Abstract
We have previously demonstrated that Akt was required for repetitive ischemia (RI)-induced coronary collateral growth (CCG) in healthy rats but was not activated by RI in the metabolic syndrome (JCR:LA-cp rats) where CCG was impaired. Here we hypothesized that failure of angiotensin type I receptor (AT1R) blockers to restore Akt activation is a key determinant of their inability to completely restore CCG in the metabolic syndrome. Therefore, we investigated whether adenovirus-mediated delivery of constitutively active Akt (MyrAkt-Adv) in conjunction with AT1R blockade (candesartan) was able to restore RI-induced CCG in JCR:LA-cp rats. Successful myocardial MyrAkt-Adv delivery was confirmed by a >80% transduction efficiency and an approximately fourfold increase in Akt expression and activation. CCG was assessed by myocardial blood flow measurements in the normal and collateral-dependent zones. MyrAkt-Adv alone significantly increased RI-induced CCG in JCR:LA-cp rats (∼30%), but it completely restored CCG in conjunction with administration of candesartan. In contrast, dominant negative Akt (DN-Akt-Adv) reversed the beneficial effect of candesartan on CCG in JCR:LA-cp rats. We conclude that optimal restoration of coronary collateral growth in JCR:LA-cp rats requires a combination of AT1R blockade with constitutive Akt activation. These findings may carry implications for metabolic syndrome patients in need of coronary revascularization.
Keywords: transient, coronary artery occlusion
transient, repetitive ischemia (RI), associated with stable angina pectoris, renders the myocardium tolerant to ischemia/reperfusion, in part by promoting collateral development (12, 17). Coronary collateral growth (CCG) could provide a minimally invasive alternative to current coronary revascularization therapies, percutaneous transluminal coronary angioplasty, and coronary artery bypass grafting. Despite significant research in this area, factors critical in the regulation of CCG remain undefined, especially in pathological states associated with high prevalence of obstructive coronary artery disease (CAD), such as type II diabetes, insulin resistance, hypertension, hyperlipidemia, obesity, or the metabolic syndrome, where CCG would be most beneficial.
Several growth factors, including VEGF and FGF, have been shown to be required for collateral growth in healthy animal models but have failed to induce collateral growth in clinical trials in patients with established CAD (9, 13). The role of other factors, such as angiotensin II (ANG II), in collateral growth is controversial. Angiotensin type 1 receptor (AT1R) blockers inhibited angiogenesis in a model of hindlimb ischemia (37). In contrast, several groups reported that concurrent treatment with angiotensin-converting enzyme (ACE) inhibitors and AT1R blockers caused an increase in angiogenesis (34), implying a beneficial effect of inhibiting ANG II-mediated signaling. A recent report (18) indicated that while ACE inhibition in hypertensive animals restored impaired mesenteric collateral growth, AT1R inhibition had no effect. Our previous study was the first to investigate the effect of AT1R blockade on CCG in normal, healthy rats [Wistar-Kyoto rats (WKY)] vs. rats that mimic the human metabolic syndrome (JCR:LA-cp). We (22) demonstrated that AT1R blockade partially restored CCG in the metabolic syndrome but abrogated CCG in normal, healthy animals. While AT1R blockade was associated with reduction in myocardial oxidative stress in both animal models, these levels were permissive for CCG in the JCR:LA-cp rats but too low to allow for CCG in the WKY animals (22).
In addition, AT1R blockade inhibited RI-induced activation of signaling pathways, which we have shown to be required for CCG, Akt, and p38 MAPK, in WKY animals but partially restored their activation in JCR:LA-cp animals (22). Specifically, AT1R blockade restored p38 MAPK activation to a profile observed in response to RI in WKY animals, which was associated with CCG (22). However, while AT1R blockade activated Akt at day 3 of the RI protocol to a level similar to that in WKY animals, it failed to elicit the sustained Akt activation observed to be associated with CCG in WKY animals (22). Thus the inability of AT1R blockers to restore Akt activation to a temporal pattern shown to be associated with CCG may underlie the inability of AT1R blockers to fully restore CCG in the metabolic syndrome. Consequently, we hypothesized that restoration of Akt activation in the metabolic syndrome JCR:LA-cp rats, in combination with AT1R blockade, would restore CCG in the these animals to levels seen in response to RI in normal, healthy rats. Furthermore, although we have previously demonstrated an association between impaired RI-induced CCG in the metabolic syndrome, and lack of Akt activation, we have not established a definitive role for Akt in collateral growth in the metabolic syndrome. Lastly, an association between prolonged and/or excessive Akt activation and impaired angiogenesis has been reported (14); thus the true benefit of sustained Akt activation in CCG remains undefined.
Therefore, in the present study, we focused on establishing a clinically relevant paradigm for maximal restoration of CCG in the metabolic syndrome. Specifically, the goal of this study was to assess whether delivery of constitutively active Akt to the myocardium may induce CCG as an alternative to conventional methods of coronary revascularization, with specific relevance to a subpopulation of metabolic syndrome patients, those suffering from stable angina and already being treated with AT1R blockers. We determined whether in vivo delivery of constitutively active Akt (MyrAkt) alone or in conjunction with AT1R blockade induced maximal restoration of RI-induced CCG in the rat model of the metabolic syndrome. Furthermore, we investigated the importance of Akt signaling in the regulation of CCG by delivering dominant negative Akt (DN-Akt) to rats treated with AT1R blockers.
MATERIALS AND METHODS
Rat model of CCG and RI.
Three- to four-month-old, male WKY or JCR:LA-cp (27) rats were obtained from Charles Rivers Laboratories (Wilmington, MA) and the breeding colony at the University of Alberta (Edmonton, AB, Canada). A pneumatic occluder was implanted over the left anterior descending coronary artery (LAD) as described previously (24, 35). The RI protocol consisted of short, repetitive, and transient ischemic episodes: 8, 40-s occlusions, once every 20 min (2 h, 20 min total) followed by a nonischemic (rest) period of 5 h, 40 min. This 8-h cycle was repeated three times per day. The JCR:LA-cp rat is an outbred closed strain originating from a cross between the lean LA/N and the spontaneously hypertensive obese (SHROB) rat and incorporating the autosomal recessive cp gene (27). Homozygous normal (+/+) or heterozygous (cp/+) rats are phenotypically lean and metabolically normal. However, rats homozygous for the cp gene (cp/cp or JCR:LA-cp) exhibit the obese phenotype and metabolic abnormalities. As reported previously, at 3–4 mo, JCR:LA-cp rats are hypertensive, obese, hyperlipidemic, hyperglycemic, and insulin resistant (21, 22), thus mimicking the human metabolic syndrome. All surgical procedures were in accordance with the Animal Welfare Act and approved by the Institutional Animal Care and Use Committee of the University of South Alabama.
Myocardial and collateral-dependent blood flow measurements.
At day 0 of RI, 5 × 105 gold-labeled (red in color) color microspheres were injected into the lumen of the left ventricle (LV) lumen during LAD occlusion. At day 10 of RI, 5 × 105 samarium-labeled (blackberry in color) microspheres were injected into the LV lumen during LAD occlusion. Due to their diameter (15 μM), the microspheres lodge in precapillary vessels and thus remain in the tissue. Heart tissue from the normal, nonischemic zone (NZ) and the collateral-dependent, ischemic zone (CZ), which is the LAD perfusion territory, as well as a reference blood sample (via a carotid catheter) were then collected, weighed, and sent to BioPal (Worcester, MA) for analysis. When neutron activated, gold and samarium emit at different frequencies; thus the signals from each type of microsphere can be measured separately in the same animal. This enables us to obtain measurements of coronary blood flow at day 0 and day 10 of RI in the same animal. Boundaries of the CZ were determined both visually at the time of initial surgery as blanching upon LAD occlusion (occluder inflation) followed by reactive hyperemia upon reperfusion (occluder deflation) and by fluorescent microspheres (15 μM), which were injected into the LV at the same time and in the same manner as the first set of color microspheres (gold). Blood flow in the NZ and the CZ (ml·min−1·g−1) was calculated as described previously, according to the formula: blood flow = [(radioactive count in myocardial tissue) × (blood withdrawal rate)/(radioactive count in blood)]/(myocardial tissue weight) (21). Results were expressed as the CZ-to-NZ (CN/NZ) flow ratio at day 10 of RI. Experiments were n = 3 [enhanced green fluorescent protein (EGFP)-Adv-treated animals] or n = 6 [MyrAkt-Adv29- and DN-Akt-Adv(Thr308Ala/Ser473Ala)29-treated animals]. Results were analyzed by two-way ANOVA followed by Bonferroni correction. P < 0.05 determined statistical significance. These measurements were performed in WKY and JCR:LA-cp rats in the following groups: sham (animal was instrumented and given MyrAkt-Adv, DN-Akt-Adv, or EGFP-Adv but did not undergo RI), RI + EGFP-Adv, RI + candesartan (3 mg/100 ml in drinking water for 21 days) + EGFP-Adv, RI + MyrAkt-Adv or DN-Akt-Adv, and RI + candesartan + MyrAkt-Adv or DN-Akt-Adv. The adenoviral constructs (DN-Akt-Adv, MyrAkt-Adv, and EGFP-Adv) were injected at 2 × 1012 plaque-forming units (PFU) in isotonic saline by direct injection into the LV cavity at the time of initial surgery (day 0 RI) during a 20-s aortic occlusion followed by a 40-s LAD occlusion. Body weight was measured at day 0 and day 10 of RI, and heart weight (total, LV, and RV) was measured at day 10 of RI (after death) in every animal.
