Abstract
Endothelial cells play an important role in the recruitment of immune cells to a disease locus through the induced expression of chemokines and cell adhesion molecules (CAMs). The proinflammatory lysophospholipid, lysophosphatidic acid (LPA), which is elevated in multiple inflammatory diseases, is a potent activator of the RhoA/Rho kinase signaling pathway and has been shown to induce the expression of CAMs in endothelial cells. The present study was undertaken to map signal transduction downstream of LPA and to investigate the contributions of the Rho kinase isoforms ROCK1 and ROCK2 to adhesion molecule expression in human umbilical vein endothelial cells. LPA activated Rho kinase within minutes and subsequently the NF-κB pathway through phosphorylation of the p65 subunit. The lipid also induced the late expression of intracellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1). Pharmacologic inhibition of Rho kinase signaling blocked LPA-induced p65 phosphorylation and suppressed ICAM-1 and VCAM-1 expression. Inhibition of the NF-κB pathway had no impact on LPA-induced Rho kinase activation, but inhibited adhesion molecule expression. Small interfering RNA-facilitated knockdown of each isoform identified ROCK2 as the mediator of LPA-driven phosphorylation of NF-κB p65 and of ICAM-1 and VCAM-1 mRNA and protein induction. Taken collectively, our data are consistent with Rho kinase being upstream of NF-κB in driving LPA-mediated adhesion molecule expression. This study also provides the first evidence of the critical involvement of ROCK2 in LPA-induced CAM expression through activation of the NF-κB pathway in human endothelial cells.
Keywords: Adhesion, Endothelium, Leukocyte, NF-Kappa B, Signal Transduction, IACM-1, LPA, Rho Kinase, VCAM-1, Lysophosphatidic Acid
Introduction
The trans-endothelial migration of activated leukocytes into inflamed tissue is an important mechanism in the pathogenesis of inflammatory diseases. Endothelial cells play a key role in this step by up-regulating the synthesis and secretion of chemokines and the cell surface expression of adhesion molecules (CAMs)2 (1). The adhesion molecules ICAM-1 and VCAM-1 bind leukocytes expressing the major integrins lymphocyte function associated antigen-1 (LFA-1) and the VLA-4 (very late activation antigen-4), respectively, and are induced on endothelial cells by inflammatory stimuli (2, 3). Both are required for the attachment of circulating leukocytes to the endothelium and mediate not only transcellular migration but also the induction of intracellular signals resulting in transient junctional disruption (1) and the paracellular passage of leukocytes across the endothelial monolayer.
Lysophosphatidic acid (LPA), a pleiotropic proinflammatory lipid mediator, is elevated in multiple disease states. High concentrations of the lipid have been detected in the bronchoalveolar lavage fluid of preclinical animal models of allergic asthma (4). LPA plays a role in regulating the secretion of pro- and anti-inflammatory mediators by airway epithelial cells and in modulating airway epithelial barrier integrity (5). Additionally, synovial fluid obtained from rheumatoid arthritis patients contain significant amounts of LPA and the LPA-synthesizing enzyme autotaxin driving cytokine secretion by and migration of resident synoviocytes (6, 7). The lipid mediator has also been implicated in the initiation of neuropathic pain (8). Blood platelets also store LPA and are an important source of the lipid. Serum and plasma contain nanomolar levels of LPA and de novo generation or release from platelet stores during inflammation may result in higher LPA levels at disease loci. LPA engages a family of at least five closely related G protein-coupled receptors LPA1–5 (9). It has been reported that HUVECs express LPA1 and LPA3 receptors and are responsive to LPA with induced expression of chemokines and cell adhesion molecules (CAMs) (10–12). In vitro and in vivo studies employing Rho kinase inhibitors, isoform-specific LPA receptor inhibitors, and targeted receptor deletions link LPA acting through the LPA1 receptor, to the downstream activation of Rho kinase (8, 13, 14).
Rho kinase, a serine/threonine kinase, was initially characterized as a mediator of the formation of RhoA-induced stress fibers and focal adhesions (15). Activation of the Rho kinase pathway leads to the phosphorylation of downstream substrates including the 20-kDa myosin light chain (MLC) (16), the myosin phosphatase subunit (MYPT-1) (17), and CPI-17A (18), which have been postulated to control a variety of fundamental cellular functions such as proliferation, survival, contraction, migration, and the transcriptional regulation of gene expression. However, abnormal activation of the pathway has been shown to induce multiple diseases (19). Accumulated evidence has demonstrated that Rho kinase activation induces inflammatory responses in various cell types including endothelial cells (20–22). However the involvement of the Rho kinase pathway in LPA-mediated expression of CAMs in endothelial cells is not fully understood.
Two isoforms of Rho kinase have been described and are widely referred to in the published literature as ROCK1 and ROCK2 (also known as ROKβ and ROKα, respectively) (23). They are both important regulators of the cytoskeleton, mediating RhoA effects on stress fiber formation, membrane ruffling, cell contraction, adhesion, and motility (24). The two isoforms are highly homologous, sharing an overall sequence homology of 65% at the amino acid level and a 92% homology within the kinase domain (25). Although ROCK1 and ROCK2 are ubiquitously expressed and highly homologous, several mechanisms have been reported that differentially regulate ROCK isoform activities. For example, ROCK1, but not ROCK2, is cleaved and activated by caspase-3 at the conserved cleavage site DETD1113 during apoptosis (26). On the other hand, ROCK2 is cleaved and activated by the proapoptotic protease granzyme B at the cleavage site IGLD1131 (27). This consensus sequence for granzyme B is not present in ROCK1.
