Abstract
This study examined sleep–wake patterns in 3 matched comparison groups of preschool-aged children: children with autism (AUT), children with developmental delay (DD) without AUT, and children who are developing typically (TYP). Sleep was assessed via actigraphy and parent-report diaries for 7 consecutive 24-hr periods across 3 time points: at enrollment (n = 194), 3 months later (n = 179), and 6 months after enrollment (n = 173). At each recording period, children in the AUT group slept less per 24-hr period, on average, and were less likely to awaken at night than children in the other two groups. In contrast, children in the DD group had more frequent and longer duration nighttime awakenings than children in the AUT group. Overall, children in the 2 neurodevelopmentally disordered groups demonstrated more night-to-night variability in their sleep–wake measures than children in the TYP group.
Although the functions of sleep are not clearly understood, there is general agreement that sufficient, quality sleep is essential for healthy development in childhood (Dahl, 1996; Meltzer & Mindell, 2007). However, what characterizes adequate and sufficient sleep in preschool-age children, especially in those with neurodevelopmental disorders such as autism (AUT), has been insufficiently studied. In general, studies document higher rates of sleep problems in young children with neurodevelopmental disorders compared with typically developing (TYP) children (Goodnight, Bates, Staples, Pettit, & Dodge, 2007; Gruber, Sadeh, & Raviv, 2000; Patzold, Richdale, & Tonge, 1998; Polimeni, Richdale, & Francis, 2005; Quine, 2001; Richdale, 1999; Richdale & Prior, 1995; Sadeh, Gruber, & Raviv, 2002; Wiggs, 2001). In AUT specifically, previous studies reported that children with AUT awakened more frequently at night (Krakowiak, Goodlin-Jones, Hertz-Picciotto, Croen, & Hansen, 2008), had earlier morning rise times (Polimeni et al., 2005), and shorter 24-hr sleep durations (Goodlin-Jones, Tang, Liu, & Anders, 2008). A majority of these studies relied on parent report to describe sleep duration, disruptions of sleep, and behavioral concomitants associated with sleep disturbances. In addition, most studies of preschoolers with AUT did not utilize developmentally matched control groups of children without AUT, making it difficult to interpret whether reported differences were related to a diagnosis of autistic disorder or other aspects of developmental delay (DD). This study moves the field forward by using a more objective sleep measure (actigraphy) and by incorporating two carefully matched comparison groups.
The two comparison groups for children with autistic disorder (AUT group) were children with DD without AUT (DD group) and TYP children (TYP group). Both the AUT and DD groups were matched on levels of cognitive and adaptive functioning so that differences could be attributed to diagnostic group. The average chronologic age in the TYP group was approximately 6 months younger than the average ages in the two neurodevelopmentally disordered groups, although age ranges were comparable for all three groups. Because sleep–wake organization changes with age (Iglowstein, Jenni, Molinari, & Largo, 2003; Iglowstein, Latal Hajnal, Molinari, Largo, & Jenni, 2006), children in the TYP group were recruited at chronologically younger ages in an attempt to better match any possible immaturity in sleep–wake organization with the two DD groups. Since there are insufficient data in the literature to determine exactly how immaturity in sleep–wake organization may manifest itself in the neurodevelopmentally disordered groups, the 6-month mean age difference was chosen as a best estimate.
This study assessed sleep via actigraphy and focused on sleep patterns, rather than parent-perceived sleep problems. In addition, sleep studies of TYP children also have documented that variability in sleep patterns affect child daytime functioning and parental stress (Bates, Viken, Alexander, Beyers, & Stockton, 2002; Doo & Wing, 2006). Similarly, studies of children with AUT have reported that difficult behavioral profiles, including sleep variability, are significant stressors for families (Davis & Carter, 2008). Therefore, this study expands on previous reports by examining patterns of between- and within-child sleep–wake variation across the three diagnostic groups.
This is the third report in a series using this sample of children. The first report focused on both the organization of sleep–wake patterns and on carefully predefined sleep problems from the cross-sectional perspective, reporting results from Time 1 data only (Goodlin-Jones et al., 2008). The second report was longitudinal in design and focused on the classification of sleep problems as previously defined in the cross-sectional report, assessing sleep problem stability and variability over three recording periods spanning a 6-month time period (Goodlin-Jones et al., 2009). This report also uses the longitudinal design but examines the patterns and variability of the actigraphically derived sleep–wake behaviors (not the sleep problems) over the 6-month period. All three reports are based on the same three matched comparison groups of preschool-aged children: children with AUT disorder, DD without AUT, and a chronologically younger group of TYP children.
We expected more disruptive sleep behaviors (i.e., more nighttime awakening and longer sleep latency times) in children in the AUT and DD groups compared with children in the TYP group, and that the three diagnostic groups would maintain their sleep pattern differences over time. We also predicted that children in the AUT group would show greater variability in their sleep measures than children in either the DD or TYP groups.
METHOD
Sample
Using the research recruitment database of the UC Davis M.I.N.D. Institute, advertisements in local pediatric offices, and word of mouth, children were recruited for a study “to learn more about sleep and waking patterns,” not for a study about sleep problems. The M.I.N.D. Institute research database contains a list of carefully diagnosed and documented potential research participants whose parents are willing to be contacted by M.I.N.D. investigators. The database includes children with AUT, other neurodevelopmental disorders, and TYP children. We enrolled children from the list if their parents expressed interest in learning more about their child’s sleep patterns. Only one preschool-aged child per family was enrolled. Children with a chronic medical illness or a current or prior clinical sleep disorder were excluded. Two children in the DD group with well-controlled seizure disorders, on maintenance anticonvulsant medication, were included. None of the children in the AUT and TYP groups were known to have seizures nor were on any psychotropic medications. The UC Davis institutional review board approved the protocol, and all parents signed informed consents.