In n = 3 animals per group in groups treated with either DN-Akt-Adv or MyrAkt-Adv, blood flow was measured at day 10 of RI before and after intravenous administration of adenosine (5 × 105 M). Because adenosine did not alter blood flow, these animals were combined with the remaining n = 3 animals, which were not treated with adenosine, for statistical analysis.
Echocardiography.
All measurements were performed while the animals were under sevofluorene (1–2%) anesthesia with continuous monitoring of body temperature, blood pressure, and heart rate. Left ventricular end-diastolic diameter and end-systolic diameter, septal wall diastolic thickness, and left ventricular free (posterior) wall diastolic thickness were measured by two-dimensional guided M-mode echocardiography from the parasternal long-axis view by using a 12- to 3- MHz vascular probe (Vevo 770, 1,000 fps; Visual Sonics, Toronto, ON, Canada). Left ventricular end diastolic and systolic volumes and ejection fraction were calculated using Vevo software (Visual Sonics). All measurements were n = 3 animals per group at day 0 and 10 of RI and were analyzed by two-way ANOVA followed by Bonferroni correction. P < 0.05 determined statistical significance.
Western blot analysis.
Hearts were excised at day 0, 3, or 10 of RI, the CZ separated from the NZ, snap-frozen, and homogenized in lysis buffer containing 0.1% SDS and 1% Triton as described previously.(18, 21, 22). Phospho-specific anti-Akt (Ser473) and p70S6 kinase, anti-total Akt and p70S6 kinase (Cell Signaling, Danvers, MA), or anti-AT1R antibodies (Abcam, Cambridge, MA) were used for Western blotting. Bands were visualized by enhanced chemiluminescence (Amersham, Piscataway, NJ) and quantified using Un-Scan-It Image software. All experiments were n = 3 animals per group for each time point (day 0, 3, or 10 of RI) and were analyzed by two-way ANOVA followed by Bonferroni correction. P < 0.05 determined statistical significance.
For quantitative determination of Adv-mediated transduction efficiency, nitrocellulose membranes containing samples from three EGFP-Adv and three nontreated WKY and JCR hearts (NZ and CZ) harvested postmortem at day 3 or 10 of RI were probed with anti-EGFP antibodies (Thermo Scientific Pierce, Pittsburgh, PA) and then reprobed with anti-β-myosin heavy chain (β-MHC) (Abcam, Cambridge, MA), anti-smooth muscle (SM)-α-actin (Sigma Aldrich, St. Louis, MO), or anti-Tie-2 (Santa Cruz Biotechnology, Santa Cruz, CA) antibodies. EGFP-to-β-MHC, EGFP-to-SM-α-actin, and EGFP-to-Tie-2 ratios were obtained and expressed as means ± SE % of EGFP-transduced cells of each of the three cell types.
Immunohistochemistry (immunofluorescence).
Snap-frozen, OCT-imbedded cardiac tissue was cut into 10-μm sections. Slides were stained with the anti-β-MHC, anti-SM-α-actin, or anti-Tie-2 primary antibodies, followed by Alexa568-conjugated secondary antibodies (Invitrogen, Carlsbad, CA). Red (Alexa568) and green (EGFP) fluorescence was visualized, quantified and representative images collected using a Nikon fluorescent microscope equipped with Nikon Elements software. Transduction efficiency for cardiac myocytes was quantified by counting all yellow myocytes, indicative of anti-β-MHC (red) and EGFP (green) costaining on merged images and red fluorescent myocytes on anti-β-MHC-stained images per microscopic field in 20 individual fields per slide (10 in the CZ and 10 in the NZ) in 10 slides total. Counts were averaged, and the ratio of yellow myocytes to red myocytes was obtained. This strategy was repeated for vascular smooth muscle cells (VSMCs; on anti-SM-α-actin-stained images) and endothelial cells (ECs; on anti-Tie-2-stained images) from 10 separate coronary vessels of varying diameters (100–500 μM) per slide (5 in the CZ and 5 in the NZ) in a total of 10 slides.
Myocardial oxidative stress measurements.
Myocardial oxidative stress [superoxide anion radical (O2·−)] production was evaluated using X-band electroparamagnetic resonance in a Bruker EMX spectrometer using 1-hydroxy-3-carboxy-pyrrolidine (Alexis, Cornerstone, CT) as a spin-trap, as previously described (22). Briefly, animals underwent two consecutive periods of ischemia/reperfusion and were killed, the heart was removed, and the CZ was separated from the NZ. Tissue was then homogenized by sonication on ice, and supernatants were collected. 1-Hydroxy-3-carboxy-pyrrolidine (238 μg/100 mg tissue) was added immediately. O2·− concentration was calculated from arbitrary units (3.4 × 106 arbitrary units/nM). Electroparamagnetic resonance measurements were n = 3 animals per group. Data were analyzed by two-way ANOVA followed by Bonferroni correction. P < 0.05 determined statistical significance.
RESULTS
Significant differences in body weight, cardiac structure, and function between WKY and JCR:LA-cp rats are presented in Tables 1, 2, and 3. These results demonstrate that the JCR:LA-cp animals exhibit hypertension and cardiac hypertrophy but retain normal cardiac function. Of direct relevance to this study, these results also demonstrate that while the AT1R blocker candesartan lowered blood pressure (afterload), as expected, it did not have a significant effect on LV hypertrophy (Table 1). Neither DN-Akt-Adv nor MyrAkt-Adv significantly altered cardiac structure or function, as indicated by measurements of heart weight, LV-to-RV weight ratio, LV weight, wall thickness, end-diastolic and end-systolic diameter, and ejection fraction of treated compared with nontreated animals (Table 1).
Table 1.