To date, however, the contributions of the ROCK isoforms to inflammatory responses like CAM expression in endothelial cells have not been investigated. Here, we have shown for the first time that LPA-induced ICAM-1 and VCAM-1 expression is mediated by activation of the Rho kinase-NF-κB pathway in human endothelial cells. In addition, the targeted knockdown of ROCK2 with siRNA, but not ROCK1, blocked LPA-induced NF-kB activation, thereby attenuating adhesion molecule expression. These findings suggest that ROCK2 is essential for LPA-mediated CAM expression in human endothelial cells and could therefore be a key regulator of the trans-endothelial migration of leukocytes in inflammatory disease.
EXPERIMENTAL PROCEDURES
Reagents and Chemicals
H1152P was purchased from Calbiochem. LPA and Bay 11-7082 were obtained from BIOMOL International (Plymouth Meeting, PA). Anti-ROCK1 and anti-ROCK2 antibodies were purchased from Bethel Laboratories (Montgomery, TX). Anti-pMYPT-1 (Thr850) antibody was obtained from Millipore (Bedford, MA). Anti-MYPT-1, anti-VCAM-1, and anti-GAPDH antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-ppMLC (Thr18/Ser19), anti-MLC-2, anti-pNF-κB p65 (Ser536), and anti-NF-κB p65 antibodies were purchased from Cell Signaling Technology (Beverly, MA). Anti-ICAM-1 antibody was purchased from R&D Systems (Minneapolis, MN).
Cell Culture and Stimulation
Primary HUVECs were purchased from LONZA (Basel, Switzerland) and cultured in endothelial growth medium-2 (EGM-2) medium containing 2% fetal bovine serum (LONZA). 1 × 106 HUVECs/well were seeded into six-well tissue culture-treated plates and grown to confluence. For all studies, cells were serum-starved overnight in EGM medium containing 0.5% serum and then pretreated for 1 h where indicated with the stated concentrations of inhibitors prior to activation with 50 μm LPA for the indicated duration. Cell lysates were analyzed by Western blotting and enzyme-linked immunosorbent assay (ELISA).
siRNA Transfection
We used ON-TARGETplus SMART pool siRNAs (containing a mixture of four siRNAs) targeting human ROCK1 sequences CUACAAG-UGUUGCUAGUUU, UAGCAAUCGUAGA-UACUUA, CCAGGAAGGUAUAUGCUAU, and GCCAAUGACUUACUUAGGA; and human ROCK2 sequences GCAACUGGCUCGUU-CAAUU, UAGAAUAUGUGGCCUAGAA, GAAACUAAUAGGACACUAA, and CAAAC-UUGGUAAAGAAUUG. siRNAs and nonspecific control siRNA duplexes were synthesized, desalted, and purified by Thermo Fisher Scientific, Inc. (Waltham, MA). 5 × 105 HUVECs/well were plated in six-well tissue culture treated plates and cultured to confluence in EGM-2 medium. 50 nm siRNA, 4 μl of RNAiMax reagent (Invitrogen), and 200 μl of Opti-MEM (Invitrogen) were preincubated for 20 min prior to mixing with 800 μl of EGM-2 culture medium to form a transfection mixture. Cells in each well were then exposed to 1,000 μl of transfection mixture for 24 h before being returned to the EGM-2 culture medium. 48 h after initiation of siRNA transfection, cells were serum-starved overnight in EGM medium containing 0.5% serum prior to treatment with 50 μm LPA. The knockdown efficiency was assessed by Western blotting. Downstream signaling events were examined in cell lysates by Western blotting and ELISA.
Measurement of Total and Phospho-NF-κB p65 by ELISA
Following LPA stimulation, cells were lysed in cell lysis buffer (Cell Signaling Technology). Cell lysates were analyzed by PathScan phospho-NF-κB p65 (Ser536) and total-NF-κB sandwich ELISA kits according to the manufacturer's instructions (Cell Signaling Technology).
Quantitative Real-Time PCR
Total RNAs were isolated using the ABI PRISM 6100 Nucleic Acid Prepstation and total RNA lysis reagents (Applied Biosystems, Foster City, CA), according to the manufacturer's instructions. The qScriptTM One-Step quantitative real-time PCR kit (Quanta Bioscience, Gaithersburg, MD) was used for reverse transcription and amplification from predeveloped TaqMan gene expression assays specific to each gene of interest. Amplified reactions were detected and quantified on an ABI PRISM 7900 sequence detection system (Applied Biosystems). Cycling parameters were denaturation at 50 °C for 10 min and 95 °C for 10 min followed by 40 amplification cycles of 95 °C for 15 s and 60 °C for 1 min. Relative gene quantities were obtained using the comparative cycle threshold method after normalization to the human B2M gene.
