Abstract
Androgen receptor (AR) is the major therapeutic target for the treatment of prostate cancer (PCa). Anti-androgens to reduce or prevent androgens binding to AR are widely used to suppress AR-mediated PCa growth; however, the androgen depletion therapy is only effective for a period of time. Here we found a natural product/Chinese herbal medicine cryptotanshinone (CTS), with a structure similar to dihydrotestosterone (DHT), can effectively inhibit the DHT-induced AR transactivation and prostate cancer cell growth. Our results indicated that 0.5 µM CTS effectively suppresses the growth of AR-positive PCa cells, but has little effect on AR negative PC-3 cells and non-malignant prostate epithelial cells. Furthermore, our data indicated that CTS could modulate AR transactivation and suppress the DHT-mediated AR target genes (PSA, TMPRSS2, and TMEPA1) expression in both androgen responsive PCa LNCaP cells and castration resistant CWR22rv1 cells. Importantly, CTS selective inhibits AR without repressing the activities of other nuclear receptors, including ERα, GR, and PR. The mechanistic studies indicate that CTS functions as an AR inhibitor to suppress androgen/AR-mediated cell growth and PSA expression by blocking AR dimerization and the AR–coregulator complex formation. Furthermore, we showed that CTS effectively inhibits CWR22Rv1 cell growth in the xenograft animal model. The previously un-described mechanisms of CTS may explain how CTS inhibits the growth of PCa cells and help us to establish new therapeutic concepts for the treatment of PCa.
Keywords: androgen receptor, anti-androgen, cryptotanshinone, danshen, prostate cancer
1. INTRODUCTION
Prostate cancer (PCa) is the most commonly diagnosed cancer among American men and remains the second leading cause of cancer death in 2010; approximately 217,730 men were diagnosed with PCa, and 32,050 men were expected to die from this disease in the United States [1]. Androgen and androgen receptor (AR) functions play a pivotal role in the carcinogenesis and progression of PCa, as well as in normal prostate development [2–5]. The AR is a member of the nuclear receptor (NR) superfamily as a ligand-dependent transcription factor [6]. Since cloning of the AR cDNA in 1988 [7], it has been extensively studied to elucidate how androgens activate the AR signaling pathway so as to be responsible for the progression of PCa. Huggins and Hodges in 1941 found androgen deprivation therapy (ADT) to be an effective therapy for PCa [8]. Currently, anti-androgens, in combination with surgical or medical castration, are widely used for the treatment of PCa. Both steroidal and non-steroidal anti-androgens are presently available and have shown clinical benefits as chemotherapeutic agents for PCa [9]. However, most patients relapse after an initial response to ADT, eventually developing castration resistant prostate cancer (CRPC) [10]. The possible mechanisms of CRPC development could be due to the altered sensitivity of AR to antiandrogens, mutations of AR, gene amplification of AR, changes of AR coregulators, and growth factor/kinase-activated AR activity. Therefore, it is desirable to develop anti-androgens or anti-AR potential drugs for use in PCa therapy [11].
Dihydrotestosterone (DHT) has a typical steroid structure and can bind to AR to control the development of the secondary sex characteristics and sex organs in males. Finasteride is a synthetic anti-androgen initially approved by the U.S. Food and Drug Administration (FDA) in 1992 as a treatment for benign prostatic hyperplasia (BPH). It was found that the parent structure of finasteride is a 3-Oxo-4-aza-5α-androst structure modified from the original steroid structure. The structure similarity of those compounds urged us to find an anti-PCa drug or natural compound with a similar steroid structure to that of DHT or finasteride.
Salvia miltiorrhiza Bunge (Danshen) is an herb commonly used in traditional oriental medicine for the treatment of cardiovascular diseases, hepatitis, menstrual disorders, diabetes, and chronic renal failure [12–13]. More than 50 tanshinones have been isolated from Danshen. Cryptotanshinone (CTS) is one of the principal active constituents in Danshen extract, its chemical name is (R)-1,2,6,7,8,9-Hexahydro-1,6,6-trimethyl-phenanthro (1,2-b) furan-10,11-dione, and has a structure very close to DHT. In an early report [14], CTS at 7 uM was shown to inhibit the growth of DU145 PCa cells by inhibiting the STAT3 signal pathway. Due to the lack of STAT3 activation in LNCaP and PC-3 cells, the earlier report also concluded that CTS does not effectively inhibit the growth of those two cells. However, this earlier report never focused on testing the CTS effects on androgen-stimulated AR biological events and androgen/AR regulated cancer cell growth. To date, CTS was also reported to show a variety of biological activities, such as anti-angiogenic [15], antioxidant [16], anti-inflammatory [17], and anti-human hepatocellular carcinoma effects [18]. In addition, CTS was reported to decrease 17α-hydroxy progesterone and reduce androgen synthesis [19]. However, there is no related report about the effect of CTS on AR inhibition.
In this study, we are the first group to discover the ability of CTS to regulate AR transactivation. We also analyzed CTS's inhibitory effects on mRNA expressions of AR target genes that are DHT-mediated in different AR positive cells. To test the functional activity of AR, we further examined the protein levels of prostate-specific antigen (PSA), as the PSA is an AR target gene. We used AR positive PCa cell lines (LNCaP and CWR22Rv1), AR negative PCa cell line (PC-3), and non-malignant prostate epithelial cell line (RWPE-1) as model systems to investigate the differential cell growth inhibition effects of CTS. Our data showed that CTS could inhibit the growth of LNCaP and CWR22Rv1 cells, but had little effect on AR negative PC-3 cells and non-malignant prostate epithelial RWPE-1 cells. Together, our data showed CTS could effectively inhibit AR activity via inhibiting the AR dimerization and AR-coregulator complex formation. CTS is a potential anti-AR compound for the therapeutic treatment for PCa.
2. MATERIALS AND METHODS
2.1. Reagents
Commercial compounds and reagents include 5α-dihydrotestosterone, dexamethasone (DEX), RU486, progesterone, hydroxflutamide (HF), 17β-estradiol (E2) and ICI 182,780 (ICI) [Sigma, St Louis, MO], ethanol (EtOH), ethyl acetate (EtOAc), petroleum ether, methanol, and chloroform (CHCl3) [Sinopharm Chemical Reagent Co, Ltd. (SCRC), Shanghai]. All other chemicals and solvents used in this study were of reagent grade or high performance liquid chromatography (HPLC) grade.
2.2. Plant Extracts Preparation
Fresh whole plants of Danshen were purchased from a Chinese medicinal herb market in Jiangsu. Whole air-dried roots of Danshen (100 g) were extracted with 95% EtOH at effluent temperature for 2 hrs twice. The solvent was evaporated to obtain crude extract (9.31g), which was applied to the silica gel column chromatography, eluted by n-Hexane-EtOAc mixture and petroleum ether-EtOAc ether as mobile phases to obtain different fractions, based on the TLC pattern. Cryptotanshinone (16 mg) was separated and purified under silica gel column chromatography with a solvent system of petroleum-EtOAc mixture, and identified by comparison of NMR and MS spectral data with reference values [20, 21].
2.3. Cell Culture
Cells were cultured at 5% CO2 and 37°C. LNCaP is an androgen-responsive and androgen dependent-human PCa cell line with a mutant AR (T877A); CWR22Rv1 is an androgen-responsive but androgen-independent human PCa cell line, which expresses endogenous AR; DU145 and PC-3 are androgen-independent human PCa cell lines that lack expression of AR. LNCaP, CWR22Rv1, DU145, and PC-3 cell lines were maintained in RPMI-1640 medium containing 10% fetal bovine serum (FBS) and antibiotics (100 units/mL penicillin, 100 µg/mL streptomycin).
RWPE-1, the non-malignant human prostate cell line was maintained in keratinocyte serum-free medium (Invitrogen, catalog no. 10724) and supplements (Invitrogen, catalog no. 37000-015).
HEK 293 cell line was generated by transformation of human embryonic kidney cell cultures, and maintained in Dulbecco’s modified Eagle’s medium (Life Technologies) supplemented with 10% FBS and antibiotics.
2.4. Plasmids
The plasmids used were pSG5AR, full-length cDNA of wild-type human AR; MMTV-Luc (MMTV) a luciferase reporter plasmid; and plasmids pSG5 progesterone receptor (pSG5PR), pSG5 glucocorticoid receptor (pSG5 GR), PIRES-flag-ARt877a, pSG5, pSG5AR(N-DBD), pcDNA3.1-ERα, pGL3 (ERE)3-Luc, pRL-TK pCMX-VP16-ARA70, Gal4-AR-LBD, and Gal4-RE-Luc were constructed as previously described [22–25].
2.5. Luciferase Assays
Luciferase activity, transfections, and reporter gene assays, were performed by using Lipofectamin 2000 (Invitrogen) according to the manufacturer’s protocol. HEK 293 cells, lacking functional AR or ERα, were transfected with wild-type AR or ERα expression plasmid and reporter gene. Briefly, 2×104 HEK 293 cells were plated on 24-well dishes with 10% charcoal stripped (CS)-FBS DMEM medium for 24 hrs, medium was refreshed, and each well of cells were transfected with 0.6 µg of pSG5-AR, pSG5-PR, pSG5-GR (for AR, PR, or GR transfections, respectively), 0.3 µg MMTV-Luc, and 1 ng pRL-TK-Luc, or with 0.3 µg cDNA3.1-ERα, pGL3 (ERE)3-Luc, and 1 ng pRL-TK-Luc for ERα transfections for 24 hrs. After transfection, the medium was refreshed to 10% CS-FBS medium and cells treated with various concentrations of CTS in the presence or absence of 1 nM DHT and/or 5 µM HF for 24 hrs for AR transfections, and treated with serial concentrations of CTS or 10 µM anti-estrogen (ICI 182,780) in the absence or presence of 10 nM E2 for 24 hrs for ER transfections. For the PR and GR reporter activity assay, 10 nM progesterone, or DEX were added, respectively. To inhibit the progesterone-PR or DEX-GR activities, 10 µM RU486 was added.