Of the 194 children recorded at Time 1, 179 children were studied at Time 2, and 173 children completed all three recording sessions, accounting for a 10.8% non-completion rate. The composition of the sample at the time of enrollment in terms of demographic, family characteristics, and dropout rate (stratified by diagnostic group) is presented in Table 1. Sample demographics and socioeconomic status (SES) suggest that participating families were representative of the greater Sacramento, California community. There were no significant differences in gender, ethnicity, or diagnosis between those who completed the study and those who did not.
TABLE 1.
Overall Sample Characteristics by Diagnostic Group at Intake
| Variable | AUTa | DDb | TYPc |
|---|---|---|---|
| Gender (% male) | 81 | 74 | 70 |
| Age at intake (in months) | |||
| M | 47 | 46 | 41* |
| SD | 10 | 12 | 11 |
| Range | 28–68 | 24–70 | 24–62 |
| Ethnicity (% Caucasian) | 59 | 47 | 70 |
| Mullen ELC | |||
| M | 60 | 55 | 101 |
| SD | 18 | 7 | 17 |
| Range | 49–140 | 49–74 | 72–148 |
| Vineland ABC | |||
| M | 62 | 62 | 98 |
| SD | 11 | 12 | 17 |
| Range | 41–87 | 31–104 | 65–151 |
| M college graduate (%) | 64 | 39* | 72 |
| Married (%) | 86 | 79* | 94 |
| Dropout (%) | 9 | 12 | 12 |
Note. AUT = autism; DD = developmental delay without AUT; M = mother; TYP = typically developing; ELC = Early Learning Composite; ABC = Adaptive Behavior Composite.
n = 68.
n = 57.
n = 69.
p < .05.
The ages of children at initial enrollment ranged from 2.0 to 5.5 years (M = 3:70 years, SD = 0:93). As described earlier, children in the AUT group were matched with children in the DD group on developmental level and adaptive capacity so that differences in sleep–wake patterns could be attributed to diagnostic group differences rather than to developmental differences. In an attempt to control for possible maturational effects on sleep–wake organization during the preschool period, children in the TYP group were specifically recruited at the younger end of the chronological age spectrum so as to better match their sleep–wake patterns with the neurodevelopmentally delayed groups. The mean age of children in the TYP group on entry to the study was 41 months compared with mean ages of 47 and 46 months for children in the AUT and DD groups, respectively (p < .05)
The comprehensive, initial research diagnostic evaluation has been described previously (Goodlin-Jones et al., 2008). Only children who met diagnostic cutoff criteria for AUT on the Autism Diagnostic Observation Schedule (ADOS) and on all domains of the Autism Diagnostic Interview–Revised (ADI–R) were included in the AUT group. Children within the DD group carried diagnoses of Down’s syndrome, Global Developmental Delay, Di George syndrome, and idiopathic DDs. To rule out the presence of undiagnosed AUT, all DD group members received ADOS and ADI–R evaluations. None met the cutoff for an autism spectrum disorder (ASD). Children in all three groups completed the Mullen Scales of Early Learning, and TYP children had Early Learning Composite (ELC) scores above 75.
The AUT and DD groups did not differ significantly from each other on mean baseline IQ (ELC) and Vineland Adaptive Behavior Composite (ABC) scores (see Table 1). As expected, both neurodevelopmentally disordered groups scored significantly lower, on average, than the TYP group on the developmental and adaptive measures, although the TYP group was significantly chronologically younger. However, both neurodevelopmental groups and the TYP group encompassed a range of developmental scores so that, for example, children in the AUT group comprised a range of Mullen ELC scores from 49 to 140, and children in the TYP group ranged from 72 to 148 (see Table 1). Family composition and SES variables such as parent age, employment status, and household size did not differ among the three diagnostic groups. The only exception was that there were significantly fewer college graduates and married mothers in the DD group; mothers of children in the AUT and TYP groups were not significantly different on any of the comparisons.
Procedures
Sleep measures
Actigraphy and a parent sleep–wake diary assessed sleep. The actigraph, a Mini Mitter Actiwatch (AW64; Mini Mitter, Bend, OR) was worn for seven consecutive days and nights on the non-dominant ankle for each of the 3 recording weeks. Because children with AUT are likely to be hypersensitive to wearing actigraphs or changes in their usual routine, our occupational therapist designed a foam rubber ankle bracelet in which the actigraph was embedded. During a practice session in the laboratory, ankle bracelets were applied repeatedly to both ankles until the child was comfortable. Parents participated in this practice session and received instruction about proper placement at home. Only two children were excluded from the study for failure to wear the ankle bracelet. There was no evidence that actigraphy was more cumbersome for children in the AUT group than for children in the other two groups.
During each 7-day week, parents completed a daily sleep diary first thing each morning for the previous 24 hr as a check for the actigraph sleep start and sleep end times. Sleep diaries were also examined to determine concordance between actigraph records and diary observations. Actigraph recordings and diaries were obtained following the initial intake evaluation (Time 1), 3 months later (Time 2), and again after 3 months (Time 3). Thus, each child was studied over a 6-month period. During each of the 3 recording weeks, a research assistant maintained daily phone contact with parents to maximize compliance. Over 90% of actigraph recordings and sleep diary records were scored.
Sleep variables
Actigraph data were scored using the manufacturer’s algorithm set at medium sensitivity. A secondary “smoothing” filter was applied to the output so that single, isolated, 1-min waking epochs were re-coded to sleep—that is, all nighttime awakenings had to meet actigraph criteria of wakefulness for at least 2 min before being scored as an awakening. The validity of this secondary smoothing algorithm has been reported previously (Sitnick, Goodlin-Jones, & Anders, 2008).
Actigraph sleep variables included bedtime (recorded clock time from the diary) and 24-hr sleep (sum of nighttime sleep duration plus nap duration in minutes). The quality of the sleep derived from the actigraph was indicated by sleep efficiency (time in bed asleep divided by the total time in bed), sleep onset latency time (number of minutes from bedtime to sleep start time), wake after sleep onset (WASO) duration (total minutes awake after sleep onset), and WASO number (the number of nighttime awakenings after sleep onset).
