Abstract
Objective
To compare the IVF outcomes of mild ovarian stimulation with conventional ovarian stimulation in poor responders.
Methods
From 2004 to 2009, 389 IVF cycles in 285 women showed poor responses (defined as either a basal FSH level ≥12 mIU/mL, or the number of retrieved oocytes ≤3, or serum E2 level on hCG day <500 pg/mL) were analyzed, retrospectively. In total, 119 cycles with mild ovarian stimulation (m-IVF) and 270 cycles with conventional ovarian stimulation (c-IVF) were included. Both groups were divided based on their age, into groups over and under 37 years old.
Results
The m-IVF group was lower than the c-IVF group in the duration of stimulation, total doses of gonadotropins used, serum E2 level on hCG day, the number of retrieved oocytes, and the number of mature oocytes. However, there was no significant difference in the number of good embryos, the number of transferred embryos, the cancellation rate, or the clinical pregnancy rate. In the m-IVF group over 37 years old, the clinical pregnancy rate and live birth rate were higher when compared with the c-IVF group, but this result was not statistically significant.
Conclusion
In poor responder groups, mild ovarian stimulation is more cost effective and patient friendly than conventional IVF. Therefore, we suggest that mild ovarian stimulation could be considered for poor responders over 37 years old.
Keywords: Mild Ovarian Stimulation, Poor Response, In Vitro Fertilization, Human
Introduction
Poor ovarian response to controlled ovarian hyperstimulation (COH) was first described by Garcia et al. [1] in 1983, and its prevalence was reported to be 5-24% of patients undergoing IVF [2].
Since studies began, different authors have used different criteria to define poor ovarian response. In general, poor responder women are the patients who show the number of retrieved oocytes of ≤3 and a peak E2 level of ≤500 pg/mL during COH for IVF. In the European Society for Human Reproduction and Embryology/American Society for Reproductive Medicine, a consensus was reached on the minimal criteria needed to define poor ovarian response. Thus, to define a poor ovarian response to COH, at least two of the following three features must be present: 1) advanced maternal age (≥40 years) or any other risk factor for poor ovarian response, 2) a previous poor ovarian response (≤3 oocytes with a conventional stimulation protocol), 3) an abnormal ovarian reserve test [3].
Several methods have been suggested for enhancing the outcome of these patients, because they have high cycle cancellation rates and a low chance of successful pregnancy. Adjuvant therapies for COH such as growth hormone therapy or pyridostigmine, oral L-arginine, and transdermal testosterone failed to improve pregnancy rates in poor ovarian responders. In addition, short protocols using GnRH antagonists as well as short or long protocols using GnRH agonists also did not show any improvements in pregnancy rates [4].
Mild ovarian stimulation, when used for patients with normal ovarian response, not only decreases the incidence of ovarian hyperstimulation syndrome (OHSS), a serious unwanted complication of COH, but also reduces the patient's discomfort and cost by using a lower dose of gonadotropin. In 2007, Baart et al. [5] reported that the number of retrieved oocytes by mild ovarian stimulation may be fewer, yet better in terms of oocyte and embryo quality. Devroey reported in 2004 that mild ovarian stimulation improved endometrial receptivity [6].
In some studies, poor responders received either IVF after a natural cycle or IVF after mild ovarian stimulation. In 2004, Kolibianakis et al. [7] reported lower pregnancy rates in elderly patients with high basal FSH levels undergoing mild ovarian stimulation. However, in vitro fertilization after natural cycle or mild ovarian stimulation is still currently under investigation.
In this study, we evaluated the utility of mild ovarian stimulation in poor responders by comparing clinical outcomes between mild ovarian stimulation and conventional ovarian stimulation in these patients.
Methods
1. Study population
This is a retrospective study including 285 infertile women who responded poorly to COH between January 2004 and June 2009. Poor ovarian response to COH was defined as a serum basal FSH level more than 12 mIU/mL, a number of retrieved oocytes not exceeding three, and E2 level less than 500 pg/mL on the day of hCG administration. Exclusion criteria of this study include the following factors: age over 43 years old, basal FSH level exceeding 25 mIU/mL, severe male factor infertility requiring testicular sperm extraction, and preimplantation genetic diagnosis.
During the study period, 285 female poor responders underwent 389 IVF cycles. Pregnancy rates, live birth rates, as well as clinical utility were compared between cycles with mild ovarian stimulation (m-IVF), the study group, and cycles with conventional ovarian stimulation (c-IVF), the control group. In order to compare differences in response according to age, poor responders were further divided into two groups by an age cutoff of 37 years old, because follicular count reduces rapidly after that age.