WKY and JCR:LA-cp rats were treated with MyrAkt-Adv or DN-Akt-Adv as indicated but did not undergo the RI protocol
| Sham (No Adv) |
DN-Akt-Adv |
MyrAkt-Adv |
||||
|---|---|---|---|---|---|---|
| Day 0 | Day 10 | Day 0 | Day 10 | Day 0 | Day 10 | |
| WKY (n = 3) | ||||||
| BW, g | 325 ± 13 | 328 ± 7 | 318 ± 8 | 319 ± 15 | 325 ± 12 | 329 ± 11 |
| MAP, mmHg | 108 ± 13 | 106 ± 12 | 107 ± 6 | 98 ± 19 | 105 ± 12 | 105 ± 11 |
| HW, g | — | 1.07 ± 0.02 | — | 1.05 ± 0.01 | — | 1.06 ± 0.03 |
| LVW, g | — | 0.80 ± 0.02 | — | 0.80 ± 0.03 | — | 0.80 ± 0.01 |
| LVW/RVW | — | 2.96 ± 0.02 | — | 3.2 ± 0.05 | — | 3.07 ± 0.02 |
| LVWT,d, mm | 1.9 ± 0.01 | 2.01 ± 0.1 | 1.92 ± 0.03 | 1.92 ± 0.02 | 2.03 ± 0.04 | 2.02 ± 0.04 |
| IVSWT,d, mm | 1.56 ± 0.06 | 1.55 ± 0.2 | 1.55 ± 0.02 | 1.55 ± 0.04 | 1.55 ± 0.01 | 1.55 ± 0.02 |
| LVEDD, mm | 7.5 ± 0.02 | 7.6 ± 0.07 | 7.6 ± 0.08 | 7.6 ± 0.07 | 7.4 ± 0.01 | 7.4 ± 0.03 |
| LVESD, mm | 4.2 ± 0.1 | 4.2 ± 0.02 | 4.3 ± 0.01 | 4.3 ± 0.01 | 4.2 ± 0.04 | 4.3 ± 0.07 |
| LVEDV, ml | 302 ± 4 | 302 ± 2 | 301 ± 4 | 302 ± 8 | 303 ± 7 | 302 ± 2 |
| LVESV, ml | 74 ± 4 | 77 ± 9 | 76 ± 7 | 73 ± 8 | 71 ± 8 | 74 ± 6 |
| EF, % | 75 ± 2 | 75 ± 4 | 75 ± 5 | 76 ± 2 | 77 ± 1 | 75 ± 3 |
| JCR:LA-cp (n = 3) | ||||||
| BW, g | 697 ± 12* | 703 ± 14* | 700 ± 8* | 709 ± 10* | 694 ± 14* | 707 ± 4* |
| MAP, mmHg | 163 ± 10* | 158 ± 8* | 157 ± 6* | 161 ± 11* | 165 ± 14* | 165 ± 4* |
| HW, g | — | 1.25 ± 0.01* | — | 1.26 ± 0.02* | — | 1.25 ± 0.02* |
| LVW, g | — | 1.03 ± 0.03* | — | 1.04 ± 0.02* | — | 1.03 ± 0.02* |
| LVW/RVW | — | 4.68 ± 0.02* | — | 4.72 ± 0.03* | — | 4.68 ± 0.05* |
| LVWT,d, mm | 2.66 ± 0.02* | 2.64 ± 0.04* | 2.65 ± 0.03* | 2.64 ± 0.05* | 2.66 ± 0.04* | 2.66 ± 0.04* |
| IVSWT,d, mm | 2.1 ± 0.06* | 2.09 ± 0.5* | 2.1 ± 0.07* | 2.10 ± 0.05* | 2.1 ± 0.04* | 2.09 ± 0.02* |
| LVED, mm | 6.2 ± 0.1* | 6.2 ± 0.07* | 6.2 ± 0.01* | 6.2 ± 0.1* | 6.2 ± 0.04* | 6.3 ± 0.03* |
| LVESD, mm | 3.3 ± 0.07* | 3.2 ± 0.03* | 3.4 ± 0.04* | 3.3 ± 0.06* | 3.2 ± 0.01* | 3.2 ± 0.03* |
| LVEDV, ml | 200 ± 7* | 199 ± 9* | 200 ± 3* | 201 ± 8* | 200 ± 9* | 200 ± 7* |
| LVESV, ml | 49 ± 3* | 51 ± 7* | 49 ± 4* | 52 ± 4* | 53 ± 8* | 55 ± 8* |
| EF, % | 75 ± 4 | 74 ± 6 | 76 ± 3 | 74 ± 4 | 73 ± 8 | 72 ± 7 |
Values are means ± SE. Rats were treated with Advs as indicated but did not undergo the repetitive ischemia (RI) protocol. BW, body weight; MAP, mean arterial pressure; HW, heart weight, LVW, left ventricular weight; RVW, right ventricular weight; LVWT,d, left ventricular free wall thickness (diastole); IVWT,d, septal wall thickness (diastole); LVEDD, left ventricular end diastolic diameter, LVESD, left ventricular end systolic diameter; LVEDV, left ventricular end diastolic volume; LVESV, left ventricular end systolic volume; EF, ejection fraction.
P < 0.05 vs. Wistar-Kyoto (WKY) rats.
Table 2.
WKY and JCR:LA-cp rats were treated with MyrAkt-Adv or DN-Akt-Adv as indicated and underwent the RI protocol
| RI (No Adv) |
DN-Akt-Adv + RI |
MyrAkt-Adv + RI |
||||
|---|---|---|---|---|---|---|
| Day 0 RI | Day 10 RI | Day 0 RI | Day 10 RI | Day 0 RI | Day 10 RI | |
| WKY (n = 3) | ||||||
| BW, g | 323 ± 7 | 328 ± 10 | 323 ± 5 | 326 ± 11 | 325 ± 1 | 329 ± 10 |
| MAP, mmHg | 103 ± 8 | 106 ± 8 | 106 ± 12 | 106 ± 9 | 111 ± 11 | 112 ± 15 |
| HW, g | — | 1.06 ± 0.02 | — | 1.05 ± 0.03 | — | 1.06 ± 0.01 |
| LVW, g, | — | 0.78 ± 0.02 | — | 0.75 ± 0.03 | — | 0.80 ± 0.04 |
| LVW/RVW | — | 2.79 ± 0.02 | — | 3.03 ± 0.03 | — | 3.07 ± 0.04 |
| LVWT,d, mm | 2.01 ± 0.04 | 2.01 ± 0.02 | 1.99 ± 0.05 | 2.01 ± 0.03 | 1.98 ± 0.02 | 2.01 ± 0.03 |
| IVSWT,d, mm | 1.55 ± 0.02 | 1.55 ± 0.4 | 1.55 ± 0.01 | 1.54 ± 0.04 | 1.55 ± 0.07 | 1.56 ± 0.04 |
| LVEDD, mm | 7.3 ± 0.04 | 7.3 ± 0.05 | 7.4 ± 0.02 | 7.4 ± 0.03 | 7.4 ± 0.09 | 7.5 ± 0.03 |
| LVESD, mm | 4.2 ± 0.01 | 4.2 ± 0.02 | 4.3 ± 0.1 | 4.3 ± 0.02 | 4.1 ± 0.02 | 4.1 ± 0.05 |
| LVEDV, ml | 302 ± 8 | 303 ± 6 | 303 ± 6 | 308 ± 10 | 304 ± 2 | 306 ± 7 |
| LVESV, ml | 74 ± 3 | 74 ± 6 | 74 ± 2 | 75 ± 2 | 75 ± 2 | 74 ± 1 |
| EF, % | 75 ± 5 | 76 ± 6 | 76 ± 3 | 76 ± 6 | 75 ± 2 | 76 ± 1 |
| JCR:LA-cp (n = 3) | ||||||
| BW, g | 699 ± 10* | 701 ± 17* | 685 ± 18* | 699 ± 16* | 694 ± 5* | 703 ± 9* |
| MAP, mmHg | 168 ± 12* | 168 ± 6* | 159 ± 11* | 167 ± 17* | 162 ± 13* | 165 ± 12* |
| HW, g | — | 1.25 ± 0.02* | — | 1.25 ± 0.01* | — | 1.24 ± 0.04* |
| LVW, g | — | 1.03 ± 0.04* | — | 1.03 ± 0.02* | — | 1.02 ± 0.03* |
| LVW/RVW | — | 4.68 ± 0.03* | — | 4.68 ± 0.06* | — | 4.64 ± 0.06* |
| LVWT,d, mm | 2.59 ± 0.06* | 2.60 ± 0.2* | 2.66 ± 0.01* | 2.67 ± 0.05* | 2.69 ± 0.05* | 2.69 ± 0.01* |
| IVSWT,d, mm | 2.11 ± 0.01* | 2.11 ± 0.5* | 2.11 ± 0.03* | 2.12 ± 0.01* | 2.1 ± 0.03* | 2.11 ± 0.02* |
| LVEDD, mm | 6.2 ± 0.01* | 6.2 ± 0.03* | 6.2 ± 0.04* | 6.2 ± 0.08* | 6.3 ± 0.02* | 6.3 ± 0.03* |
| LVESD, mm | 3.3 ± 0.04* | 3.3 ± 0.06* | 3.2 ± 0.02* | 3.3 ± 0.04* | 3.2 ± 0.07* | 3.2 ± 0.03* |
| LVEDV, ml | 195 ± 2* | 193 ± 5* | 203 ± 5* | 203 ± 8* | 199 ± 1* | 196 ± 4* |
| LVESV, ml | 55 ± 5* | 55 ± 2* | 60 ± 3* | 59 ± 2* | 54 ± 11* | 53 ± 8* |
| EF, % | 72 ± 5 | 72 ± 2 | 70 ± 6 | 71 ± 6 | 73 ± 9 | 73 ± 5 |
Values are means ± SE. Rats underwent 0 or 10 days of RI.
P < 0.05 vs. WKY.
Table 3.
