Abstract
Objective
Ovarian angiogenesis plays an important role in folliculogenesis. However, little is known about the expression of angiogenic factors during follicular development according to female age. Stromal cell derived factor-1α (SDF-1α) plays a role in granulosa cell survival and embryo quality as an angiogenic chemokine. Leptin is also involved in folliculogenesis and angiogenesis. This study examined expression of SDF-1α and leptin, and their effects on the expression of angiogenic factors in the ovary during follicular development according to female age.
Methods
Ovaries were collected from C57BL mice of two age groups (6-9 weeks and 24-26 weeks) at 6, 12, 24, and 48 hours after 5 IU pregnant mare's serum gonadotropin (PMSG) injection. The expression of ovarian SDF-1α and leptin mRNA was evaluated by RT-PCR. In the organ culture experiment, the ovaries were cultured in transwell permeable supports with Waymouth's medium treated with various doses of SDF-1α (50-200 ng/mL) or leptin (0.01-1 µg/mL) for 7 days. Then, mRNA expression of vascular endothelial growth factor (VEGF), endothelial nitric oxide synthase (eNOS), and visfatin were examined in the cultured ovaries.
Results
Expression of SDF-1α and leptin in the ovary was significantly lower in the aged mouse group compared to the young mouse group (p<0.05). Expression of these two factors increased with follicular development after PMSG administration. SDF-1α treatment stimulated visfatin expression in a dose-dependent manner, while leptin treatment significantly increased eNOS expression.
Conclusion
These results suggest that decrease of ovarian SDF-1α and leptin expression may be associated with aging-related reduction of ovarian function. SDF-1α and leptin may play a role in follicular development by regulating the expression of angiogenic factors in mouse ovaries.
Keywords: Ovary, Angiogenesis, Aging, Stromal Cell Derived Factor-1 Alpha, Leptin, Mouse
Introduction
It is well known that ovarian angiogenesis plays an important role in a series of events of folliculogenesis such as follicular growth and the selection of dominant follicle by delivering hormones, hormone precursors, oxygen and nutrients [1,2]. For this reason, the selected follicles possess a more elaborate microvasculature than other follicles and mature follicles have the highest vascular density [3,4]. These facts indicate that an active blood supply to the ovary seems to be essential for the induction of oocytes with good quality [5].
Advancing female age is an important factor contributing to an increasing incidence of infertility, but remains a fastidious problem in infertility treatment. With the advance of a female's age, chromosomal abnormality and production of abnormal oocytes increase and for the same reason the number of ovulated oocyte decreases and oocyte quality worsens [6-8]. A recent review suggested a possible mechanism for age-related nuclear and cytoplasmic damage that it may occur as a result of inadequate ovarian angiogenesis in primordial follicles as well as in ovarian stroma vessels [9]. However, little is known about the expression of angiogenic factors according to female age during folliculogenesis.
Stromal cell derived factor-1α (SDF-1α), one of important chemotactic chemokines [10], induces endothelial progenitor cells from bone marrow and is involved in angiogenesis by regulating the expression of vascular endothelial growth factor (VEGF) [11,12]. In addition, it has been reported that SDF-1α recruits immune cells into preovulatory follicles and is involved in ovarian function by improving the survival of granulosa cells and oocyte quality [13,14].
Leptin, a 16 kDa small protein, initially known as adipokine secreted by adipose tissues, is involved in weight control, but recently, it has been reported that leptin involves in hematopoiesis and vascular formation by promoting the expression of VEGF and angiogenesis with VEGF and fibroblast growth factor (FGF) [15-18].
Furthermore, evidences have accumulated that leptin is involved in folliculogenesis by suppressing the apoptosis of granulosa cells in the immature rat, by promoting the maturation to fertile stages and by improving nuclear and cytoplasmic maturations of oocytes [19-22]. These results imply that leptin has promoting effects on both cell division and angiogenesis and it can be postulated that it effectively activates ovarian neovascularization.
Therefore, this study was carried out to examine the ovarian expression of SDF-1α and leptin during follicular development according to female age and also to investigate the effects of these factors on the expressions of angiogenic factors by ovarian tissue cultures in vitro.
Methods
This study was approved by the Institutional Review Board of Pusan National University Hospital, Korea.
1. Animals
In all experiments, C57BL inbred mice were purchased from the Korea Experimental Animal Center (Daegu, Korea). The mice were maintained on a light-dark cycle, with the light on at 5:00 AM and off at 7:00 PM, and with food and water available ad libitum.
