Abstract
The maturation process of mammalian oocytes accompanies an extensive rearrangement of the cytoskeleton and associated proteins. As this process requires a delicate interplay between the cytoskeleton and its regulators, it is often targeted by various external and internal adversaries that affect the congression and/or segregation of chromosomes. Asymmetric cell division in oocytes also requires specific regulators of the cytoskeleton, including formin-2 and small GTPases. Recent literature providing clues regarding how actin filaments and microtubules interact during spindle migration in mouse oocytes are highlighted in this review.
Keywords: Oocyte, Spindle, Formin, GTP Phosphohydrolases, Human, Mice
Meiotic maturation in oocytes
Meiotic maturation of mammalian oocytes is an elaborate process which requires timely organization of the cell division apparatus. Oocytes within fetal ovaries are arrested at the prophase of meiosis I (PI), thus remaining tetraploid until puberty. Meiosis resumes when the cyclic stimulation of gonadotropins reaches the ovaries when organisms are sexually mature. Meiosis I is completed at the time of ovulation and Meiosis II at fertilization. The haploid genome forms the female pronucleus (PN) and produces a diploid zygote when fused with the male PN. This process involves spindle migration prior to cytokinesis to produce unequal daughter cells--one oocyte and one polar body (PB) [1]. It is also unique in that mammalian oocytes are devoid of centrosomes [2]. In the absence of centrosomes, components that are normally associated with these structures are located in satellite centers called microtubule organizing centers (MTOCs). More than 80 MTOCs are present in mouse oocytes and they contain components of centrosomes that are required for microtubule polymerization, such as γ-tubulin and pericentrin [3-5].
During PI, short and unstable microtubules are scattered throughout the cytoplasm. γ-tubulin ring structures are also scattered in the cytoplasm and they are responsible for the polymerization of these microtubules [1,2]. Upon germinal vesicle breakdown (GVBD), oocytes enter metaphase I (MI). Condensed chromosomes begin to interact with microtubules at many sites and this interaction is not limited to kinetochores [6]. In small cells, chromosomes are efficiently captured by microtubules and this is believed to be the mechanism of spindle assembly. However, in large cells like oocytes, chromosome congression has been shown to involve a contractile actin network along with microtubules [7]. Without the formation of a dynamic actin network, some chromosomes may fail to congress, leading to aneuploidy [7]. Once the chromosomes are all aligned and associated with microtubules, microtubules form bipolar arrays constituting the spindle. The meiotic spindle of mammalian oocytes exhibits a morphology typical of acentromeric cells, i.e., barrel-shaped without astral microtubules [2]. It is believed that kinetochore fibers are not involved in the formation of the first meiotic spindle [6]. This is a fundamental difference between meiosis and mitosis, as kinetochore fibers collaborate with motor proteins to move the chromosome-spindle complex in mitotic cells.
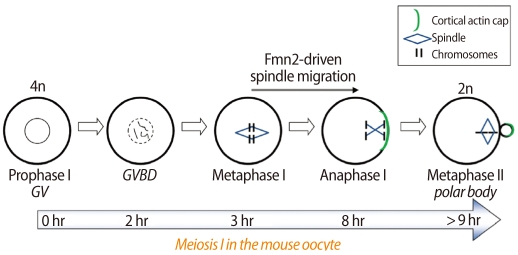
In oocytes, MI lasts for about 6-11 hours and spindle migration to the egg cortex occurs during this time [6] (Figure 1). When the chromosome-spindle complex moves toward the egg cortex, it always involves a spindle pole more closely located to the cortex. Spindle movement induces a cortical differentiation represented by the accumulation of actin filaments and a lack of microvilli [8]. After PB extrusion, chromosomes realign and proceed to metaphase II (MII).
Figure 1.
A diagram depicting the timeline of meiosis I in the mouse oocyte. Fmn2 seems to polymerize actin filaments, which are required for the migration of the chromosome-spindle complex during MI. Fmn2, formin-2; GV, germinal vesicle stage; GVBD, germinal vesicle breakdown; MI, metaphase I.