Data Analysis
Statistical analyses used SAS Version 9.1 (SAS Institute, 2002–2003, Cary, NC) and included descriptive statistics for all categorical and continuous variables. Random-effects regression models (Laird & Ware, 1982) were used (a) to estimate patterns of change in actigraph variables measuring sleep and awakening amounts and timing over the course of the study, (b) to test whether diagnosis and other covariates were related to the initial level or rate of change in sleep variables, and (c) to examine the between- and within-child variability and their heterogeneity across diagnostic groups. This approach allowed the use of all available data for each child. The models assumed that each child’s individual trajectory followed the mean path, except for random effects that caused the initial level to be higher or lower and the rate of change to be faster or slower. Preliminary analyses showed that there was very little variability in the rates of change. Therefore, only random intercepts were fit, and the observed measurements were assumed to differ from a child’s true path only by independent, identically distributed errors at each time of observation. The initial set of models included fixed effects for diagnosis, age at baseline, and recording period (time in months) since baseline. A second set of models added the interaction of diagnosis with time and age. The interaction terms tested whether the rate of change in sleep variables varied by diagnosis and baseline age, respectively. We also examined a series of nested models in which additional parameters for ethnicity, mother’s age, marital status, and education were included. Parameters that did not add significantly to the model were removed; therefore, the reported models include only significant predictors. Natural log transformation was employed for the sleep onset latency variable in an attempt to normalize the distribution. The random-effects model partitioned variability into two components: (a) the deviation of each child’s individual mean from the population’s mean (between-child variation) and (b) the deviation of a measurement taken on a particular night for a child from the child’s overall mean (within-child variation). Separate variances for the random effects (assessing the between-child variance structure) and for the residual error (assessing the within-child variance) were estimated for each group, and tests to assess whether the between- and within-child variances differed across diagnoses were performed. Residual analyses and graphical diagnostics were used to check the validity of the model assumptions. We concluded from these analyses that model assumptions were adequately met.
Because the frequency distribution of responses for both WASO duration and WASO number exhibited a large spike at zero, with 21% of nights without any awakenings, the traditional mixed-effect models described earlier could not be used for these two variables. Instead, a two-part mixed-effects model with correlated random effects accounting for clumping at zero was used (Tooze, Grunwald, & Jones, 2002). This two-part model predicted both the probability of an awakening and, given that a child awakened, the distribution of awakenings (the number and duration of awakenings). In addition, the two-part model accounted for the positive correlation between the probability of awakening and the distribution of those awakenings. The same covariates used in the random-effects models were used in the two-part model, and a similar model-building strategy was adopted. The continuous part of the models was fitted to the log-transformed WASO variables to normalize their distribution.
RESULTS
Sleep–Wake Patterns Over Time
Means, standard deviations, and lower and upper quartiles for each of the variables by diagnostic group were computed for all participants at each recording period (see Table 2). In general, all children went to bed, on average, around 9:00 p.m. and slept for 10.5 to 11 hr in a 24-hr day. Sleep efficiency was greater than 90% for all groups. Figure 1 graphically portrays the estimated average paths and the between- and within-child variability for each diagnostic group for bedtime and 24-hr sleep. Table 3 summarizes the random-effects models assessing the relation of diagnosis, recording period, and age on the sleep–wake variables from which the trajectories in Figure 1 are derived.
TABLE 2.
Summary of Actigraph Sleep–Wake Variables Over 6 Months
| Actigraph Variable | Time 1a M ± SD (Q1–Q3) | Time 2b M ± SD (Q1–Q3) | Time 3c M ± SD (Q1–Q3) |
|---|---|---|---|
| Bedtime (hours:minutes) | |||
| AUT | 21:01 ± 1:08 (20:18–21:31) | 21:00 ± 1:14 (20:11–21:40) | 20:47 ± 1:37 (20:06–21:44) |
| DD | 21:08 ± 1:01 (20:36–21:41) | 21:07 ± 1:02 (20:37–21:42) | 20:59 ± 1:19 (20:24–21:33) |
| TYP | 20:46 ± 0:51 (20:10–21:05) | 20:55 ± 0:54 (20:21–21:17) | 20:50 ± 0:59 (20:21–21:15) |
| Sleep onset latency (minutes) | |||
| AUT | 39 ± 28 (23–46) | 33 ± 23 (16–44) | 36 ± 31 (17–47) |
| DD | 42 ± 31 (24–50) | 41 ± 28 (23–54) | 42 ± 26 (26–51) |
| TYP | 35 ± 19 (25–41) | 36 ± 19 (20–45) | 38 ± 21 (21–46) |
| WASO duration (minutes) | |||
| AUT | 19 ± 17 (8–25) | 17 ± 16 (6–26) | 16 ± 16 (5–23) |
| DD | 29 ± 22 (14–36) | 25 ± 21 (9–34) | 23 ± 24 (6–38) |
| TYP | 18 ± 12 (9–24) | 18 ± 14 (7–24) | 15 ± 12 (7–19) |
| WASO number | |||
| AUT | 2.5 ± 1.7 (1.4–3.0) | 2.1 ± 1.5 (1.0–2.7) | 2.1 ± 1.7 (0.9–3.0) |
| DD | 3.7 ± 2.4 (2.3–5.0) | 3.3 ± 2.5 (1.5–4.3) | 3.1 ± 2.5 (0.9–4.6) |
| TYP | 3.1 ± 1.8 (1.7–4.3) | 3.2 ± 2.5 (1.4–4.0) | 2.9 ± 2.2 (1.3–4.0) |
| Sleep efficiency (%) | |||
| AUT | 91 ± 5 (90–94) | 92 ± 4 (91–96) | 92 ± 5 (90–96) |
| DD | 90 ± 5 (86–93) | 91 ± 5 (87–95) | 91 ± 6 (88–95) |
| TYP | 92 ± 3 (90–95) | 92 ± 4 (89–95) | 92 ± 4 (90–95) |
| Total sleep in 24 hr (hours:minutes) | |||
| AUT | 10:36 ± 0:51 (10:06–11:09) | 10:30 ± 0:44 (9:50–11:05) | 10:39 ± 1:41 (9:54–10:56) |
| DD | 11:06 ± 0:55 (10:21–11:46) | 10:56 ± 0:51 (10:08–11:37) | 11:06 ± 1:16 (10:15–11:26) |
| TYP | 11:14 ± 0:44 (10:42–11:39) | 10:58 ± 0:46 (10:23–11:32) | 10:59 ± 0:45 (10:30–11:34) |
Note. Time = each recording time separated by 3 months; Q1 = lower quartile (cuts off lowest 25% of data); Q3 = upper quartile (cuts off highest 25% of data); AUT = autism; DD = developmental delay without AUT; TYP = typically developing; WASO = wake after sleep onset.
n = 194.
n = 179.
n = 173.