WKY and JCR:LA-cp rats were treated with candesartan plus MyrAkt-Adv or DN-Akt-Adv as indicated and underwent the RI protocol
| RI + Cand (No Adv) |
DN-Akt-Adv + RI + Cand |
MyrAkt-Adv + RI + Cand |
||||
|---|---|---|---|---|---|---|
| Day 0 RI | Day 10 RI | Day 0 RI | Day 10 RI | Day 0 RI | Day 10 RI | |
| WKY (n = 3) | ||||||
| BW, g | 325 ± 13 | 325 ± 17 | 316 ± 9 | 317 ± 17 | 320 ± 2 | 326 ± 10 |
| MAP, mmHg | 91 ± 2†‡ | 85 ± 9†‡ | 94 ± 9†‡ | 87 ± 12†‡ | 92 ± 11†‡ | 85 ± 7†‡ |
| HW, g | — | 1.04 ± 0.03 | — | 1.04 ± 0.03 | — | 1.05 ± 0.02 |
| LVW, g | — | 0.78 ± 0.03 | — | 0.78 ± 0.04 | — | 0.79 ± 0.05 |
| LVW/RVW | — | 3.00 ± 0.05 | — | 3.00 ± 0.03 | — | 3.03 ± 0.05 |
| LVWT,d, mm | 1.89 ± 0.01 | 1.89 ± 0.06 | 1.97 ± 0.04 | 2.98 ± 0.06 | 2.06 ± 0.04 | 2.05 ± 0.02 |
| IVSWT,d, mm | 1.54 ± 0.02 | 1.54 ± 0.2 | 1.54 ± 0.03 | 1.55 ± 0.04 | 1.55 ± 0.03 | 1.55 ± 0.02 |
| LVEDD, mm | 7.5 ± 0.04 | 7.5 ± 0.03 | 7.4 ± 0.02 | 7.4 ± 0.05 | 7.5 ± 0.08 | 7.5 ± 0.02 |
| LVESD, mm | 4.3 ± 0.02 | 4.3 ± 0.02 | 4.3 ± 0.02 | 4.3 ± 0.03 | 4.2 ± 0.03 | 4.2 ± 0.05 |
| LVEDV, ml | 304 ± 3 | 302 ± 1 | 303 ± 9 | 304 ± 6 | 304 ± 7 | 305 ± 7 |
| 72 ± 3 | 72 ± 4 | 72 ± 4 | 75 ± 3 | 75 ± 3 | 75 ± 5 | |
| EF, % | 76 ± 3 | 76 ± 2 | 76 ± 4 | 75 ± 4 | 75 ± 4 | 75 ± 6 |
| JCR:LA-cp (n = 3) | ||||||
| BW, g | 704 ± 10* | 710 ± 6* | 699 ± 11* | 708 ± 16* | 699 ± 14* | 703 ± 9* |
| MAP, mmHg | 134 ± 6*†‡ | 128 ± 5*†‡ | 139 ± 11*†‡ | 125 ± 16*†‡ | 139 ± 15*†‡ | 127 ± 4*†‡ |
| HW, g | — | 1.23 ± 0.03* | — | 1.25 ± 0.02* | — | 1.24 ± 0.02* |
| LVW, g | — | 1.01 ± 0.01* | — | 1.03 ± 0.01* | — | 1.02 ± 0.03* |
| LVW/RVW | — | 4.59 ± 0.05* | — | 4.68 ± 0.04* | — | 4.64 ± 0.02* |
| LVWT,d, mm | 2.64 ± 0.02* | 2.63 ± 0.6* | 2.66 ± 0.06* | 2.66 ± 0.02* | 2.59 ± 0.01* | 2.58 ± 0.06* |
| IVSWT,d, mm | 2.09 ± 0.06* | 2.01 ± 0.3* | 2.08 ± 0.04* | 2.08 ± 0.04* | 2.12 ± 0.07* | 2.13 ± 0.06* |
| LVEDD, mm | 6.4 ± 0.08* | 6.5 ± 0.03* | 6.2 ± 0.05* | 6.2 ± 0.1* | 6.5 ± 0.09* | 6.5 ± 0.03* |
| LVESD, mm | 3.3 ± 0.02* | 3.3 ± 0.02* | 3.3 ± 0.01* | 3.3 ± 0.04* | 3.2 ± 0.08* | 3.2 ± 0.02* |
| LVEDV, ml | 192 ± 12* | 198 ± 3* | 202 ± 4* | 198 ± 2* | 195 ± 5* | 200 ± 10* |
| LVESV, ml | 50 ± 9* | 55 ± 2* | 52 ± 7* | 52 ± 6* | 54 ± 6* | 53 ± 4* |
| EF, % | 74 ± 5 | 72 ± 2 | 74 ± 5 | 74 ± 4 | 72 ± 5 | 74 ± 6 |
Values are means ± SE. Rats underwent 0 or 10 days of RI and were treated with candesartan (Cand).
P < 0.05 vs. WKY;
P < 0.05 vs. sham;
P < 0.05 vs RI.
Adenovirus-mediated delivery of constitutively active Akt (MyrAkt-Adv) improves CCG in JCR:LA-cp rats.
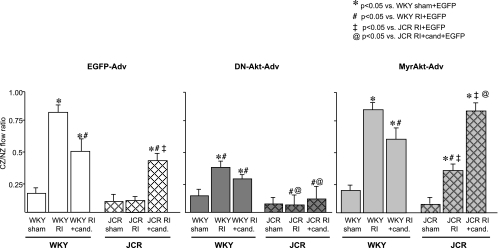
Our previous studies (22) have demonstrated that RI induced CCG in healthy WKY animals (at day 10 of RI CZ/NZ flow ratio was 0.84 ± 0.02 vs. 0.13 ± 0.02 in sham) but completely failed to induce CCG in the JCR:LA-cp animals (at day 10 of RI CZ/NZ flow ratio was 0.12 ± 0.02). In the present study, MyrAkt-Adv (2 × 1012 PFU) caused a significant but modest increase in collateral-dependent blood flow in JCR:LA-cp rats (at day 10 of RI CZ/NZ flow ratio was 0.38 ± 0.03) but had no effect on collateral-dependent blood flow in the WKY animals (at day 10 of RI CZ/NZ flow ratio was 0.82 ± 0.03; Fig. 1). EGFP-Adv was used to control for nonspecific effects of Adv delivery. There was no significant difference in the CZ/NZ flow ratio between EGFP-Adv-treated animals (Fig. 1) and nontreated animals (either WKY or JCR for all groups) (22), indicating that EGFP-Adv administration alone had no effect on CCG. Consistent with our previous findings (13, 22), although basal coronary flows tended to be slightly lower in the JCR animals, the difference was not statistically significant. In other words, neither NZ nor CZ flows at day 0 of RI were significantly different between WKY and JCR:LA-cp animals. Furthermore, NZ flows at day 10 of RI were not significantly different between WKY and JCR:LA-cp animals in any treatment group nor was there any difference in NZ flows within the individual rat strains regardless of treatment (data not shown).
Fig. 1.
Wistar-Kyoto rats (WKY) and JCR:LA-cp rats were treated with enhanced green fluorescent protein (EGFP)-Adv, MyrAkt-Adv, or dominant negative (DN)-Akt-Adv as indicated and underwent 10 days of repetitive ischemia (RI). Coronary flow was measured in the collateral-dependent, ischemic zone (CZ) and the normal, nonischemic zone (NZ) using microspheres during left anterior descending coronary artery occlusion and expressed as the ratio between CZ and NZ flows at day 10 of RI. *P < 0.05 vs. WKY sham + EGFP-Adv; #P < 0.05 vs. WKY RI + EGFP-Adv; ‡P < 0.05 vs. JCR RI + EGFP-Adv; @P < 0.05 vs. JCR RI + candesartan + EGFP-Adv.
Adenovirus-mediated delivery of constitutively active Akt (MyrAkt-Adv) with concomitant AT1R blockade results in complete restoration of CCG in the JCR:LA-cp rats.
We (22) have previously demonstrated that the AT1R blocker, candesartan (3 mg/100 ml in drinking water for 21 days) significantly improved CCG in the JCR:LA-cp rats but failed to restore it completely (at day 10 of RI CZ/NZ flow ratio was 0.45 ± 0.03 vs. 0.84 ± 0.02 for WKY rats). In contrast, candesartan caused a moderate decrease in collateral-dependent blood flow in the WKY animals (at day 10 of RI CZ/NZ flow ratio was 0.58 ± 0.04; ref. 22). In the present study, MyrAkt-Adv did not improve CCG in candesartan-treated WKY animals (at day 10 of RI CZ/NZ flow ratio was 0.58 ± 0.06; Fig. 1). However, concomitant treatment with candesartan and MyrAkt-Adv completely restored RI-induced CCG in the JCR:LA-cp rats (at day 10 of RI CZ/NZ flow ratio was 0.83 ± 0.03; Fig. 1). EGFP-Adv had no significant effect (Fig. 1). Neither candesartan alone nor MyrAkt-Adv had any significant effect on basal coronary flow (NZ flow at day 0 or 10 of RI or CZ flow on day 0 of RI).