As we have reviewed above, meiosis in oocytes has several unique features. Herein we will review recent literature shedding light on the regulation of the cytoskeleton during meiotic maturation, focusing on small GTPases and formins.
Small GTPases in mouse oocytes
Small GTPases are homologous to the alpha subunit of G-protein-coupled receptors, but they act independently by hydrolyzing GTP to GDP in the cytoplasm [9]. They carry multiple functions in cell organization and movement by regulating both microtubule and actin filaments. There are five major subfamilies in the small GTPase superfamily, and in mouse oocytes, members of the Rho and Ran subfamilies are implicated in cytoskeletal functions [8].
Cdc42 and Rac are members of the Rho subfamily. In mouse oocytes, the role of these factors are well demonstrated by the use of specific inhibitors or by overexpression of mutant forms. Cdc42 is localized in the meiotic spindle as well as in the central spindle [10]. When Cdc42 is suppressed by overexpressing a mutant form, the spindle remains centrally located in mouse oocytes [11]. Interestingly, both spindle poles are stretched toward the cortex under this condition, thereby exhibiting unusually long morphology. These oocytes also cannot complete cytokinesis [11]. Other research also showed that siRNA injection to downregulate Cdc42 expression reduced the rate of PB extrusion [12]. IQ-domain GTPase-activating proteins I (IQGAP1) has recently been suggested as a downstream mediator of Cdc42 [10].
In the case of Rac, the GTP-bound active form is polarized at the cortical actin cap. Inhibition of Rac arrests oocytes at prometaphase I (PMI) resulting in misaligned chromosomes and elongated spindles. No spindle migration is observed. This result suggests a role for Rac in spindle stability and anchoring to the egg cortex [13].
Ran belongs to the Ran subfamily of small GTPases, and its function in meiosis is unique in that it acts as a diffusible signal emanating from the chromosomes to the cortex. When DNA-coated beads are injected into mouse oocytes, they behave like meiotic chromosomes and produce an ectopic cortical actin cap [14]. The formation of an ectopic actin cap is dependent on the distance between the injected DNA and the cortex. Ran-GTP is concentrated near chromosomes during the metaphase, and the inhibition of its activity causes disappearance of the cortical actin cap. It has been hypothesized that a Ran-GTP gradient drives the asymmetric positioning of the spindle [14]. However, Ran activity does not seem to affect spindle formation [15].
Formins in mouse oocytes
1. Formin-2
Formins are actin nucleators with highly conserved domains called formin homology (FH) domains [16,17]. Cappuccino, a formin in Drosophila, is involved in establishing egg polarity by regulating cytoplasmic streaming driven by microtubules [18]. There are multiple members of this domain in mammals, and among these, formin-2 (Fmn2), mDia1, mDia2, and mDia3 have been implicated in oocyte biology [19,20].
In mammalian cells, formin proteins are invariably involved in the nucleation of actin cables. The mechanisms of regulation are diverse and so are the types of cells associated with certain formin proteins [16]. In mouse oocytes, Fmn2 has been extensively studied. This interest originated from a knockout mouse study which showed that spindle migration during meiosis I is defective in oocytes from Fmn2-/- female mice [21]. From this study, a tug-and-pull interaction between microtubule and actin filaments mediated by Fmn2 was further suspected but no method was then able to visualize such interaction.
A breakthrough came in several independent works that demonstrated a role for Fmn2 as a scaffold for the cytoskeleton during spindle migration in oocytes. Azoury et al. [22] used a probe for an actin filament called Utrophin-EGFP. This fusion protein is made of the calponin-homology domain of utrophin, an actin-binding protein, and EGFP reporter. It has been shown to specifically bind to filamentous actin (F-actin) in many systems [23]. The sensitivity of this probe under live confocal microscopy finally enabled the visualization of F-actin cages, which are organized as spindle-like structures on the meiotic spindle. As meiosis I proceeds, F-actin accumulates between one spindle pole and the cortex, linking the chromosome-spindle complex to the egg cortex [22]. This is assumed to generate a mechanical force pulling the chromosome-spindle complex and thus making asymmetric division happen. In Fmn2-/- oocytes, the F-actin cage on the meiotic spindle is entirely missing [22]. We recently showed that Fmn2 protein is localized on the spindle during meiosis I [19]. Thus, this structure of the F-actin cage on the spindle seems to require the nucleation and elongation of actin filaments by Fmn2.