Briefly, 5×104 LNCaP cells or CWR22Rv1 cells were plated on 24-well dishes with 10% CS-FBS RPMI-1640 medium for 24 hrs, medium was refreshed and cells transfected with 0.3 µg MMTV-ARE-Luc and 1 ng pRL-TK-Luc for 24 hrs. After transfection, the medium was changed to 10% CS-FBS medium for treatment with various concentrations of CTS in the presence or absence of 1 nM DHT and/or 5 µM HF for 24 hrs. These cells were then harvested and assayed for luciferase activity using the Dual Luciferase Assay System. Data were expressed as relative luciferase activity normalized to the internal Renilla luciferase control.
For the mammalian 2-hybrid assay to determine the AR N-C interaction and AR-AR coregulator interaction, HEK 293 cells were plated on 24-well dishes with 10% CS-FBS DMEM medium for 24 hrs. Cells were transfected with pGal4-RE-Luc reporter plasmid, pGal4-ARDBD-LBD (AR DNA binding domain and ligand binding domain), pCMX-VP16-AR or pCMX-VP16-ARA70 plasmids as indicated in the figure. After 24 hrs transfection, the medium was refreshed to 10% CS-FBS medium and cells were treated with 1 nM DHT and/or CTS for an additional 24 hrs. tk-RL luciferase was co-transfected as the internal control. Cells were then harvested for the dual luciferase assay (Promega, WI).
2.6. RT-PCR and Real-time PCR
Total RNA was extracted from PCa cells using Trizol (Invitrogen). Reverse transcription was performed using the Superscript first-stand synthesis kit (Invitrogen). Quantitative real-time PCR analyses using the comparative CT method were performed on an ABI PRISM 7700 Sequence Detector System using the SYBR Green PCR Master Mix kit (Perkin Elmer, Applied Biosystems, Wellesley, MA, USA) according to the manufacturer’s instructions. After an initial incubation at 50°C for 2 min and 10 min at 95°C, amplification was performed for 40 cycles at 95°C for 20 s, 65°C for 20 s, and 72°C for 30 s. Specific primer pairs were determined with the Primer-Express program (Applied Biosystems). The PSA primer pairs were 5'-AGG CCT TCC CTG TAC ACC AA-3' and 5'-GTC TTG GCC TGG TCA TTT CC-3'. The TMPRSS2 primer pairs were 5'-GTA CAC TGT TTC CAT GTT ATG-3' and 5'-AAT AAG AAG GAG TCA TTT GAG-3'. The TMEPA1 primer pairs were 5'-CCT TCT CTT CCC CTT TCC ATC TCC-3' and 5'-GTC CCG CCA ACC CCA AAT CTA TCT-3'. The normalization control used was β-actin, and the primers used were 5'-TCA CCC ACA CTG TGC CCC ATC TAC GA-3' and 5'-CAG CGG AAC CGC TCA TTG CCA ATG G-3'.
2.7. Western Blot Analysis
2.0×106 cells (per 100-mm dish) were washed with 1x PBS and scraped into a lysis buffer. Protein concentrations were measured with the BCA protein reagent (Pierce Chemical, Rockford, IL). Approximately 50 µg of protein/lane were loaded and run on a 10% polyacrylamide gel with a Tris/glycine running buffer system and then transferred onto a polyvinylidene difluoride membrane. The blots were probed with primary anti-AR (N-20) and anti-PSA (C-19) antibodies with dilutions of 1:500 to 1:1,000 and incubated at room temperature for 2 hrs. The secondary antibody [rabbit anti-goat IgG, 1:5,000 dilution (Santa Cruz Biotechnology) or rabbit anti-mouse IgG, 1:5,000 dilution (Pierce Chemical, Rockford, IL)] was used at room temperature for 1 hr. Immunoblot analyses were performed with horseradish peroxidase-conjugated anti-rabbit or anti-mouse IgG antibodies using enhanced chemiluminescence Western blotting detection reagents (Amersham Biosciences). The quantification of Western blotting results was done by Image Lab statistic software (Bio-red).
2.8. Cell Growth Assay in Vitro
To determine cell growth, 2.0×104 LNCaP cells, 1.5×104 CWR22RV1 cells, 6.0×103 PC-3 cells, or 2.0×104 RWPE-1 cells were plated in triplicate in 24-well culture plates. Cell medium was replenished and cell growth was determined by MTT assay (Sigma) and direct cell count. Serum-free medium containing MTT (0.5 µg/ml) was added into each well. After 2 hrs incubation at 37°C all crystals had solubilized and the optical density of the solution was determined spectrophotometrically at 570 nm [26].
2.9. Cytotoxicity Assay (the IC50 value determination) in Vitro
Cytotoxicity assay was performed according to the protocol in our laboratory [26]. To determine the IC50 value, 1.0×106 LNCaP cells, 5.0×105 CWR22RV1 cells, 6.0×104 DU145 and 6.0×104 PC-3 cells were plated in triplicate in CS-FBS RPMI medium in 24-well culture plates. The cells were incubated with serial concentrations of CTS for 2 days and cell viability was determined in triplicate by MTT assay. CTS + medium only (no cells) were included as controls. CTS treated cells were compared to untreated cell control wells. IC50 value was analyzed with the program CompuSyn (Developer).
2.10. Competitive ligand binding assay
To perform the competitive ligand binding assay, 3×105 LNCaP cells were plated into 6-well plates at Day 0. The medium was then replaced by 10% CS-FBS medium on Day 1. On Day 2, the tritium labeled R1881 (Amersham) was added into the culture medium at a final concentration of 1 nM except the background control cells. Unlabeled DHT with the concentrations of 0.1, 1, 10, 100, 1000 nM were used to compete for the labeled 3H-R1881 binding as specific ligand binding competition control. CTS at the concentration ranges from 0.1, 0.25, 1, 2.5, 10, 25 µM were used to determine its AR binding capability. After incubation at 5% CO2 and 37°C for 1 hr, the cells were washed twice with PBS, trypsinized, and then add 10 ml of scintillation fluid added to the vial and measured by scintillation counter.
2.11. Xenograft animal model
Male nude mice (6 weeks of age) were purchased from Shanghai Experimental Animal Center (Chinese Academy of Science). The CWR22Rv1 cells re-suspended in serum free medium at 1×107 cells/ml concentration were diluted with matrigel in 1:1 ratio into the final concentration of 5×106/ml. 1x106 (in 200 µl) cells per site of injection were injected subcutaneously into the right flank of each nude mice at 7 weeks of age. When the tumors were palpable (>50mm3) after one week of implantation, the mice were randomly assigned into 3 experimental groups (n=6), treated with either vehicle (100 µl corn oil), low dose CTS (5 mg/Kg), or high dose CTS (25 mg/Kg) by intra-peritoneal (i.p.) injection every two days for 4 weeks. The mouse body weights were monitored weekly during the 4 weeks of treatment. The mice were sacrificed at 12 weeks old 24 hrs after the last treatment and the harvested tumors were weighed to determine the CTS in vivo anti-cancer effect.
2.12. Statistics
Data are presented as the means ± SDs for the indicated number of separate experiments. The statistical significance of differences between two groups of data was analyzed by paired t-test and P-values <0.05 were considered significant. In the in vivo animal experiment, measurements of tumor volume and body weights among the three groups were analyzed through one-way ANOVA coupled with the Newman-Keuls test.
3. RESULTS
3.1. CTS Specifically Inhibits the DHT-mediated AR Transactivation, but not the ER, PR, and GR-mediated Transactivations
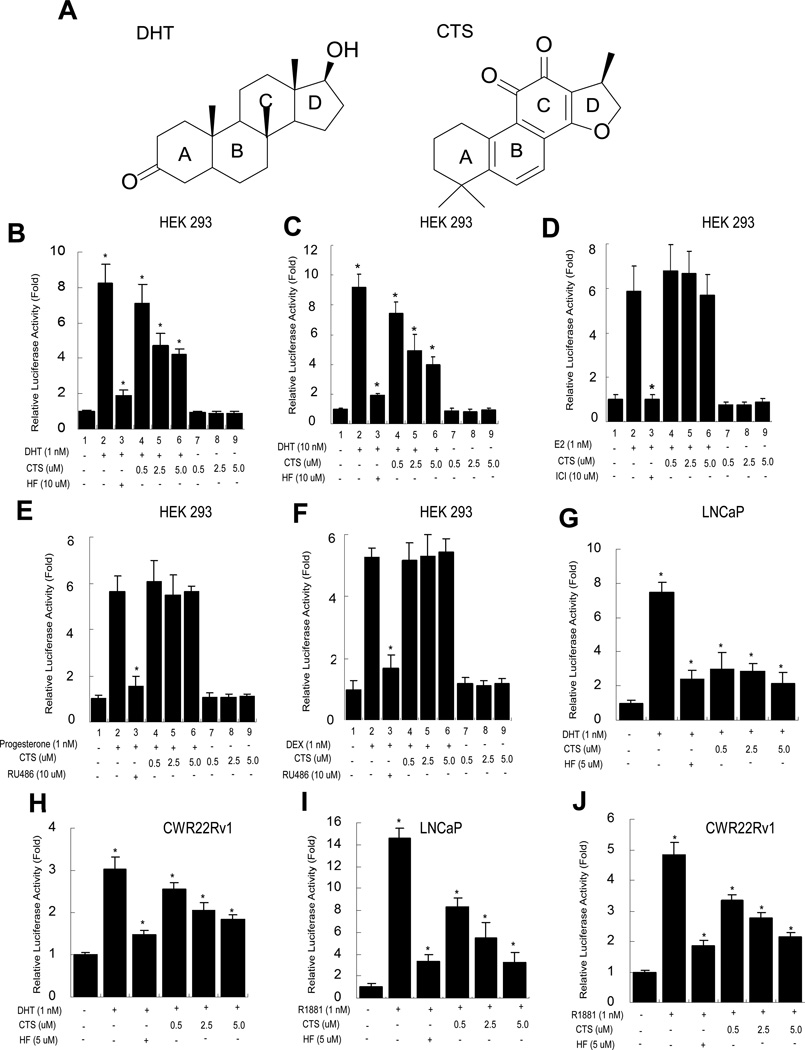
NRs have the ability to directly bind DNA, regulate the expression of specific genes, and play key roles in cancer development. Among the NR superfamily, androgen/AR signaling was found to be crucial for PCa development. DHT is a most effective androgen to activate the AR functions. In chemical structure (Fig. 1A), the parent compound of DHT and a number of steroids contain three cyclohexane rings (designated as rings A, B, and C in the left panel) and one cyclopentane ring (the D ring) and are composed of seventeen carbon atoms, which is similar to the structure of CTS. To test whether CTS could modulate AR function, we first investigated the ability of CTS to regulate AR transactivation activity in the HEK 293 cells. The relative luciferase activity was determined within cells transiently transfected with AR and the reporter construct (mouse mammary tumor virus (MMTV)-Luc) containing AR response element (ARE). Our results showed AR transactivation activity was induced by DHT, but blocked by HF. Interestingly, CTS showed the suppression of AR transactivation ability induced by either 1 nM or 10 nM of DHT (Fig. 1B, 1C). Next, we evaluated the effect of CTS on estrogen-induced ER transcriptional activity in HEK 293. HEK 293 cells were transfected with ER and an ER response element (ERE)-luciferase reporter. ER transactivation activity was induced by 1 nM E2, but blocked by 1µM antiestrogen ICI 182,780. CTS did not have a significant inhibitory effect on E2-mediated ER transactivation (Fig. 1D). As comparison, we also tested whether CTS can inhibit the PR and GR mediated transactivation. As shown in Fig. 1E and 1F, the antagonist RU486 can, but CTS cannot inhibit the P-PR and Dex-GR activities (lanes 3 vs 4–6, and 3 vs 4–6).