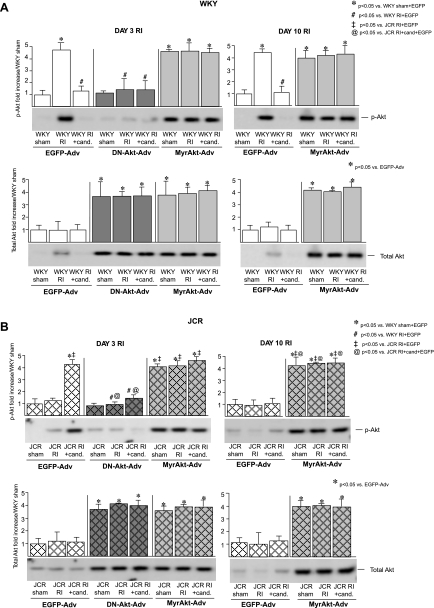
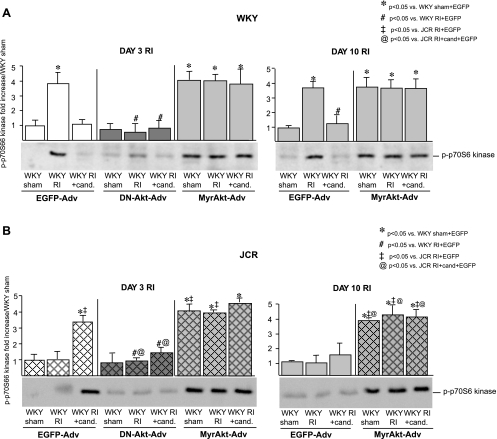
Successful MyrAkt delivery was confirmed by Western blotting for total Akt expression and Akt phosphorylation. Administration of 2 × 1012 PFU of MyrAkt-Adv resulted in a significant increase in total Akt expression (∼4 fold vs. EGFP-Adv) in whole heart homogenates from both WKY and JCR:LA-cp rats 48–72 h after injection (day 3 of the RI protocol) and remained elevated throughout day 10 of the RI protocol (Fig. 2). Akt phosphorylation also increased significantly 72 h postinjection (∼4-fold vs. EGFP-Adv), corresponding with the previously reported RI-induced increase in Akt phosphorylation beginning at day 3 of RI (22) and remained elevated for the duration of the 10-day RI protocol in all WKY and JCR:LA-cp rats receiving MyrAkt-Adv (Fig. 2). To confirm that Akt phosphorylation resulted in Akt activation, we evaluated the phosphorylation of p70S6 kinase, an immediate downstream target of Akt, and found a similar increase in phosphorylation (∼3.5-fold vs. EGFP-Adv or no Adv) with the temporal pattern of phosphorylation identical to that of Akt (Fig. 3). Total p70S6 kinase expression did not change in any treatment group (data not shown). EGFP-Adv administration had no effect on Akt expression, phosphorylation, or p70S6 kinase phosphorylation (Figs. 2 and 3).
Fig. 2.
A: WKY rats were treated with EGFP-Adv, MyrAkt-Adv, or DN-Akt-Adv as indicated and underwent 3 or 10 days of RI as indicated. Top: representative Western blots with phospho-specific anti-Akt (pAkt, Ser473) antibodies and cumulative data as area × density (arbitrary units) for pAkt in the CZ. *P < 0.05 vs. WKY sham + EGFP-Adv; #P < 0.05 vs. WKY RI + EGFP-Adv. Bottom: representative Western blots with and anti-total Akt (Total Akt) antibodies and cumulative data as area × density (arbitrary units) for total Akt in the CZ. *P < 0.05 vs. WKY sham + EGFP-Adv; #P < 0.05 vs. WKY RI + EGFP-Adv. B: same as A except JCR:LA-cp rats were treated with EGFP-Adv, MyrAkt-Adv, or DN-Akt-Adv as indicated and underwent 3 or 10 days of RI as indicated. *P < 0.05 vs. WKY sham + EGFP-Adv; ‡P < 0.05 vs. JCR RI + EGFP-Adv; @P < 0.05 vs. JCR RI + candesartan + EGFP-Adv.
Fig. 3.
A: WKY rats were treated with EGFP-Adv, MyrAkt-Adv, or DN-Akt-Adv and underwent 3 or 10 days of RI as indicated. Representative Western blots with phospho-specific anti-p70S6 kinase antibodies and cumulative data as area × density (arbitrary units) for pp70S6 kinase in the CZ. *P < 0.05 vs. WKY sham + EGFP-Adv; #P < 0.05 vs. WKY RI + EGFP-Adv. B: same as A except JCR:LA-cp rats were treated with EGFP-Adv, MyrAkt-Adv, or DN-Akt-Adv as indicated and underwent 3 or 10 days of RI as indicated. *P < 0.05 vs. WKY sham + EGFP-Adv; ‡P < 0.05 vs. JCR RI + EGFP-Adv; @P < 0.05 vs. JCR RI + candesartan + EGFP-Adv.
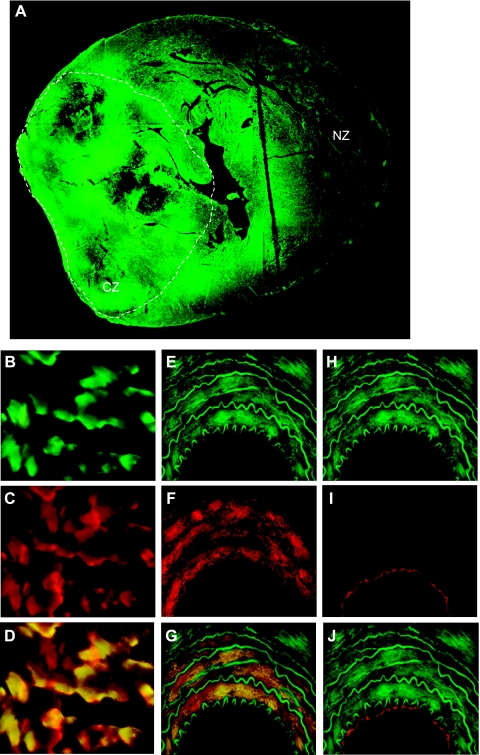
Transduction efficiency was evaluated by administering EGFP-Adv, which has an identical viral backbone as MyrAkt-Adv. Cell-specific markers, β-MHC, the expression of which is confined specifically to cardiac myocytes, SM-α-actin, the expression of which is confined specifically to VSMC or Tie-2, the expression of which is confined specifically to ECs, were used to determine percentage of EGFP-transduced cardiac myocytes, VSMCs, and ECs. Transduction efficiencies were as follows: myocytes (83 ± 4% in the CZ, 66 ± 2% in the NZ), VSMC (82 ± 3% in the CZ, 61 ± 4% in the NZ), and EC (3 ± 1% in the CZ, 4 ± 2% in the NZ) (data not shown). High Adv-mediated transduction efficiency of cardiac myocytes and vascular cells in the CZ with the exception of ECs was confirmed by immunofluorescence using anti-β-MHC, anti-SM-α-actin, or anti-Tie-2 antibodies (see Fig. 6). No adverse effects, such as cardiomyopathy or heart failure, which can be associated with long-term Adv delivery, were observed. There were no significant changes in heart weight, LV-to-RV weight ratio, LV weight, wall thickness, end-diastolic or end-systolic diameter, or ejection fraction compared with non-Adv-treated animals (Table 1). Similarly, the Advs had no effect on mortality (4% for JCR:LA-cp and 0% for WKY rats that survived the initial surgery).
Fig. 6.
Red and green fluorescence in cardiac cross sections. A: EGFP in the whole heart (×2). Dashed line approximates the CZ. B: EGFP in CZ myocytes (×40). C: β-myosin heavy chain-stained CZ myocytes (×40). D: merge of B and C. E: EGFP in a coronary artery wall (×40). F: smooth muscle-α-actin-stained coronary artery wall (×40) G: merge of E and F. H: EGFP in a coronary artery wall (×40). I: Tie-2-stained coronary artery wall (×40). J: merge of H and I.
Adenovirus-mediated delivery of dominant negative Akt (DN-Akt-Adv) reverses the beneficial effect of AT1R blockade on CCG in the JCR:LA-cp rats.