FIGURE 1.
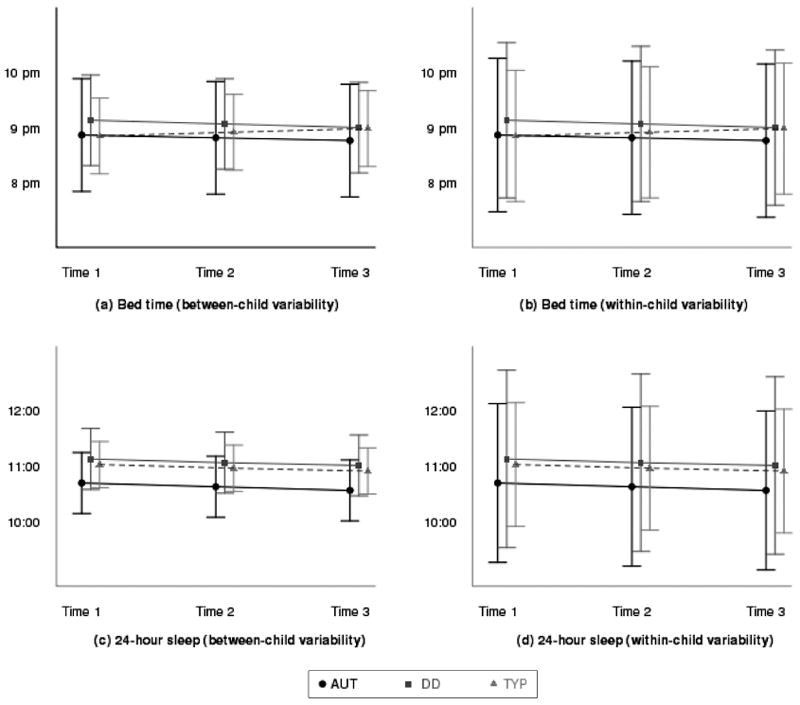
Estimated trajectories of sleep behaviors and between-child and within-child variability by diagnosis for bedtime and 24-hr sleep at enrollment (Time 1), 3 months later (Time 2), and 6 months after enrollment (Time 3). Note. Figure portrays the estimated average paths for bedtime and 24-hr sleep over 6 months. The horizontal lines depict the estimated mean trajectories over time for each group. The error bars in Figure 1a and 1c represent the variability around the mean of each diagnostic group (between-child standard deviations). The error bars in Figure 1b and 1d represent the night-to-night variability for each diagnostic group (within-child standard deviations). Children in the typically developing (TYP) group had a trend to gradually go to bed later over time (p < .10), and this change was significantly different from both of the neurodevelopmentally disordered groups (both ps < .05). All children had a slight decline in their average 24-hr sleep over time (p < .05). As expected, within-child variability, determined by the size of the error bars in Figure 1b and 1d, was greater than between-child variability, as depicted in Figure 1a and 1c, for all three diagnostic groups. AUT = autism; DD = developmental delay without AUT.
TABLE 3.
Summary of the Random-Effects Modelsa (Parameter Estimates and Standard Errors) Assessing the Relation of Diagnostic Group, Recording Period, and Chronologic Age With Baseline Level and Rates of Change of Actigraph Variables
| Sleep Variable
| ||||
|---|---|---|---|---|
| Group | Bedtimeb (hours:minutes) | Sleep Onset Latencyc | Sleep Efficiencyd (%) | 24-Hr Sleepd (hours:minutes) |
| Estimated trajectory for TYP children | ||||
| Baseline | 8:42 (0:06) | 3.22 (0.06) | 91.96 (0.38) | 11:02 (0:04) |
| 3 months | 8:46 (0:06) | 3.20 (0.06) | 92.29 (0.36) | 10:58 (0:04) |
| 6 months | 8:50 (0:06) | 3.19 (0.06) | 92.62 (0.38) | 10:55 (0:04) |
| Estimated difference between children with AUT and TYP children | ||||
| Baseline | 0:01 (0:10) | −0.15 (0.10) | −1.07 (0.62)* | −0:21 (0:06)*** |
| 3 months | −0:06 (0:09) | −0.15 (0.10) | −1.07 (0.62)* | −0:21 (0:06)*** |
| 6 months | −0:13 (0:10) | −0.15 (0.10) | −1.07 (0.62)* | −0:21 (0:06)*** |
| Estimated difference between children with DD without AUT and TYP children | ||||
| Baseline | 0:17 (0:10)* | 0.05 (0.10) | 1.98 (0.65)** | 0:06 (0:07) |
| 3 months | 0:09 (0:09) | 0.05 (0.10) | −1.98 (0.65)** | 0:06 (0:07) |
| 6 months | 0:00 (0:10) | 0.05 (0.10) | −1.98 (0.65)** | 0:06 (0:07) |
| Estimated difference between AUT and DD children | ||||
| Baseline | −0:16 (0:11) | −0.20 (0.12) | 0.91 (0.73) | −0:27 (0:07)*** |
| 3 months | −0:15 (0:10) | −0.20 (0.12) | 0.91 (0.73) | −0:27 (0:07)*** |
| 6 months | −0:13 (0:11) | −0.20 (0.12) | 0.91 (0.73) | −0:27 (0:07)*** |
Note. TYP = typically developing; AUT = autism; DD = developmental delay without AUT.