Schuh and Ellenberg [24] also took advantage of Fmn2-/- mice to visualize F-actin structures that position the spindle. The same actin probe, Utrophin-EGFP, was used to generate an improved image of actin filaments [23]. This work provided two important clues to understanding the mechanism of asymmetric spindle positioning in mouse oocytes. First, they showed that spindle positioning requires a dynamic network of actin filaments that are nucleated by Fmn2. Secondly, myosin-II, a motor protein for F-actin, is enriched in the spindle poles to pull actin filaments nucleated by Fmn2 [24].
Li et al. [25] took a different approach in visualizing the dynamic actin network in mouse oocytes. Lifeact, a newly devised probe for actin filaments was adopted [26]. However, the limitation of this probe is evident when compared to the results generated with Utrophin-EGFP, as Lifeact was only able to generate a rather diffused localization of an actin "cloud" near the spindle [25].
Nonetheless, all three studies provided solid evidence that actin filaments are formed on the meiotic spindle and that Fmn2 is primarily responsible for this F-actin structure. While these studies could not provide the precise localization of Fmn2, a later study showed its localization by immunofluorescence staining [19]. Fmn2 is localized on the meiotic spindle both in MI and MII, and nocodazole treatment to depolymerize the spindle also disrupted Fmn2 localization. Therefore, an intact spindle structure seems to be a prerequisite for Fmn2 localization. Fmn2 has also been shown to interact directly with microtubules in vitro [19]. An in vitro microtubule interaction assay using different domains of Fmn2 protein showed that the FH2 domain precipitates with polymerized microtubules. Thus, it is plausible that the spindle in mouse oocytes directly interacts with Fmn2, which at the same time nucleates the actin filaments necessary for asymmetric spindle positioning. The possibility of Fmn2 as a cytoskeletal scaffold linking the actin filaments and spindle is therefore presented from a series of studies using Fmn2 deficient mice.
2. mDia subfamily of formins
mDia genes are the mammalian homologs of Drosophila diaphanous. mDia proteins, unlike Fmn2, contain Rho-binding domains in the N-terminus, along with the conserved FH domains. GTPase-binding domain overlaps with another domain called FH3, which is thought to participate in autoregulation of mDia proteins [27]. As seen above, the potential role for Fmn2 in oocyte maturation has been extensively investigated. We have also shown that mDia1 and mDia2 exhibit specific localization in mouse oocytes. mDia1 exhibits a similar localization with Fmn2, on the microtubules of mouse oocytes [19]. This is reminiscent of mDia1 localization in mitotic cells where it has also been shown to be associated with microtubules of all kinds [28]. mDia2 is localized in spindle poles during meiosis along with γ-tubulin, suggesting that mDia2 is a component of the spindle pole complex. Similar localization has been noted in NIH3T3 cells [19]. In other cell systems, mDia2 has been implicated in the stabilization of microtubules and in the ability to induce actin scaffolding of the contractile ring during cytokinesis [29]. The expression and localization of mDia3 in mouse oocytes has not been elucidated. However, it is noteworthy that oocytes from patients with polycystic ovary syndrome (PCOS) express several-fold higher levels of mDia3 (DIAPH2 in humans) [20]. Further investigation is warranted to determine if overexpression of mDia3 has any adverse effect on mammalian oocytes.
All three mDia members have a recognized GTPase-binding domain at the N-terminus. mDia1 has been shown to be activated by Rho only, while mDia2 and mDia3 have been shown to be activated by Rho, Rac, and Cdc42 [30]. Whether mDia proteins expressed in mouse oocytes are influenced by these small GTPases needs further investigation.