Fig. 1.
CTS selectively inhibits DHT-mediated AR transactivation, but not the ER, PR, and GR activity. A: Structure similarity of DHT and CTS. Left: DHT structure Right: CTS structure, similar to DHT, contains three cyclohexane rings (designated as rings A, B, and C in the left panel) and one furan ring (the D ring) composed of seventeen carbon atoms. B & C: Inhibition of CTS on the androgen-induced AR transcriptional activity in HEK 293 cells. D-F: No effects of CTS on the transcriptional activities of estrogen-induced ERα, Dex-induced GR, and progesterone-induced PR in HEK 293 cells. G: Inhibition of CTS on the DHT-induced AR transcriptional activity of a gain-function mutant AR (T877A) in prostate cancer LNCaP cells. H: Inhibition of CTS on the DHT-induced AR transcriptional activity in CWR22Rv1 cells. MMTV-Luc or ERE-Luc activities were determined. Data represent mean ± SD from three independent experiments. I & J: Inhibition of CTS on the R1881 induced AR transcriptional activity in LNCaP and CWR22Rv1 cells.
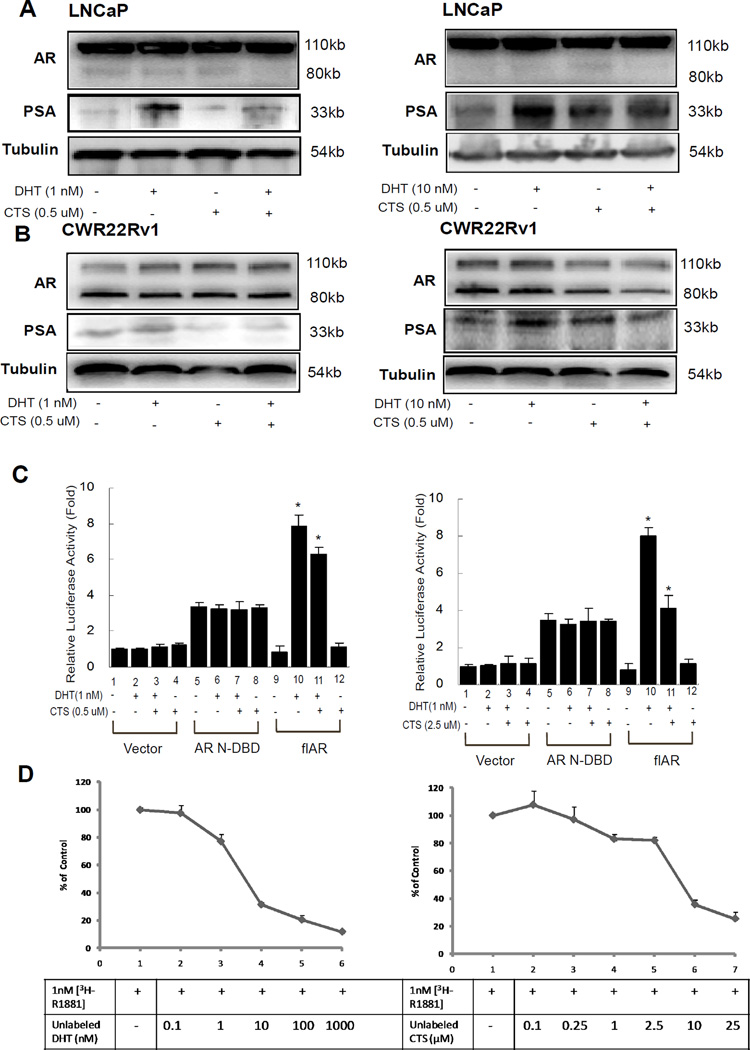
To further confirm the ability of CTS in regulating AR transactivation, we used the AR positive PCa LNCaP and CWR22Rv1 cells [27]. Both of these cells were transfected with MMTV-ARE-Luc. As shown in Fig. 1, AR transactivation activities were induced by DHT, and could be blocked by antiandrogen HF. Meanwhile, AR activity induced by DHT was effectively suppressed by treatment of 0.5, 2.5 or 5 µM CTS. (Fig. 1G, 1H). Consistently, the AR activity induced by an alternative ligand, R1881, can also be inhibited by CTS treatment (Fig. 1I, 1J).
3.2. CTS Treatment Inhibits the Growth of AR Positive PCa Cells, but not the AR Negative PC-3 Cells or Non-malignant Prostate Epithelial Cells
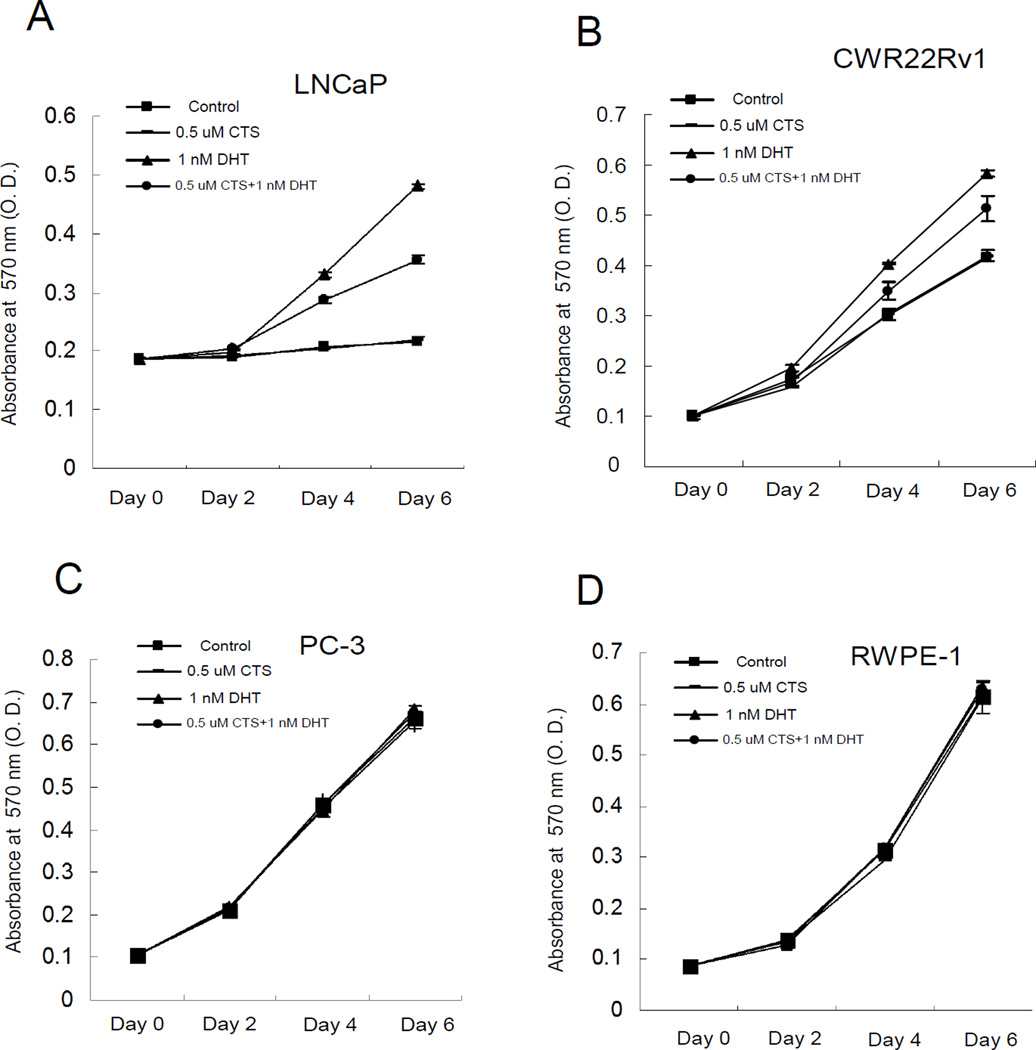
We then investigated the effects of CTS on the inhibition of DHT-induced cell proliferation in AR positive cells (LNCaP and CWR22Rv1) and compared this with the AR negative PCa cells (PC-3) and non-malignant prostate epithelial cells (RWPE-1). 0.5 µM CTS reduced DHT-induced cell growth in LNCaP cells by 45%, and consistently it showed around 45% inhibition of DHT induced growth in CWR22Rv1 cells (Fig. 2A, 2B). On the other hand, CTS didn’t inhibit the growth of AR negative cells (PC-3) or non-malignant prostate epithelial cells (RWPE-1) with or without DHT (Fig. 2C, 2D). Although 0.5 µM CTS does not completely inhibit AR activity (Fig. 1B, 1C), the ~45% inhibition of DHT-induced AR activity by CTS could effectively control the cancer cell growth. We acknowledge that CTS may also regulate other signaling pathways, such as STAT3 or growth factor signaling, to control cancer cell growth. We will investigate those underlying mechanisms in the future. Together, our data showed that CTS, as low as 0.5 µM, could inhibit the androgen-induced AR activity and effectively control the growth of AR positive prostate cancer cells.