2. Assessment of ovarian SDF-1α and leptin after superovulation induction
Pregnant mare's serum gonadotropin (PMSG) (Sigma, St Louis, MO, USA) of 5 IU was injected intraperitoneally into female mice of two age groups (6-9 weeks old and 24-26 weeks old) in order to synchronize the estrus cycles. Ovarian tissues were collected 6, 12, 24, and 48 hours post PMSG injection and the expressions of SDF-1α and leptin mRNA were assessed by the reverse transcriptase-polymerase chain reaction (RT-PCR) method.
3. Ovarian tissue culture and treatment of SDF-1α and leptin
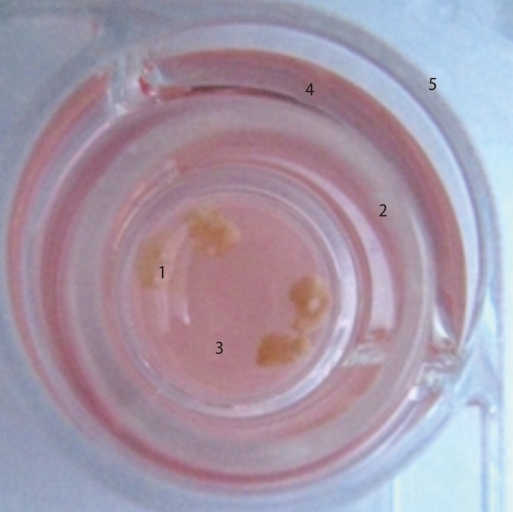
To induce ovulation and synchronize the estrus cycles, 5 IU of PMSG was injected and 48 hours later followed by the injection of 5 IU of human chorionic gonadotropin (hCG, Sigma) in the 9-11 week-old female mice. Two days after hCG injection, the ovarian tissues were collected, and the adipose tissue and connective tissues surrounding the ovary were removed using a 26-G needle. The isolated ovarian tissues were washed three times with normal saline and two times with culture medium. Then the ovarian tissues were cultured in Waymouth's medium (Invitrogen, Carlsbad, CA, USA) on Transwell permeable supports (0.4 µm Millicell-CM; Millipore, Bedford, MA, USA) for 7 days in 37℃, 5% CO2 incubator (Figure 1).
Figure 1.
Organ culture system of ovaries. 1, ovaries; 2, transwell permeable support (Millipore filter); 3, medium; 4, plain medium; 5, 4-well dish.
Waymouth's medium was supplemented with 10% FBS, 0.23 mM pyruvic acid, 3 mg/mL BSA, 1x ITS supplement (Invitrogen) and penicillin/streptomycin. Various concentrations of SDF-1α (50, 100 and 200 ng/mL, Cell Science, Inc., Canton, MA, USA) and leptin (0.01, 0.1 and 1 µg/mL, BioSource International, Inc., Camarillo, CA, USA) were added in the culture medium throughout the whole incubation period. These two factors were not added for the controls. The culture medium was replaced with fresh medium in every 2 days. Expressions of mRNAs of angiogenic factors such as VEGF, endothelial nitric oxide synthase (eNOS), and visfatin were assessed by RT-PCR method.
4. RNA preparation and RT-PCR
Total RNA was extracted using Trizol reagent (Invitrogen) according to the manufacturer's protocol. Complementary DNA (cDNA) was synthesized from 5 µg of total RNA with AMV Reverse Transcriptase (Promega, Madison, WI, USA) using a random hexamer (Bioneer, Daejon, Korea) at 42℃ for 1 hour. Each cDNA was subjected to polymerase chain reaction (PCR) amplification using gene-specific primers (Table 1). GAPDH expression was used as control. PCR products were visualized by electrophoresis on 1.2% agarose gel. The PCR bands were quantified and normalized relative to the control band with Image J (National Institutes of Health Image Software, ver. 1.35d, NIH, Bethesda, MD, USA). Two samples were prepared for each tissue and double experiments were performed. The experiments were repeated 3 times.
Table 1.
Primers sequences used for polymerase chain reaction amplification and conditions
SDF-1α, stromal cell derived factor-1α; eNOS, endothelial nitric oxide synthase; VEGF, vascular endothelial growth factor; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
5. Statistics
Data analysis was performed using SPSS ver.12.0 (SPSS Inc., San Rafael, CA, USA), with a value of p<0.05 being considered significant. Differences between groups were tested using Mann-Whitney U-tests. All data were expressed as mean±SE.
