Abstract
Objective
Laser-assisted intracytoplasmic sperm injection (LA-ICSI), also known as micro-opening or thinning of the zona pellucida (ZP) prior to ICSI, may help to reduce mechanical damage to the oocyte during the procedure. The aim of the present study was to evaluate and analyze the efficacy of our institutional LA-ICSI program, which features laser-assisted ZP thinning prior to ICSI, in comparison with conventional ICSI (C-ICSI), performed on patients with different clinical characteristics.
Methods
Patients undergoing a total of 212 ICSI cycles were randomly divided into an LA-ICSI group (106 cycles) and a conventional ICSI group (106 cycles). To reduce tissue damage, we thinned the ZP by approximately 70%, using a laser, before ICSI. Patients thus treated formed the LA-ICSI group. Comparisons included the morphological quality of transferred embryos, blastocyst development of the remaining embryos, and clinical pregnancy, in terms of ICSI method and patient characteristics.
Results
Fertilization, development of remaining embryos, and pregnancy rate were significantly higher in the LA-ICSI group compared with the C-ICSI group. Fertilization, embryonic development, and the pregnancy rate were all improved in younger patients (<38 years of age) and in those who underwent a low number of IVF-ET attempts (<3 trials). In addition, the pregnancy rate was increased in older patients.
Conclusion
LA-ICSI may be useful in improving the chance of pregnancy in all ICSI patients.
Keywords: Intracytoplasmic Sperm Injection, Laser-assisted Intracytoplasmic Sperm Injection, In Vitro Fertilization-embryo Transfer, Zona Pellucida Thinning, Clinical Outcome, Human
Introduction
In the time since the first successful pregnancy after ICSI was reported in 1992 [1], ICSI has become a routine method of fertilization if male factor infertility is evident, and the treatment is also used in instances of non-male factor infertility if repeated attempts at IVF-ET fail [2-5]. However, ICSI has several drawbacks. These include mechanical damage to the cytoplasmic membrane, the cytoskeleton, and the meiotic spindle that occurs during membrane penetration and sperm deposition [6,7]. In fact, oocyte degeneration and abnormal fertilization constitute the principal reasons for the cancellation of assisted reproductive techniques (ART) cycles. Additionally, some oocytes are very fragile, and the zona pellucida can be very resistant to penetration, resulting in sudden breakage of the membrane during ICSI [7-9]. To overcome these problems, several groups have developed piezo- or laser-assisted ICSI techniques (LA-ICSI). Piezo-ICSI, which uses a blunt injection needle operated by a piezo-electric device, efficiently prevents damage to both mouse and human oocytes [10,11]. However, this technique has become unpopular owing to a risk of mercury contamination and practical difficulties. Lasers have already been used as convenient and safe tools in assisted hatching and pre-implantation genetic diagnosis (PGD). Rienzi et al. [12] reported that LA-ICSI, featuring micro-opening or drilling of the ZP prior to ICSI, reduced the degeneration rate of human oocytes and increased embryo development rates in patients who had experienced prior ICSI failures caused by poor oocyte survival [13]. Several groups have reported similar results in selected patients with histories of poor ICSI outcomes, for whom only limited numbers of oocytes were available [13,14]. In addition, when used to overcome fertilization problems, laser-assisted ZP thinning prior to routine ICSI both improved oocyte survival and increased the hatching rate in vitro [15]. However, the clinical utility of LA-ICSI remains controversial because comparisons of fertilization, embryo quality, and clinical outcomes have not been performed in the general patient populations eligible for routine ICSI. In addition, the study groups have had included few cases. Therefore, the objective of the present work was to evaluate the possible benefits of LA-ICSI, thus thinning of the ZP prior to ICSI, as part of a routine ICSI program in non-randomized patients. We compared the data with those collected from conventional ICSI (C-ICSI) patients. The clinical outcomes of both groups were re-analyzed in terms of the age and history of female patients, to identify any possible clinical significance of laser use.
Methods
This study was approved by the Institutional Review Board of the CHA Gangnam Medical Center (Seoul, Korea) and was conducted between February and September of 2009. Patients experiencing a total of 212 ICSI cycles were randomly divided into two groups (106 cycles of LA-ICSI and 106 cycles of C-ICSI).
