Abstract
Implantation of an embryo occurs during the mid-secretory phase of the menstrual cycle, known as the "implantation window." During this implantation period, there are significant morphologic and functional changes in the endometrium, which is followed by decidualization. Many immune cells, such as dendritic and natural killer (NK) cells, increase in number in this period and early pregnancy. Recent works have revealed that antigen-presenting cells (APCs) and NK cells are involved in vascular remodeling of spiral arteries in the decidua and lack of APCs leads to failure of pregnancy. Paternal and fetal antigens may play a role in the induction of immune tolerance during pregnancy. A balance between effectors (i.e., innate immunity and helper T [Th] 1 and Th17 immunity) and regulators (Th2 cells, regulatory T cells, etc.) is essential for establishment and maintenance of pregnancy. The highly complicated endocrine-immune network works in decidualization of the endometrium and at the fetomaternal interface. We will discuss the role of immune cells in the implantation period and during early pregnancy.
Keywords: Implantation, Endometrium, Decidua, Dendritic Cells, Macrophages, Lymphocytes, Natural Killer Cells, Regulatory T Cells, Th17 Cells, Human
Introduction
The endometrium is the site where the blastocyst is implanted and a key place not only for supporting fetal growth through supplementation of oxygen and nutrients but also for protecting the embryo and later the fetus from microbial invasion during pregnancy. Implantation of the embryo occurs during the mid-secretory phase of the menstrual cycle, known as the "implantation window." During this implantation period, there are significant morphologic and functional changes in the endometrium, which is followed by decidualization. These events in the endometrium are mainly controlled by ovarian steroid hormones-estrogen and progesterone [1].
The uterine endometrium consists of two main cellular components, the stromal cells and the glandular cells. During the implantation window, the fibroblast-like endometrial stromal cells are transformed into larger and rounded decidual cells (decidualization). In the glandular cells, secretory glandules develop and large apical protrusions (pinopodes) and microvilli emerge as well [2]. Furthermore, ovarian steroid hormones regulate the expression of various cytokines, chemokines, growth factors, and adhesion molecules in the secretory endometrium [1].
Around the implantation period, there is a major change in the proportion and number of endometrial immune cells [3,4]. This peri-implantation period seems to be the earliest period in which the mother can recognize that she is pregnant [5]. The serum hCG concentration increases during this period, and it is likely that the maternal immune cells recognize fetal antigens from the implantation period [6]. It is now widely accepted that immunologic tolerance is inevitable for establishment and maintenance of pregnancy [7].
In this article, the role of immune cells in the endometrium of peri-implantation period and following period will be discussed.
Peripheral blood and endometrial lymphocytes during a menstrual cycle
It has been reported that the number and proportion of immune cells in the peripheral blood and endometrium change between the follicular and luteal phases of the ovarian cycle. However, exact figures on the fluctuation of peripheral blood lymphocytes remain unknown due to the contradictory results of different studies [8,9].
Recently, our group published a relatively large-scale study that was designed to sample peripheral blood serially during a menstrual cycle [10]. In the luteal phase, the percentage of CD3+ T and CD3+CD4+ helper T (Th) cells decreased, but the natural killer (NK) cell percentage and NK cell cytotoxicity increased. However, other lymphocyte subpopulations (B and natural killer T [NKT] cells) and the ratios of Th1/Th2 cytokines producing Th cells did not fluctuate. One important characteristic of peripheral blood immune cells is that they have been suggested as a major source of endometrial immune cells.