Whole Cell Extracts and Western Blot Analysis
Cells were washed twice with cold phosphate-buffered saline, and whole cell proteins were isolated using M-PERTM mammalian protein extraction reagent (Thermo Fisher Scientific, Inc.) and lithium dodecyl sulfate sample buffer (Invitrogen) containing Halt Protease and a phosphatase inhibitor mixture (Thermo Fisher Scientific, Inc.). Cell lysates were scraped from the dishes, sonicated on ice, and centrifuged at 14,000 × g for 10 min to remove insoluble material. Samples were separated by SDS-PAGE and electrotransferred onto polyvinylidene difluoride membranes (Millipore). After blocking, blots were incubated overnight at 4 °C with the following primary antibodies in CanGetSignal solution 1 (TOYOBO, Japan): anti-pMYPT-1 (Thr850) (1:2,000), anti-MYPT-1 (1:2,000), anti-VCAM-1 (1:2,000), anti-GAPDH (1:2,000), anti-ppMLC (Thr18/Ser19) (1:2,000), anti-MLC-2 (1:2,000), anti-pNF-κB p65 (Ser536) (1:2,000), anti-NF-κB p65 (1:2,000), and anti-ICAM-1 (1:1,000) antibodies. After washing three times in 0.05% Tween 20 in Tris-buffered saline, primary antibodies were detected with the appropriate horseradish peroxidase-labeled secondary antibodies (diluted 1:10,000 in CanGetSignal solution 2) for 2 h at room temperature. Proteins were visualized by SuperSignal West Dura Extended Duration Substrate (Thermo Fisher Scientific, Inc.) according to the manufacturer's instructions.
Analysis of Cell Viability
The effect of siRNAs on cell viability was measured by CellTiter-Glo luminescent cell viability assay (Promega, Madison WI) according to the manufacturer's instructions.
Statistical Analysis
Results are expressed as the means and S.E. Multigroup comparison was performed by one-way analysis of variance, followed by the Newman-Keuls test as a post hoc analysis. Calculations were performed using GraphPad Prism program software (GraphPad Software, San Diego, CA).
RESULTS
LPA Activates the Rho Kinase and NF-κB Pathways and Induces CAM Expression in HUVECs
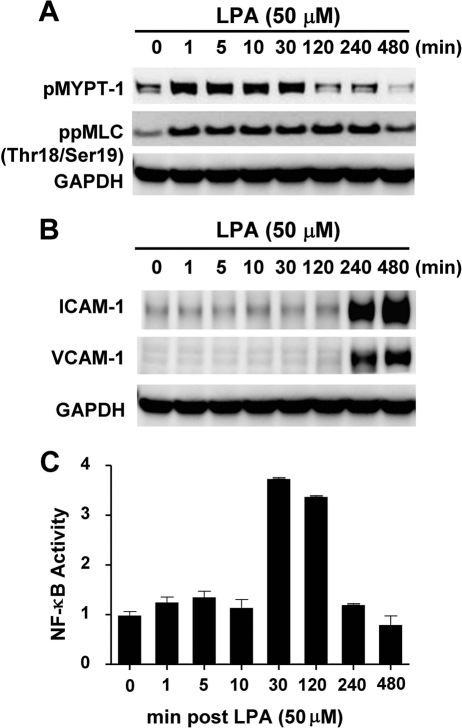
HUVECs were cultured to confluence in growth medium (EGM-2) containing 2% fetal bovine serum prior to being serum-starved overnight in 0.5% fetal bovine serum. The serum starvation was performed to return cells to a resting state and to minimize any endogenous signaling events promoted by inflammatory mediators contained in the serum. Cells were then incubated with 50 μm LPA, and whole cell lysates were harvested at the indicated times. Activation of Rho kinase signaling was assessed by measuring the phosphorylation of two canonical substrates, MLC and MYPT-1 by Western blot analysis using phosphospecific antibodies (Fig. 1A). The pathway was rapidly activated as evidenced by the maximal phosphorylation of both substrates within 5 min (Fig. 1A). Some basal activity of the pathway was also seen in the absence of LPA stimulation (0 min, Fig. 1A). The blot was then stripped and reprobed with ICAM-1 and VCAM-1 antibodies. As shown in Fig. 1B, the expression of both ICAM-1 and VCAM-1 was very delayed, being significantly and coordinately induced at 4 h and sustained for up to 8 h. GAPDH was used as a normalization control. Activation of the NF-κB pathway was measured by ELISA over the same time course and expressed as a ratio of phosphorylated NF-κB p65 (Ser536) to total NF-κB p65 (Fig. 1C). An LPA-mediated peak activation of NF-κB p65 occurred later than Rho kinase activation but prior to induction of CAM expression, peaking at 30 min before returning to baseline levels by 4 h (Fig. 1C).
FIGURE 1.
LPA activates Rho kinase and NF-κB pathways and induces expression of ICAM-1 and VCAM-1 protein in HUVECs. HUVECs were cultured in EGM-2 growth medium and serum-starved overnight in 0.5% serum prior to addition of 50 μm LPA. At the indicated times, cells were harvested and lysates were prepared for either Western blotting or ELISA as described under “Experimental Procedures.” Western blot analysis of Rho kinase substrates ppMLC and pMYPT-1 (A) and adhesion molecules ICAM-1 and VCAM-1 (B) at the indicated times after LPA addition. ELISAs of phospho NF-κB P65 and total NF-κB P65 in HUVEC lysates were performed at the indicated times after LPA addition (C). NF-κB activity is expressed as a ratio of these two measurements. Values are means (n = 3) ± S.E.