Clinical implications of defective meiosis in human oocytes
Meiotic errors in human oocytes are considered the leading culprit of reproductive failures and show a high association with maternal age [31]. Because of the high incidence of trisomy in aged mothers, chromosome nondisjunction and failed recombination have been investigated as age-dependent factors of pregnancy failures [32]. Molecules involved in synapsis and recombination in homologous chromosomes are thus implicated as early targets of meiotic errors [31]. In contrast, gross morphological defects such as maturation arrest may have alternative causes involving errors from the dynamic organization and movement of the cytoskeleton [21]. Thus, small GTPases and formins are both good candidates for an investigation into the association between altered cytoskeleton dynamics and aneuploidy.
While these molecules have been heavily investigated in vertebrate oocyte and cell systems, information on their association with aneuploidy in human oocytes is scarce. In human follicular cells, Cdc42 has been implicated as one of the predictor genes of oocyte competence [33,34]. Higher expression of Cdc42 in follicle cells is positively correlated with pregnancy success. However, whether Cdc42 is expressed in human oocytes is not known.
Formins, on the other hand, are implicated in several clinical conditions. Such diversity in diseases probably comes from the generic nature of formins as actin nucleators. The DIAPH2 (mDia3) gene on the X chromosome was disrupted in a patient with premature ovarian syndrome [35]. An autosomal dominant mutation in the DIAPH1 (mDia1) gene is associated with nonsyndromic hearing loss in an extended kindred [36]. FMN2 and DIAPH2 mRNAs are both increased several-fold in oocytes from polycystic ovary syndrome (PCOS) patients [20]. Including these two, many genes involved in spindle dynamics, chromosome alignment, and centrosome functions are aberrantly expressed in PCOS oocytes [20]. It is thus likely that meiotic defects are contributing factors in the reduced developmental competence of PCOS oocytes. Further investigation is warranted to assess whether formins are determining factors of oocyte quality in humans.
Conclusion
As we have partly discussed in this review, the mouse oocyte has served as an excellent system to portray the complex nature of cytoskeleton dynamics. Visualizing F-actin networks on meiotic spindles for the first time has advanced our understanding of the collaboration between actin and microtubules during oocyte maturation. While the primary involvement of Fmn2 in this interaction is suspected, the possible interactions between mDia and small GTPases need to be further pursued. Understanding the dynamic regulation of the cytoskeleton in oocyte maturation will help prevent aneuploidy originating from cytoskeletal defects in human oocytes.
Footnotes
This work was supported by a grant of the Korean Healthcare Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (A080045).
No potential conflict of interest relevant to this article was reported.
References
- 1.Brunet S, Maro B. Cytoskeleton and cell cycle control during meiotic maturation of the mouse oocyte: integrating time and space. Reproduction. 2005;130:801–811. doi: 10.1530/rep.1.00364. [DOI] [PubMed] [Google Scholar]
- 2.Compton DA. Spindle assembly in animal cells. Annu Rev Biochem. 2000;69:95–114. doi: 10.1146/annurev.biochem.69.1.95. [DOI] [PubMed] [Google Scholar]
- 3.Schuh M, Ellenberg J. Self-organization of MTOCs replaces centrosome function during acentrosomal spindle assembly in live mouse oocytes. Cell. 2007;130:484–498. doi: 10.1016/j.cell.2007.06.025. [DOI] [PubMed] [Google Scholar]