2. Study design
1) Controlled ovarian hyperstimulation
An ovarian hyperstimulation protocol was initiated after confirming the lack of any growing follicle greater than 10 mm in diameter by ultrasound, and basal serum E2 not exceeding 50 pg/mL on day 2 or 3 of the menstrual cycle.
Ovarian hyperstimulation was achieved either by short or long protocols using GnRH agonist leuprolide (Lucrin®, Abott Korea Ktd., Seoul, Korea) or short protocols using 0.25 mg GnRH antagonists (Cetrotide®, Merck Serno, Geneva, Switzerland or Orgalutran® Schering-Plough Organon, Oss, the Netherlands).
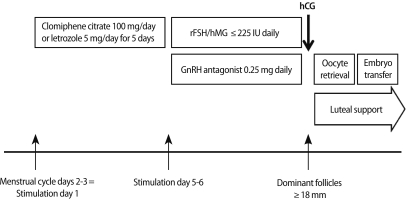
In the m-IVF group, patients were either given 100 mg of clomiphene citrate (Clomiphene®, Youngpoong Pharma, Seoul, Korea) or 5 mg letrozole (Femara®, Novartis, East Hanover, NJ, USA) daily for five days, or no medication, followed by administration of 150-225 IU of either recombinant FSH (Gonal-F®, Merck Serno) or human menopausal gonadotropin (human menopausal gonadotropin, Menopur®, Ferring Pharmaceuticals Korea Co. Ltd., Seoul, Korea) daily (Figure 1). In the c-IVF group, more than 300 IU of FSH or hMG were given.
Figure 1.
Schematic diagram of mild ovarian stimulation.
2) Oocyte retrieval and embryo transfer
For follicular maturation, 250 µg of recombinant hCG (Ovidrel®, Merck Serno) was injected subcutaneously when a dominant follicle reached 18 mm in diameter. Oocytes were retrieved approximately 34-36 hours after hCG administration. Depending on the quality of the sperms retrieved, either conventional IVF or ICSI was used to fertilize eggs.
After an incubation period of two or three days, good quality embryos were selected and transferred into the uterine cavity. Luteal support for those who had embryos transferred, either 50 mg injection of progesterone (Progesteron®, Watson Pharmaceuticals Inc., Morristown, NJ, USA) or 400 mg transvaginal progesterone (Yenatron®, Acraf S.p.A., Roma, Italy) were administered.
3) Confirmation of pregnancy
Clinical pregnancy was confirmed by a serum β-hCG level on the 12th day from ovum pickup measuring at least 5 mIU/mL, and the presence of a gestational sac in the follow-up ultrasound at 5-6 weeks of gestational age. Abortion was defined as fetal loss before 20 weeks gestation despite the presence of a gestational sac in the first trimester ultrasound.
3. Statistical analysis
Statistical analysis was performed with SPSS ver. 12.0 (SPSS Inc. Chicago, IL, USA). A student's t-test and chi-square test were performed for data analysis. A p-value of less than 0.05 was considered to be statistically significant.
Results
Of the total 389 cycles of IVF performed on poor responders, 119 cycles were mild ovarian stimulation cycles, and 270 cycles were conventional ovarian stimulation cycles.
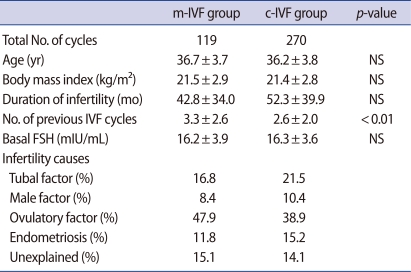
Table 1. compares the two groups' clinical features. The number of previous IVF cycles was significantly higher in the study group, whereas there were no differences in age, body mass index, duration of infertility, cause of infertility, or basal FSH levels. The total number of doses of gonadotropin used during hyperstimulation was significantly lower in the study group than in the control group (901.6±587.0 IU vs. 3,165.6±1,459.2 IU, p<0.001). Similarly, the total stimulation period (8.5±2.9 days vs. 10.1±2.7 days, p<0.001) was significantly shorter in the study group. The endometrial thickness was significantly thicker in the control group on the day of hCG administration (10.1±2.7 mm vs. 8.1±2.2 mm).