Fig. 2.
Differential growth inhibition effects of CTS on different prostate cells. Cells were treated with EtOH and CTS (0.5 µM) in the absence or presence of 1 nM DHT. Medium with indicated treatments was refreshed every 2 days (the half-life of CTS is 22.5±1.5 hr, data not shown) for a total of 7 days. A: CTS inhibits the DHT-induced growth of LNCaP cells. B: CTS inhibits the DHT-induced growth of CWR22Rv1 cells. C: No effects of CTS on the growth of AR-negative PC-3 cells. D: No effects of CTS on non-malignant prostate RWPE1 cells. Data represent mean ± SD of three independent experiments with three replicates in each experiment.
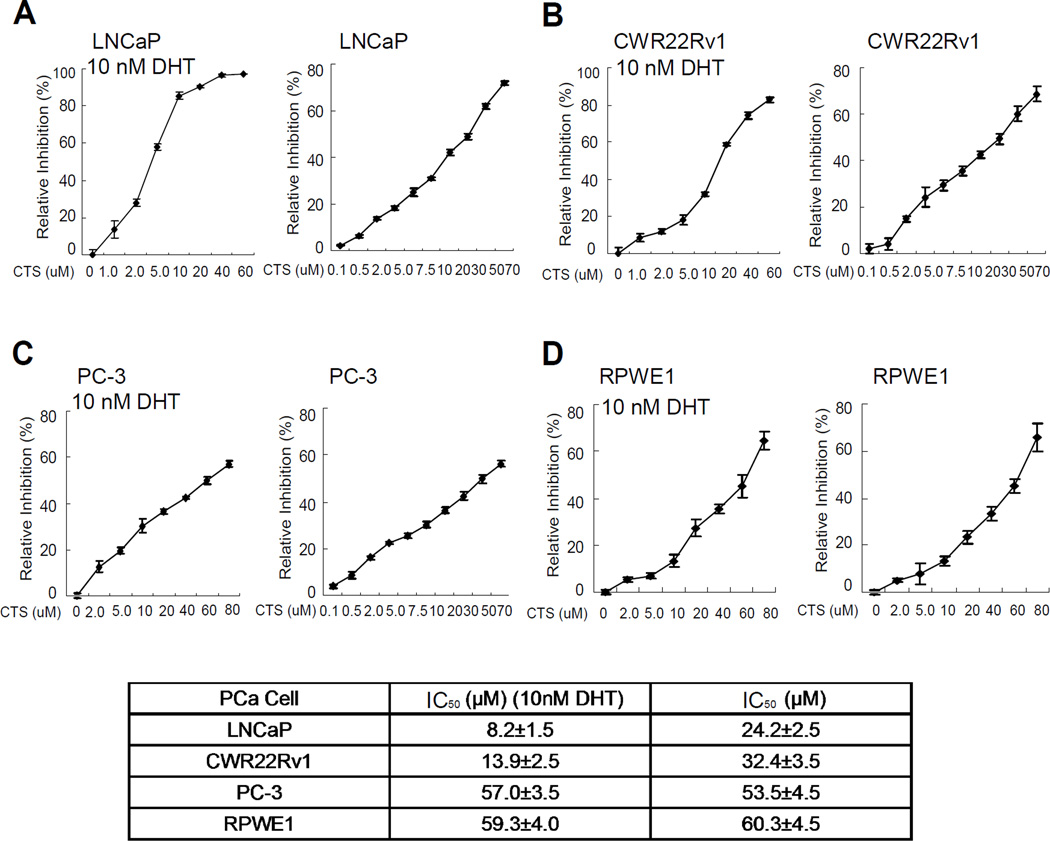
The half maximal inhibitory concentration (IC50) is a measure of the effectiveness of a compound in inhibiting biological or biochemical function. IC50 values can be determined by constructing a dose-response curve and examining the effect of different concentrations of antagonist on reversing agonist activity [28]. The IC50 concentrations for CTS on the PCa cells were evaluated by half-inhibition of cell growth at 48 hr of CTS treatments with or without 10 nM DHT. In the presence of DHT, the IC50 concentrations for CTS in LNCaP, CWR22Rv1, PC-3 were 8.2±1.5 µM, 13.9±2.5 µM, and 57.0±3.5 µM, respectively (Fig. 3A-C). In the absence of DHT, the IC50 concentrations for CTS in LNCaP, CWR22Rv1, and PC-3 were 24.2±2.5 µM, 32.4±3.5 µM, 53.5±4.5 µM, respectively (Fig. 3D-F). We used multiple assays and reproducibly found that CTS could effectively inhibit the AR positive LNCaP and CWR22Rv1 cell growth. Therefore, we tested the mechanisms by which CTS controls AR activity and cancer cell growth in AR positive PCa cells.
Fig. 3.
IC50 of CTS in different prostate cancer cells. We determined the cell half-inhibition (IC50) of CTS in LNCaP, CWR22rv1, and PC3. Cells were seeded on 24 well plates in medium with 10%FBS for 24 hr. Medium was then refreshed to medium with 10 %CS-FBS for another 24 hr, and cells were treated with serial concentrations of CTS with or without 1 nM DHT for 2 days. Cells growth and IC50 value were determined by MTT assay. Half-inhibition of CTS was shown in Fig. 3A–3C when cells were treated with 10 nM DHT, and in Fig. 3D–3F when cells were not treated with DHT. Data represent mean ± SD of two independent experiments with three replicates in each experiment.
3.3. CTS Inhibits the DHT-induced AR Target Gene Expression in LNCaP and CWR22Rv1 Cells
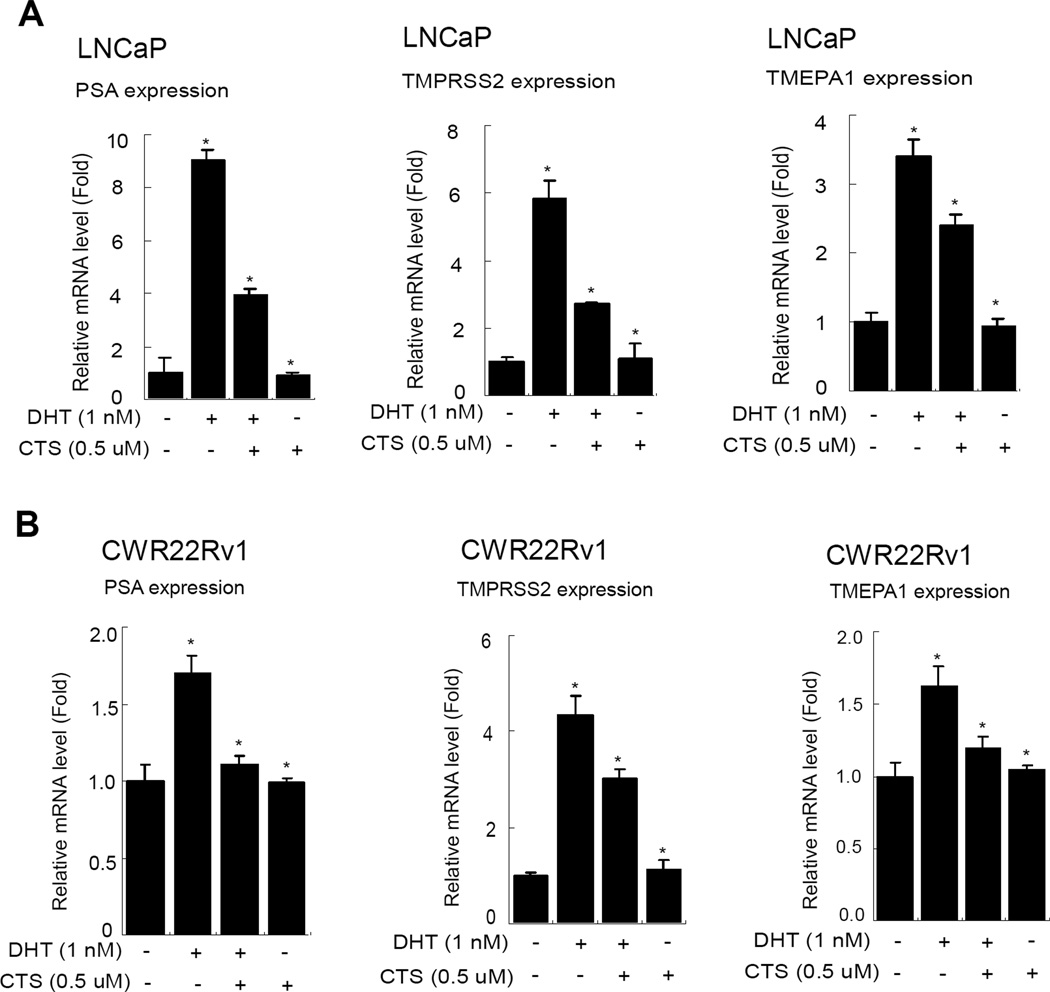
In Fig. 1., we observed that 0.5 µM CTS could effectively inhibit AR/MMTV-Luc activity in PCa cells. To further illustrate the ability of CTS to regulate the AR downstream genes, we assayed AR target genes expression in AR positive PCa cells, LNCaP and CWR22Rv1 cells. Our data showed that the AR target gene (PSA, TMPRSS2, and TMEPA1) mRNA levels were induced by 1 nM DHT, and 0.5 µM CTS could effectively suppress the DHT-induced AR target genes in LNCaP and CWR22Rv1 cells (Fig. 4).
Fig. 4.
