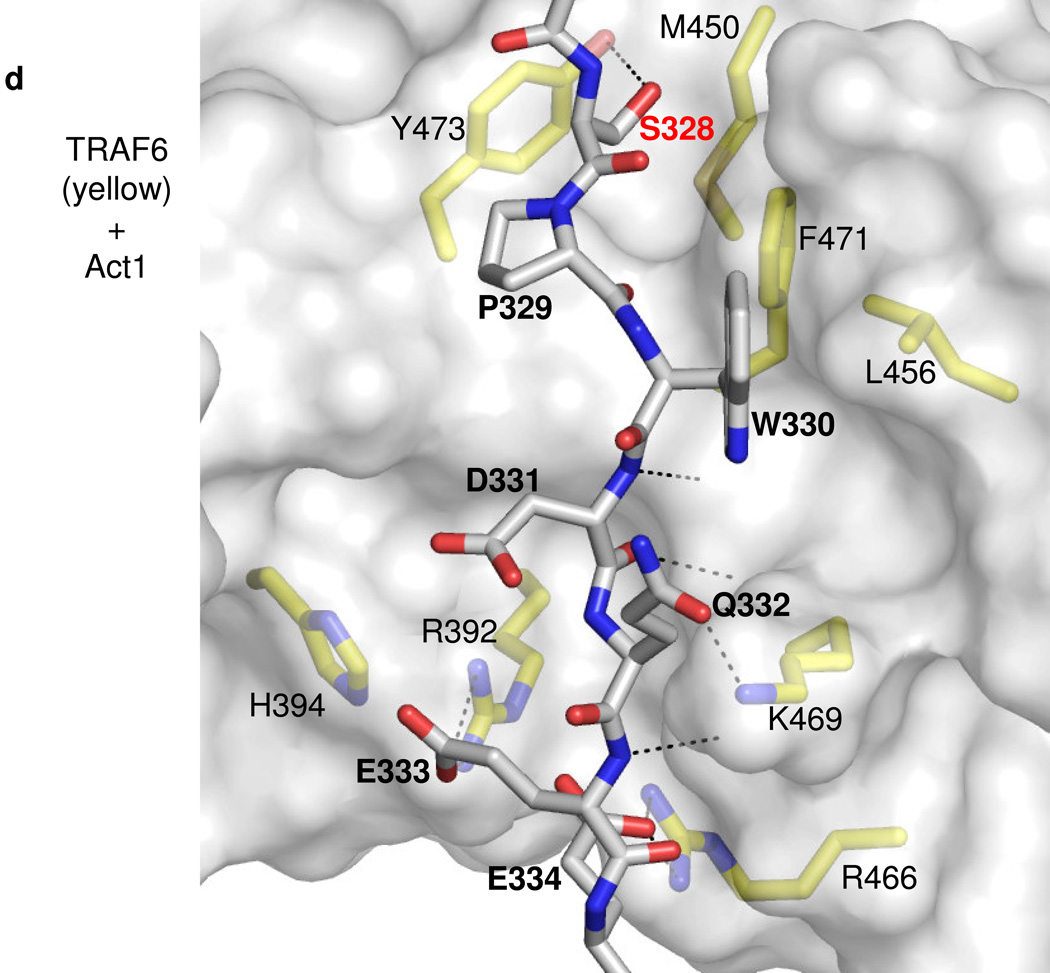
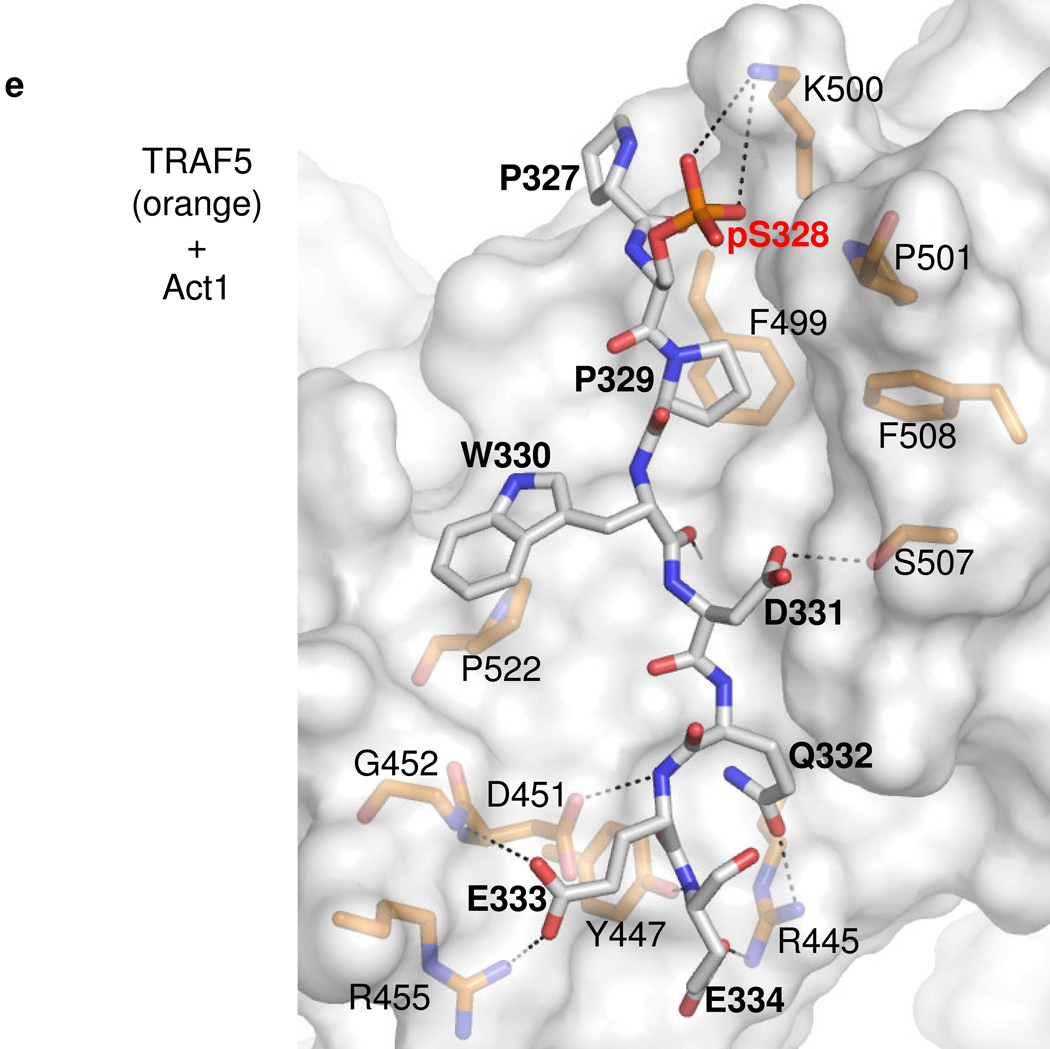
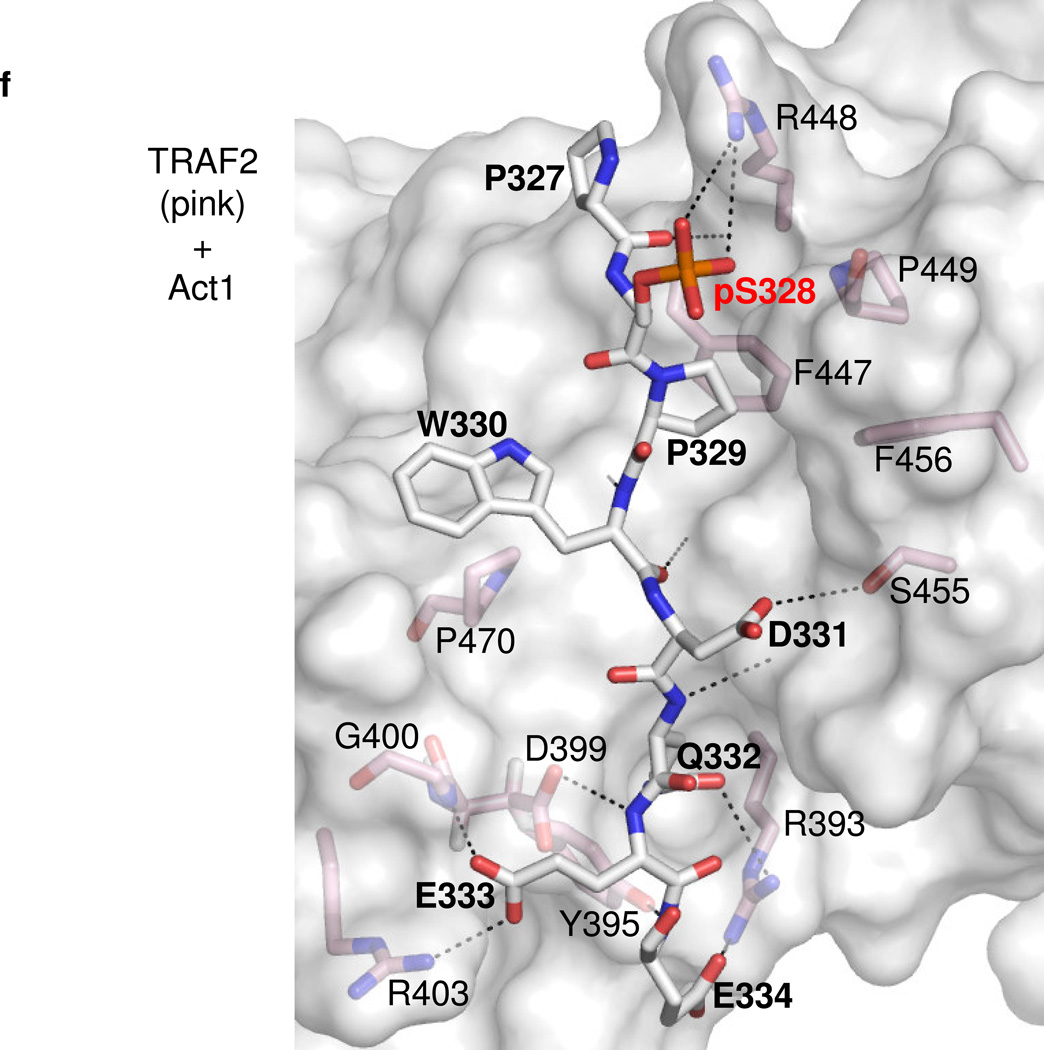
D. –F. Details of representative models of the Act1 TRAF-binding motifs docked to TRAF domains of (D) TRAF6, (E) TRAF5 and (F) TRAF2 respectively. Act1-interacting sidechains of the TRAF domains are shown and labeled in standard text. Residues of Act1 are labeled in bold, while the key Act1 Serine/phosphoserine is labeled in red bold. Putative hydrogen bonds or salt bridges between Act1 and TRAF domain sidechains are shown as dashed lines. Hydrogen bonds between Act1 backbone atoms and TRAF domain backbone atoms are also shown as dashed lines but, for clarity, the corresponding TRAF domain backbone atoms are not shown explicitly.