1. LA- and C-ICSI procedures
Starting on the third day of the cycle, controlled ovarian hyperstimulation was achieved using recombinant FSH (rFSH; Gonal-F, Merck-Serono, Modugno Bari, Italy). To prevent any premature LH surge, we employed a GnRH agonist (Lucrin, Abbott, Seoul, Korea) using the long protocol, or a GnRH antagonist (Cetorelix, Cetrotide, Merck-Serono) given after 5-6 days of stimulation. Ovulation was triggered by the injection of 10,000 IU hCG (Profasi, Merck-Serono) when at least two follicles were over 18 mm in diameter. Transvaginal oocyte retrieval was performed 34-36 hours after hCG administration.
For LA-ICSI, an oocyte was held with the polar body in the 12-o'clock position. The ZP was thinned by approximately 70% at the 3-o'clock position (where the injection needle was to penetrate) using a laser pulse 100 µ sec in duration (ZILOS-tk class I laser, Hamilton Thorne Research, Beverly, MA, USA). An ICSI needle was introduced into the oocyte through the pre-thinned region of the ZP, and the overall process was termed LA-ICSI. The control group received conventional ICSI and was termed the C-ICSI group. After both C-ICSI and LA-ICSI, oocytes injected with a spermatozoon were incubated (under 5% CO2 and 5% O2, both v/v, and at 37℃) in drops of Quinn's Advantage Cleavage medium (Sage, Trumbull, CT, USA) under oil.
2. Evaluation of oocyte integrity, fertilization, embryo development, and pregnancy
Fertilization and degeneration of injected oocytes were monitored 16-18 hours after ICSI. Fertilized embryos were transferred to drops of fresh cleavage medium. At day 3, embryo quality was graded into five levels based on the percentage of fragmentation and the number and size of blastomeres [14]; two or three embryos were selected for embryo transfer. After transfer to the uterus, the remaining embryos were cultured to day 7 for evaluation of blastocyst formation. To artificially prepare the endometrium, E2 valerate (4-6 mg/day; Progynova, Schering, Bayer, New Zealand) was administrated and progesterone (100 mg/day; Samil Pharm. Co., Ltd., Seoul, Korea) was injected when the endometrium attained 8 mm in thickness. Pregnancy was evaluated by measurement of the serum β-hCG level 12 days after embryo transfer and a clinical pregnancy was defined as thepresence of a gestational sac with positive heart activity at 5-7 weeks.
3. Analysis of clinical outcomes by patient profiles
All clinical outcomes, including fertilization and degeneration rates, development of remaining embryos into blastocysts, clinical pregnancy rate and abortion rate were evaluated. First, clinical outcomes in the LA-ICSI and C-ICSI groups were compared among all patients. Second, the data were re-analyzed with respect to female age (<38 years and ≥38 years). The age of patients was distinguished based on research results of a study that used laser for zona thining [16]. Last, the data were re-analyzed in terms of the number of prior IVF-ET attempts (<3 and ≥3).
4. Statistical analysis
Unless otherwise specified, data are expressed as means±SE. Clinical outcomes were analyzed using the chi-square test and Student's t-test. A p<0.05 was considered to be statistically significant.
Results
1. Comparisons of the clinical outcomes of the LA-ICSI and C-ICSI groups
The clinical profiles of patients in both groups are summarized in Table 1. The average age , sperm strict morphology using Kruger's strict criteria and the cycle date of hCG injection were similar in both groups. Also, neither the E2 concentration on the day of hCG injection nor the total number of retrieved oocytes differed between groups. In C-ICSI patients, 941 MII oocytes retrieved from 106 cycles were injected with sperm; 714 (75.9%) became fertilized and 40 (4.3%) degenerated. A total of 953 MII oocytes retrieved from 106 cycles were injected in the LA-ICSI group; 777 (81.5%) became fertilized and 27 (3.0%) degenerated. Figure 1 shows that, although the degeneration rate did not differ between groups, the fertilization rate in the LA-ICSI group was higher than that in the C-ICSI group (81.5% vs. 75.9%; p<0.05). Also, the blastocyst formation rate from embryos remaining after transfer and the clinical pregnancy rate were significantly higher in the LA-ICSI group (36.2% and 50.0% vs. 29.1% and 30.2%, respectively; p<0.05), even though the number of transferred embryos and the scores of such embryos at day 3 were similar in both groups. Use of LA-ICSI slightly increased the implantation rate, but the increase was not statistically significant.
Table 1.
Clinical profiles of the laser-assisted intracytoplasmic sperm injection (LA-ICSI) and conventional ICSI (C-ICSI) groups
Values are presented as means ± SE.
NS, not significant.
Figure 1.
Comparison of clinical outcomes between the laser-assisted intracytoplasmic sperm injection (LA-ICSI) and conventional ICSI (C-ICSI) groups. ap<0.05.