Models were adjusted for demographic characteristics (ethnicity, mother’s age, marital status, and education).
Race was a significant predictor; the trajectories were estimated for a Caucasian child; non-Caucasian children went to bed 19 min later.
Sleep onset latency time was log-transformed for analyses.
Chronological age at baseline was a significant predictor; the trajectories were estimated for a child who was 44 months old at baseline.
p < .10.
p < .01.
p < .001.
Bedtimes
At baseline (see Table 3), children in the DD group tended to go to bed 17 min later (on average) than children in the TYP group (p < .10). This trend did not hold up over subsequent recording because of a significant interaction between time and diagnostic group (Figure 1a and 1b, slope difference)—that is, bedtimes for children in the TYP group became later over time, whereas bedtimes for children in the AUT and DD groups remained stable over the 6-month period. In addition, Caucasian children, regardless of diagnostic group, had 19-min earlier bedtimes than non-Caucasian children (p < .01).
24-Hr Sleep
As reported initially for Time 1 (Goodlin-Jones et al., 2008), children in the AUT group slept less in 24 hr, on average, than children in the TYP group by 21 min and less than the children in DD group by 27 min (see Table 3). There were no differences across groups in the rate of change in 24-hr sleep over the recording period, with all children maintaining their differences and sleeping progressively less in 24 hr (p < .05; Figure 1c and 1d). In addition, older children slept less (p < .001).
Sleep Efficiency and Sleep Onset Latency
The nighttime sleep pattern differences by diagnostic group reported initially (Goodlin-Jones et al., 2008) also were maintained over the two subsequent recording periods (see Table 3). On all three occasions, children in the DD group slept less efficiently than children in the TYP group (p < .01); there was a trend for children in the AUT group to sleep less efficiently than children in the TYP group as well (p < .10). Sleep efficiency improved with age and time since baseline (both ps < .01), for all participants, regardless of their diagnostic group. Sleep onset latency times were comparable across groups at Time 1, and this pattern did not change over time.
Nighttime Awakenings
Table 4 summarizes the results of the two-part random-effects models with correlated random effects for the WASO variables. The first part of the model estimates the probability of WASO occurrence; this probability was significantly lower for the AUT group than for the TYP (p < .05) and DD groups (p < .01), and decreased over the course of the study for all groups (p < .01).
TABLE 4.
Summary of Two-Part Random-Effects Models (Parameter Estimates and Standard Errors) Assessing the Probability of Awakening at Night (WASO Occurrence) and the Intensity of Awakening (WASO Number and Duration)
| Model Terma | WASO Number | WASO Duration |
|---|---|---|
| WASO occurrence (logistic) | ||
| AUT–TYP difference | −0.50 (0.21)* | −0.49 (0.21)* |
| DD–TYP difference | 0.20 (0.23) | 0.17 (0.22) |
| AUT–DD difference | −0.70 (0.23)** | −0.64 (0.22)** |
| Time | −0.09 (0.02)*** | −0.09 (0.02)*** |
| Variance of random effects | 1.16 (0.20)** | 1.03 (0.18)** |
| WASO intensity (lognormal scale) | ||
| AUT–TYP difference | −0.21 (0.07)** | −0.16 (0.09) |
| DD–TYP difference | 0.06 (0.07) | 0.24 (0.10)* |
| AUT–DD difference | −0.27 (0.07)*** | −0.39 (0.10)*** |
| Time | −0.01 (0.00)** | −0.02 (0.01)* |
| Variance of random effects | 0.14 (0.02)*** | 0.23 (0.03)*** |
| Residual variance | 0.42 (0.01)*** | 0.92 (0.03)*** |
| Covariance | 0.36 (0.05)*** | 0.38 (0.06)*** |
Note. Analyses used a two-part logistic-log normal-normal mixed-effect model with correlated random effects. WASO = wake after sleep onset; AUT = autism; TYP = typically developing; DD = developmental delay without AUT.
Models were adjusted for demographic characteristics (ethnicity, mother’s age, marital status, and education).
p < :05.
p < :01.
p < :001.
The second part of the two-part model estimates the intensity of WASO variables (WASO number and duration) for the children who awakened at least once during the night. Both the number and the duration of the nighttime awakenings significantly decreased over time for all groups (p < .05). Among the children who were awake, AUT children awakened significantly less often than children in the TYP and DD groups (p < .01 and p < .001, respectively). Children in the DD group spent significantly more time awake than children in the other two groups on all three occasions. The estimated correlations between the occurrence and random effects were 0.91 for WASO number and 0.78 for WASO duration, indicating that, after accounting for covariate differences, children with a greater tendency to awaken were more likely to stay awake longer.
Between-Child and Within-Child Variability in Nighttime Variables
Variability in actigraph measures was predominantly due to within-child variation, with estimated within-child standard deviations at least two times greater than between-child standard deviations (see Table 5 and Figure 1). However, after adjusting for covariates, a consistent pattern of between-child variability was detected. In general, the two neurodevelopmentally disordered groups were more variable than the TYP group. For bedtime, children in the DD group resembled children in the TYP group in that both groups had less variability than children in the AUT group. For all the other sleep variables, variability from the estimated average path predicted for their diagnostic group was greater for children in the AUT and DD groups than children in the TYP group. In addition, children in the AUT and DD groups demonstrated greater within-child variability: Single measurements for a child in these groups deviated more from the child’s average pattern than those for a child in the TYP group, except for sleep onset latency times where no significant difference in variability across groups was detected.
TABLE 5.