Table 1.
Basal characteristics of IVF patients undergoing mild ovarian stimulation and conventional ovarian stimulation
The values are expressed as mean±SD.
m-IVF, mild ovarian stimulation; c-IVF, conventional ovarian stimulation; NS, not significant; No, number.
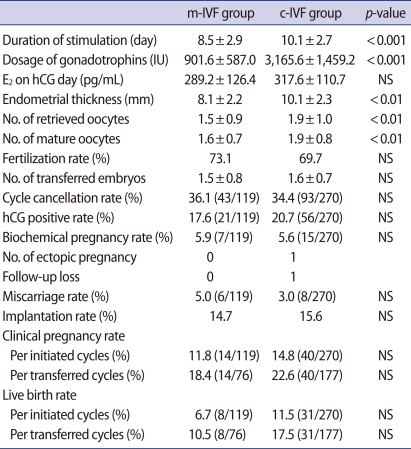
The total number of oocytes retrieved and the number of oocytes that matured were each significantly fewer in the study group. However, there were no differences in the fertilization rates, number of transferred embryos, or number of good quality embryos between the two groups (Table 2). Although clinical pregnancy rates per initiated cycle and per transferred cycle in the control group (14.8% and 22.6%, respectively) were higher than in the study group (11.8% and 18.4%, respectively), these values were not statistically significant. Similarly, live birth rates per initiated cycle and per transferred cycle were higher in the control group (11.5% and 17.5%, respectively) than in the study group (6.7% and 10.5%, respectively), but did not show statistical significance.
Table 2.
IVF outcomes in patients with mild ovarian stimulation and conventional ovarian stimulation
The values are expressed as mean±SD.
m-IVF, mild ovarian stimulation; c-IVF, conventional ovarian stimulation; NS, not significant; No, number.
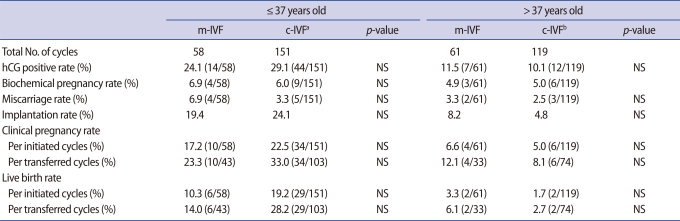
Clinical pregnancy rates and live birth rates in patients below age 37 showed decreased values in the study group, regardless of the rates per initiated cycle or per transferred cycle. However, this also failed to show statistical significance. On the other hand, in the subgroup of patients over age 37, these values were higher in the study group than in the control group; however, there was no statistical significance (Table 3).
Table 3.
IVF outcomes in patients with mild ovarian stimulation and conventional ovarian stimulation according to age
m-IVF, mild ovarian stimulation; c-IVF, conventional ovarian stimulation; No, number; NS, not significant.
aInclude 1 ectopic pregnancy; bInclude 1 follow-up loss.
Discussion
It has been reported in a number of studies that mature oocyte counts of 5-15 may lead to a good clinical outcome of IVF, and furthermore, oocyte counts of less than 5 predict significantly reduced clinical pregnancy rates as well as live birth rates compared to the normal response group [8,9]. Many possible methods have been proposed to improve pregnancy rates for such poor responders, yet none has been very promising to date.
In 1996, Land et al. [10] showed an increase in follicular count and the number of retrieved oocytes in a group of poor responders who had received 450 IU of hMG daily, compared to a group that received 225 IU. However, the number of embryos was similar between the two groups, and the clinical pregnancy rate was unexpectedly lower in the group that received 450 IU. As a high dose gonadotropin does not increase the number of embryos nor improve pregnancy rates, a low-dose-gonadotropin administration protocol has emerged, which has not only retained pregnancy rates but has also saved costs.
Mild ovarian stimulation in normal ovarian response groups is known to be a patient-friendly method that reduces the incidence of ovarian hyperstimulation syndrome, and at the same time, reduces unnecessary discomfort to patients by using a lower dose of gonadotropin, and also reduces medical expenses [11]. Many studies have reported that mild ovarian stimulation, compared to conventional ovarian stimulation, improves embryo quality and implantation rates. Recently, it has been suggested that mild ovarian stimulation could reduce the proportion of mosaic embryos and aneuploidies [12]. While many investigators have investigated the usefulness of mild ovarian stimulation, few studies have focused on mild ovarian stimulation in poor responders.