- 4.Carabatsos MJ, Combelles CM, Messinger SM, Albertini DF. Sorting and reorganization of centrosomes during oocyte maturation in the mouse. Microsc Res Tech. 2000;49:435–444. doi: 10.1002/(SICI)1097-0029(20000601)49:5<435::AID-JEMT5>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 5.Gueth-Hallonet C, Antony C, Aghion J, Santa-Maria A, Lajoie-Mazenc I, Wright M, et al. Gamma-tubulin is present in acentriolar MTOCs during early mouse development. J Cell Sci. 1993;105:157–166. doi: 10.1242/jcs.105.1.157. [DOI] [PubMed] [Google Scholar]
- 6.Brunet S, Maria AS, Guillaud P, Dujardin D, Kubiak JZ, Maro B. Kinetochore fibers are not involved in the formation of the first meiotic spindle in mouse oocytes, but control the exit from the first meiotic M phase. J Cell Biol. 1999;146:1–12. doi: 10.1083/jcb.146.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lénárt P, Bacher CP, Daigle N, Hand AR, Eils R, Terasaki M, et al. A contractile nuclear actin network drives chromosome congression in oocytes. Nature. 2005;436:812–818. doi: 10.1038/nature03810. [DOI] [PubMed] [Google Scholar]
- 8.Verlhac MH, Dumont J. Interactions between chromosomes, microfilaments and microtubules revealed by the study of small GTPases in a big cell, the vertebrate oocyte. Mol Cell Endocrinol. 2008;282:12–17. doi: 10.1016/j.mce.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 9.Van Aelst L, D'Souza-Schorey C. Rho GTPases and signaling networks. Genes Dev. 1997;11:2295–2322. doi: 10.1101/gad.11.18.2295. [DOI] [PubMed] [Google Scholar]
- 10.Bielak-Zmijewska A, Kolano A, Szczepanska K, Maleszewski M, Borsuk E. Cdc42 protein acts upstream of IQGAP1 and regulates cytokinesis in mouse oocytes and embryos. Dev Biol. 2008;322:21–32. doi: 10.1016/j.ydbio.2008.06.039. [DOI] [PubMed] [Google Scholar]
- 11.Na J, Zernicka-Goetz M. Asymmetric positioning and organization of the meiotic spindle of mouse oocytes requires CDC42 function. Curr Biol. 2006;16:1249–1254. doi: 10.1016/j.cub.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 12.Cui XS, Li XY, Kim NH. Cdc42 is implicated in polarity during meiotic resumption and blastocyst formation in the mouse. Mol Reprod Dev. 2007;74:785–794. doi: 10.1002/mrd.20571. [DOI] [PubMed] [Google Scholar]
- 13.Halet G, Carroll J. Rac activity is polarized and regulates meiotic spindle stability and anchoring in mammalian oocytes. Dev Cell. 2007;12:309–317. doi: 10.1016/j.devcel.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 14.Deng M, Suraneni P, Schultz RM, Li R. The Ran GTPase mediates chromatin signaling to control cortical polarity during polar body extrusion in mouse oocytes. Dev Cell. 2007;12:301–308. doi: 10.1016/j.devcel.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 15.Dumont J, Petri S, Pellegrin F, Terret ME, Bohnsack MT, Rassinier P, et al. A centriole- and RanGTP-independent spindle assembly pathway in meiosis I of vertebrate oocytes. J Cell Biol. 2007;176:295–305. doi: 10.1083/jcb.200605199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chesarone MA, DuPage AG, Goode BL. Unleashing formins to remodel the actin and microtubule cytoskeletons. Nat Rev Mol Cell Biol. 2010;11:62–74. doi: 10.1038/nrm2816. [DOI] [PubMed] [Google Scholar]
- 17.Faix J, Grosse R. Staying in shape with formins. Dev Cell. 2006;10:693–706. doi: 10.1016/j.devcel.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 18.Manseau L, Calley J, Phan H. Profilin is required for posterior patterning of the Drosophila oocyte. Development. 1996;122:2109–2116. doi: 10.1242/dev.122.7.2109. [DOI] [PubMed] [Google Scholar]
- 19.Kwon S, Shin H, Lim HJ. Dynamic interaction of formin proteins and cytoskeleton in mouse oocytes during meiotic maturation. Mol Hum Reprod. 2010 Oct 22; doi: 10.1093/molehr/gaq088. [Epub] DOI: 10.1093/molehr/gaq088. [DOI] [PubMed] [Google Scholar]
- 20.Wood JR, Dumesic DA, Abbott DH, Strauss JF., 3rd Molecular abnormalities in oocytes from women with polycystic ovary syndrome revealed by microarray analysis. J Clin Endocrinol Metab. 2007;92:705–713. doi: 10.1210/jc.2006-2123. [DOI] [PubMed] [Google Scholar]