CTS Inhibits the AR target gene expression in LNCaP and CWR22Rv1 cells. Cells were treated with ethanol or CTS (0.5 µM) in the absence or presence of 1 nM DHT for 2 days. We used real-time RT-PCR to analyze the mRNA expressions of AR target genes, PSA, TMPRSS2, and TMEPA1, in LNCaP and CWR22Rv1 cells. The respective mRNA levels of these genes in each treatment group were displayed as fold changes compared to the untreated group. Data are shown as the mean ± SD of three independent experiments with three replicates in each experiment.
3.4. CTS Does not Alter AR Protein Expression and Stability
Our data showed that CTS inhibited the AR mediated activity and target gene expression, yet the mechanism remained unclear. We then determined the effect of CTS on the protein levels of AR using Western blot analysis. Our data showed the protein levels of AR were not significantly altered in LNCaP and CWR22Rv1 cells treated with 0.5 µM CTS and with 1 nM or 10 nM DHT (Fig. 5A, 5B), but the growth of LNCaP and CWR22Rv1 cells were inhibited by CTS (Fig. 2). The PSA protein expression was used as control to show inhibition effects of CTS. Consistent with Fig. 4 data, we found the protein levels of PSA were induced by DHT treatment and CTS inhibited these DHT-induced PSA protein expressions (Fig. 5A, 5B).
Fig. 5.
CTS inhibited AR transactivation is not via changing AR protein expression or stability. A and B: Western blot analyses of PSA and AR levels in control and CTS treated LNCaP or CWR22Rv1 cells in the absence or presence of DHT. 50 µg of total protein from cells was applied onto a 10% sodium dodecylsulfate-polyacrylamide gel and subjected to electrophoresis followed by Western blot using anti-AR or anti-PSA antibodies. The values shown in Supplementary Fig. 1 represent changes in density of the bands normalized to α-tubulin using the Image Lab statistics software (the representative graph quantitation are shown). All of the data were validated by three independent experiments. C. CTS inhibits the transactivation of the full-length AR (flAR), but not the constitutive activated N-terminal AR. pSG5-ARN-DBD and pSG5-flAR were transfected into HEK 293 cells. After 24 hrs transfection, cells were treated with or without 10 nM DHT and 2.5 µM CTS. D. CTS cannot effectively bind to AR. We used competitive ligand binding assay to determine whether CTS can specifically bind to the AR. LNCaP cell medium was changed to RPMI with10% CS-FBS for 24 hr and 1 nM 3H-R1881 was then added into culture medium with or without DHT or CTS for 1 hr. Unlabeled DHT with the concentrations of 0.1, 1, 10, 100, 1000 nM were used to compete for the 3H-R1881 binding as positive control (left). CTS at the concentration ranges of 0.1, 0.25, 1, 2.5, 10, 25 µM were used to determine the potential AR antagonist effects (right).
3.5. CTS Does not Directly Inhibit the Transactivation of the N-terminal AF1 Domain of AR
It has been reported that there are different AR N-terminal splicing variants with constitutive AF-1 activity in the absence of androgen treatment [29–32]. Our data indicated that CTS could inhibit the full-length AR transactivation; therefore, we were interested in testing whether CTS could effectively inhibit N-terminal AF1 transactivation of AR. Both the full-length AR and AR N-DNA binding domain (N-DBD) cDNAs were transfected into HEK 293 cells. After 24 hrs transfection, cells were treated with or without 1 nM DHT and 0.5 or 2.5 µM CTS (as indicated in Figure). Our data indicated that N-terminal AR possesses a constitutive AF-1 transactivation without being influenced by ligand treatment (Fig. 5C, lane 5, 6). However, CTS treatment could not affect the AF-1 transactivation (Fig. 5C, lane 7, 8). As a control, CTS can inhibit the DHT-induced full-length AR transactivation (Fig. 5C, lane 11 vs 10). Together, our data of Fig. 5 suggested that CTS does not direct affect the AR protein stability or the N-terminal AF-1 function domain of AR to inhibit the AR functions.
Since the inhibition effect of CTS required the ligand binding domain (LBD), it is important to know whether CTS can bind to AR and functions as an anti-androgen. To address this question, competitive ligand binding assays using 3H-R1881 was performed in LNCaP cells. To show the specific ligand binding competition curve, the unlabelled DHT at concentrations of 0.1, 1, 10, 100, and 1000 nM were used to compete for the 1 nM 3H-R1881 binding (Fig. 5D, left panel). It was known that R1881 has a slightly higher binding affinity than that of DHT, thus, it was not surprising to observe that 10 nM DHT can be more effective than 1 nM DHT to compete for the 3H-R1881 binding to AR. Our results have showed that 0.5 µM and 2.5 µM of CTS can inhibit AR activity, PCa cell growth, but can not compete with the 3H-R1881 binding to AR (Fig. 5D, right panel). As shown in Fig. 3, in the presence of DHT, the IC50 concentrations for CTS in LNCaP was 8.2±1.5 µM, and the higher concentrations of CTS may elicit cytotoxicity. Therefore, it is expected that the ligand binding curve dropped at 10 and 25 µM of CTS treatments. Together, our results suggest that CTS does not function as an antagonist to inhibit AR activity in PCa cells.
3.6. CTS Inhibits the AR N/C-Dimerization and the Formation of AR-Coregulator Complex
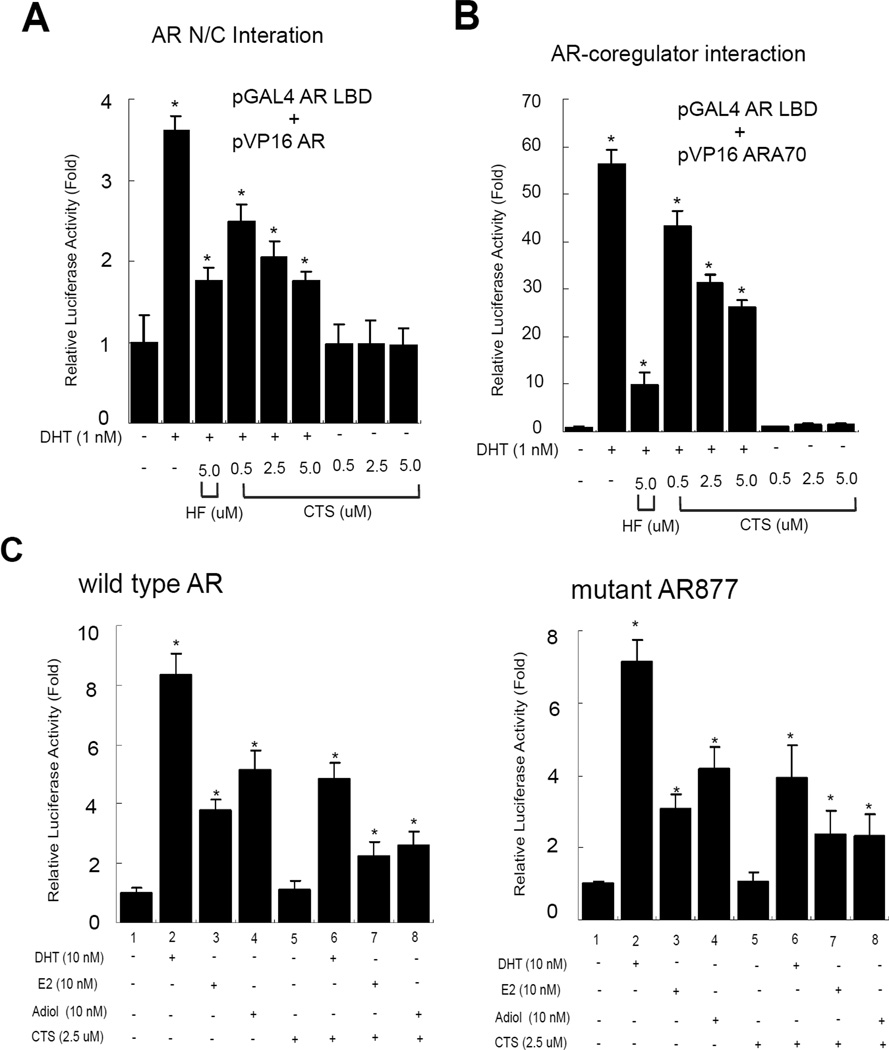
AR is a transcriptional factor that belongs to the NR superfamily, containing a conserved NH2- (N) terminal functional domain, a DBD, a hinge region, and a COOH-(C) terminal LBD. After ligand binding, AR is dissociated from chaperones, phosphorylated, and translocated into the nucleus. AR then binds to DNA response elements on target gene promoters as a dimer and recruits some selective type I coregulators to enhance target gene expression. Early reports showed that AR N and C terminal (N-C) interaction was important for full AR functions [33–34]. To further delineate the mechanism by which CTS inhibits AR transactivation, we tested whether CTS could affect the AR N-C interaction using mammalian 2-hybrid interaction assay. Cells were transfected with Gal4-RE-Luc, Gal4-DBD fused-AR LBD and VP16 fused-ARN. Again DHT could stimulate the AR N-C interaction, and CTS showed dose-dependent suppression of AR N-C interaction (Fig. 6A). In addition to the N-C dimerization, we also tested the ability of CTS to affect the interaction between AR and co-regulator ARA70 using mammalian 2-hybrid interaction assay [22, 35]. The HEK 293 cells were transfected with Gal4-RE-Luc, VP16-fused ARA70, and Gal4-AR-LBD. As shown in Fig. 6B, the interaction between Gal4-AR-LBD and VP16-ARA70 was induced by DHT, but blocked by HF and CTS showed dose-dependent suppression of AR and ARA70 interaction ability induced by DHT. Together, our data of Fig. 5 and Fig. 6 A-B suggest that CTS does not directly affect the AR protein stability, nor the N-terminal AF-1 function N domain of AR to inhibit the AR functions. CTS could inhibit AR through modulating the AR N-C dimerization and AR-coregulator complex formation.
Fig. 6.