To further investigate the importance of Akt signaling in the regulation of CCG, we administered the DN-Akt-Adv (2 × 1012 PFU) to candesartan-treated JCR:LA-cp rats. Administration of DN-Akt-Adv resulted in complete reversal of the increase in RI-induced collateral-dependent blood flow achieved by candesartan alone (at day 10 of RI CZ/NZ flow ratio was 0.12 ± 0.08 vs. 0.45 ± 0.03 for RI + candesartan) (22) (Fig. 1). DN-Akt-Adv also significantly decreased RI-induced CCG in the WKY animals with or without candesartan (at day 10 of RI CZ/NZ flow ratio was 0.30 ± 0.02 and 0.39 ± 0.03, respectively, vs. 0.58 ± 0.04 and 0.84 ± 0.02 without DN-Akt-Adv, respectively) (22) (Fig. 1).
Successful DN-Akt-Adv delivery was confirmed by Western blotting for total Akt expression and Akt phosphorylation. Comparable to MyrAkt-Adv, administration of 2 × 1012 PFU of DN-Akt-Adv resulted in a significant increase in total Akt expression (∼4-fold vs. EGFP-Adv) 72 h after injection (Fig. 2) and remained elevated throughout day 10 of the RI protocol as reported previously (21). DN-Akt-Adv significantly decreased Akt and p70S6 kinase phosphorylation in candesartan-treated JCR:LA-cp and WKY rats at day 3 [∼4- and ∼3.5-fold, respectively, vs. EGFP-Adv (Figs. 2 and 3) and through day 10 of RI] (21). Total p70S6 kinase expression did not change (data not shown). As with MyrAkt-Adv, no adverse effects were observed (Table 1).
We (21) have previously shown that vascular reactivity does not affect measurements of CCG in the coronary vasculature in either the WKY or the JCR:LA-cp rats. However, since Akt is a known regulator of endothelial nitric oxide synthase (eNOS) activation, to exclude possible confounding effects of vascular reactivity on assessment of CCG in this study, n = 3 animals from each MyrAkt-Adv- and DN-Akt-Adv-treated group were treated with a vasodilator adenosine (5 × 105 M) before blood flow measurements at day 10 of the RI protocol. There was no difference in blood flow between these animals and the animals that did not receive adenosine (data not shown).
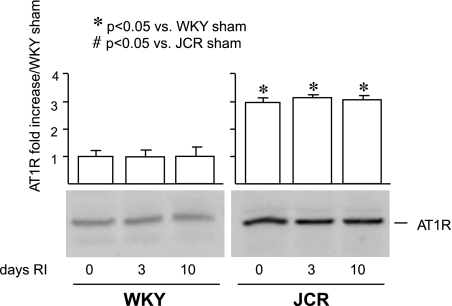
Basal AT1R levels are elevated in JCR:LA-cp rats.
Since this study utilized AT1R blockers, we assessed basal as well as RI-induced AT1R levels in an effort to explain the effect of candesartan on CCG. Baseline AT1R levels were elevated in the JCR:LA-cp animals (∼3-fold) but were not altered by RI in either rat strain for the duration of the RI protocol (Fig. 4).
Fig. 4.
WKY and JCR:LA-cp rats underwent 0, 3, or 10 days of RI as indicated. Representative Western blots with anti-angiotensin type I receptor (AT1R) antibodies and cumulative data as area × density (arbitrary units) for AT1R in the CZ. *P < 0.05 vs. WKY sham; #P < 0.05 vs. JCR sham.
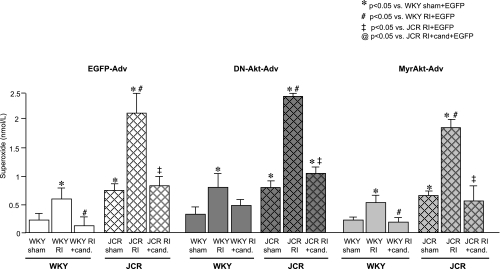
Finally, since we have previously shown that candesartan lowered oxidative stress in both rat strains (22), we examined the effects of MyrAkt-Adv and DN-Akt-Adv on myocardial O2·−. MyrAkt-Adv elicited a small decrease and DN-Akt-Adv a small increase in myocardial O2·− in both the CZ and the NZ, but these effects were not statistically significant (Fig. 5). EGFP-Adv had no effect on myocardial O2·− (data not shown) (22).
Fig. 5.
Cumulative results for superoxide anion radical (O2·−) levels in the CZ of WKY and JCR:LA-cp rats treated with EGFP-Adv, MyrAkt-Adv, or DN-Akt-Adv as indicated at day 3 of RI. *P < 0.05 vs. WKY sham + EGFP-Adv; ‡P < 0.05 vs. JCR RI + EGFP-Adv; @P < 0.05 vs. JCR RI + candesartan + EGFP-Adv.
DISCUSSION
The most important and clinically relevant finding in our study is that sustained Akt activation with concomitant AT1R blockade resulted in complete restoration of CCG in a rat model that mimics the human metabolic syndrome and transient, repetitive ischemia characteristic of stable angina pectoris. Sustained Akt activation alone improved, but did not fully restore, RI-induced CCG in the rat model of the metabolic syndrome. This is likely due to the existence of AT1R -dependent but Akt-independent signaling pathways that are also important for CCG. Consistent with this notion, we (22) have previously shown that activation of the p38 MAPK pathway, which is independent of Akt, was fully restored by AT1R blockade in the JCR:LA-cp rat. In contrast, blockade of Akt activation significantly impaired RI-induced CCG in normal, healthy animals and reversed the beneficial effect of AT1R blockade on RI-induced CCG in the metabolic syndrome.
These findings are in agreement with our recent results (21) demonstrating that Akt was required for CCG in normal, healthy animals and with a report (16) that a genetic knockout of an upstream regulator of Akt, PI3-kinase, reduced collateral growth induced by hindlimb ischemia. Also, although a process distinct from collateral growth, ischemia- and VEGF-induced angiogenesis was impaired in Akt knockout mice (1). Short-term Akt activation (2 wk) induced physiological hypertrophy accompanied by increased vascular density and enhanced expression of proangiogenic growth factors (31). In contrast, Akt activation has been associated with impaired angiogenesis: angiogenesis in response to hindlimb ischemia was increased in Akt1−/− mice (19). Long-term Akt activation (6 wk) led to pathological hypertrophy associated with reduced capillary density, blunted p70S6 kinase activation, and reduced VEGF expression (31).
The controversy surrounding Akt and its effects on vascular growth might relate to the magnitude and duration of its activation; however, the mechanisms responsible for these differential effects are unclear. Prolonged Akt activation could negatively regulate insulin signaling through insulin response substrate-1 (20, 32), which has been shown to mediate protein synthesis including VEGF synthesis (36). The implications of these findings on Akt-dependent regulation of CCG in the metabolic syndrome, however, are difficult to predict since the metabolic syndrome is characterized by impaired insulin signaling and may not be affected by insulin response substrate-1 downregulation. On the other hand, the magnitude of Akt activation may also modulate its effect on vascular growth. Akt is known to increase NO production, and NO has been shown to decrease cell proliferation in favor of promoting cell differentiation (3). Thus one explanation for the negative effects of Akt activation on collateral growth may be that excessive Akt activation in the early phase of collateral formation when VSMC de-differentiate (30) to proliferate and migrate is detrimental to CCG. Since the level of Akt activation achieved by the MyrAkt-Adv construct in our study was similar to that achieved by RI alone and compatible with CCG in the normal, healthy animal model (Fig. 2), we believe that excessive Akt activation was not a factor. In addition, in this study constitutively active Akt was highly expressed for ∼7 days, thus remaining within the 2-wk window associated with beneficial effects of Akt activation on angiogenesis. On the other hand, MyrAkt-Adv had no significant effect on basal coronary flow. We hypothesize that this is also due to the duration of MyrAkt expression in our study, which alone, without RI as a stimulus for coronary collateralization, was not sufficient to elicit increased coronary blood flow. Likewise, MyrAkt-Adv did not induce cardiac hypertrophy, likely because this process occurs over a longer period of time.
Another consideration relevant to our findings is the location of MyrAkt and DN-Akt expression, which impacts Akt activation. Based on EGFP expression, our adenoviral constructs efficiently infected cardiac myocytes and vascular smooth muscle but not the endothelium (Fig. 6). Since myocytes comprise 80% of cells in the whole heart by volume, the observed Akt expression and activation reflect changes primarily in myocytes with some contribution from fibroblasts and VSMCs. This could be related to the potential role of Akt in secretion of growth factors and matrix-degrading enzymes from myocytes and to a smaller extent VSMC survival, proliferation, migration, and de-differentiation. These processes are critical for CCG, and while they have not been shown to be regulated by Akt in collateral growth, they are known to be regulated by Akt in other systems.