Computational modeling of Act1-TRAF interactions
Act1 serine-311, which is targeted by IKKi, is located adjacent to a TRAF-interacting consensus motif (murine Act1 residues 310–317 and human Act1 residues 327–334). To obtain insights into how phosphorylation of this serine may affect Act1 interaction with TRAFs, we computationally docked a human Act1327–334 peptide to crystal structures of TRAF domains from TRAF6 and TRAF2, and to a homology model of the TRAF domain of TRAF5, which has high sequence similarity to that of TRAF2. We were guided by structures of these TRAF domains bound to TRAF-interacting motifs from a variety of ligands including CD40 (TRAF6 ligand) and TNFR2 (TRAF2 ligand)34–36. In these ligands, the TRAF-interacting motifs make a small number of backbone-backbone interactions with strand β7 of the TRAF domains but primarily lie across one of the sheets of the TRAF domain β-sandwich fold. In both TRAF6 and TRAF2, the residue at the so-called P−2 position of the ligand motif (typically P/S/T/A) makes close contact with a hydrophobic pocket on one side of the interacting surface (residues from β6 and β7), while the P0 residue (typically occupied by Q/E) and following acidic/polar residues interact with basic and polar sidechains from the other side of the surface (residues from β3 and β4).
We were able to dock the human Act1 TRAF-interacting sequence (PSPWDQEE, corresponding to murine SSPQDQEE) to TRAF6, TRAF5 and TRAF2, in configurations resembling those of structurally characterized TRAF ligands. Because the Act1 sequence contains multiple residues to be potentially compatible with the P−2 and P0 positions, the Act1 sequence may dock in different registers to TRAF6 and TRAF5/2. In our best models of the Act1-TRAF6 interaction, human Act1-W330 occupies the P0 position and makes favorable hydrophobic interactions with nonpolar residues that form a bulge on the TRAF6 surface (Fig. 7c). In other TRAF6 ligands, the P0 position is occupied by a glutamate sidechain36, which makes similar hydrophobic contacts while its carboxylate group is stabilized by hydrogen bonding to TRAF backbone amides (Fig. 7c,d). In addition, the equivalent residue in murine Act1 is a glutamine (Q313), which presumably interacts with TRAF6 in a similar manner as the glutamate residues of other TRAF6 ligands. Human Act1-S328 (murine Act1-S311) occupies the P−2 position and closely contacts the corresponding hydrophobic pocket. The serine sidechain may be partially stabilized by hydrogen bonding to TRAF6-Y473, which forms one wall of the pocket. Our modeling suggests that phosphorylation of this serine by IKKi may destabilize the binding of Act1 to TRAF6, because the bulky, charged phosphoserine would sterically clash with the small P−2 binding pocket.
In contrast, our modeling of the Act1-TRAF5/2 (Fig. 7c,e,f) interaction suggests that the P−2 position is filled by human Act1-P329 (murine Act1-P312), and the P0 position is occupied by an acidic residue, D331 (murine Act1-D314). This binding mode positions the sidechain of the key serine S328 (murine Act1-S311) so that it protrudes away from the TRAF domain surface but is proximal to a basic sidechain (human TRAF5-K500 or TRAF2-R448, which are conserved in their murine homologues). Phosphorylation of S328 (murine Act1-S311) allows for formation of a salt bridge between the phosphate and the basic residue, increasing the affinity of the Act1 motif for TRAF5/2. Our modeling results therefore suggest that phosphorylation of Act1 promotes redistribution from TRAF6 to TRAF5 (and/or TRAF2) in a two-fold manner. First, the phosphorylation reduces the affinity for TRAF6 due to incompatibility between the phosphate group and the P−2 binding pocket. Second, the phosphorylation gives rise to additional favorable electrostatic interactions with the surface of TRAF5 or TRAF2. These hypotheses are fully consistent with our other studies on the effect of IKKi on the balance of Act1-mediated signaling pathways.