Pharmacologic Inhibition of Rho Kinase Signaling Suppresses LPA-Induced Activation of NF-κB p65 as well as ICAM-1 and VCAM-1 Expression in HUVECs
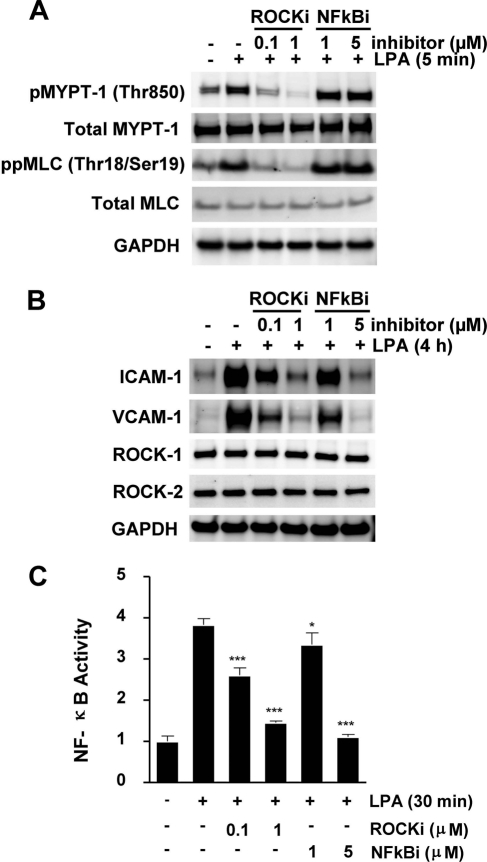
In this set of experiments, we mapped the LPA-mediated downstream signaling in HUVECs employing H1152P, a Rho kinase inhibitor, and Bay 11-7082, an NF-κB inhibitor. LPA treatment in these studies was for the duration that induced peak activation of the substrate being measured, based on data from Fig. 1, A–C. As shown in Fig. 2A, H1152P suppressed LPA-mediated phosphorylation of both Rho kinase substrates MLC and MYPT-1 in a concentration-dependent manner, whereas NF-κB inhibition had no impact on Rho kinase signaling. Both H1152P and the specific inhibitor for the NF-κB pathway Bay 11–7082 suppressed LPA-induced ICAM-1 and VCAM-1 expression in HUVECs (Fig. 2B). Neither LPA treatment nor exposure to inhibitors had any impact on the expression levels of the two Rho kinase isoforms ROCK1 and ROCK2 (Fig. 2B). Additionally, both inhibitors blocked LPA-mediated NF-κB p65 phosphorylation in a dose-dependent manner (Fig. 2C). These data suggest that Rho kinase has a crucial role in LPA-induced CAM expression through the activation of NF-κB pathway in HUVECs.
FIGURE 2.
LPA-induced expression of ICAM-1 and VCAM-1 protein in HUVECs is mediated by Rho kinase activation upstream of NF-κB. Serum-starved HUVECs were preincubated for 1 h with the specified concentrations of either H1152P, a Rho kinase inhibitor, or Bay 11-7082, an NF-κB inhibitor (NFkBi), prior to treatment with LPA for the indicated durations. Cell lysates were analyzed by Western blot analysis of pMYPT-1 and ppMLC using phosphospecific antibodies (A) and ICAM-1, VCAM-1, ROCK1, and ROCK2 protein (B). Total MYPT-1, total MLC, and GAPDH were used as normalization controls (C). NF-κB activities were assessed for the different treatments, as described in the legend to Fig. 1. Values are means (n = 3) ± S.E. *, p < 0.05 and ***, p < 0.0001 compared with no compound treatment. ROCKi, ROCK inhibitor.
Knockdown of ROCK1 and ROCK2 Is Essential to Block LPA-mediated Phosphorylation of MYPT-1 and MLC
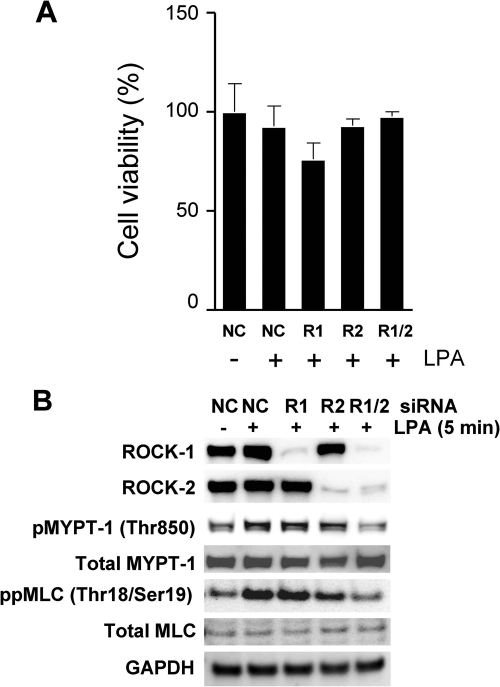
To evaluate the contributions of Rho kinase isoforms (ROCK1 and ROCK2) to LPA-mediated signaling pathways, we used an siRNA approach to achieve the targeted knockdown of each isoform in HUVECs. Cells were treated with siRNA (50 nm) directed either against each ROCK isoform or with a negative control (NC) siRNA. None of the siRNA treatments had any significant impact on cell viability (Fig. 3A). Compared with cells exposed to negative control siRNA, a >90% knockdown of targeted protein was observed in cells exposed to target siRNA for 72 h (Fig. 3B), and this level of knockdown was sustained for up to 96 h (data not shown). Although the combination of ROCK1/ROCK2 knockdown suppressed LPA-mediated MYPT-1 and MLC phosphorylation compared with NC siRNAs (Fig. 3B), the knockdown of the individual ROCK isoforms failed to suppress MYPT-1 and MLC phosphorylation (Fig. 3B).
FIGURE 3.