Akt has been shown to mediate cell proliferation (32), protein synthesis (25), and VSMC differentiation in the absence of p38 MAPK and ERK1/2 activation (8). Our previous study demonstrated that p38 MAPK was not activated at days 5–10 of CCG (22). Thus sustained Akt activation in the present study could promote VSMC re-differentiation characteristic of the later stages of collateral development (30). Akt has also been recently implicated in the regulation of matrix metalloproteinase 9 (MMP9) in hepatic metastasis (5) and associated with increased MMP2 expression in de-differentiated VSMC (23). Increased MMP2 and 9 expression and activation in growing collaterals have been reported (4). MMP2 and 9 are secreted by VSMC, adventitial fibroblasts, and cardiac myocytes and mediate degradation of the components of the internal and the external elastic laminae (15), processes necessary for cell migration and thus collateral remodeling (30).
NO and VEGF have also been shown to be critical for collateral growth (30). Ample evidence indicates that VEGF activates eNOS by Akt-dependent phosphorylation on Ser1179 (2, 6). Our adenoviral constructs did not infect ECs and thus could not have regulated eNOS. However, all three NOS isoforms (eNOS, neuronal NOS, and inducible NOS) have been found in VSMC and cardiac myocytes (7). Our results further show that DN-Akt-Adv elicited a small but statistically insignificant increase in myocardial O2·− in both WKY and JCR rats, while MyrAkt-Adv caused a small but statistically insignificant decrease in myocardial O2·− (Fig. 5). The small magnitude of these changes may be related to the fact that Akt-Advs did not transduce ECs with any appreciable efficiency and thus could not have effected eNOS activation in ECs. These results further suggest that although myocytes and VSMC express eNOS, NO produced via Akt-dependant regulation of eNOS in these cells is too small in magnitude to have a significant impact on O2·− and may instead be used for regulation of other events, for example, VEGF expression. eNOS-dependent NO has been shown to induce VEGF synthesis by VSMC (7). NO induces VEGF synthesis by stabilizing HIF-1α and thus increasing VEGF expression, via an Akt-dependent pathway in VSMC and cardiac myocytes (7).
The role of the renin-angiotensin system in angiogenesis and collateral growth is controversial. AT1R blockade resulted in reduced collateral development and angiogenesis in a model of hindlimb ischemia (29). Hindlimb ischemia-induced angiogenesis and perfusion were significantly improved in AT2R knockout mice (33). Infusion of subpressor doses of ANG II increased NO and VEGF production as well as hindlimb perfusion in wild-type and AT2R knockout mice (33). In contrast, ACE inhibitors improved perfusion in a model of hindlimb ischemia, which was accompanied by increased VEGF and NO production (10). We believe that these disparate findings are critically related to the myocardial oxidative stress in the animal at the onset of treatment and, consequently, the activation of redox-sensitive signaling pathways involved in the regulation of collateral growth. The AT1R is a potent activator of the vascular and myocardial oxidases, including the NAD(P)H oxidase and mitochondrial oxidases. We (22) have previously shown elevated basal oxidative stress in the JCR:LA-cp animals, which is consistent with our novel observations that basal AT1R levels are elevated in the JCR:LA-cp animals (Fig. 4). In contrast to its effect on myocardial oxidative stress, transient, repetitive ischemia did not alter AT1R expression in either rat strain. This suggests that the RI-induced AT1R-dependent increase in oxidative stress is achieved primarily through activation of AT1R-mediated signaling rather than alterations in receptor number. We (22) have shown that AT1R blockade reduced basal and RI-induced oxidative stress below levels conducive to CCG and prevented Akt activation in healthy animals. In contrast, AT1R blockade reduced myocardial oxidative stress to levels permissive for CCG and associated with partial restoration of Akt activation in the JCR:LA-cp rats (22). Similar results were obtained when JCR:LA-cp and WKY rats were treated with the antioxidant apocynin (21, 22). These findings are likely due to the fact that Akt is a redox-sensitive kinase (36) and, like many redox-sensitive signaling pathways, requires neither too much nor too little oxidative stress for activation. Complete obliteration of O2·− or H202 abolished ANG II-dependent Akt activation in cultured VSMC (36). Our observations are also in agreement with earlier studies in which the ACE inhibitor captopril and the AT1R antagonist ibesartan reduced the incidence of myocardial lesions in the JCR:LA-cp rats without a significant effect on insulin and glucose metabolism or lipid levels (26, 28, 38), as well as a study demonstrating that AT1R blockade reduced oxidative stress and increased Akt activation in the aorta and VSMC from spontaneously hypertensive rats (11). Our study did not identify the reason why AT1R blockade failed to elicit sustained Akt activation despite reducing oxidative stress. However, we did not measure oxidative stress throughout the RI protocol. Thus it is possible that altered temporal regulation of oxidative stress by AT1R blockers is partially responsible.
In combination with our previous findings, these results suggest that sustained Akt activation is one of the redox-sensitive mediators required for CCG and that its activation is critically, but not exclusively, dependent on the amount of AT1R-dependent oxidative stress generation. AT1R blockade in the metabolic syndrome phenotype, with elevated basal oxidative stress, reduces oxidative stress (22) to within the boundaries of a window permissive for activation of redox-sensitive signaling pathways, including transient activation of Akt. In contrast, in the normal phenotype, AT1R blockade also reduces oxidative stress (22) but to levels that fall below the lower boundary of the window needed for activation of redox-sensitive signaling pathways, including Akt. Our finding that AT1R blockade alone cannot completely restore CCG in the metabolic syndrome, which correlates with incomplete (transient) Akt activation (22), also suggests that AT1R-independent signaling and/or redox-independent alterations in AT1R-dependent signaling, which regulate Akt activation in normal animals, may be compromised in the metabolic syndrome.
Our results are consistent with CCG-mediated cardioprotection. Further support for this notion is provided by our observation that candesartan did not reduce LV hypertrophy in the JCR:LAcp animals in our study. Thus structural cardiac remodeling was not the reason for improved coronary blood flow. This may be due to the fact that at the time of intervention, these animals have long-standing, established LV hypertrophy, which cannot be reversed by a relatively short candesartan treatment. In agreement with these results, we have observed a similar lack of effect of afterload reduction by a Ca2+ channel blocker on CCG in the metabolic syndrome (22).
Candesartan alone did not improve basal coronary flow in the metabolic syndrome animals, suggesting that the AT1R-mediated regulation of CCG is critically dependent on the oxidative state of the myocardium at the time of treatment. Basal (non-RI-induced) level of oxidative stress in JCR:LA-cp rats is equivalent to that induced by RI in WKY animals. However, regardless of oxidative stress levels, RI is a necessary stimulus for CCG, which may account for the fact that candesartan did not alter basal coronary flow in JCR:LA-cp rats.