Discussion
IL-17 is mainly produced by pathogenic TH17 cells and is implicated in the pathogenesis of many autoimmune inflammatory diseases. Because IL-17 uses a unique receptor, identification of signaling pathways that couple receptor activation with physiologic end points and understanding of their mechanisms of action are crucial for the development of new therapeutic strategies to attenuate this major pro-inflammatory pathway. We here report a new function of IKKi in IL-17-mediated signaling linked with neutrophilia and pulmonary inflammation. IKKi deficiency abolished IL-17-induced airway inflammation via the impaired expression of pro-inflammatory and neutrophil-mobilizing cytokines and chemokines. We show that IL-17 stimulation induces the interaction of IKKi with Act1 resulting in phosphorylation of Act1, which has a critical impact on a subset of IL-17-induced activities. The identification IKKi as a critical upstream kinase in the IL-17 pathway opens new avenues for the development of novel anti-inflammatory therapies.
While IL-17 induces transcriptional activation of inflammatory genes, it also impacts their production at the post-transcriptional level14. Many cytokine and chemokine mRNAs are known to exhibit short half-lives through mechanisms that depend upon sequences located with the 3’UTR. The best characterized instability sequences contain adenine and uridine-rich elements (AREs) characterized by a pentamer “AUUUA” or a nonamer “UUAUUUAUU”37,38. These instability sequences are recognized by RNA binding proteins such as Tristetraprolin (TTP) that promote mRNA degradation via recruitment of decapping, deadenylase, and exonuclease activities. We have recently reported that the sequence motif in the CXCL1 mRNA 3’UTR that confers IL-17 sensitivity does not contain the AUUUA motif and is not recognized by TTP37. In this regard, we now report that IL17-sensitive instability is mediated by the arginine serine rich splicing factor SF2/ASF. Interestingly, mRNA decay mechanisms can be regulated via MAPK-dependent signaling including p38, Erk and Jnk39–42. It is intriguing that in IKKi-deficient cells, IL-17-induced MAPK but not NF-κB activation was substantially reduced, which might contribute to the inability of IL-17 to stabilize chemokine mRNAs in these cells.
In this regard, we have previously shown that Act1 forms a complex with TRAF6 upon IL-17 stimulation to mediate IL-17-mediated NF-κB activation, but not mRNA stabilization14,26. Previously Act1 was show to be required for IL-17-induced immediate-early genes and Act1 is essential for IL-17-mediated NF-κB activation43. Moreover, we found that TRAF2/5 are required for IL-17-induced mRNA stabilization. The serine in the Act1 molecule targeted by IKKi is located adjacent to a putative TRAF-interacting consensus motif (P/S/T/A-X-Q/E-acidic/polar) on Act126. Our computational modeling suggests that phosphorylation of Act1-S311 modulates of the relative affinities for TRAF6 and TRAF5 (and/or TRAF2), enabling signaling through these additional TRAF components. These results suggest that IKKi-mediated Act1 phosphorylation might have a critical impact on the selective interaction of Act1 with different TRAFs and on specific Act1-dependent IL-17 signaling pathways. IKKi deficiency and mutation of Act1-Ser 311 indeed abolished IL-17-induced Act1-TRAF2/5 complex formation and chemokine mRNA stabilization, whereas the Act1-TRAF6-NF-κB axis was retained. Therefore, IKKi is an important kinase that controls the bifurcation of TRAF2/5- versus TRAF6-dependent signaling pathways through its impact on Act1 phosphorylation, promoting IL-17-induced inflammatory gene expression at the posttranscriptional levels. It is also important to point out that the IKKi-mediated phosphorylation of Act1 may affect additional aspects of Act1 and Act1-mediated downstream signaling. However, it is unlikely that IKKi-mediated phosphorylation of Act1 is required for the E3 activity of Act1 (ref. 26), since neither IKKi deficiency nor S311A mutation affected IL-17-induced NF-κB activation, which is dependent on the E3 activity of Act1. These findings not only increase our mechanistic understanding of IL-17 signaling but also provide useful information for future cell-based assays in the development and analysis of candidate IKKi inhibitors.
While we found that only the modified form of Act1 was detected in the immune complex of IKKi, it remains unclear how IKKi is activated upon IL-17 stimulation and recruited to Act1. It is known that Act1 is recruited to IL-17R through SEFIR-SEFIR domain interaction. Intriguingly, the IL-17R SEFIR alone was not sufficient to support IL-17-dependent signaling. Rather, an additional sequence located C-terminally to SEFIR was also necessary15. It was further shown that the extended SEFIR region in the IL-17RC is required for interaction with a phosphorylated form of Act1. Consistent with this, we observed that IKKi is able to interact with IL-17R in the absence of Act1 and the SEFIR domain of IL-17R is not required for this interaction, suggesting that IKKi is possibly recruited to the receptor through sequences C-terminal of the SEFIR in the IL-17R (unpublished data). On the other hand, we found that IL-17-induced IKKi auto-phosphorylation was reduced in Act1-deficient cells, indicating that Act1 is probably still required for IKKi activation in response to IL-17 stimulation. The activated IKKi then in turn phosphorylates Act1. Future studies are required to elucidate the detailed molecular mechanism for the activation and recruitment of IKKi to IL-17R-Act1 in response to IL-17 stimulation.