In this study on poor responders, similar to the results shown in other studies on normal responders, the number of retrieved oocytes and matured oocytes were significantly lower in the mild ovarian stimulation group than in the conventional ovarian stimulation group. There was no significant difference in the number of good quality embryos, fertilization rates, or the number of transferred embryos between two groups. This implies that when a mild ovarian stimulation protocol was applied in the poor responders, despite a small number of retrieved oocytes, good quality embryos were produced that improved the fertilization rate, clinical pregnancy rate, and live birth rate. This was particularly true in the subgroup of women aged 37 or more, which in study group, showed higher pregnancy rates and live birth rates than control group. Judging by these results, a high dose gonadotropin protocol failed to prove its usefulness in IVF procedures on patients over 37 years of age [13]. However, in this study, pregnancy rates were lower in the study group than was expected for the general population, and this may be due to differences in numbers of previous IVF procedures and differences in endometrial thickness.
Patients in the study group had previously undergone several more cycles of IVF procedures, and this may have resulted in lowered clinical pregnancy rates and live birth rates. Comparing the number of cycles undergone until the first IVF, the study group was 6.7%, 18 cycles, whereas the control group was 75.6%, 96 cycles, and the difference was statistically significant. It may be reflected that clinicians have a tendency to avoid mild ovarian stimulation in women with the first cycle. The reason for this is that although mild ovarian stimulation can yield results as good as conventional ovarian stimulation, many studies report higher cycle cancellation rates [14].
Furthermore, the endometrial thickness on ultrasound measured thinner on the day of hCG administration in the study group than in the control group. This is because in the control group, the clomiphene-gonadotropin combination method was used in 7 cycles (4.1%), whereas in the study group, it was used in 91 cycles (76.5%). Clomiphene is known to cause endometrial thinning in 15-50% of patients due to the antiestrogenic effect of the compound itself, and unfortunately, result in a lower pregnancy rate [15]. Thin endometrium has been shown to affect pregnancy rates in previous studies. The preovulatory endometrium is important in achieving pregnancy, and in 1993, Dickey et al. [16] found that when the preovulatory endometrium is thinner than 6 mm, pregnancy is virtually impossible, and when it is thinner than 8 mm, there is an increased risk of preclinical miscarriage. Also, we observed that endometrial thickness was thinner for those undergoing the clomiphene-gonadotropin combination method than the letrozol-gonadotropin combination method (7.6 mm vs. 9.5 mm, p<0.01). Therefore, the clinical pregnancy rate was lower with the clomiphene-gonadotropin combination method than the letrozol-gonadotropin combination method.
In summary, for poor responders with high basal FSH, mild ovarian stimulation resulted in IVF outcomes similar to conventional ovarian stimulation. In poor responders older than 37 years old in particular, mild ovarian stimulation yielded slightly better pregnancy rates than conventional ovarian stimulation. Mild ovarian stimulation can save costs by using a smaller amount of gonadotropin and can reduce patients' burden of undergoing frequent injections. In conclusion, in terms of embryo quality, pregnancy rate, and cost effectiveness, mild ovarian stimulation is a promising alternative to conventional ovarian stimulation for poor ovarian responders.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Garcia JE, Jones GS, Acosta AA, Wright G., Jr Human menopausal gonadotropin/human chorionic gonadotropin follicular maturation for oocyte aspiration: phase II, 1981. Fertil Steril. 1983;39:174–179. doi: 10.1016/s0015-0282(16)46815-9. [DOI] [PubMed] [Google Scholar]