Measures of Variability in Sleep Behaviors Across Diagnostic Groups
| Estimated Standard Deviations
| ||||
|---|---|---|---|---|
| Source of Variability | Bedtime | Sleep Onset Latency | Sleep Efficiency | 24-Hr Sleep |
| Between-childa | ||||
| AUT | 1:01 | 0.35c | 13.26 | 0:33 |
| DD | 0:49 | 0.35c | 13.77 | 0:33 |
| TYP | 0:41 | 0.17c | 7.32 | 0:25 |
| Within-childb | ||||
| AUT | 1:23 | 1.01c | 51.04 | 1:26 |
| DD | 1:24 | 0.95c | 44.89 | 1:36 |
| TYP | 1:11 | 0.91c | 25.07 | 1:07 |
Note. Sleep Onset Latency time was log-transformed to normalize distribution of scores; AUT = autism; DD = developmental delay without AUT; TYP = typically developing.
Standard deviations capture the degree to which each child’s individual mean deviates from their diagnostic group mean.
Standard deviations capture the degree to which the child’s actigraph measures, taken on a particular night, vary from the child’s overall mean.
Standard deviations not significantly different across diagnosis groups.
Figure 1 graphically portrays the within- and between-child variability for bedtime and 24-hr sleep. The within-child variability, determined by the size of the error bars in Figure 1b and 1d, is greater than the between-child variability, as depicted in Figure 1a and 1c, for all three diagnostic groups. In addition, the variability for individuals in the two neurodevelopmentally disordered groups is greater than that exhibited by individuals in the TYP group.
DISCUSSION
Previous studies have reported that children with AUT have significantly more disrupted sleep than TYP children, emphasizing the importance of assessing sleep in children with ASDs (Krakowiak et al., 2008; Polimeni et al., 2005). However, studies often relied only on parental reports and not on more objective observations of sleep. Moreover, some reports have not included comparison groups of DD children without AUT or TYP children, making it difficult to determine whether the problematic sleep behaviors were related to AUT, to the DDs that often accompany AUT, or to other factors such as chronologic age.
This study attempted to measure sleep–wake patterns using multiple methods over time. By recording sleep actigraphically and via parental report over 6 months, our data provide information about sleep–wake organization and its stability over a relatively short period of time. In this sample of preschool-age children recruited from a community pool of children to a study of typical sleep patterns rather than a study of sleep disorders, children in all three diagnostic groups had bedtimes and morning rise times that were fairly comparable. Moreover, children with autistic disorder did not differ significantly from TYP children in the organization of their nighttime sleep. However, their 24-hr sleep was significantly shorter than children in the other two groups at all three recording periods. It was the children in the DD group whose nighttime sleep was most disrupted at each recording period, characterized by significantly more frequent and longer nighttime awakenings. Both within- and between-child variability for all sleep–wake variables were greater, for the most part, in children with neurodevelopmental disorders than in TYP children; however, within-child variability was greater than between-child variability in all three groups.
Since both the shorter sleep periods of children in the AUT group and the more frequent and longer nighttime awakenings that characterized the sleep of children in the DD group were persistent over the 6-month period, it is possible that these patterns are characteristic of, and reflect, real group differences. On the other hand, the shorter sleep periods of children in the AUT group might also reflect their older chronologic ages when compared with the younger ages of the children in the TYP group. Age, however, does not explain the fragmented sleep of children in the DD group since they were older than children in the TYP group and the same age as children in the AUT group. The question of whether these results are intrinsic to the diagnostic groups, or whether other factors such as age are involved, cannot be answered by our design. Whatever the cause, we speculate that less 24-hr sleep, characteristic of children in the AUT group, might become problematic for parents. Future studies of sleep problems in children with AUT should match comparison groups on chronologic age and include measures of 24-hr sleep.
Our finding that children with autistic disorder did not demonstrate the predicted disruption of nighttime sleep or difficulty in falling asleep at bedtime are discrepant from previous studies that report longer sleep onset times and more sleep fragmentation in children with ASD (Cotton & Richdale, 2006; Honomichl, Goodlin-Jones, Burnham, Gaylor, & Anders, 2002; Krakowiak et al., 2008). It is possible that these studies may have included children at older ages or with the broader AUT phenotype. The similar bedtimes and sleep onset latency times of children in all three groups likely precludes group differences in circadian organization at these ages. However, our study is not the first study to report relatively comparable sleep in children with ASD when compared to controls (Elia et al., 2000; Miano et al., 2007). Parents of children with AUT tend to report elevated rates of sleep problems, but assessment of concurrent sleep behaviors do not consistently complement parental concerns (Goodlin-Jones et al., 2009; Hering, Epstein, Elroy, Iancu, & Zelnik, 1999; Miano et al., 2007).
Nevertheless, our results have demonstrated diagnostic group differences. The shorter 24-hr sleep durations in children with autistic disorder and the more fragmented nighttime sleep in the children with DD without AUT were robust, persisting for 6 months. The focus on 24-hr sleep and on the 6-month continuity of sleep–wake variables has not been reported previously. We also have replicated previous reports of maturational changes in sleep–wake state organization in our TYP group children (improved sleep efficiency and shorter and fewer nighttime awakenings) and documented similar trends in our neurodevelopmentally disordered children.
There are two clinical implications for the findings of this study. First, clinicians need to be more sensitive to 24-hr sleep patterns. Although the mean difference in 24-hr sleep between the AUT, DD, and TYP groups appeared relatively minor (approximately 30 min), previous studies have documented that such a difference is clinically and behaviorally significant, especially if it persists (Sadeh, Gruber, & Raviv, 2003; Waterhouse, Atkinson, Edwards, & Reilly, 2007). The persistently shorter 24-hr sleep periods noted in the AUT group might reflect irregularities of the melatonin-serotonin system not measured in this study, or in other arousal mechanisms (Wirojanan et al., 2009). It is also possible that the AUT group’s shorter 24-hr sleep reflects the expected reduction in sleep for an older age group—that is, their 24-hr sleep times were on track for their chronologic age in comparison to the TYP group who were younger in chronologic age. If this explains the differences in 24-hr sleep durations between children in the AUT and TYP groups, it is important to consider that children in the DD group who, like children in the AUT group, were chronologically older than children in the TYP group nevertheless demonstrated 24-hr sleep durations comparable to the children in the TYP group, suggesting that the maturation of their sleep–wake mechanisms might be delayed. More research on developmental trajectories of 24-hr sleep–wake organization is needed.