IKKi is an IKK-related kinase. MS analysis showed that IKKi can phosphorylate Act1 on S311 in vitro, indicating that IKKi may act as a direct kinase of Act1. Mutation of S311 in Act1 (S311A) impaired IL-17-mediated gene expression and CXCL1 mRNA stabilization, demonstrating the importance of IL-17-induced IKKi-mediated Act1 phosphorylation in IL-17 signaling. Although IKKi was able to phosphorylate Act1 in vitro, other kinases may also be involved in the phosphorylation of Act1 in response to IL-17 stimulation. Importantly, S311A mutation did not completely abrogate IL-17-mediated Act1 modification, suggesting that additional phosphorylation/modification sites are present in Act1. Furthermore, IKKi might also phosphorylate substrates other than Act1 in response to IL-17 stimulation. Together with TBK1, IKKi has been shown to play an important role in the innate immune response, where it functions as a kinase for IRF3 and IRF7 signaling pathways during viral infection29,31,32. We did explore the possible link from IKKi to IRF activation in response to IL-17 stimulation. However, we failed to detect any IL-17-induced IKKi-mediated activation of IRF signaling pathways (unpublished data). The novel function of IKKi in IL-17 signaling indicates the multi-faceted nature of this kinase, whose spatial/temporal regulation and activation are likely crucial for the appropriate functions of IKKi in various signaling pathways and consequent immune responses.
Another important question is whether IKKi also participates in signaling pathways mediated by other IL-17 family members. We have previously shown that Act1 is required for IL-25 (also known as IL-17E)-mediated signaling and inflammation. IL-25 is the most divergent member of the IL-17 family; it is expressed in mouse T lymphocytes of the CD4+ subset with a TH2 profile and human innate effector eosinophils and basophils. IL-25 plays an important role in the initiation and propagation of the TH2 immune response. IL-25-induced BAL infiltration and lung inflammation were much reduced in IKKi-deficient mice compared to that in wild-type mice (unpublished data, Bulek and Li). Therefore, future studies are required to investigate how IKKi participates in Act1-dependent signaling induced by other IL-17 family members.
Methods
Mice
IKKi-deficient and wild type C57BL/6 control mice were generous gift from T. Maniatis (Department of Molecular and Cellular Biology, Harvard University, Cambridge, MA, USA). The experiments were performed with mice aged 6–8 weeks. The institutional committee reviewed and approved the animal experiments.
Preparation of primary murine trachea epithelial cells (MTECs)
This primary cell culture method has been described previously44 and results in differentiated pseudostratified and ciliated airway epithelial cells. The epithelial cells were isolated from murine trachea and cultured on a native basement membrane for 14 days at an air-liquid interface. The single difference between the present culturing method and original method involves the formation of the native basement membrane directly on tissue culture insert (0.4 µm pores) in the absence of the collagen layer. This improves visibility of the cells by phase-contrast microscopy and improves recovery of cellular extracts.
Intranasal instillation of IL-17
Mice were anesthetized with isoflurane. Carrier-free, murine rIL-17 (R&D Systems) resuspended in sterile saline (0.9%) was instilled into nasal opening in 50 µl (1µg) aliquot per mouse.
Bronchoalveolar lavage and tissue collection
A total of 0.7 ml of HL-1 medium (BioWhittaker) was used to obtain BAL fluid through trachea using a blunt needle and 1-ml syringe. Cytospin slide preparations where obtained using Shandon CytoSpin III Cytocentrifuge (Shandon/ Thermo Scientific). Differential leukocyte counts were obtained on cytospin slide preparation after Diff Quik Giemsa stain (Hema3). Lungs were collected and snap frozen immediately in liquid nitrogen container. Total RNA was obtained by using TRIzol (Invitrogen) and OMNI TH tissue homogenizer (Omni International). H&E staining was obtained on lung tissue after fixation in 10% neutral buffered formalin and paraffin embedding.
Kinase assay
Cells were washed twice with ice-cold PBS, and lysed using Co-IP buffer for 20 min on ice. Cell lysate (600 µl) was incubated with rabbit anti-Act1 and protein G-agarose beads overnight at 4°C with constant agitation. The beads were washed twice with Co-IP buffer following by two additional washes with kinase buffer (20 mM HEPES, pH 7.5, 10 mM MgCl2, 25m M NaCl, 20 mM β-glycerophosphate, and 10 mM sodium orthovanadate). Washed beads were incubated with 30 µl kinase buffer including 50 µM of cold ATP, 5 µCi of radioactive ATP (γ-32P-ATP) and with/without GST-IKKi (Active Motif) at 30°C for 30 min. The reaction was stopped by the addition of 4x sample buffer and heating for 5 min at 95°C. Proteins were subjected to SDS-PAGE, followed by autoradiography.
In-gel digestions, nano-ESI-MS/MS and MS data analysis
The gel piece cut from each band was destained with 50% 100 mM NH4HCO3/50% acetonitrile solution for 2~8 h. 10 mM TCEP (tris(2-carboxyethyl)phosphine) was used to incubate gel bands for 30 min followed by 50 mM Iodoacetymide incubation of 30 min at dark. 200 µl ACN (acetonitrile) and 100 mM NH4HCO3 were then used alternatively to dehydrate and rehydrate these gel pieces 3 times. 50 µl of freshly made trypsin (20 ng/µl) (Promega sequencing grade modified) in 25 mM NH4HCO3 was added to each of the samples and digested at 37 °C for overnight. Tryptic digested peptides were extracted with 50% ACN/5% formic acid, dried, and dissolved in 12 µl 0.1% formic acid solution for MS analysis.