Both Rho kinase isoforms ROCK1 and ROCK2 phosphorylate MYPT-1 and MLC and can compensate for the loss of the other. HUVECs were incubated with 50 nm οf either a nonspecific control siRNA (NC) or a ROCK1-specific, ROCK2-specific, or dual ROCK1/ROCK2 siRNAs for 24 h in a transfection medium. The medium was then replaced with standard growth medium (EGM-2), and cells were incubated for an additional 24 h. Cells were then serum-starved overnight prior to LPA exposure for 5 min. A, cell viability was assessed for each treatment with CellTiter-Glo luminescent cell viability assay (Promega) following the manufacturer's instructions. Values are means (n = 4) ± S.E. B, efficiency of gene knockdown was assessed by Western blotting with ROCK1- and ROCK2-specific antibodies (top two panels). Phosphorylation of MYPT-1 and MLC was assessed by Western blotting with phosphospecific antibodies. Total MYPT-1, total MLC, and GAPDH were used as normalization controls.
ROCK-2 Drives LPA-mediated NF-κB Pathway Activation in HUVECs
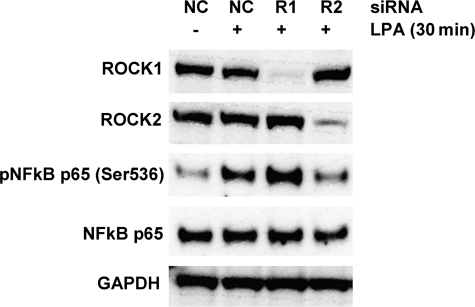
We then explored the contributions of each ROCK isoform toward LPA-mediated activation of NF-κB. At 72 h post-transfection, a >90% knockdown of each ROCK isoform was observed (Fig. 4, top two columns). Treatment with LPA for 30 min following a 72 h exposure to nonspecific siRNA, resulted in significant phosphorylation of the NF-κB p65 subunit (Fig. 4). Knockdown of ROCK1 had no impact on LPA-mediated p65 phosphorylation, whereas knockdown of ROCK2 very significantly suppressed p65 phosphorylation (Fig. 4). These data suggest that ROCK-2, but not ROCK-1, is an important regulator of LPA-mediated NF-κB activation in HUVECs.
FIGURE 4.
ROCK2 activates NF-κB by phosphorylating the p65 subunit. siRNA-mediated gene knockdowns were performed as described in the legend to Fig. 3, and cells were then exposed to LPA for 30 min. Effectiveness of gene knockdown was assessed by Western blotting with ROCK1- and ROCK2-specific antibodies (top two panels). Phospho-NF-κB p65 was measured with a phosphospecific antibody and total NF-κB p65 and GAPDH were used as normalization controls.
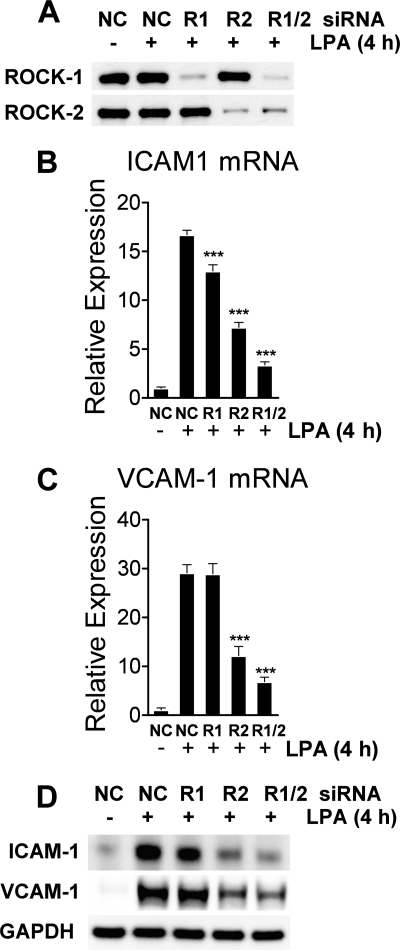
ROCK-2 Knockdown Suppresses LPA-induced Expression of ICAM-1 and VCAM-1 mRNA and Protein in HUVECs
Finally, we assessed the impact of ROCK isoform knockdown on ICAM-1 and VCAM-1 mRNA and protein expression in HUVECs. At 72 h post-transfection, ROCK1 and ROCK2 siRNAs achieved a selective and almost complete knockdown of their gene target (Fig. 5A). In cells exposed to NC siRNA, a 4-h LPA treatment induced ICAM-1 mRNA expression 15-fold (Fig. 5B) and VCAM-1 mRNA expression 30-fold (Fig. 5C) above untreated cells. Whereas ROCK-1 knockdown decreased LPA-induced ICAM-1 mRNA by 24%, ROCK-2 knockdown had greater impact, reducing ICAM-1 mRNA by 60% (Fig. 5B). In addition, ROCK-2 knockdown also significantly decreased LPA-induced VCAM-1 expression by 60% compared with NC siRNA treatment (Fig. 5C). Protein expression of ICAM-1 and VCAM-1 mirrored mRNA expression, with ROCK2 knockdown most significantly reducing ICAM-1 and VCAM-1 protein and with ROCK1 knockdown having little to no influence on adhesion molecule expression (Fig. 5D). There was no significant synergistic impact of a dual ROCK1/ROCK2 knockdown on adhesion molecule expression (Fig. 5D).
FIGURE 5.