2. Comparisons of clinical outcomes of the LA-ICSI and C-ICSI groups with respect to female age
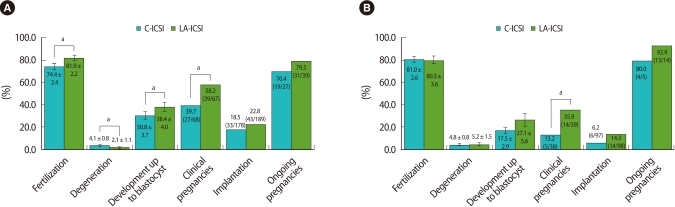
To investigate the specific efficacy of LA-ICSI, the data from both groups were re-analyzed in terms of female age. As shown in Figure 2, LA-ICSI showed improved pregnancy rates in both age groups (58.2% vs. 39.7% in those <38 years old; 35.9% vs. 13.2% in those ≥38 years old, p<0.05) when compared with C-ICSI. In addition, LA-ICSI increased the fertilization (81.9% vs. 74.4%, respectively; p<0.05) and developmental rates of the remaining embryos (38.4% vs. 30.8%, respectively; p<0.05) and also reduced the degeneration rate (2.1% vs. 4.1%) in younger females (<38 years of age) (Figure 2A) when compared to the C-ICSI group. LA-ISCI did not affect these parameters in older patients (≥38 years of age) (Figure 2B).
Figure 2.
Comparison of clinical outcomes between the laser-assisted intracytoplasmic sperm injection (LA-ICSI) and conventional ICSI (C-ICSI) groups in females of different age groups (A: <38 years old, B: ≥38 years old). ap<0.05.
3. Comparisons of clinical outcomes of the LA-ICSI and C-ICSI groups in terms of repeated IVF failure
In the present study, 42 IVF-ICSI patients who had experienced repeated IVF failure (≥3 IVF-ET attempts) were divided into two groups and the clinical outcomes after C-ICSI and LA-ICSI were analyzed. As shown in Figure 3A, all clinical outcomes, including embryonic development, clinical pregnancy, and implantation rates, were slightly higher in the LA-ICSI group than in the C-ICSI group (13.3% vs. 25.9%, 30.0% vs. 50.0%, and 14.0% vs. 21.8%, respectively), but these differences were not significant. However, when the 170 ICSI cycles of patients with a low number of prior IVF-ET attempts were analyzed, fertilization, embryonic development, and clinical pregnancy rates were significantly improved in the LA-ICSI group compared with the C-ICSI group (82.4% vs. 75.6%, 37.6% vs. 30.7%, and 50.0% vs. 30.2%; all p<0.05, respectively) (Figure 3B).
Figure 3.
Comparison of clinical outcomes between the laser-assisted intracytoplasmic sperm injection (LA-ICSI) and conventional ICSI (C-ICSI) groups according to the number of prior IVF-ET attempts (A: ≥3 attempts, B: <3 attempts). ap<0.05.
Discussion
To overcome a low fertilization rate caused by oocyte degeneration or abnormal embryonic development [4,6], the ICSI procedure has been modified over the past 20 years to reduce mechanical oocyte damage. LA-ICSI either creates a hole in or thins the ZP [9], and this may permit easy entrance of the needle into oocytes that are abnormal in terms of thickness or ZP resistance. Hence, many researchers have suggested that LA-ICSI can reduce the damage suffered by oocytes during ICSI, with consequent benefits for embryonic development [14,17]. However, most prior studies used ZP penetration methods or analyzed only small numbers of specific patients with poor ICSI histories. To date, the efficacy of LA-ICSI in patients of varying characteristics has not been well studied. In the present work, we conducted a prospective comparison (LA-ICSI vs. C-ICSI) on patients undergoing our routine ICSI procedure, which features a conjugated IVF-ET program, and next re-analyzed clinical outcomes in terms of patient age and the number of prior IVF-ET attempts. The results show that LA-ICSI may not mitigate damage to injected oocytes, but enhanced both the fertilization rate and blastocyst formation by residual embryos. Although the scores of transferred embryos did not differ between groups, we hypothesized that the quality of embryos selected for transfer in the LA-ICSI group may have been better than that of C-ICSI group embryos, resulting in a satisfactory clinical pregnancy rate (Figure 1). In fact, the implantation rate was not increased significantly and was only slightly improved in the LA-ICSI group.