- 2.Loutradis D, Drakakis P, Milingos S, Stefanidis K, Michalas S. Alternative approaches in the management of poor response in controlled ovarian hyperstimulation (COH) Ann N Y Acad Sci. 2003;997:112–119. doi: 10.1196/annals.1290.013. [DOI] [PubMed] [Google Scholar]
- 3.Ferraretti AP, La Marca A, Fauser BC, Tarlatzis B, Nargund G, Gianaroli L, et al. ESHRE consensus on the definition of 'poor response' to ovarian stimulation for in vitro fertilization: the Bologna criteria. Hum Reprod. 2011;26:1616–1624. doi: 10.1093/humrep/der092. [DOI] [PubMed] [Google Scholar]
- 4.Kyrou D, Kolibianakis EM, Venetis CA, Papanikolaou EG, Bontis J, Tarlatzis BC. How to improve the probability of pregnancy in poor responders undergoing in vitro fertilization: a systematic review and meta analysis. Fertil Steril. 2009;91:749–766. doi: 10.1016/j.fertnstert.2007.12.077. [DOI] [PubMed] [Google Scholar]
- 5.Baart EB, Martini E, Eijkemans MJ, Van Opstal D, Beckers NG, Verhoeff A, et al. Milder ovarian stimulation for in-vitro fertilization reduces aneuploidy in the human preimplantation embryo: a randomized controlled trial. Hum Reprod. 2007;22:980–988. doi: 10.1093/humrep/del484. [DOI] [PubMed] [Google Scholar]
- 6.Devroey P, Bourgain C, Macklon NS, Fauser BC. Reproductive biology and IVF: ovarian stimulation and endometrial receptivity. Trends Endocrinol Metab. 2004;15:84–90. doi: 10.1016/j.tem.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 7.Kolibianakis E, Zikopoulos K, Camus M, Tournaye H, Van Steirteghem A, Devroey P. Modified natural cycle for IVF does not offer a realistic chance of parenthood in poor responders with high day 3 FSH levels, as a last resort prior to oocyte donation. Hum Reprod. 2004;19:2545–2549. doi: 10.1093/humrep/deh452. [DOI] [PubMed] [Google Scholar]
- 8.De Vries MJ, De Sutter P, Dhont M. Prognostic factors in patients continuing in vitro fertilization or intracytoplasmic sperm injection treatment and dropouts. Fertil Steril. 1999;72:674–678. doi: 10.1016/s0015-0282(99)00334-9. [DOI] [PubMed] [Google Scholar]
- 9.Sharma V, Allgar V, Rajkhowa M. Factors influencing the cumulative conception rate and discontinuation of in vitro fertilization treatment for infertility. Fertil Steril. 2002;78:40–46. doi: 10.1016/s0015-0282(02)03160-6. [DOI] [PubMed] [Google Scholar]
- 10.Land JA, Yarmolinskaya MI, Dumoulin JC, Evers JL. High-dose human menopausal gonadotropin stimulation in poor responders does not improve in vitro fertilization outcome. Fertil Steril. 1996;65:961–965. doi: 10.1016/s0015-0282(16)58269-7. [DOI] [PubMed] [Google Scholar]
- 11.Pelinck MJ, Vogel NE, Hoek A, Arts EG, Simons AH, Heineman MJ. Minimal stimulation IVF with late follicular phase administration of the GnRH antagonist cetrorelix and concomitant substitution with recombinant FSH: a pilot study. Hum Reprod. 2005;20:642–648. doi: 10.1093/humrep/deh685. [DOI] [PubMed] [Google Scholar]
- 12.Verberg MF, Macklon NS, Nargund G, Frydman R, Devroey P, Broekmans FJ, et al. Mild ovarian stimulation for IVF. Hum Reprod Update. 2009;15:13–29. doi: 10.1093/humupd/dmn056. [DOI] [PubMed] [Google Scholar]
- 13.Gougeon A, Ecochard R, Thalabard JC. Age-related changes of the population of human ovarian follicles: increase in the disappearance rate of non-growing and early-growing follicles in aging women. Biol Reprod. 1994;50:653–663. doi: 10.1095/biolreprod50.3.653. [DOI] [PubMed] [Google Scholar]
- 14.Weghofer A, Margreiter M, Bassim S, Sevelda U, Beilhack E, Feichtinger W. Minimal stimulation using recombinant follicle-stimulating hormone and a gonadotropin-releasing hormone antagonist in women of advanced age. Fertil Steril. 2004;81:1002–1006. doi: 10.1016/j.fertnstert.2003.09.050. [DOI] [PubMed] [Google Scholar]
- 15.Mitwally MF, Casper RF. Aromatase inhibition reduces gonadotrophin dose required for controlled ovarian stimulation in women with unexplained infertility. Hum Reprod. 2003;18:1588–1597. doi: 10.1093/humrep/deg311. [DOI] [PubMed] [Google Scholar]
- 16.Dickey RP, Olar TT, Taylor SN, Curole DN, Harrigill K. Relationship of biochemical pregnancy to pre-ovulatory endometrial thickness and pattern in patients undergoing ovulation induction. Hum Reprod. 1993;8:327–330. doi: 10.1093/oxfordjournals.humrep.a138045. [DOI] [PubMed] [Google Scholar]