Results
1. Expressions of ovarian SDF-1α and leptin genes according to female age
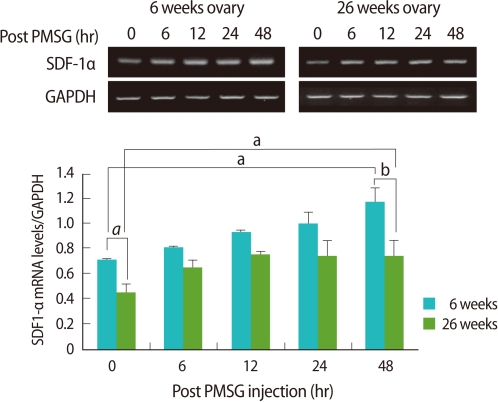
Ovarian expression of SDF-1α mRNA was significantly low in the old mice when compared to the young (p<0.05). Regardless of age, ovarian expression of SDF-1α mRNA post PMSG injection increased as the follicles developed, and the increase tendency was significantly lower in the old mice than the young (p<0.01) (Figure 2).
Figure 2.
Reverse transcriptase-polymerase chain reaction (RT-PCR) detection for stromal cell derived factor-1α(SDF-1α expression in ovaries of young (6-9 weeks old) and aged (24-26 weeks old) female mice. Whole ovaries were collected 0, 6, 12, 24, and 48 hours after pregnant mare's serum gonadotropin (PMSG) injection (n=4/point in time). The graphical representation of SDF-1α intensity normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) is shown below a representative example of RT-PCR (n=3). ap<0.05 and bp<0.01.
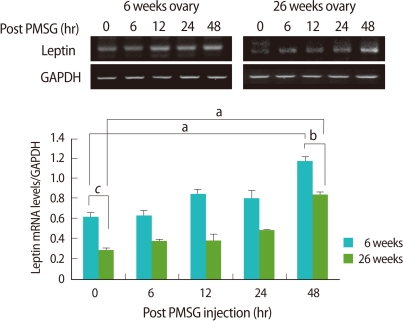
Ovarian leptin mRNA also expressed significantly low in the old mice when compared to the young (p<0.005). As follicles grew after PMSG injection, leptin mRNA expression in the ovary was increased regardless of age, and the tendency of the increase was significantly low in the old mice than the young (p<0.05) (Figure 3).
Figure 3.
Reverse transcriptase-polymerase chain reaction (RT-PCR) detection for leptin expression in ovaries of young (6-9 weeks old) and aged (24-26 weeks old) female mice. Whole ovaries were collected 0, 6, 12, 24, and 48 hours after pregnant mare's serum gonadotropin (PMSG) injection (n=4/point in time). The graphical representation of leptin intensity normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) are shown below a representative example of RT-PCR (n=4). ap<0.05, bp< 0.01, and cp<0.005.
2. Effects of SDF-1α and leptin on expressions of ovarian angiogenic factors
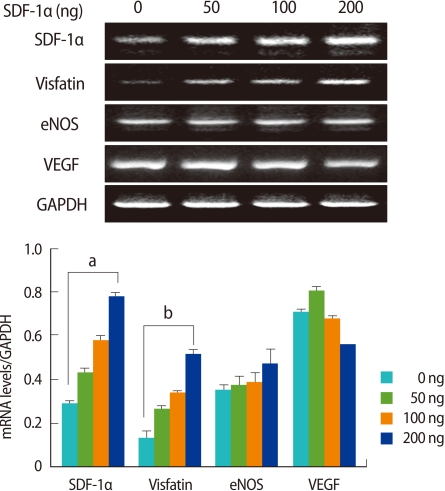
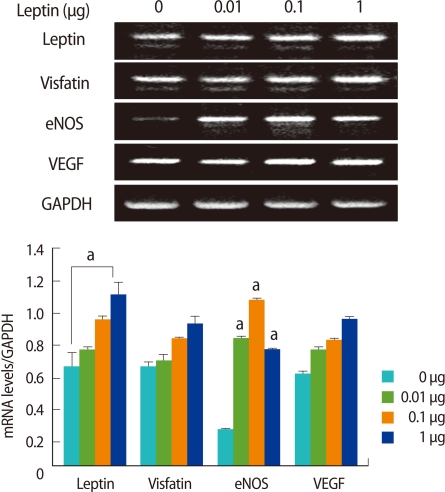
To determine the effects of SDF-1α and leptin on the expressions of ovarian angiogenic factors, various concentrations of SDF-1α and leptin were treated in ovarian tissue culture, and the expressions of VEGF, eNOS, and visfatin mRNA were measured. SDF-1α significantly enhanced the expressions of visfatin and SDF-1α mRNAs in a dose-dependent manner (p<0.01), however, it had no effect on the expressions of eNOS or VEGF mRNA (Figure 4). Regardless of the concentration, leptin enhanced the mRNA of leptin itself and eNOS expressions, and in particular, eNOS mRNA expression was significantly increased (p<0.005) (Figure 5).