A significant number of patients with established obstructive CAD, consequently at risk for myocardial infarction and in need of coronary revascularization, are currently treated with AT1R blockers for management of hypertension and complications associated with type II diabetes and the metabolic syndrome. Thus our finding that, in combination with AT1R blockade, sustained Akt activation is both necessary and sufficient for complete restoration of CCG may be of specific clinical significance to metabolic syndrome patients with episodes of transient cardiac ischemia, such as stable angina. However, the clinical applicability of our findings depends on resolution of a myriad of important issues. The large difference in heart size between rats and humans would likely affect transduction efficiency. The extended length of time required for coronary collateralization in larger animals may not be compatible with known deleterious effects of prolonged Akt activation. Also, cardiac function in our metabolic syndrome animals is normal. This is frequently not the case in patients that require coronary revascularization. Thus these studies would have to be conducted in larger animal models of coronary collateral growth, the metabolic syndrome, and heart failure. Finally, gender differences may have a profound effect on the role Akt plays in the regulation of CCG and would have to be examined.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant R01-HL-093052.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Ackah E, Yu J, Zoellner S, Iwakiri Y, Skurk C, Shibata R, Ouchi N, Easton R, Galasso G, Birnbaum M, Walsh K, Sessa W. Akt1/PKBalpha is critical for ischemic and VEGF-mediated angiogenesis. J Clin Invest 115: 2119–2127, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Boo YC, Sorescu G, Boyd N, Shiojima I, Walsh K, Du J, HJ Shear stress stimulates phosphorylation of endothelial nitric-oxide synthase at Ser1179 by Akt-independent mechanisms: role of protein kinase A. J Biol Chem 277: 3388–3396, 2002 [DOI] [PubMed] [Google Scholar]
- 3. Bussolati B, Dunk C, Grohman M, Kontos CD, Mason J, Ahmed A. Vascular endothelial growth factor receptor-1 modulates vascular endothelial growth factor-mediated angiogenesis via nitric oxide. Am J Pathol 159: 993–1008, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cai WJ, Koltai S, Kocsis E, Scholz D, Kostin S, Luo X, Schaper W, Schaper J. Remodeling of the adventitia during coronary arteriogenesis. Am J Physiol Heart Circ Physiol 284: H31–H40, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Chen JS, Wang Q, Fu XH, Huang XH, Chen XL, Cao LQ, Chen LZ, Tan HX, Li W, Bi J, Zhang LJ. Involvement of PI3K/PTEN/AKT/mTOR pathway in invasion and metastasis in hepatocellular carcinoma: association with MMP-9. Hepatol Res 39: 177–186, 2009 [DOI] [PubMed] [Google Scholar]
- 6. Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 399: 601–605, 1999 [DOI] [PubMed] [Google Scholar]
- 7. Dulak J, Józkowicz A. Regulation of vascular endothelial growth factor synthesis by nitric oxide: facts and controversies. Antioxid Redox Signal 5: 123–132, 2003 [DOI] [PubMed] [Google Scholar]
- 8. Hayashi K, Takahashi M, Kimura K, Nishida W, Saga H, Sobue K. Changes in the balance of phosphoinositide 3-kinase/protein kinase B (Akt) and the mitogen-activated protein kinases (ERK/p38MAPK) determine a phenotype of visceral and vascular smooth muscle cells. J Cell Biol 145: 727–740, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hughes GC, Annex BH. Angiogenic therapy for coronary artery and peripheral arterial disease. Expert Rev Cardiovasc Ther 3: 521–535, 2005 [DOI] [PubMed] [Google Scholar]
- 10. Iglarz M, Silvestre JS, Duriez M, Henrion D, Lévy BI. Chronic blockade of endothelin receptors improves ischemia-induced angiogenesis in rat hindlimbs through activation of vascular endothelial growth factor-no pathway. Arterioscler Thromb Vasc Biol 21: 1598–1603, 2001 [DOI] [PubMed] [Google Scholar]
- 11. Kawahara S, Umemoto S, Tanaka M, Umeji K, Matsuda S, Kubo M, Matsuzaki M. Up-regulation of Akt and eNOS induces vascular smooth muscle cell differentiation in hypertension in vivo. J Cardiovasc Pharmacol 45: 367–374, 2005 [DOI] [PubMed] [Google Scholar]
- 12. Koerselman J, van der Graaf Y, de Jaegere P, Grobbee D. Coronary collaterals: an important and underexposed aspect of coronary artery disease. Circulation 107: 2507–2511, 2003 [DOI] [PubMed] [Google Scholar]
- 13. Lee SU, Wykrzykowska JJ, Laham RJ. Angiogenesis: bench to bedside, have we learned anything? Toxicol Pathol 34: 3–10, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Liao JK. Fine-tuning the angiogenic response to vascular endothelial growth factor. Circ Res 103: 229–230, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu P, Sun M, Sader S. Matrix metalloproteinases in cardiovascular disease. Can J Cardiol, Suppl B: 25B–30B, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Madeddu P, Kraenkel N, Barcelos L, Siragusa M, Campagnolo P, Oikawa A, Caporali A, Herman A, Azzolino O, Barberis L, Perino A, Damilano F, Emanueli C, Hirsch E. Phosphoinositide 3-kinase gama gene knockout impairs postischemic neovascularization and endothelial progenitor cell function. Arterioscler Thromb Vasc Biol 28: 68–76, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Maulik N, Das D. Potentiation of angiogenic response by ischemic and hypoxic preconditioning of the heart. Cell Mol Med 6: 13–24, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Miller SJ, Norton LE, Murphy MP, Dalsing MC, Unthank JL. The role of the renin-angiotensin system and oxidative stress in spontaneously hypertensive rat mesenteric collateral growth impairment. Am J Physiol Heart Circ Physiol 292: H2523–H2531, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Miyauchi H, Minamino T, Tateno K, Kunieda T, Toko H, Komuro I. Akt negatively regulates the in vitro lifespan of human endothelial cells via a p53/p21-dependent pathway. EMBO J 23: 212–220, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. O'Neill BT, Abel ED. Akt1 in the cardiovascular system: friend or foe? J Clin Invest 115: 2059–2064, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Reed R, Potter B, Smith E, Jadhav R, Villalta P, Jo H, Rocic P. Redox-sensitive Akt and Src regulate coronary collateral growth in metabolic syndrome. Am J Physiol Heart Circ Physiol 296: H1811–H1821, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Reed R, Kolz C, Potter B, Rocic P. The mechanistic basis for the disparate effects of angiotensin II on coronary collateral growth. Arterioscler Thromb Vasc Biol 28: 61–67, 2008 [DOI] [PubMed] [Google Scholar]
- 23. Risinger GM, Jr, Hunt TS, Updike DL, Bullen EC, Howard EW. Matrix metalloproteinase-2 expression by vascular smooth muscle cells is mediated by both stimulatory and inhibitory signals in response to growth factors. J Biol Chem 281: 25915–25925, 2006 [DOI] [PubMed] [Google Scholar]
- 24. Rocic P, Kolz C, Reed R, Potter B, Chilian W. Optimal reactive oxygen species concentration and p38 MAP kinase are required for coronary collateral growth. Am J Physiol Heart Circ Physiol 292: H2729–H2736, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Ruggero D, Sonenberg N. The Akt of translational control. Oncogene 24: 7426–7434, 2005 [DOI] [PubMed] [Google Scholar]
- 26. Russell JC, Graham SE, Amy RM, Dolphin PJ. Inhibition of myocardial lesions in the JCR:LA-corpulent rat by captopril. J Cardiovasc Pharmacol 31: 971–977, 1998 [DOI] [PubMed] [Google Scholar]
- 27. Russell JC, Kelly SE, Proctor SD. The JCR:LA-cp rat: an animal model of the metabolic syndrome exhibiting micro- and macro-vascular disease. In: Animal Models of Diabetes, edited by Shafrir E. Baton Rouge, LA: CRC, 2007 [Google Scholar]
- 28. Russell JC, Kelly SE, Vine DF, Proctor SD. Irbesartan-mediated reduction of renal and cardiac damage in insulin resistant JCR:LA-cp rats. Br J Pharmacol 158: 1588–1596, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sasaki K, Murohara T, Ikeda H, Sugaya T, Shimada T, Shintani S, Imaizumi T. Evidence for the importance of angiotensin II type 1 receptor in ischemia-induced angiogenesis. J Clin Invest 109: 603–611, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schaper W. Collateral circulation: past and present. Basic Res Cardiol 104: 5–21, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shiojima I, Sato K, Izumiya Y, Schiokofer S, Ito M, Liao R, Colucci W, Walsh K. Disruption of coordinated cardiac hypertrophy and angiogenesis contributes to the transition to heart failure. J Clin Invest 115: 2108–2118, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shiojima I, Walsh K. Regulation of cardiac growth and coronary angiogenesis by the Akt/PKB signaling pathway. Genes Dev 20: 3347–3365, 2006 [DOI] [PubMed] [Google Scholar]
- 33. Silvestre JS, Tamarat R, Senbonmatsu T, Icchiki T, Ebrahimian T, Iglarz M, Besnard S, Duriez M, Inagami T, Lévy BI. Antiangiogenic effect of angiotensin II type 2 receptor in ischemia-induced angiogenesis in mouse hindlimb. Circ Res 90: 1072–1079, 2002 [DOI] [PubMed] [Google Scholar]
- 34. Toblli JE, Cao G, DeRosa G, Di Gennaro F, Forcada P. Angiotensin-converting enzyme inhibition and angiogenesis in myocardium of obese Zucker rats. Am J Hypertens 17: 172–180, 2004 [DOI] [PubMed] [Google Scholar]
- 35. Toyota E, Warltier D, Brock T, Ritman E, Kolz C, O'Malley P, Rocic P, Focardi M, Chilian W. Vascular endothelial growth factor is required for coronary collateral growth in the rat. Circulation 112: 2108–2113, 2005 [DOI] [PubMed] [Google Scholar]
- 36. Vander Haar E, Lee SI, Bandhakavi S, Griffin TJ, Kim DH. Insulin signaling to mTOR mediated by the Akt/PKB substrate PRAS40. Nat Cell Biol 9: 316–323, 2007 [DOI] [PubMed] [Google Scholar]
- 37. Walther T, Menrad A, Orzechowski HD, Siemeister G, Paul M, Schirner M. Differential regulation of in vivo angiogenesis by angiotensin II receptors. FASEB J 17: 2061–2067, 2003 [DOI] [PubMed] [Google Scholar]