Mass spectrometry
MS Experiments were done using: an LTQ-FT mass spectrometer (Thermo Finnigan). Nano-Reverse Phase Liquid Chromatography (RPLC) separations were performed on a Dionex Ultimate U3000 HPLC (Dionex) with a 5 cm × 75 µm Pico Frit C18 column (New Objective) directly connected to a New Objectives nanospray emitter (10 µm, New Objectives,). Chromatography was performed using mobile phase A (0.1% formic acid in water) and B (80% cetonitrile, 0.04% formic acid in water) with a linear gradient of 1% per min, starting with 100% of solution A at a flow rate of 0.3 µl/min. All data were acquired in positive ion mode. CID (collision induced dissociation) and was used to fragment peptides in FT-MS. In these experiments, full MS scans (m/z 300–2000) were followed by eight subsequent MS2 scans on the top eight most abundant peptide ions using a normalized collision energy of 35%.
Tandem MS data were analyzed by use of MassMatrix and Mascot software. Act1 tandem MS data were searched against protein databases containing Act1 sequence and a mouse whole protein database separately. Carbamidomethylation of cysteine was included as a fixed modification. N terminal acetylation and phosphorylation of S, T and Y were selected as variable modifications. Two missed cleavages were allowed for searching the data. The mass tolerance was set to 10 ppm for the precursor ion search and 1 Da for the product ion. The maximum length for matched peptides was 55 amino acids.
Energy-minimized Act1/TRAF docking
Human Act1(121–130) peptides were docked to human TRAF2(311–501), TRAF5(366–556) or TRAF6(347–501) by a combined rigid body/torsion angle dynamics simulated annealing protocol in Xplor-NIH45. Intermolecular distance restraints were adapted from conserved polar and hydrophobic interactions found in existing crystal structures of peptides bound to TRAF2 or TRAF6 (TRAF2 PDB codes: 1CA934, 1D0035, 1D0135, 1D0A35, 1CZY35, 1CZZ35, 1QSC; TRAF6 PDB codes: 1LB536, 1LB636). A TRAF5 homology model was obtained from the SwissModel Repository46,47. The combined rigid body/torsion angle dynamics simulated annealing protocol48 allowed the TRAF domain and Act1 peptide each to freely translate and rotate while TRAF domain side chains at the TRAF/Act1 interface were allowed to freely sample torsional space during the cooling phase. The entire Act1 peptide backbone and all Act1 side chains were allowed to freely sample torsional space during the cooling phase. The TRAF domain backbone was treated as a rigid body throughout the protocol. The energy terms utilized by the docking protocol consisted of a distance restraint term (Edist), a van der Waals repulsion term (Evdw) to avoid steric clashes, a radius of gyration term (Egyr) to minimize expansion of the protein peptide complex and a torsion angle database energy term (Etor) to favor preferred rotamer conformations for interfacial side chains49. For each TRAF domain/Act1 peptide complex, 200 structures were generated. The top 2% of structures, judged by lowest total energies with no distance restraint violations, were selected as a representative ensemble for the docking solution.
Acknowledgements
This work was supported by Senior Investigator Award to Xiaoxia Li from American Asthma Foundation and National Institutes of Health Grant (R01HL098935-01A1). R.C.P. was supported by American Heart Association postdoctoral fellowship 09POST2010041. This work made use of the High Performance Computing Resource in the Core Facility for Advanced Research Computing at Case Western Reserve University. IKKi-deficient and wild type C57BL/6 control mice were generous gift from T. Maniatis (Department of Molecular and Cellular Biology, Harvard University, Cambridge, MA, USA).
Footnotes
Author contributions
KB, CL, SS, MFG, TH, WQ and DS performed experiments. KB and CL were responsible for experimental design, performance, and interpretation and for writing the manuscript. LW and MC carried out the MS study. RP and SM performed the structure modeling. AA, LM and VH helped with the primary murine airway epithelial cell culture. XL and TH designed and interpreted experiments and wrote the manuscript. All authors reviewed the final manuscript.