Figure 4.
Effect of stromal cell derived factor-1α(SDF-1α) on visfatin, endothelial nitric oxide synthase (eNOS), and vascular endothelial growth factor (VEGF) mRNA expression in cultured mouse ovaries. Ovaries collected from 11-week-old-female mice were placed into culture and treated with SDF-1α (0, 50, 100, and 200 ng/mL) for 7 days. SDF-1α, visfatin, eNOS, and VEGF mRNA levels were detected by reverse transcriptase-polymerase chain reaction (RT-PCR). The graphical representation of the intensity of each gene normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH, is shown below a representative example of RT-PCR (n=3). ap<0.005 and bp<0.01 (vs. 0 ng/mL).
Figure 5.
Effect of leptin on visfatin, endothelial nitric oxide synthase (eNOS), and vascular endothelial growth factor (VEGF) mRNA expression in cultured mouse ovaries. Ovaries from 11 weeks old female mice were placed into culture and treated with leptin (0, 0.01, 0.1, and 1 µg/mL) for 7 days. Leptin, visfatin, eNOS, and VEGF mRNA levels were detected by reverse transcriptase-polymerase chain reaction (RT- PCR). The graphical representation of the intensity of each gene normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) are shown below a representative example of RT-PCR (n=3). ap<0.005 (vs. 0 µg/mL).
Discussion
The present study was carried out to investigate SDF-1α and leptin gene expressions in the ovary using different age groups of female mice to understand the age-related changes of ovarian angiogenic factor gene expressions. This study shows for the first time that ovarian SDF-1α and leptin mRNA expressions decreased with increasing age. This result suggests that these angiogenic factors might be involved in the decrease in ovarian function and ovarian aging with advancing female age.
The age-related decline of fertility in women is attributable to the decreases in number and quality of oocytes and follicles [23], and this reduction is primarily responsible for the apoptosis of oocytes and granulosa cells surrounding the oocyte [24]. SDF-1α is mainly secreted by fibroblast and endothelial cells, but it has been reported that it is also locally secreted from ovarian granulosa cells and introduced into the follicular fluid [13,25]. Leptin is also synthesized mainly by granulosa and theca cells in the ovaries [26,27] and it inhibits the apoptosis of follicle cells [19]. These results indicate that apoptosis of granulosa cells increases with advancing female age and thereby the expressions of SDF-1α and leptin are decreased in the ovaries. However, the present study did not demonstrate which part these factors are expressed within the ovary or whether their expressions in granulosa cells decrease with age. Therefore further study on the localization of SDF-1α and leptin expression in the ovaries by immunohistochemistry is needed to warrant the result of our present study.
Another limitation of this study is that the expression of SDF-1α and leptin was assessed at the mRNA transcript level. Considering the physiological role of proteins, their expressions should be evaluated at the protein level by western blot. For this reason, we made several attempts at western blotting, but failed to obtain an acceptable data due to very little expression of these factors.
In this study, another notable finding is that the expressions of SDF-1α and leptin mRNAs are enhanced as the follicle grows after the induction of ovulation by PMSG administration. This result suggests that the expressions of SDF-1α and leptin mRNAs are induced by gonadotropins, and as expressed, they have potential effects on follicle development. The interactions between granulosa and theca cells are necessary to the antral follicle developments which are essential in oocyte maturation [28], and Skinner et al. [25] reported that SDF-1α and VEGF expressions are controlled by granulosa and theca cells and that they are the representative candidate factors that are concerned with the development of antral follicles. Recently, Nishigaki et al. [29] reported that the concentration of SDF-1α significantly decreased in follicles less than 14 mm in diameter when compared to those of 15 mm or more in diameter, and as the diameter and volume of follicle are increased, the concentrations of VEGF and SDF-1α in the follicular fluid are augmented and the recovery rates of oocytes are also increased. These results indicate that SDF-1α can play an important role in follicle growth and development.