The second clinical implication from this study is that, although the diagnostic groups maintained similar sleep behaviors over the 6-month period, these patterns do not necessarily imply that children in the groups had a sleep disorder. Moreover, close inspection of the between- and within-child variability revealed far more variable sleep in children in the AUT and DD groups. Therefore, it is evident that merely assessing average sleep behaviors does not capture the complexities of sleep–wake state organization that may be most stressful for families. The presence of more night-to-night variability has been observed in other children with clinical diagnoses, such as attention deficit hyperactivity disorder (ADHD) (Gruber et al., 2000; Paavonen et al., 2009). Unstable sleep behavior is hypothesized to impact daytime functioning in a manner similar to insomnia or chronic short sleep. Further research is required to elucidate the pathway from sleep patterns to sleep disorders, including measures of day-today variability, daytime sleepiness, and impaired daytime behavior and performance.
There are limitations to this study. Although children with specific sleep disorders were excluded, it is possible that families with children who had more sleep irregularities or parents who were concerned about their child’s sleep were more likely to enroll. Thus, the findings may not reflect a random community sample. Similarly, our results are not applicable for families referred for clinical sleep problems. In addition, our reported nighttime awakening durations and numbers should be interpreted with caution. Previous actigraphy validity studies have reported adequate validity for estimates of sleep but have highlighted poorer actigraph validity estimates of waking frequencies and durations (Ancoli-Israel et al., 2003; Sitnick et al., 2008; Tryon, 2004). This limitation is especially salient in this study, as estimates in night waking may have a large margin of error. Finally, because the sleep of children with AUT was characterized by less sleep per 24 hr, future studies of sleep in children with AUT should attend more closely to daytime sleep behaviors.
Acknowledgments
This work was supported, in part, by Grant No. RO-1-MH068232, awarded to Thomas F. Anders from the National Institute of Mental Health. We are grateful to Stephanie Sitnick, Sara Waters, and Anny Wu for their assistance, and to the parents and children who participated.
Footnotes
Publisher's Disclaimer: Full terms and conditions of use: http://www.informaworld.com/terms-and-conditions-of-access.pdf
This article may be used for research, teaching and private study purposes. Any substantial or systematic reproduction, re-distribution, re-selling, loan or sub-licensing, systematic supply or distribution in any form to anyone is expressly forbidden.
The publisher does not give any warranty express or implied or make any representation that the contents will be complete or accurate or up to date. The accuracy of any instructions, formulae and drug doses should be independently verified with primary sources. The publisher shall not be liable for any loss, actions, claims, proceedings, demand or costs or damages whatsoever or howsoever caused arising directly or indirectly in connection with or arising out of the use of this material.
Contributor Information
Thomas F. Anders, Department of Psychiatry and Behavioral Sciences UC Davis M.I.N.D. Institute, Sacramento, CA
Ana-Maria Iosif, Division of Biostatistics, Department of Public Health Sciences UC Davis, Sacramento, CA.
A. J. Schwichtenberg, Department of Psychiatry and Behavioral Sciences UC Davis M.I.N.D. Institute, Sacramento, CA
Karen Tang, Department of Psychiatry and Behavioral Sciences UC Davis M.I.N.D. Institute, Sacramento, CA.
Beth L. Goodlin-Jones, Department of Psychiatry and Behavioral Sciences UC Davis M.I.N.D. Institute, Sacramento, CA
References
- Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26:342–392. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- Bates JE, Viken RJ, Alexander DB, Beyers J, Stockton L. Sleep and adjustment in preschool children: Sleep diary reports by mothers relate to behavior reports by teachers. Child Development. 2002;73:62–74. doi: 10.1111/1467-8624.00392. [DOI] [PubMed] [Google Scholar]
- Cotton S, Richdale A. Brief report: Parental descriptions of sleep problems in children with autism, Down syndrome, and Prader–Willi syndrome. Research in Developmental Disabilities. 2006;27:151–161. doi: 10.1016/j.ridd.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Dahl RE. The impact of inadequate sleep on children’s daytime cognitive function. Seminars in Pediatric Neurology. 1996;3(1):44–50. doi: 10.1016/s1071-9091(96)80028-3. [DOI] [PubMed] [Google Scholar]
- Davis NO, Carter A. Parenting stress in mothers and fathers of toddlers with austism spectrum disorders: Associations with child characteristics. Journal of Autism and Developmental Disorders. 2008;38:1278–1291. doi: 10.1007/s10803-007-0512-z. [DOI] [PubMed] [Google Scholar]
- Doo S, Wing YK. Sleep problems of children with pervasive developmental disorders: Correlation with parental stress. Developmental Medicine and Child Neurology. 2006;48:650–655. doi: 10.1017/S001216220600137X. [DOI] [PubMed] [Google Scholar]
- Elia M, Ferri R, Musumeci SA, Del Gracco S, Bottitta M, Scuderi C, et al. Sleep in subjects with autistic disorder: A neurophysiological and psychological study. Brain Development. 2000;22:88–92. doi: 10.1016/s0387-7604(99)00119-9. [DOI] [PubMed] [Google Scholar]
- Goodlin-Jones B, Schwichtenberg AJ, Iosif A, Tang K, Lui J, Anders T. Six-month persistence of sleep problems in young children with autism, developmental delay and typical development. Journal of the American Academy of Child and Adolescent Psychiatry. 2009;48:847–854. doi: 10.1097/CHI.0b013e3181a8135a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodlin-Jones B, Tang K, Liu J, Anders TF. Sleep patterns in preschool-age children with autism, developmental delay, and typical development. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47:930–938. doi: 10.1097/CHI.ObO13e3181799f7c. [DOI] [PubMed] [Google Scholar]