Reference List
- 1.Bettelli E, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 2.Cho JS, et al. IL-17 is essential for host defense against cutaneous Staphylococcus aureus infection in mice. J. Clin. Invest. 2010;120:1762–1773. doi: 10.1172/JCI40891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conti HR, et al. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J. Exp. Med. 2009;206:299–311. doi: 10.1084/jem.20081463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harrington LE, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 5.Iwakura Y, Ishigame H. The IL-23/IL-17 axis in inflammation. J. Clin. Invest. 2006;116:1218–1222. doi: 10.1172/JCI28508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 7.Langrish CL, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park H, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alcorn JF, Crowe CR, Kolls JK. TH17 cells in asthma and COPD. Annu. Rev. Physiol. 2010;72:495–516. doi: 10.1146/annurev-physiol-021909-135926. [DOI] [PubMed] [Google Scholar]
- 10.Kolls JK, Khader SA. The role of Th17 cytokines in primary mucosal immunity. Cytokine Growth Factor Rev. 2010;21:443–448. doi: 10.1016/j.cytogfr.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kolls JK. Th17 cells in mucosal immunity and tissue inflammation. Semin. Immunopathol. 2010;32:1–2. doi: 10.1007/s00281-010-0198-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakae S, et al. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity. 2002;17:375–387. doi: 10.1016/s1074-7613(02)00391-6. [DOI] [PubMed] [Google Scholar]
- 13.Chang SH, Park H, Dong C. Act1 adaptor protein is an immediate and essential signaling component of interleukin-17 receptor. J. Biol. Chem. 2006;281:35603–35607. doi: 10.1074/jbc.C600256200. [DOI] [PubMed] [Google Scholar]
- 14.Hartupee J, Liu C, Novotny M, Li X, Hamilton T. IL-17 enhances chemokine gene expression through mRNA stabilization. J. Immunol. 2007;179:4135–4141. doi: 10.4049/jimmunol.179.6.4135. [DOI] [PubMed] [Google Scholar]
- 15.Ho AW, Gaffen SL. IL-17RC: a partner in IL-17 signaling and beyond. Semin. Immunopathol. 2010;32:33–42. doi: 10.1007/s00281-009-0185-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang F, et al. Requirement for both JAK-mediated PI3K signaling and ACT1/TRAF6/TAK1-dependent NF-kappaB activation by IL-17A in enhancing cytokine expression in human airway epithelial cells. J. Immunol. 2007;179:6504–6513. doi: 10.4049/jimmunol.179.10.6504. [DOI] [PubMed] [Google Scholar]
- 17.Kao CY, et al. Up-regulation of CC chemokine ligand 20 expression in human airway epithelium by IL-17 through a JAK-independent but MEK/NF-kappaB-dependent signaling pathway. J. Immunol. 2005;175:6676–6685. doi: 10.4049/jimmunol.175.10.6676. [DOI] [PubMed] [Google Scholar]
- 18.Qian Y, et al. The adaptor Act1 is required for interleukin 17-dependent signaling associated with autoimmune and inflammatory disease. Nat. Immunol. 2007;8:247–256. doi: 10.1038/ni1439. [DOI] [PubMed] [Google Scholar]
- 19.Shen F, et al. IL-17 receptor signaling inhibits C/EBPbeta by sequential phosphorylation of the regulatory 2 domain. Sci. Signal. 2009;2:ra8. doi: 10.1126/scisignal.2000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shen F, Gaffen SL. Structure-function relationships in the IL-17 receptor: implications for signal transduction and therapy. Cytokine. 2008;41:92–104. doi: 10.1016/j.cyto.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Toy D, et al. Cutting edge: interleukin 17 signals through a heteromeric receptor complex. J. Immunol. 2006;177:36–39. doi: 10.4049/jimmunol.177.1.36. [DOI] [PubMed] [Google Scholar]
- 22.Novatchkova M, Leibbrandt A, Werzowa J, Neubuser A, Eisenhaber F. The STIR-domain superfamily in signal transduction, development and immunity. Trends Biochem. Sci. 2003;28:226–229. doi: 10.1016/S0968-0004(03)00067-7. [DOI] [PubMed] [Google Scholar]
- 23.Swaidani S, et al. The critical role of epithelial-derived Act1 in IL-17- and IL-25-mediated pulmonary inflammation. J. Immunol. 2009;182:1631–1640. doi: 10.4049/jimmunol.182.3.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li X, et al. Act1, an NF-kappa B-activating protein. Proc. Natl. Acad. Sci. USA. 2000;97:10489–10493. doi: 10.1073/pnas.160265197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwandner R, Yamaguchi K, Cao Z. Requirement of tumor necrosis factor receptor-associated factor (TRAF)6 in interleukin 17 signal transduction. J. Exp. Med. 2000;191:1233–1240. doi: 10.1084/jem.191.7.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu C, et al. Act1, a U-box E3 ubiquitin ligase for IL-17 signaling. Sci. Signal. 2009;2:ra63. doi: 10.1126/scisignal.2000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peters RT, Liao SM, Maniatis T. IKKepsilon is part of a novel PMA-inducible IkappaB kinase complex. Mol. Cell. 2000;5:513–522. doi: 10.1016/s1097-2765(00)80445-1. [DOI] [PubMed] [Google Scholar]
- 28.Shimada T, et al. IKK-i, a novel lipopolysaccharide-inducible kinase that is related to IkappaB kinases. Int. Immunol. 1999;11:1357–1362. doi: 10.1093/intimm/11.8.1357. [DOI] [PubMed] [Google Scholar]