Ovarian folliculogenesis is inhibited in leptin-deficient mice [21], and the decrease of serum leptin concentration is associated with ovarian dysfunction [30,31]. In addition, fertility is restored by leptin treatment in ob/ob infertile mice [32], and the treatment of leptin during superovulation induction significantly increases the number of offspring in rabbit [33]. Moreover, in a previous report [34], we confirmed that the number and quality of the ovulated oocytes improved by the administration of leptin during superovulation induction with PMSG in old mice. Likewise, many studies have demonstrated the essential roles of leptin in the maintenance of normal ovarian functions such as folliculogenesis and oocyte maturation, and our finding that leptin expression is increased as follicular growth reconfirms the above fact.
As a result of RT-PCR analyses of 17 kinds of chemokines in 6- to 8-week-old normal mice, Zhou et al. [14] reported that MCP-1, RANTES, and IP-10 mRNAs are expressed high but SDF-1α mRNA is not expressed. This is somewhat different from our finding that SDF-1α is expressed in the ovaries and this discrepancy might arise from the differences in mouse strain or primers used in RT-PCR of SDF-1α. They used the CD-1 mouse strain and designed SDF-1α primers based on human nucleotide sequences.
Angiogenesis actively occurs in the ovary, and the follicular microvasculature develops under the control of ovarian angiogenic factors [35]. VEGF is one of the representative angiogenic factors and it plays an important role in folliculogenesis and the activities of granulosa cells [13,25]. It has been reported that SDF-1α promotes the expression of VEGF as well as angiogenesis with VEGF in cord vein endothelial cells [36], and that neovascularization during folliculogenesis is dependent on the synergistic regulatory actions of SDF-1α and VEGF [29]. Leptin also promotes VEGF expression as well as angiogenesis with VEGF and FGF [15,16]. However, VEGF expression was not significantly increased by SDF-1α and leptin in the ovarian tissue culture of the present study (Figures 4, 5). The reason why VEGF expression is not induced by these two factors is unclear. Further studies on the effects of SDF-1α and leptin in ovarian VEGF expression are needed to determine whether VEGF expression is controlled by these two factors or mainly by in vitro culture conditions.
However, in the present ovarian tissue culture, SDF-1α and leptin induced the expressions of visfatin and eNOS, respectively, in a concentration-dependent manner. Visfatin is secreted by the adipose tissue and it is an adipokine that has an insulin-like effect [37]. It has recently been discovered that it is also secreted by various cells including macrophages and amniotic epithelial cells and is concerned with proinflammatory responses and angiogenesis [38-40]. Recently, it has also been reported that there is a correlation between the concentration of visfatin in the follicular fluid and the number of oocytes retrieved in hyperstimulated women [41]. eNOS is nitric oxide synthase presented in blood vessel, and nitric oxide is known as an angiogenesis-related factor that controls the vasodilation and the expression of VEGF [42,43]. In the end, these results indicate that SDF-1α and leptin are involved in angiogenesis in the ovary. Therefore, it can be concluded that the decrease in the ovarian expressions of SDF-1α and leptin along with age inhibits angiogenesis and due to this decrease, apoptosis of granulosa and theca cells is promoted and in turn gives rise to a decrease in the number and quality of oocytes, eventually resulting in fertility decline. Further studies testing this hypothesis are needed.
Of interest, the treatment of ovarian tissue with SDF-1α and leptin resulted in the increased expression of SDF-1α and leptin in the ovary, respectively. This result indicates that each expression of these factors might be primarily stimulated in the ovary in a paracrine or autocrine manner although there is a possibility that ovarian expressions of these factors may be regulated by negative feedback inhibition when their expressions are increased.