Laser drilling of the ZP is used principally for laser-assisted hatching (LAH). Although LAH may enhance embryo implantation by mechanically facilitating the hatching process and permitting embryo-endometrium contact, LAH is associated with the risks associated with all invasive approaches. Mantoudis et al. [18] developed a partial LAH technique involving ZP thinning without bleaching of the inner membrane; the pregnancy rate improved significantly . LAH has also been offered to selected patients who were older, who had experienced prior IVF-ET failures, or who responded poorly to gonadotropins. As with LAH, LA-ICSI has been used to treat selected patients who failed previous ICSI cycles or who produced limited numbers of MII oocytes [12-14]. By re-analyzing patients with specific indications for LA-ICSI, we found that the technique significantly improved clinical outcomes in both older and younger females. However, LA-ICSI may not improve clinical outcomes of patients with histories of prior IVF-ET failures (≥3 attempts); this was in contrast to the results in patients with fewer prior attempts (Figure 3). Several reports have indicated that opening of the ZP prior to ICSI, and assisted hatching before embryo transfer are associated with twin pregnancies [12,16,19-21]. However, in the present study, we did not fully penetrate the ZP before ICSI to avoid this problem. Our approach may thus not affect the multiple pregnancy rate. The results indicate that LA-ICSI is valuable in routine practice.
In conclusion, LA-ICSI may reduce damage to oocytes during fertilization, in turn leading to improved embryonic development. Enhanced clinical outcomes after use of LA-ICSI were evident in selected patients who had experienced repeated IVF failure, or who were older, as well as when all patients undergoing routine ICSI were evaluated as two unselected groups. Also, LA-ICSI is a simple procedure. Therefore, we suggest that the LA-ICSI procedure be used for all ICSI patients to improve clinical outcomes.
Footnotes
This work was supported by a grant from the Korea Healthcare Technology R&D Project, Ministry for Health, Welfare & Family affairs, Republic of Korea (A084923).
No potential conflict of interest relevant to this article was reported.
References
- 1.Palermo G, Joris H, Devroey P, Van Steirteghem AC. Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet. 1992;340:17–18. doi: 10.1016/0140-6736(92)92425-f. [DOI] [PubMed] [Google Scholar]
- 2.Ebner T, Moser M, Sommergruber M, Jesacher K, Tews G. Complete oocyte activation failure after ICSI can be overcome by a modified injection technique. Hum Reprod. 2004;19:1837–1841. doi: 10.1093/humrep/deh325. [DOI] [PubMed] [Google Scholar]
- 3.Ola B, Afnan M, Sharif K, Papaioannou S, Hammadieh N, Barratt CL. Should ICSI be the treatment of choice for all cases of in-vitro conception? Considerations of fertilization and embryo development, cost effectiveness and safety. Hum Reprod. 2001;16:2485–2490. doi: 10.1093/humrep/16.12.2485. [DOI] [PubMed] [Google Scholar]
- 4.Oehninger S, Gosden RG. Should ICSI be the treatment of choice for all cases of in-vitro conception? No, not in light of the scientific data. Hum Reprod. 2002;17:2237–2242. doi: 10.1093/humrep/17.9.2237. [DOI] [PubMed] [Google Scholar]
- 5.Bhattacharya S, Hamilton MP, Shaaban M, Khalaf Y, Seddler M, Ghobara T, et al. Conventional in-vitro fertilisation versus intracytoplasmic sperm injection for the treatment of non-male-factor infertility: a randomised controlled trial. Lancet. 2001;357:2075–2079. doi: 10.1016/s0140-6736(00)05179-5. [DOI] [PubMed] [Google Scholar]
- 6.Ebner T, Yaman C, Moser M, Sommergruber M, Jesacher K, Tews G. A prospective study on oocyte survival rate after ICSI: influence of injection technique and morphological features. J Assist Reprod Genet. 2001;18:623–628. doi: 10.1023/A:1013171505702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu J, Nagy Z, Joris H, Tournaye H, Smitz J, Camus M, et al. Analysis of 76 total fertilization failure cycles out of 2732 intracytoplasmic sperm injection cycles. Hum Reprod. 1995;10:2630–2636. [PubMed] [Google Scholar]