- 29.Fitzgerald KA, et al. IKKepsilon and TBK1 are essential components of the IRF3 signaling pathway. Nat. Immunol. 2003;4:491–496. doi: 10.1038/ni921. [DOI] [PubMed] [Google Scholar]
- 30.Hemmi H, et al. The roles of two IkappaB kinase-related kinases in lipopolysaccharide and double stranded RNA signaling and viral infection. J. Exp. Med. 2004;199:1641–1650. doi: 10.1084/jem.20040520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kawai T, et al. IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat. Immunol. 2005;6:981–988. doi: 10.1038/ni1243. [DOI] [PubMed] [Google Scholar]
- 32.Sharma S, et al. Triggering the interferon antiviral response through an IKK-related pathway. Science. 2003;300:1148–1151. doi: 10.1126/science.1081315. [DOI] [PubMed] [Google Scholar]
- 33.Tebo JM, et al. Interleukin-1-mediated stabilization of mouse KC mRNA depends on sequences in both 5'- and 3'-untranslated regions. J. Biol. Chem. 2000;275:12987–12993. doi: 10.1074/jbc.275.17.12987. [DOI] [PubMed] [Google Scholar]
- 34.Park YC, Burkitt V, Villa AR, Tong L, Wu H. Structural basis for self-association and receptor recognition of human TRAF2. Nature. 1999;398:533–538. doi: 10.1038/19110. [DOI] [PubMed] [Google Scholar]
- 35.Ye H, Park YC, Kreishman M, Kieff E, Wu H. The structural basis for the recognition of diverse receptor sequences by TRAF2. Mol. Cell. 1999;4:321–330. doi: 10.1016/s1097-2765(00)80334-2. [DOI] [PubMed] [Google Scholar]
- 36.Ye H, et al. Distinct molecular mechanism for initiating TRAF6 signalling. Nature. 2002;418:443–447. doi: 10.1038/nature00888. [DOI] [PubMed] [Google Scholar]
- 37.Datta S, et al. IL-17 regulates CXCL1 mRNA stability via an AUUUA/tristetraprolin-independent sequence. J. Immunol. 2010;184:1484–1491. doi: 10.4049/jimmunol.0902423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hartupee J, Li X, Hamilton T. Interleukin 1alpha-induced NFkappaB activation and chemokine mRNA stabilization diverge at IRAK1. J. Biol. Chem. 2008;283:15689–15693. doi: 10.1074/jbc.M801346200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fukunaga R, Hunter T. MNK1, a new MAP kinase-activated protein kinase, isolated by a novel expression screening method for identifying protein kinase substrates. EMBO J. 1997;16:1921–1933. doi: 10.1093/emboj/16.8.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hitti E, et al. Mitogen-activated protein kinase-activated protein kinase 2 regulates tumor necrosis factor mRNA stability and translation mainly by altering tristetraprolin expression, stability, and binding to adenine/uridine-rich element. Mol. Cell Biol. 2006;26:2399–2407. doi: 10.1128/MCB.26.6.2399-2407.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rowlett RM, et al. MNK kinases regulate multiple TLR pathways and innate proinflammatory cytokines in macrophages. Am. J. Physiol Gastrointest. Liver Physiol. 2008;294:G452–G459. doi: 10.1152/ajpgi.00077.2007. [DOI] [PubMed] [Google Scholar]
- 42.Ueda T, Watanabe-Fukunaga R, Fukuyama H, Nagata S, Fukunaga R. Mnk2 and Mnk1 are essential for constitutive and inducible phosphorylation of eukaryotic initiation factor 4E but not for cell growth or development. Mol. Cell Biol. 2004;24:6539–6549. doi: 10.1128/MCB.24.15.6539-6549.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sonder SU, et al. IL-17-induced NF-kappaB activation via CIKS/Act1: physiologic significance and signaling mechanisms. J. Biol. Chem. 2011;286:12881–12890. doi: 10.1074/jbc.M110.199547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lauer ME, et al. Differentiated murine airway epithelial cells synthesize a leukocyte-adhesive hyaluronan matrix in response to endoplasmic reticulum stress. J. Biol. Chem. 2008;283:26283–26296. doi: 10.1074/jbc.M803350200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schwieters CD, Kuszewski JJ, Tjandra N, Clore GM. The Xplor-NIH NMR molecular structure determination package. J. Magn Reson. 2003;160:65–73. doi: 10.1016/s1090-7807(02)00014-9. [DOI] [PubMed] [Google Scholar]
- 46.Kiefer F, Arnold K, Kunzli M, Bordoli L, Schwede T. The SWISS-MODEL Repository and associated resources. Nucleic Acids Res. 2009;37:D387–D392. doi: 10.1093/nar/gkn750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kopp J, Schwede T. The SWISS-MODEL Repository of annotated three-dimensional protein structure homology models. Nucleic Acids Res. 2004;32:D230–D234. doi: 10.1093/nar/gkh008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Clore GM, Schwieters CD. Docking of protein-protein complexes on the basis of highly ambiguous intermolecular distance restraints derived from 1H/15N chemical shift mapping and backbone 15N-1H residual dipolar couplings using conjoined rigid body/torsion angle dynamics. J. Am. Chem. Soc. 2003;125:2902–2912. doi: 10.1021/ja028893d. [DOI] [PubMed] [Google Scholar]
- 49.Clore GM, Kuszewski J. Chi(1) rotamer populations and angles of mobile surface side chains are accurately predicted by a torsion angle database potential of mean force. J. Am. Chem. Soc. 2002;124:2866–2867. doi: 10.1021/ja017712p. [DOI] [PubMed] [Google Scholar]