A. CTS inhibits the interaction of AR N-terminus and C-terminus using mammalian 2-hybrid interaction assay. B. CTS inhibits the interaction of AR and AR coregulator using mammalian 2-hybrid assay. C. CTS inhibits the E2 and Adiol-induced full-length (flAR) and transactivation. MMTV-Luc reporter was activated through AR in the presence of 10 nM DHT, E2 or Adiol (lanes 2–4). 2.5 µM CTS could effectively inhibit the DHT, E2, and Adiol stimulated AR activity (lanes 6–8). The solvent (EtOH) treated AR-baseline transcriptional activity was counted as 1 fold (lane 1). Data were averaged from three independent experiments.
3.7. CTS can block E2 or Adiol-mediated AR activity in PCa cells
After androgen ablation therapy by surgical or chemical castration, it has been reported that other hormones may possibly activate AR to affect the cancer cell growth and recurrence in PCa patients [36–38]. Our earlier reports suggested that 17β-estradiol (E2) and androst-5-Δene-3,7-diol (Δ5-androstenediol or Adiol) are natural hormones that could activate the AR transactivation in the absence of testicular androgens, testosterone and DHT. Furthermore, two potent antiandrogens, hydroxyflut amide (Eulexin) and bicalutamide (Casodex), fail to block completely the E2 or Adiol-induced AR transactivation in PCa cells [36–37]. Compared to wild type AR, our earlier studies also found that the gain-of-function mutant AR T877A could be more accessible by E2 and Adiol [36–37]. Therefore, it is of great interest to know whether CTS could inhibit the E2 or Adiol mediated transactivation. In our earlier studies, we characterized and titrated for the effective concentration for E2 and Adiol to activate AR transactivation and found it is 10 nM [22, 37]. Our data consistently showed that 0.5 µM CTS treatments could effectively inhibit the Adiol-or E2-mediated transactivations of wild type AR as well as mutant AR, and 2.5 µM CTS treatment has more profound effects (Fig. 6C). Our data indicated that CTS could be developed as a new therapeutic agent to block E2 or Adiol’s androgenic action in PCa cells.
3.8 CTS can effectively inhibit PCa growth in vivo using xenograft mouse PCa model
It is important to know the therapeutic efficiency of CTS in an in vivo animal PCa model. To test the CTS anti-cancer effect in vivo, we used the CWR22Rv1 xenograft PCa model [39]. The CWR22Rv1 cell line was established from the re-growth tumor on androgen dependent CWR22 cell xenograft mouse after castration, and showed androgen independent characteristics [39]. Therefore, it was widely used as an in vivo model in nude mice to evaluate the therapeutic effect in CRPC [40, 41], which is more important in clinics.
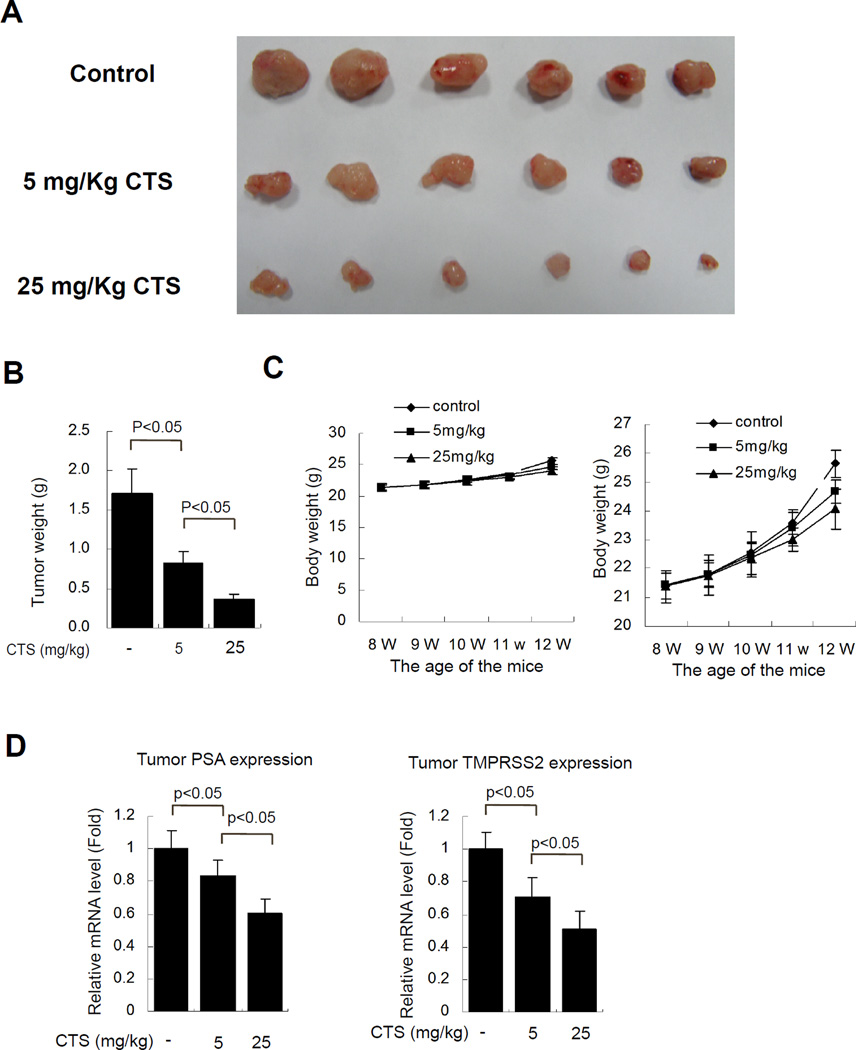
When CWR22Rv1 xenograft tumors became palpable, the mice were randomly assigned to 3 groups and treated with control oil (with 5% DMSO), a low dose (5 mg/Kg CTS), or a higher dose (25 mg/Kg CTS), every two days by i.p. injection. After 4 weeks of treatments, the mice were sacrificed and tumors were collected. Our data showed that CTS treatments could effectively reduce the tumor size at both low and high doses (Fig. 7A, and 7B, P<0.05, versus control) without affecting the mouse body weight (Fig. 7C). Together, these results suggest that CTS has a great potential as a clinical therapy for PCa patients.
Fig. 7. CTS treatments inhibit cancer growth using the in vivo CWR22Rv1 xenograft PCa model.
To test the therapeutic effect of CTS, 1x106 CWR22Rv1 cells per site were injected subcutaneously into nude mice at 7 weeks (W) of age. One week after implantation, when tumors established to the size >50 mm3, mice were i.p. injected with vehicle (DMSO), low dose of CTS (5 mg/kg), or high dose of CTS (25 mg/mice), every two days for 4 weeks (from 8 to 12 weeks old). The mice were then sacrificed and the tumors were collected for data analysis. A. CTS treatments inhibit CWR22Rv1 tumor size in a dose dependent manner. B. CTS treatments inhibit CWR22Rv1 tumor weights (N=6 for each group, P<0.05). C. The mouse body weights were not affected by mock or CTS treatments. The right panel showed an enlarged measurement scale of body weights. As the mock control mice carried a bigger tumor, thus the BW is around 1.2 g slightly heavier than the CTS treated group at the end of experiment.
4. DISCUSSION
4.1 CTS is a natural compound, and has a chemical structure similar to DHT
In recent years, drug developers have rediscovered the potential value of herbal medicines, and their incorporation into medical care has been encouraged by the World Health Organization’s Traditional Medicines Strategy [42–43]. DHT is a commonly known androgenic or reproductive hormone, and is responsible for the formation of secondary sex characteristics in men. In chemical structure (Fig. 1A), the parent compound of DHT and a number of steroids contain three cyclohexane rings (designated as rings A, B, and C in the left panel) and one cyclopentane ring (the D ring), which are composed of seventeen carbon atoms. CTS is a natural product, isolated from the herbal plant Salvia miltiorrhiza Bunge. Interestingly, when compared with DHT, CTS has a very similar chemical structure. The difference in their chemical structures is that oxygen in the D ring of CST is replaced by a carbon in DHT. We also compare the structure of CTS with finasteride, an inhibitor for 5 α-reductase which converts testosterone to DHT. Finasteride has a 3-Oxo-4-aza-5α-androst mother structure, which is modified from the similar steroid structure. The main difference between the two structures is that one nitrogen in the A ring of finasteride is replaced by a carbon in DHT. Due to chemical structure similarities, this leads us to further examine the relationship between the biological activity of CTS and androgen. The current study found that CTS can function as anti-AR agent to block the AR activity by inhibiting AR N-C dimerization and coregulator recruitment.
4.2 Earlier report did not test the effect of CTS on AR transactivation and AR mediated cell growth
An earlier report suggested that the IC50 of CTS in DU145 is around 7 µM [14]. However, the report did not address the CTS effect on DHT-induced AR activity and cell growth of PCa cells. The PCa DU145 cells expresses STAT3, which was inhibited by CTS, causing reduced cell growth. CTS was also reported to reduce androgen synthesis for the prenatally androgenized male rats [19]. However, there is no report regarding the effect of CTS on AR activities and in other PCa cells. AR is crucial for PCa development and recurrence [44]. The AR is a ligand-activated transcription factor that mediates the biological responses to androgens. After the ligand binds to the AR, and then the ligand–receptor complex translocates to the nucleus and binds specific androgen response elements on the chromosome. Our study demonstrated that CTS, a potential anti-AR agent, inhibited AR function in PCa. Since CTS contains effective anti-AR activity but has poor water solubility, we could modify CTS and screen better potential anti-AR compounds derived from CTS in the future.
Our data showed that CTS affected PCa cells growth through an AR dependent pathway with the growth inhibition effects on AR positive LNCaP and CWR22Rv1 cells, but not AR negative prostate cancer PC-3 cells, or non-malignant prostate RWPE1 cells. The dependence on AR activity and growth factor signals could be different between non-malignant prostate cells and prostate cancer cells. It was reported that RWPE1 cell growth is slightly stimulated by the synthetic androgen, Mibolerone; however, the viability of RWPE1 cells may not be androgen dependent. The specialized culture media for RPWE-1 cells does not contain fetal bovine serum, but is supplemented with 5% bovine pituitary extract and epidermal growth factors. It was reported that there is less than 0.1 pM androgen in 5% bovine pituitary extract [45]. Therefore, androgen/AR function may not be the key factor to determine the cell viability in the non-malignant prostate RWPE1 cells. It is likely that the growth and viability of RPWE-1 cells are more dependent on growth factors. Furthermore, using epithelial and stromal tissue recombination experiments, it is has been reported that the epithelial AR is not a determining factor for the normal prostate epithelia cell viability or basic glandular formation [46], and prostate epithelial AR is more responsible for the secreted functions and prostate luminal enfolding inside the prostate ducts [47]. In addition, the physiological differences between normal cells and cancer cells may also explain the differential response toward CTS treatment in RWPE1 and PCa cells [48].