- 8.Nagy ZP, Liu J, Joris H, Bocken G, Desmet B, Van Ranst H, et al. The influence of the site of sperm deposition and mode of oolemma breakage at intracytoplasmic sperm injection on fertilization and embryo development rates. Hum Reprod. 1995;10:3171–3177. doi: 10.1093/oxfordjournals.humrep.a135881. [DOI] [PubMed] [Google Scholar]
- 9.Palermo GD, Alikani M, Bertoli M, Colombero LT, Moy F, Cohen J, et al. Oolemma characteristics in relation to survival and fertilization patterns of oocytes treated by intracytoplasmic sperm injection. Hum Reprod. 1996;11:172–176. doi: 10.1093/oxfordjournals.humrep.a019012. [DOI] [PubMed] [Google Scholar]
- 10.Huang T, Kimura Y, Yanagimachi R. The use of piezo micromanipulation for intracytoplasmic sperm injection of human oocytes. J Assist Reprod Genet. 1996;13:320–328. doi: 10.1007/BF02070146. [DOI] [PubMed] [Google Scholar]
- 11.Yanagida K, Katayose H, Yazawa H, Kimura Y, Konnai K, Sato A. The usefulness of a piezo-micromanipulator in intracytoplasmic sperm injection in humans. Hum Reprod. 1999;14:448–453. doi: 10.1093/humrep/14.2.448. [DOI] [PubMed] [Google Scholar]
- 12.Rienzi L, Greco E, Ubaldi F, Iacobelli M, Martinez F, Tesarik J. Laser-assisted intracytoplasmic sperm injection. Fertil Steril. 2001;76:1045–1047. doi: 10.1016/s0015-0282(01)02861-8. [DOI] [PubMed] [Google Scholar]
- 13.Demirol A, Benkhalifa M, Sari T, Gurgan T. Use of laser-assisted intracytoplasmic sperm injection (ICSI) in patients with a history of poor ICSI outcome and limited metaphase II oocytes. Fertil Steril. 2006;86:256–258. doi: 10.1016/j.fertnstert.2005.11.078. [DOI] [PubMed] [Google Scholar]
- 14.Abdelmassih S, Cardoso J, Abdelmassih V, Dias JA, Abdelmassih R, Nagy ZP. Laser-assisted ICSI: a novel approach to obtain higher oocyte survival and embryo quality rates. Hum Reprod. 2002;17:2694–2699. doi: 10.1093/humrep/17.10.2694. [DOI] [PubMed] [Google Scholar]
- 15.Moser M, Ebner T, Sommergruber M, Gaisswinkler U, Jesacher K, Puchner M, et al. Laser-assisted zona pellucida thinning prior to routine ICSI. Hum Reprod. 2004;19:573–578. doi: 10.1093/humrep/deh093. [DOI] [PubMed] [Google Scholar]
- 16.Balakier H, Mandel R, Sojecki A, Motamedi G, Zaver S, Librach C. Laser zona thinning in women aged <or=37 years: a randomized study. Fertil Steril. 2009;91:1479–1482. doi: 10.1016/j.fertnstert.2008.07.1729. [DOI] [PubMed] [Google Scholar]
- 17.Valojerdi MR, Eftekhari-Yazdi P, Karimian L, Ashtiani SK. Effect of laser zona pellucida opening on clinical outcome of assisted reproduction technology in patients with advanced female age, recurrent implantation failure, or frozen-thawed embryos. Fertil Steril. 2008;90:84–91. doi: 10.1016/j.fertnstert.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 18.Mantoudis E, Podsiadly BT, Gorgy A, Venkat G, Craft IL. A comparison between quarter, partial and total laser assisted hatching in selected infertility patients. Hum Reprod. 2001;16:2182–2186. doi: 10.1093/humrep/16.10.2182. [DOI] [PubMed] [Google Scholar]
- 19.Alikani M, Noyes N, Cohen J, Rosenwaks Z. Monozygotic twinning in the human is associated with the zona pellucida architecture. Hum Reprod. 1994;9:1318–1321. doi: 10.1093/oxfordjournals.humrep.a138701. [DOI] [PubMed] [Google Scholar]
- 20.da Costa AA, Abdelmassih S, de Oliveira FG, Abdelmassih V, Abdelmassih R, Nagy ZP, et al. Monozygotic twins and transfer at the blastocyst stage after ICSI. Hum Reprod. 2001;16:333–336. doi: 10.1093/humrep/16.2.333. [DOI] [PubMed] [Google Scholar]
- 21.Schieve LA, Meikle SF, Peterson HB, Jeng G, Burnett NM, Wilcox LS. Does assisted hatching pose a risk for monozygotic twinning in pregnancies conceived through in vitro fertilization. Fertil Steril. 2000;74:288–294. doi: 10.1016/s0015-0282(00)00602-6. [DOI] [PubMed] [Google Scholar]