Taken together, ovarian SDF-1α and leptin expression decrease with advancing female age and are promoted by gonadotropins and show an inclination to increase with follicular growth. These results indicate that ovarian SDF-1α and leptin have an important role in ovarian aging and function including folliculogenesis.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Geva E, Jaffe RB. Role of vascular endothelial growth factor in ovarian physiology and pathology. Fertil Steril. 2000;74:429–438. doi: 10.1016/s0015-0282(00)00670-1. [DOI] [PubMed] [Google Scholar]
- 2.Redmer CA, Reynolds LP. Angiogenesis in the ovary. Rev Reprod. 1996;1:182–192. doi: 10.1530/ror.0.0010182. [DOI] [PubMed] [Google Scholar]
- 3.Ravindranath N, Little-Ihrig L, Phillips HS, Ferrara N, Zeleznik AJ. Vascular endothelial growth factor messenger ribonucleic acid expression in the primate ovary. Endocrinology. 1992;131:254–260. doi: 10.1210/endo.131.1.1612003. [DOI] [PubMed] [Google Scholar]
- 4.Zeleznik AJ, Schuler HM, Reichert LE., Jr Gonadotropin-binding sites in the rhesus monkey ovary: role of the vasculature in the selective distribution of human chorionic gonadotropin to the preovulatory follicle. Endocrinology. 1981;109:356–362. doi: 10.1210/endo-109-2-356. [DOI] [PubMed] [Google Scholar]
- 5.Fraser HM. Regulation of the ovarian follicular vasculature. Reprod Biol Endocrinol. 2006;4:18. doi: 10.1186/1477-7827-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tarín JJ, Pérez-Albalá S, Cano A. Cellular and morphological traits of oocytes retrieved from aging mice after exogenous ovarian stimulation. Biol Reprod. 2001;65:141–150. doi: 10.1095/biolreprod65.1.141. [DOI] [PubMed] [Google Scholar]
- 7.te Velde ER, Peasron PL. The variability of female reproductive aging. Hum Reprod Update. 2002;8:141–154. doi: 10.1093/humupd/8.2.141. [DOI] [PubMed] [Google Scholar]
- 8.Thouas GA, Trounson AO, Jones GM. Effect of female age on mouse oocyte developmental competence following mitochondrial injury. Biol Reprod. 2005;73:366–373. doi: 10.1095/biolreprod.105.040956. [DOI] [PubMed] [Google Scholar]
- 9.Tatone C, Amicarelli F, Carbone MC, Monteleone P, Caserta D, Marci R, et al. Cellular and molecular aspects of ovarian follicle ageing. Hum Reprod Update. 2008;14:131–142. doi: 10.1093/humupd/dmm048. [DOI] [PubMed] [Google Scholar]
- 10.Burger JA, Kipps TJ. CXCR4: a key receptor in the crosstalk between tumor cells and their microenvironment. Blood. 2006;107:1761–1767. doi: 10.1182/blood-2005-08-3182. [DOI] [PubMed] [Google Scholar]
- 11.Bachelder RE, Wendt MA, Mercurio AM. Vascular endothelial growth factor promotes breast carcinoma invasion in an autocrine manner by regulating the chemokine receptor CXCR4. Cancer Res. 2002;62:7203–7206. [PubMed] [Google Scholar]
- 12.Kryczek I, Lange A, Mottram P, Alvarez X, Cheng P, Hogan M, et al. CXCL12 and vascular endothelial growth factor synergistically induce neoangiogenesis in human ovarian cancers. Cancer Res. 2005;65:465–472. [PubMed] [Google Scholar]
- 13.Kryczek I, Frydman N, Gaudin F, Krzysiek R, Fanchin R, Emilie D, et al. The chemokine SDF-1/CXCL12 contributes to T lymphocyte recruitment in human pre-ovulatory follicles and coordinates with lymphocytes to increase granulosa cell survival and embryo quality. Am J Reprod Immunol. 2005;54:270–283. doi: 10.1111/j.1600-0897.2005.00307.x. [DOI] [PubMed] [Google Scholar]
- 14.Zhou C, Borillo J, Wu J, Torres L, Lou YH. Ovarian expression of chemokines and their receptors. J Reprod Immunol . 2004;63:1–9. doi: 10.1016/j.jri.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 15.Sierra-Honigmann MR, Nath AK, Murakami C, García-Cardeña G, Papapetropoulos A, Sessa WC, et al. Biological action of leptin as an angiogenic factor. Science. 1998;281:1683–1686. doi: 10.1126/science.281.5383.1683. [DOI] [PubMed] [Google Scholar]