ROCK2 is the dominant mediator of LPA-driven ICAM-1 and VCAM-1 mRNA and protein expression in HUVECs. siRNA-mediated gene knockdowns were performed as described in the legend to Fig. 3, and cells were then incubated with LPA for an additional 4 h. Knockdown efficacy was evaluated by Western blotting (A). ICAM-1 (B) and VCAM-1 (C) mRNA levels were measured by quantitative real-time PCR using gene-specific primers. Values are means (n = 4) ± S.E. ***, p < 0.0001 compared with nonspecific control (NC) siRNA treatment. D, following siRNA treatment and exposure to LPA for 4 h, ICAM-1 and VCAM-1 proteins were visualized by Western blotting with gene-specific antibodies. GAPDH was used as a normalization control.
DISCUSSION
Due to the propensity of LPA to stick to plastic and bind serum protein, the mediator is usually added at micromolar concentrations. In these studies, cells were therefore incubated with 50 μm LPA to overcome these limitations. LPA rapidly activated Rho kinase in HUVECs as evidenced by the maximal phosphorylation of its substrates MLC and MYPT-1 within 5 min of LPA addition (Fig. 1A). A similar phosphorylation kinetics of Rho kinase substrates has been reported in endothelial cells stimulated with thrombin (28). In addition to Rho kinase activation, we also detected activation of NF-κB p65 and the induced expression of ICAM-1 and VCAM-1 in LPA-treated endothelial cells. The LPA1 receptor has been shown to be essential for LPA-mediated induction of ICAM-1 mRNA and expression of cell-surface protein as well as the adhesion of U937 macrophages to LPA-activated HUVEC (10). The C terminus of the LPA-1 receptor interacts with the glycine-leucine-glycine-phenylalanine domain of the Rho guanine-nucleotide exchange factor (RhoGEF) to promote signaling the Rho kinase (29). Thus, LPA engaging its LPA1 receptor likely drives adhesion molecule expression via Rho kinase activation.
Rahman et al. (30) observed that in endothelial cells, thrombin induced the NF-κB p65 homodimer to bind an NF-κB site on the ICAM-1 promoter, increasing the expression of ICAM-1 mRNA, the cell-surface expression of ICAM-1 protein and the adhesion of neutrophils to endothelial cells. More recently, Anwar et al. (22) linked Rho kinase activation to thrombin-induced, p65-mediated induction of ICAM-1 expression in endothelial cells. In the current study, the sequential activation (Fig. 1) of Rho kinase (5 min) followed by NF-κB p65 (30 min) and then ICAM-1 and VCAM-1 (4 h), hinted at Rho kinase activation as being the early event driving NF-κB activation and thereby the transcriptional up-regulation of ICAM-1 and VCAM-1.
We adopted a pharmacologic approach to evaluate the sequence of signaling events downstream of LPA, culminating in cell adhesion molecule expression in endothelial cells. The Rho kinase inhibitor H1152P is an ATP-competitive, ROCK isoform-nonselective inhibitor of low nanomolar potency that is selective against the larger family of kinases (31). Bay 11-7082 blocks the phosphorylation and degradation of the inhibitory subunit ΙκB, bound to NF-κB, and thereby inhibits NF-κB signaling (32). H1152P blocked phosphorylation of the Rho kinase substrates as well as the phosphorylation of NF-κB p65 (Fig. 2, A and C). Bay 11-7082 had no influence on MLC and MYPT-1 phosphorylation but inhibited NF-κB p65 phosphorylation (Fig. 2, A and C). Both inhibitors blocked ICAM-1 and VCAM-1 expression. Collectively, the published literature (8, 13, 14) and our data support a pathway in which LPA, acting through the LPA1 receptor, promotes Rho kinase-mediated NF-κB activation and thereby the transcriptional up-regulation of adhesion molecule mRNA and protein expression in human endothelial cells.
The Rho kinase isoforms ROCK1 and ROCK2 share a 92% homology at the amino acid level within their kinase domains and are 100% identical within their ATP pockets, making the design of isoform-selective, ATP-competitive inhibitors challenging. Recently, SLx-2119 has been described as a ROCK2-selective compound (33). We took on an siRNA approach to examine the contributions of ROCK1 and ROCK2 to LPA-mediated signaling. LPA treatment of HUVECs exposed to nonspecific control siRNA and serum-starved overnight, caused rapid phosphorylation of MLC and MYPT-1 (Fig. 3B). The dual ROCK1/ROCK2 knockdown significantly inhibited phosphorylation of both Rho kinase substrates. However, the individual knockdown of either ROCK1 or ROCK2 had no impact on phosphorylation of either substrate, suggesting the ability for each isoform to compensate for the loss of the other. Similar results have been reported in pancreatic cells (34) and in smooth muscle cells (35). The RhoA-Rho kinase pathway has been historically studied in smooth muscle cells where phosphorylation of MLC drives smooth muscle contraction (36). Additionally, Rho kinase can indirectly influence the amount of phosphorylated MLC by phosphorylating and inactivating MLC phosphatase (MYPT-1) (17). Multiple studies have also linked the phosphorylation of MLC and MYPT-1 in endothelial cells to the induction of the endothelial cell contractile apparatus with subsequent paracellular gap formation and leukocyte diapedesis. Exposure of endothelial cells to mildly oxidized low density lipoprotein induced Rho kinase-dependent MLC phosphorylation, stress fiber formation, and intercellular gaps within minutes (38). Inhibition of MYPT-1 activity in intact microvessels also promoted a rapid phosphorylation of MLC, resulting in a contractile response and loss of barrier integrity (39). The pharmacologic inhibition of both isoforms would therefore be essential to block Rho kinase-mediated signaling through MLC and limit paracellular gap formation.