- 21.Leader B, Lim H, Carabatsos MJ, Harrington A, Ecsedy J, Pellman D, et al. Formin-2, polyploidy, hypofertility and positioning of the meiotic spindle in mouse oocytes. Nat Cell Biol. 2002;4:921–928. doi: 10.1038/ncb880. [DOI] [PubMed] [Google Scholar]
- 22.Azoury J, Lee KW, Georget V, Rassinier P, Leader B, Verlhac MH. Spindle positioning in mouse oocytes relies on a dynamic meshwork of actin filaments. Curr Biol. 2008;18:1514–1519. doi: 10.1016/j.cub.2008.08.044. [DOI] [PubMed] [Google Scholar]
- 23.Burkel BM, von Dassow G, Bement WM. Versatile fluorescent probes for actin filaments based on the actin-binding domain of utrophin. Cell Motil Cytoskeleton. 2007;64:822–832. doi: 10.1002/cm.20226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schuh M, Ellenberg J. A new model for asymmetric spindle positioning in mouse oocytes. Curr Biol. 2008;18:1986–1992. doi: 10.1016/j.cub.2008.11.022. [DOI] [PubMed] [Google Scholar]
- 25.Li H, Guo F, Rubinstein B, Li R. Actin-driven chromosomal motility leads to symmetry breaking in mammalian meiotic oocytes. Nat Cell Biol. 2008;10:1301–1308. doi: 10.1038/ncb1788. [DOI] [PubMed] [Google Scholar]
- 26.Riedl J, Crevenna AH, Kessenbrock K, Yu JH, Neukirchen D, Bista M, et al. Lifeact: a versatile marker to visualize F-actin. Nat Methods. 2008;5:605–607. doi: 10.1038/nmeth.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lammers M, Rose R, Scrima A, Wittinghofer A. The regulation of mDia1 by autoinhibition and its release by Rho·GTP. EMBO J. 2005;24:4176–4187. doi: 10.1038/sj.emboj.7600879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kato T, Watanabe N, Morishima Y, Fujita A, Ishizaki T, Narumiya S. Localization of a mammalian homolog of diaphanous, mDia1, to the mitotic spindle in HeLa cells. J Cell Sci. 2001;114:775–784. doi: 10.1242/jcs.114.4.775. [DOI] [PubMed] [Google Scholar]
- 29.Bartolini F, Moseley JB, Schmoranzer J, Cassimeris L, Goode BL, Gundersen GG. The formin mDia2 stabilizes microtubules independently of its actin nucleation activity. J Cell Biol. 2008;181:523–536. doi: 10.1083/jcb.200709029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lammers M, Meyer S, Kuhlmann D, Wittinghofer A. Specificity of interactions between mDia isoforms and Rho proteins. J Biol Chem. 2008;283:35236–35246. doi: 10.1074/jbc.M805634200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hunt PA, Hassold TJ. Human female meiosis: what makes a good egg go bad? Trends Genet. 2008;24:86–93. doi: 10.1016/j.tig.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 32.Hassold T, Hunt P. Maternal age and chromosomally abnormal pregnancies: what we know and what we wish we knew. Curr Opin Pediatr. 2009;21:703–708. doi: 10.1097/MOP.0b013e328332c6ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hamel M, Dufort I, Robert C, Gravel C, Leveille MC, Leader A, et al. Identification of differentially expressed markers in human follicular cells associated with competent oocytes. Hum Reprod. 2008;23:1118–1127. doi: 10.1093/humrep/den048. [DOI] [PubMed] [Google Scholar]
- 34.Hamel M, Dufort I, Robert C, Léveillé MC, Leader A, Sirard MA. Genomic assessment of follicular marker genes as pregnancy predictors for human IVF. Mol Hum Reprod. 2010;16:87–96. doi: 10.1093/molehr/gap079. [DOI] [PubMed] [Google Scholar]
- 35.Bione S, Sala C, Manzini C, Arrigo G, Zuffardi O, Banfi S, et al. A human homologue of the Drosophila melanogaster diaphanous gene is disrupted in a patient with premature ovarian failure: evidence for conserved function in oogenesis and implications for human sterility. Am J Hum Genet. 1998;62:533–541. doi: 10.1086/301761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lynch ED, Lee MK, Morrow JE, Welcsh PL, León PE, King MC. Nonsyndromic deafness DFNA1 associated with mutation of a human homolog of the Drosophila gene diaphanous. Science. 1997;278:1315–1318. [PubMed] [Google Scholar]