- 16.Cao R, Brakenhielm E, Wahlestedt C, Thyberg J, Cao Y. Leptin induces vascular permeability and synergistically stimulates angiogenesis with FGF-2 and VEGF. Proc Natl Acad Sci USA. 2001;98:6390–6395. doi: 10.1073/pnas.101564798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park HY, Kwon HM, Lim HJ, Hong BK, Lee JY, Park BE, et al. Potential role of leptin in angiogenesis: leptin induces endothelial cell proliferation and expression of matrix metalloproteinases in vivo and in vitro. Exp Mol Med. 2001;33:95–102. doi: 10.1038/emm.2001.17. [DOI] [PubMed] [Google Scholar]
- 18.Yamagishi S, Amano S, Inagaki Y, Okamoto T, Takeuchi M, Inoue H. Pigment epithelium-derived factor inhibits leptin-induced angiogenesis by suppressing vascular endothelial growth factor gene expression through anti-oxidative properties. Microvasc Res. 2003;65:186–190. doi: 10.1016/s0026-2862(03)00005-0. [DOI] [PubMed] [Google Scholar]
- 19.Almog B. Leptin attenuates follicular apoptosis and accelerates the onset of puberty in immature rat. Mol Cell Endocrinol. 2001;183:179–191. doi: 10.1016/s0303-7207(01)00543-3. [DOI] [PubMed] [Google Scholar]
- 20.Craig J, Zhu H, Dyce PW, Petrik J, Li J. Leptin enhances oocyte nuclear and cytoplasmic maturation via the mitogen-activated protein kinase pathway. Endocrinology. 2004;145:5355–5363. doi: 10.1210/en.2004-0783. [DOI] [PubMed] [Google Scholar]
- 21.González RR, Simón C, Caballero-Campo P, Norman R, Chardonnens D, Devoto L, et al. Leptin and reproduction. Hum Reprod Update. 2000;6:290–300. doi: 10.1093/humupd/6.3.290. [DOI] [PubMed] [Google Scholar]
- 22.Moschos S, Chan JL, Mantzoros CS. Leptin and reproduction: a review. Fertil Steril. 2002;77:433–444. doi: 10.1016/s0015-0282(01)03010-2. [DOI] [PubMed] [Google Scholar]
- 23.Faddy MJ, Gosden RG, Gougeon A, Richardson SJ, Nelson JF. Accelerated disappearance of ovarian follicles in mid-life: implications for forecasting menopause. Hum Reprod. 1992;7:1342–1346. doi: 10.1093/oxfordjournals.humrep.a137570. [DOI] [PubMed] [Google Scholar]
- 24.Vaskivuo TE, Anttonen M, Herva R, Billig H, Dorland M, te Velde ER, et al. Survival of human ovarian follicles from fetal to adult life: apoptosis, apoptosis-related proteins, and transcription factor GATA-4. J Clin Endocrinol Metab. 2001;86:3421–3429. doi: 10.1210/jcem.86.7.7679. [DOI] [PubMed] [Google Scholar]
- 25.Skinner MK, Schmidt M, Savenkova MI, Sadler-Riggleman I, Nilsson EE. Regulation of granulosa and theca cell transcriptomes during ovarian antral follicle development. Mol Reprod Dev. 2008;75:1457–1472. doi: 10.1002/mrd.20883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cioffi JA, Van Blerkom J, Antczak M, Shafer A, Wittner S, Snidgrass HR. The expression of leptin and its receptors in pre-ovulatory human follicles. Mol Hum Reprod. 1997;3:467–472. doi: 10.1093/molehr/3.6.467. [DOI] [PubMed] [Google Scholar]
- 27.Löffler S, Aust G, Köhler U, Spanel-Borowski K. Evidence of leptin expression in normal and polycystic human ovaries. Mol Hum Reprod. 2001;7:1143–1149. doi: 10.1093/molehr/7.12.1143. [DOI] [PubMed] [Google Scholar]
- 28.Tajima K, Orisaka M, Yata H, Goto K, Hosokawa K, Kotsuji F. Role of granulosa and theca cell interactions in ovarian follicular maturation. Microsc Res Tech. 2006;69:450–458. doi: 10.1002/jemt.20304. [DOI] [PubMed] [Google Scholar]
- 29.Nishigaki A, Okada H, Okamoto R, Sugiyama S, Miyazaki K, Yasuda K, et al. Concentrations of stromal cell-derived factor-1 and vascular endothelial growth factor in relation to the diameter of human follicles. Fertil Steril. 2011;95:742–746. doi: 10.1016/j.fertnstert.2010.10.028. [DOI] [PubMed] [Google Scholar]