Inflammatory stimuli such as thrombin and tumor necrosis factor-α induced cell adhesion molecule expression in endothelial cells via Rho kinase and NF-κB (22, 40). However, there have been no reports to date, outlining the contributions of ROCK isoforms to NF-κB-mediated expression of cell adhesion molecules. Our siRNA studies clearly identified ROCK2 as the dominant isoform driving LPA-mediated activation of NF-κB (Fig. 4) and the ensuing transcriptional up-regulation of ICAM-1 and VCAM-1 mRNA and protein (Fig. 5). Changes in NF-κB transcriptional activity have been attributed to phosphorylation of the p65 subunit by a variety of kinases and the cytosol to nuclear translocation of the subunit (41). It has been proposed that Rho kinase-induced actin polymerization could promote the dissociation of the transcriptional co-activator MAL from actin monomers, permitting its translocation from cytosol to nucleus to promote gene transcription (37). It remains to be seen whether ROCK2 activation promotes p65 nuclear translocation by a similar mechanism.
In conclusion, we have shown that both Rho kinase isoforms can drive LPA-mediated signaling through MLC with each isoform capable of compensating for the loss of the other. We have also shown that LPA induces adhesion molecule expression in endothelial cells through Rho kinase-triggered NF-κB activation. Additionally, employing siRNAs to achieve the targeted knockdown of each isoform, we have demonstrated that ROCK2 is essential for LPA-mediated NF-kB p65 phosphorylation and the subsequent expression of adhesion molecules. Thus, signaling through both isoforms needs to be blocked to prevent MLC driven events, such as loss of endothelial barrier integrity and paracellular migration, whereas selective inhibition of ROCK2 signaling would be sufficient to block transcellular leukocyte migration aided by adhesion molecule expression.
This work was supported by Pfizer, Inc.
- CAM
- cell adhesion molecule
- LPA
- lysophosphatidic acid
- ICAM-1
- intracellular adhesion molecule-1
- VCAM-1
- vascular cell adhesion molecule-1
- HUVEC
- human umbilical vein endothelial cell
- MLC
- myosin light chain
- MYPT-1
- myosin phosphatase-1
- ROCK
- Rho kinase
- siRNA
- small interfering RNA
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- ELISA
- enzyme-linked immunosorbent assay
- NC
- negative control
- EGM-2
- endothelial growth medium-2.
REFERENCES
- 1. Cook-Mills J. M., Deem T. L. (2005) J. Leukoc. Biol. 77, 487–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Marlin S. D., Springer T. A. (1987) Cell 51, 813–819 [DOI] [PubMed] [Google Scholar]
- 3. Alon R., Kassner P. D., Carr M. W., Finger E. B., Hemler M. E., Springer T. A. (1995) J. Cell Biol. 128, 1243–1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhao Y., Tong J., He D., Pendyala S., Evgeny B., Chun J., Sperling A. I., Natarajan V. (2009) Respir. Res. 10, 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhao Y., Natarajan V. (2009) Cell Signal 21, 367–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nochi H., Tomura H., Tobo M., Tanaka N., Sato K., Shinozaki T., Kobayashi T., Takagishi K., Ohta H., Okajima F., Tamoto K. (2008) J. Immunol. 181, 5111–5119 [DOI] [PubMed] [Google Scholar]
- 7. Zhao C., Fernandes M. J., Prestwich G. D., Turgeon M., Di Battista J., Clair T., Poubelle P. E., Bourgoin S. G. (2008) Mol. Pharmacol. 73, 587–600 [DOI] [PubMed] [Google Scholar]
- 8. Inoue M., Rashid M. H., Fujita R., Contos J. J., Chun J., Ueda H. (2004) Nat. Med. 10, 712–718 [DOI] [PubMed] [Google Scholar]
- 9. Noguchi K., Herr D., Mutoh T., Chun J. (2009) Curr. Opin. Pharmacol. 9, 15–23 [DOI] [PubMed] [Google Scholar]
- 10. Lin C. I., Chen C. N., Lin P. W., Chang K. J., Hsieh F. J., Lee H. (2007) Biochem. Biophys. Res. Commun. 363, 1001–1008 [DOI] [PubMed] [Google Scholar]
- 11. Lin C. I., Chen C. N., Chen J. H., Lee H. (2006) J. Cell. Biochem. 99, 1216–1232 [DOI] [PubMed] [Google Scholar]
- 12. Lee H., Lin C. I., Liao J. J., Lee Y. W., Yang H. Y., Lee C. Y., Hsu H. Y., Wu H. L. (2004) Am. J. Physiol. Cell Physiol. 287, C1657–1666 [DOI] [PubMed] [Google Scholar]