In addition to testing CTS’s biological activity, we also performed the IC50 test in DU145, LNCaP, and PC-3 cells. Indeed, we observed that the IC50 of CTS in DU145 is around 7.0 µM due to the CTS inhibition of endogenous STAT3 activity in DU145 cells (data not shown), which is consistent with the previous report [14]. Except for the DU145 cells, our data consistently showed that CTS more effectively inhibits the growth and viability of AR-positive PCa cells using multiple strategies and assays.
4.3. CTS cannot Inhibit the Constitutive AR-N Terminus Activity, but It can interfere with the N-C Dimerization and the AR Coregulator Complex Formation
Although CTS could not inhibit the constitutively AR N–terminal activation, it has been reported that the constitutively active AR splice variants, such as AR3, is present at only less than 10% amount as compared to that of full-length of AR in PCa. Importantly, the functions of those AR variants may require the heterodimer with the full-length of AR [32]. Thus, our finding that CTS could block AR N-C interaction remains important to apply the therapy of CTS in PCa. In addition, it has been shown that AR coregulators may play important roles in CRPC and the failure of antiandrogen therapy [22, 35, 49]. Our data showed CTS can inhibit the formation of AR-coregulator complex, which highlight its potential clinical value in prostate cancer therapy at refractory stages. Moreover, we showed that CTS can inhibit the E2 or Adiol induced AR activities. The inhibition of non-androgen activated AR activity by CTS in PCa may also advance its application in CRPC. Importantly, we showed the CTS could effectively inhibit the CWR22Rv1 tumor growth using an animal PCa model, indicating that CTS can work efficiently to reduce CRPC growth in vivo.
4.4. The Potential Application of CTS on PCa Metastasis and Future Therapy
Cancer metastasis is a multistep and complex process that involves dissociation of the tumor cells from the organ of origin, degradation of the extracellular matrix, cell migration, invasion of surrounding tissues, cell adhesion, and colonization to distant sites in the patients. Human PCa progression to advanced metastatic disease is associated with relapse to a castration resistant (or hormone-refractory) state due to impaired apoptotic response or growth factor pathway in the of androgen ablation stage [50–51]. AR gene amplification is found in one third of advanced PCa tumors and is believed to contribute to progression and metastasis of PCa [2, 52]. To further test the effect of CTS-inhibited DHT-regulated invasion and migration in AR positive PCa cells will be our future work.
In summary, CTS is a natural product, isolated from an herb, Salvia miltiorrhiza Bunge (Danshen). The Danshen extract has been used in clinical treatment for coronary heart disease [12, 13]. Not only does CTS show diverse biological activities, but also has only minor side effects. The data presented in this study reveal that CTS is a novel potential AR signaling inhibitor, which can block AR regulated gene expression and cell growth in androgen responsive and in CRPC cells. The inhibitory effects of CTS are due to its anti-AR effect and interference with the AR N-C dimerization and AR-coregulator complex formation. Our data also indicated that CTS could inhibit the E2 or Adiol–mediated AR transactivation in the absence of androgens and DHT. Our data have demonstrated that CTS has the potential to be developed as an anti-AR drug for the treatment of PCa patients.
Supplementary Material
The values represent changes in density of the bands normalized to α-tubulin using the Image Lab statistics software (Bio-Rad). The relative protein expression amount were compared to the vehicle control group (without CTS and DHT treatment) A. Quantification results of Fig.5A; B. Quantification results of Fig.5B
ACKNOWLEDGMENTS
This work was supported by the George H. Whipple Professor Endowment. We thank Karen Wolf for the assistance of manuscript preparation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
None Declared
REFERENCES
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer Statistics, 2010, CA. Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Heinlein CA, Chang C. Androgen receptor in prostate cancer. Endocr. Rev. 2004;25(2):276–308. doi: 10.1210/er.2002-0032. [DOI] [PubMed] [Google Scholar]
- 3.Henderson BE, Feigelson HS. Hormonal carcinogenesis. Carcinogenesis. 2000;21:427–433. doi: 10.1093/carcin/21.3.427. [DOI] [PubMed] [Google Scholar]
- 4.Marker PC, Donjacour AA, Dahiya R, Cunha GR. Hormonal, cellular, and molecular control of prostatic development. Dev Biol. 2003;253(2):165–174. doi: 10.1016/s0012-1606(02)00031-3. [DOI] [PubMed] [Google Scholar]
- 5.Bonkhoff H, Remberger K. Differentiation pathways and histogenetic aspects of normal and abnormal prostatic growth: a stem cell model. The Prostate. 1996;28(2):98–106. doi: 10.1002/(SICI)1097-0045(199602)28:2<98::AID-PROS4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 6.Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schütz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans RM. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang C, Kokontis J, Liao S. Structural analysis of complementary DNA and amino acid sequences of human and rat androgen receptors. PNAS. 1988;85:7211–7215. doi: 10.1073/pnas.85.19.7211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huggins C, Hodges CV. Studies on prostatic cancer. I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res. 1941;1:293–297. doi: 10.3322/canjclin.22.4.232. [DOI] [PubMed] [Google Scholar]
- 9.Wirth MP, Hakenberg OW, Froehner M. Antiandrogens in the Treatment of Prostate Cancer. European Urology. 2007;51(2):306–314. doi: 10.1016/j.eururo.2006.08.043. [DOI] [PubMed] [Google Scholar]
- 10.Terada N, Shimizu Y, Yoshida T, Maeno A, Kamba T, Inoue T, Nakamura E, Kamoto T, Ogawa O. Antiandrogen Withdrawal Syndrome and Alternative Antiandrogen Therapy Associated With the W741C Mutant Androgen Receptor in a Novel Prostate Cancer Xenograft. The Prostate. 2010;70:252–261. doi: 10.1002/pros.21058. [DOI] [PubMed] [Google Scholar]
- 11.Ohtsu H, Itokawa H, Xiao Z, Su CY, Shih CC, Chiang T, Chang E, Lee Y, Chiu SY, Chang C, Lee KH. Antitumor Agents 222.y Synthesis and Anti-androgen Activity of New Diarylheptanoids. Bioorg. Med. Chem. 2003;11:5083–5090. doi: 10.1016/j.bmc.2003.08.029. [DOI] [PubMed] [Google Scholar]
- 12.Chan K, Chui SH, Wong DY, Ha WY, Chan CL, Wong RN. Protective effects of Danshensu from the aqueous extract of Salvia miltiorrhiza (Danshen) against homocysteine-induced endothelial dysfunction. Life Sci. 2004;75(26):3157–3171. doi: 10.1016/j.lfs.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 13.Wang XH, Morris-Natschke SL, Lee KH. Med. Res. Rev. 2007;27:133–148. doi: 10.1002/med.20077. [DOI] [PubMed] [Google Scholar]
- 14.Shin DS, Kim HN, Shin KD, Yoon YJ, Kim SJ, Han DC, Kwon BM. Cryptotanshinone inhibits constitutive signal transducer and activator of transcription 3 function through blocking the dimerization in DU145 prostate cancer cells. Cancer Res. 2009;69(1):193–202. doi: 10.1158/0008-5472.CAN-08-2575. [DOI] [PubMed] [Google Scholar]
- 15.Hur JM, Shim JS, Jung HJ, Kwon HJ. Cryptotanshinone but not tanshinone IIA inhibits angiogenesisin vitro. Exp Mol Med. 2005;37(2):133–137. doi: 10.1038/emm.2005.18. [DOI] [PubMed] [Google Scholar]
- 16.Park EJ, Zhao YZ, Kim YC, Sohn DH. Cryptotanshinone, can protect against liver toxicity in vivo and in vitro due to its antioxidant effects. Food Chem. Toxicol. 2009;47(1):2742–2748. doi: 10.1016/j.fct.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 17.Hur JM, Shim JS, Jung HJ, Kwon HJ. Cryptotanshinone but not tanshinone IIA inhibits angiogenesisin vitro. Exp Mol Med. 2005;37(2):133–137. doi: 10.1038/emm.2005.18. [DOI] [PubMed] [Google Scholar]
- 18.Lee WY, Cheung CC, Liu KW, Fung KP, Wong J, Lai PB, Yeung JH. Cytotoxic Effects of Tanshinones from Salvia miltiorrhiza on Doxorubicin-Resistant Human Liver Cancer Cells. J. Nat. Prod. 2010;73(5):854–859. doi: 10.1021/np900792p. [DOI] [PubMed] [Google Scholar]
- 19.Li XH, Yang XM, Wu XK. Effects of Cryptotanshinone in Lowering Androgens Synthesis for the Prenatally Androgenized M ale Rats. Chinese Journal of Integrated Traditional and Western Medicine. 2008;28(11):1001–1004. [PubMed] [Google Scholar]
- 20.Danheiser RL, Casebier DS, Firooznia F. Aromatic Annulation Strategy for the Synthesis of Angularly-Fused Diterpenoid Quinones. Total Synthesis of (+)-Neocryptotanshinone, (−)-Cryptotanshinone, Tanshinone IIA, (f)-Royleanone. Org. Chem. 1995;60:8341–8350. [Google Scholar]
- 21.Sairafianpour M, Christensen J, Staerk D, Budnik BA, Kharazmi A, Bagherzadeh K, Jaroszewski JW. Leishmanicidal, Antiplasmodial, and Cytotoxic Activity of Novel Diterpenoid 1,2-Quinones from Perovskia abrotanoides: New Source of Tanshinones. J. Nat. Prod. 2001;64:1398–1403. doi: 10.1021/np010032f. [DOI] [PubMed] [Google Scholar]
- 22.Yeh S, Lin HK, Kang HY, Thin TH, Lin MF, Chang C. From HER2/Neu signal cascade to androgen receptor and its coactivators: A novel pathway by induction of androgen target genes through MAP kinase in prostate cancer cells. PNAS. 1999;96:5458–5463. doi: 10.1073/pnas.96.10.5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yeh S, Hu YC, Rahman M, Lin HK, Hsu CL, Ting HJ, Kang HY, Chang C. Increase of androgen-induced cell death and androgen receptor transactivation by BRCA1 in prostate cancer cells. PNAS. 2000;97:11256–11261. doi: 10.1073/pnas.190353897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen M, Ni J, Zhang Y, Muyan M, Yeh S. ERAP75 Functions as a Coactivator to Enhance Estrogen Receptor α Transactivation in Prostate Stromal Cells. The Prostate. 2008;68:1273–1282. doi: 10.1002/pros.20774. [DOI] [PubMed] [Google Scholar]
- 25.Chen M, Ni J, Chang HC, Lin CY, Muyan M, Yeh S. CCDC62/ERAP75 functions as a coactivator to enhance estrogen receptor beta-mediated transactivation and target gene expression in prostate cancer cells. Carcinogenesis. 2009;30:841–850. doi: 10.1093/carcin/bgn288. [DOI] [PubMed] [Google Scholar]
- 26.Ni J, Pang ST, Yeh S. Differential Retention of α-Vitamin E is Correlated With Its Transporter Gene Expression and Growth Inhibition Efficacy in Prostate Cancer Cells. The Prostate. 2007;67:463–471. doi: 10.1002/pros.20517. [DOI] [PubMed] [Google Scholar]
- 27.Tepper CG, Boucher DL, Ryan PE, Ma AH, Xia L, Lee LF, Pretlow TG, Kung HJ. Characterization of a novel androgen receptor mutation in a relapsed CWR22 prostate cancer xenograft and cell line. Cancer Res. 2002;62(22):6606–6614. [PubMed] [Google Scholar]
- 28. http://en.wikipedia.org/wiki/IC50.