- 30.Shimizu H, Shimomura Y, Nakanishi Y, Futawatari T, Ohtani K, Sato N, et al. Estrogen increases in vivo leptin production in rats and human subjects. J Endocrinol. 1997;154:285–289. doi: 10.1677/joe.0.1540285. [DOI] [PubMed] [Google Scholar]
- 31.Hong SC, Yoo SW, Cho GJ, Kim T, Hur JY, Park YK, et al. Correlation between estrogens and serum adipocytokines in premenopausal and postmenopausal women. Menopause. 2007;14:835–840. doi: 10.1097/GME.0b013e31802cddca. [DOI] [PubMed] [Google Scholar]
- 32.Chehab FF, Lim ME, Lu R. Correction of the sterility defect in homozygous obsess female mice by treatment with the human recombinant leptin. Nat Genet. 1996;12:318–320. doi: 10.1038/ng0396-318. [DOI] [PubMed] [Google Scholar]
- 33.Sirotkin AV, Rafay J, Kotwica J. Leptin controls rabbit ovarian function in vivo and in vitro: Possible interrelationships with ghrelin. Theriogenology. 2009;72:765–772. doi: 10.1016/j.theriogenology.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 34.Joo JK, Joo BS, Kim SC, Choi JR, Park SH, Lee KS. Role of leptin in improvement of oocyte quality by regulation of ovarian angiogenesis. Anim Reprod Sci. 2010;119:329–334. doi: 10.1016/j.anireprosci.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 35.Robinson RS, Woad KJ, Hammond AJ, Laird M, Hunter MG, Mann GE. Angiogenesis and vascular function in the ovary. Reproduction. 2009;138:869–881. doi: 10.1530/REP-09-0283. [DOI] [PubMed] [Google Scholar]
- 36.Yamaguchi J, Kusano KF, Masuo O, Kawamoto A, Silver M, Murasawa S, et al. Stromal cell-derived factor-1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation. 2003;107:1322–1328. doi: 10.1161/01.cir.0000055313.77510.22. [DOI] [PubMed] [Google Scholar]
- 37.Hug C, Lodish HF. Medicine. Visfatin: a new adipokine. Science. 2005;307:366–367. doi: 10.1126/science.1106933. [DOI] [PubMed] [Google Scholar]
- 38.Ognjanovic S, Bryant-Greenwood GD. Pre-B-cell colony-enhancing factor, a novel cytokine of human fetal membranes. Am J Obstet Gynecol. 2002;187:1051–1058. doi: 10.1067/mob.2002.126295. [DOI] [PubMed] [Google Scholar]
- 39.Luk T, Malam Z, Marshall JC. Pre-B cell colony-enhancing factor (PBEF)/visfatin: a novel mediator of innate immunity. J Leukoc Biol. 2008;83:804–816. doi: 10.1189/jlb.0807581. [DOI] [PubMed] [Google Scholar]
- 40.Adya R, Tan BK, Punn A, Chen J, Randeva HS. Visfatin induces human endothelial VEGF and MMP-2/9 production via MAPK and PI3K/Akt signalling pathways: novel insights into visfatin-induced angiogenesis. Cardiovasc Res. 2008;78:356–365. doi: 10.1093/cvr/cvm111. [DOI] [PubMed] [Google Scholar]
- 41.Shen CJ, Tsai EM, Lee JN, Chen YL, Lee CH, Chan TF. The concentrations of visfatin in the follicular fluids of women undergoing controlled ovarian stimulation are correlated to the number of oocytes retrieved. Fertil Steril. 2010;93:1844–1850. doi: 10.1016/j.fertnstert.2008.12.090. [DOI] [PubMed] [Google Scholar]
- 42.Sengoku K, Takuma N, Horikawa M, Tsuchiya K, Komori H, Sharifa D, et al. Requirement of nitric oxide for murine oocyte maturation, embryo development, and trophoblast outgrowth in vitro. Mol Reprod Dev. 2001;58:262–268. doi: 10.1002/1098-2795(200103)58:3<262::AID-MRD3>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 43.Murohara T, Horowitz JR, Silver M, Tsurumi Y, Chen D, Sullivan A, et al. Vascular endothelial growth factor/vascular permeability factor enhances vascular permeability via nitric oxide and prostacyclin. Circulation. 1998;97:99–107. doi: 10.1161/01.cir.97.1.99. [DOI] [PubMed] [Google Scholar]