- 13. Maruta T., Yanagita T., Matsuo K., Uezono Y., Satoh S., Nemoto T., Yoshikawa N., Kobayashi H., Takasaki M., Wada A. (2008) J. Neurochem. 105, 401–412 [DOI] [PubMed] [Google Scholar]
- 14. Hashimoto T., Yamashita M., Ohata H., Momose K. (2003) J. Pharmacol. Sci. 91, 8–14 [DOI] [PubMed] [Google Scholar]
- 15. Leung T., Manser E., Tan L., Lim L. (1995) J. Biol. Chem. 270, 29051–29054 [DOI] [PubMed] [Google Scholar]
- 16. Amano M., Ito M., Kimura K., Fukata Y., Chihara K., Nakano T., Matsuura Y., Kaibuchi K. (1996) J. Biol. Chem. 271, 20246–20249 [DOI] [PubMed] [Google Scholar]
- 17. Kimura K., Ito M., Amano M., Chihara K., Fukata Y., Nakafuku M., Yamamori B., Feng J., Nakano T., Okawa K., Iwamatsu A., Kaibuchi K. (1996) Science 273, 245–248 [DOI] [PubMed] [Google Scholar]
- 18. Kitazawa T., Eto M., Woodsome T. P., Brautigan D. L. (2000) J. Biol. Chem. 275, 9897–9900 [DOI] [PubMed] [Google Scholar]
- 19. Wettschureck N., Offermanns S. (2002) J. Mol. Med. 80, 629–638 [DOI] [PubMed] [Google Scholar]
- 20. Aihara M., Dobashi K., Iizuka K., Nakazawa T., Mori M. (2003) Int. Immunopharmacol. 3, 1619–1625 [DOI] [PubMed] [Google Scholar]
- 21. He Y., Xu H., Liang L., Zhan Z., Yang X., Yu X., Ye Y., Sun L. (2008) Arthritis Rheum. 58, 3366–3376 [DOI] [PubMed] [Google Scholar]
- 22. Anwar K. N., Fazal F., Malik A. B., Rahman A. (2004) J. Immunol. 173, 6965–6972 [DOI] [PubMed] [Google Scholar]
- 23. Nakagawa O., Fujisawa K., Ishizaki T., Saito Y., Nakao K., Narumiya S. (1996) FEBS Letters 392, 189–193 [DOI] [PubMed] [Google Scholar]
- 24. Loirand G., Guérin P., Pacaud P. (2006) Circ. Res. 98, 322–334 [DOI] [PubMed] [Google Scholar]
- 25. Mueller B. K., Mack H., Teusch N. (2005) Nat. Rev. Drug Discov. 4, 387–398 [DOI] [PubMed] [Google Scholar]
- 26. Sebbagh M., Renvoizé C., Hamelin J., Riché N., Bertoglio J., Bréard J. (2001) Nat. Cell Biol. 3, 346–352 [DOI] [PubMed] [Google Scholar]
- 27. Sebbagh M., Hamelin J., Bertoglio J., Solary E., Bréard J. (2005) J. Exp. Med. 201, 465–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Essler M., Amano M., Kruse H. J., Kaibuchi K., Weber P. C., Aepfelbacher M. (1998) J. Biol. Chem. 273, 21867–21874 [DOI] [PubMed] [Google Scholar]
- 29. Yamada T., Ohoka Y., Kogo M., Inagaki S. (2005) J. Biol. Chem. 280, 19358–19363 [DOI] [PubMed] [Google Scholar]
- 30. Rahman A., Anwar K. N., True A. L., Malik A. B. (1999) J. Immunol. 162, 5466–5476 [PubMed] [Google Scholar]
- 31. Sasaki Y., Suzuki M., Hidaka H. (2002) Pharmacol. Ther. 93, 225–232 [DOI] [PubMed] [Google Scholar]
- 32. Ohkita M., Takaoka M., Shiota Y., Nojiri R., Sugii M., Matsumura Y. (2002) Jpn. J. Pharmacol. 89, 81–84 [DOI] [PubMed] [Google Scholar]
- 33. Boerma M., Fu Q., Wang J., Loose D. S., Bartolozzi A., Ellis J. L., McGonigle S., Paradise E., Sweetnam P., Fink L. M., Vozenin-Brotons M. C., Hauer-Jensen M. (2008) Blood Coagul. Fibrinolysis 19, 709–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Garton A. J., Castaldo L., Pachter J. A. (2008) Methods Enzymol. 439, 491–500 [DOI] [PubMed] [Google Scholar]
- 35. Wang Y., Zheng X. R., Riddick N., Bryden M., Baur W., Zhang X., Surks H. K. (2009) Circ. Res. 104, 531–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kureishi Y., Kobayashi S., Amano M., Kimura K., Kanaide H., Nakano T., Kaibuchi K., Ito M. (1997) J. Biol. Chem. 272, 12257–12260 [DOI] [PubMed] [Google Scholar]
- 37. Miralles F., Posern G., Zaromytidou A. I., Treisman R. (2003) Cell 113, 329–342 [DOI] [PubMed] [Google Scholar]
- 38. Essler M., Retzer M., Bauer M., Heemskerk J. W., Aepfelbacher M., Siess W. (1999) J. Biol. Chem. 274, 30361–30364 [DOI] [PubMed] [Google Scholar]
- 39. van Nieuw Amerongen G. P., Musters R. J., Eringa E. C., Sipkema P., van Hinsbergh V. W. (2008) Am. J. Physiol. Cell Physiol. 294, C1234–1241 [DOI] [PubMed] [Google Scholar]
- 40. Wu X., Guo R., Chen P., Wang Q., Cunningham P. N. (2009) Am. J. Physiol. Renal Physiol. 297, F316–326 [DOI] [PubMed] [Google Scholar]
- 41. Vermeulen L., De Wilde G., Notebaert S., Vanden Berghe W., Haegeman G. (2002) Biochem. Pharmacol. 64, 963–970 [DOI] [PubMed] [Google Scholar]