- 29.Dehm SM, Schmidt LJ, Heemers HV, Vessella RL, Tindall DJ. Splicing of a novel androgen receptor exon generates a constitutively active androgen receptor that mediates prostate cancer therapy resistance. Cancer Res. 2008;68(13):5469–5477. doi: 10.1158/0008-5472.CAN-08-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu R, Dunn TA, Wei S, Isharwal S, Veltri RW, Humphreys E, Han M, Partin AW, Vessella RL, Isaacs WB, Bova GS, Luo J. Ligand-Independent Androgen Receptor Variants Derived from Splicing of Cryptic Exons Signify Hormone-Refractory Prostate Cancer. Cancer Res. 2009;69(1):16–22. doi: 10.1158/0008-5472.CAN-08-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guo Z, Yang X, Sun F, Jiang R, Linn DE, Chen H, Chen H, Kong X, Melamed J, Tepper CG, Kung HJ, Brodie AM, Edwards J, Qiu Y. A Novel Androgen Receptor Splice Variant Is Up-regulated during Prostate Cancer Progression and Promotes Androgen Depletion–Resistant Growth. Cancer Res. 2009;69(6):2305–2313. doi: 10.1158/0008-5472.CAN-08-3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Watson PA, Chen YF, Balbas MD, Wongvipat J, Socci ND, Viale A, Kim K, Sawyers CL. Constitutively active androgen receptor splice variants expressed in castration-resistant prostate cancer require full-length androgen receptor. PNAS. 2010;107(39):16759–16765. doi: 10.1073/pnas.1012443107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hsu CL, Chen YL, Ting HJ, Lin WJ, Yang Z, Zhang Y, Wang L, Wu CT, Chang HC, Yeh S, Pimplikar SW, Chang C. Androgen Receptor (AR) NH2- and COOH-Terminal Interactions Result in the Differential Influences on the AR-Mediated Transactivation and Cell Growth. Molecular Endocrinology. 2005;19(2):350–361. doi: 10.1210/me.2004-0190. [DOI] [PubMed] [Google Scholar]
- 34.He B, Kemppainen JA, Voegel JJ, Gronemeyer H, Wilson EM. Activation function 2 in the human androgen receptor ligand binding domain mediates interdomain communication with the NH(2)-terminal domain. J Biol Chem. 1999;274:37219–37225. doi: 10.1074/jbc.274.52.37219. [DOI] [PubMed] [Google Scholar]
- 35.Yeh S, Chang C. Cloning and characterization of a specific coactivator, ARA70, for the androgen receptor in human prostate cells. PNAS. 1996;93(11):5517–5521. doi: 10.1073/pnas.93.11.5517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yeh S, Miyamoto H, Shima H, Chang C. From estrogen to androgen receptor: A new pathway for sex hormones in prostate. PNAS. 1998;95:5527–5532. doi: 10.1073/pnas.95.10.5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miyamoto H, Yeh S, Lardy H, Messing E, Chang C. Δ5 -Androstenediol is a natural hormone with androgenic activity in human prostate cancer cells. PNAS. 1998;95:11083–11088. doi: 10.1073/pnas.95.19.11083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miyamoto H, Messing EM, Chang C. Androgen Deprivation Therapy for Prostate Cancer: Current Status and Future Prospects. The Prostate. 2004;9999:1–22. doi: 10.1002/pros.20115. [DOI] [PubMed] [Google Scholar]
- 39.Tepper CG, Boucher DL, Ryan PE, Ma AH, Xia L, Lee LF, Pretlow TG, Kung HJ. Characterization of a novel androgen receptor mutation in a relapsed CWR22 prostate cancer xenograft and cell line. Cancer Res. 2002;62:6606–6614. [PubMed] [Google Scholar]
- 40.Lin FM, Tsai CH, Yang YC, Tu WC, Chen LR, Liang YS, Wang SY, Shyur LF, Chien SC, Cha TL, Hsiao PW. A novel diterpene suppresses CWR22Rv1 tumor growth in vivo through antiproliferation and proapoptosis. Cancer Res. 2008;68(16):6634–6642. doi: 10.1158/0008-5472.CAN-08-0635. [DOI] [PubMed] [Google Scholar]
- 41.Sirotnak FM, She Y, Lee F, Chen J, Scher HI. Studies with CWR22 xenografts in nude mice suggest that ZD1839 may have a role in the treatment of both androgen-dependent and androgen-independent human prostate cancer. Clin Cancer Res. 2002;8(12):3870–3876. [PubMed] [Google Scholar]
- 42.Zhang J, Huang M, Guan S, Bi HC, Pan Y, Duan W, Chan SY, Chen X, Hong YH, Bian JS, Yang HY, Zhou S. A Mechanistic Study of the Intestinal Absorption of Cryptotanshinone, the Major Active Constituent of Salvia miltiorrhiza. JPET. 2006;317(3):1285–1294. doi: 10.1124/jpet.105.100701. [DOI] [PubMed] [Google Scholar]
- 43.Wang AM, Sha SH, Lesniak W, Schacht J. Tanshinone (Salviae miltiorrhizae Extract) Preparations Attenuate Aminoglycoside-Induced Free Radical Formation In Vitro and Ototoxicity In Vivo. Antimicrob Agents Chemother. 2003;47(6):1836–1841. doi: 10.1128/AAC.47.6.1836-1841.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chuan YC, Pang ST, Cedazo-Minguez A, Norstedt G, Pousette A, Flores-Morales A. Androgen Induction of Prostate Cancer Cell Invasion Is Mediated by Ezrin. JBC. 2006;281(40):29938–29948. doi: 10.1074/jbc.M602237200. [DOI] [PubMed] [Google Scholar]
- 45.McKeehan WL, Adams PS, Rosser MP. Direct mitogenic effects of insulin, epidermal growth factor, glucocorticoid, cholera toxin, unknown pituitary factors and possibly prolactin, but not androgen, on normal rat prostate epithelial cells in serum-free, primary cell culture. Cancer Res. 1984 May;44(5):1998–2010. [PubMed] [Google Scholar]
- 46.Cunha GR, Lung B. The possible influence of temporal factors in androgenic responsiveness of urogenital tissue recombinants from wild-type and androgen-insensitive (Tfm) mice. J Exp Zool. 1978;205(2):181–193. doi: 10.1002/jez.1402050203. [DOI] [PubMed] [Google Scholar]
- 47.Wu CT, Altuwaijri S, Ricke WA, Huang SP, Yeh S, Zhang C, Niu Y, Tsai MY, Chang C. Increased prostate cell proliferation and loss of cell differentiation in mice lacking prostate epithelial androgen receptor. PNAS. 2007;104(31):12679–12684. doi: 10.1073/pnas.0704940104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 49.Yeh S, Miyamoto H, Chang C. Hydroxyflutamide may not always be a pure antiandrogen. Lancet. 1997;349(9055):852–853. doi: 10.1016/S0140-6736(05)61756-4. [DOI] [PubMed] [Google Scholar]
- 50.Sakamoto S, McCann RO, Kyprianou N. Talin1 Promotes Prostate Cancer Invasion and Metastasis via AKT Signaling and Anoikis Resistance. NPRE. 2009 doi: 10.1158/0008-5472.CAN-09-2833. 3059.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wegiel B, Jiborn T, Abrahamson M, Helczynski L, Otterbein L, Persson JL, Bjartell A. Cystatin C Is Downregulated in Prostate Cancer and Modulates Invasion of Prostate Cancer Cells via MAPK/ Erk and Androgen Receptor Pathways. PLoS ONE. 2009;4(11):e7953. doi: 10.1371/journal.pone.0007953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rubin MA. Targeted therapy of cancer: new roles for pathologists--prostate cancer. Modern Pathology. 2008;21:44–55. doi: 10.1038/modpathol.2008.11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The values represent changes in density of the bands normalized to α-tubulin using the Image Lab statistics software (Bio-Rad). The relative protein expression amount were compared to the vehicle control group (without CTS and DHT treatment) A. Quantification results of Fig.5A; B. Quantification results of Fig.5B