- Goodnight JA, Bates JE, Staples AD, Pettit GS, Dodge KA. Temperamental resistance to control increases the association between sleep problems and externalizing behavior development. Journal of Family Psychology. 2007;21:39–48. doi: 10.1037/0893-3200.21.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber R, Sadeh A, Raviv A. Instability of sleep patterns in children with attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39:495–501. doi: 10.1097/00004583-200004000-00019. [DOI] [PubMed] [Google Scholar]
- Hering E, Epstein R, Elroy S, Iancu DR, Zelnik N. Sleep patterns in autistic children. Journal of Autism and Developmental Disorders. 1999;29:143–147. doi: 10.1023/a:1023092627223. [DOI] [PubMed] [Google Scholar]
- Honomichl RD, Goodlin-Jones BL, Burnham M, Gaylor E, Anders TF. Sleep patterns of children with pervasive developmental disorders. Journal of Autism and Developmental Disorders. 2002;32:553–561. doi: 10.1023/a:1021254914276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglowstein I, Jenni OG, Molinari L, Largo RH. Sleep duration from infancy to adolescence: Reference values and generational trends. Pediatrics. 2003;111:302–307. doi: 10.1542/peds.111.2.302. [DOI] [PubMed] [Google Scholar]
- Iglowstein I, Latal Hajnal B, Molinari L, Largo RH, Jenni OG. Sleep behaviour in preterm children from birth to age 10 years: A longitudinal study. Acta Paediatrica. 2006;95:1691–1693. doi: 10.1080/08035250600686938. [DOI] [PubMed] [Google Scholar]
- Krakowiak P, Goodlin-Jones B, Hertz-Picciotto I, Croen LA, Hansen RL. Sleep problems in children with autism spectrum disorders, developmental delays, and typical development: A population-based study. Journal of Sleep Research. 2008;17:197–206. doi: 10.1111/j.1365-2869.2008.00650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- Meltzer LJ, Mindell JA. Relationship between child sleep disturbances and maternal sleep, mood, and parenting stress: A pilot study. Journal of Family Psychology. 2007;21:67–73. doi: 10.1037/0893-3200.21.1.67. [DOI] [PubMed] [Google Scholar]
- Miano S, Bruni O, Elia M, Trovato A, Smerieri A, Verrillo E, et al. Sleep in children with autistic spectrum disorder: A questionnaire and polysomnographic study. Sleep Medicine. 2007;9:64–70. doi: 10.1016/j.sleep.2007.01.014. [DOI] [PubMed] [Google Scholar]
- Paavonen EJ, Raikkonen K, Lahti J, Komsi N, Heinonen K, Pesonen AK, et al. Short sleep duration and behavioral symptoms of attention-deficit/hyperactivity disorder in healthy 7- to 8-year-old children. Pediatrics. 2009;123:e857–e864. doi: 10.1542/peds.2008-2164. [DOI] [PubMed] [Google Scholar]
- Patzold LM, Richdale AL, Tonge BJ. An investigation into sleep characteristics of children with autism and Asperger’s disorder. Journal of Pediatric Child Health. 1998;34:528–533. doi: 10.1046/j.1440-1754.1998.00291.x. [DOI] [PubMed] [Google Scholar]
- Polimeni MA, Richdale AL, Francis AJ. A survey of sleep problems in autism, Asperger’s disorder and typically developing children. Journal of Intellectual Disability Research. 2005;49:260–268. doi: 10.1111/j.1365-2788.2005.00642.x. [DOI] [PubMed] [Google Scholar]
- Quine L. Sleep problems in primary school children: Comparison between mainstream and special school children. Childcare, Health and Development. 2001;27:201–221. doi: 10.1046/j.1365-2214.2001.00213.x. [DOI] [PubMed] [Google Scholar]
- Richdale AL. Sleep problems in autism: Prevalence, cause, and intervention. Developmental Medicine and Child Neurology. 1999;41:60–66. doi: 10.1017/s0012162299000122. [DOI] [PubMed] [Google Scholar]
- Richdale AL, Prior MR. The sleep/wake rhythm in children with autism. European Child and Adolescent Psychiatry. 1995;4:175–186. doi: 10.1007/BF01980456. [DOI] [PubMed] [Google Scholar]
- Sadeh A, Gruber R, Raviv A. Sleep, neurobehavioral functioning, and behavior problems in school-age children. Child Development. 2002;73:405–417. doi: 10.1111/1467-8624.00414. [DOI] [PubMed] [Google Scholar]
- Sadeh A, Gruber R, Raviv A. The effects of sleep restriction and extension on school-age children: What a difference an hour makes. Child Development. 2003;74:444–455. doi: 10.1111/1467-8624.7402008. [DOI] [PubMed] [Google Scholar]
- Sitnick S, Goodlin-Jones B, Anders T. The use of actigraphy to study sleep disorders in preschoolers: Some concerns about detection of nighttime awakenings. Sleep. 2008;31:395–401. doi: 10.1093/sleep/31.3.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooze J, Grunwald G, Jones R. Analysis of repeated measures data with clumping at zero. Statistical Methods in Medical Research. 2002;11:341–355. doi: 10.1191/0962280202sm291ra. [DOI] [PubMed] [Google Scholar]
- Tryon WW. Issues of validity in actigraphic sleep assessment. Sleep. 2004;27:158–165. doi: 10.1093/sleep/27.1.158. [DOI] [PubMed] [Google Scholar]
- Waterhouse J, Atkinson G, Edwards B, Reilly T. The role of a short post-lunch nap in improving cognitive, motor, and sprint performance in participants with partial sleep deprivation. Journal of Sports Science. 2007;25:1557–1566. doi: 10.1080/02640410701244983. [DOI] [PubMed] [Google Scholar]
- Wiggs L. Sleep problems in children with developmental disorders. Journal of the Royal Society of Medicine. 2001;94:177–179. doi: 10.1177/014107680109400406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirojanan J, Jaquemont S, Diaz R, Anders T, Hagerman R, Goodlin-Jones B. The efficacy of melatonin for sleep problems in children with autism and/or fragile-X syndrome. Journal of Clinical Sleep Medicine. 2009;5:145–150. [PMC free article] [PubMed] [Google Scholar]