Dendritic cells and macrophages in the endometrium and decidua
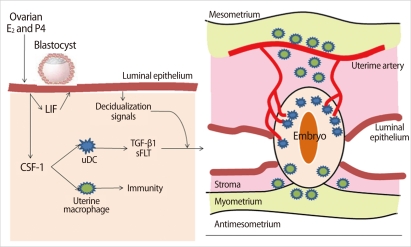
Dendritic cells (DCs) and macrophages, the major antigen presenting cells in the endometrium, seem to play an important role in the maintenance of pregnancy. After implantation of a blastocyst, DCs are recruited into the endometrium and accumulated, especially around the implanted embryo [11]. In the deciduae, DCs represent around 5-10% of all hematopoietic uterine cells. DCs are not only essential for the induction of primary immune responses but also important for the induction of immunological tolerance. The function and differentiation of DCs are regulated by the local microenvironment determined by cytokines and chemokines [11]. Levels of colony-stimulating factor (CSF)-1 synthesized by the uterine epithelium increase at the time of implantation and continue to elevate dramatically throughout the process of placentation [12]. This CSF-1 is the major regulator of the mononuclear phagocytic lineage and controls the proliferation, migration, viability, and function of DCs and macrophages and affects decidual cells and trophoblasts (Figure 1) [12]. Endometrial epithelial cells produce leukemia inhibitor factor (LIF) as well as CSF-1. LIF plays a role in embryo implantation and decidualization. DCs secrete soluble FMS-like tyrosine kinase1 (sFLT1) and transforming growth factor (TGF)-β1, which act locally and regulate angiogenesis in the endometrium and are involved in the development of regulatory T (Treg) cells (Figure 2) [12].
Figure 1.
Role of dendritic cells (DCs) and macrophages in implantation. Ovarian steroid hormones, E2, and progesterone (P4) stimulate synthesis of growth factors from endometrial epithelial cells, including colony-stimulating factor (CSF-1) and leukemia inhibitor factor (LIF). Uterine DCs are required for efficient decidualization of the endometrium. Macrophages play a largely immune role at this stage (Modified from Pollard JW. J Clin Invest 2008;118:3832-5 [12]). TGF, transforming growth factor; sFLT, soluble FMS-like tyrosine kinase.
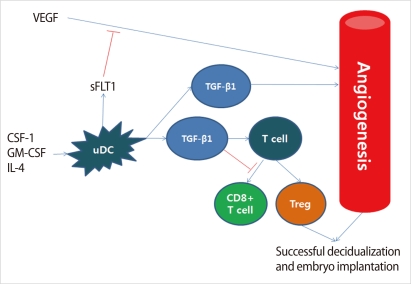
Figure 2.
Proposed roles of uterine dendritic cells (DCs) in the regulation of angiogenesis and T cell action at the maternal-fetal interface. Monocytes are recruited to the uterus and differentiate into mature tolerogenic cells such as uDCs, under the influence of colony-stimulating factor (CSF-1), GM-CSF, and interleukin (IL)-4. Uterine DCs produce soluble FMS-like tyrosine kinase 1 (sFLT1) and transforming growth factor (TGF)-β1. sFLT1, a soluble form of VEGFR1, modulates the actions of vascular endothelial growth factor (VEGF), and TGF-β1 influences endothelial cell viability and suppresses cytotoxic CD8+ T cell function and development of regulatory T (Treg) cells ( Modified from Pollard JW. J Clin Invest 2008;118: 3832-5 [12]).
In a study done in pregnant mice, depletion of uterine DCs resulted in the severe impairment of the implantation process, leading to embryo resorption [11]. The authors suggested that uterine DCs directly fine-tune decidual angiogenesis by providing two critical factors, sFlt1 and TGF-β1, that promote coordinated blood vessel maturation. Collectively, uterine DCs appear to govern uterine receptivity, independent of their predicted role in immunological tolerance, by regulating tissue remodeling and angiogenesis [11].
Macrophages are the innate immune cells that eliminate microbes in the endometrium. The number of endometrial macrophages increases in the late secretory endometrium as compared to the proliferative phase [13]. Decidual macrophages can participate in diverse activities during pregnancy [14]. Decidual macrophages comprise about 20-25% of the total decidual leukocytes and are the main subset of human leukocyte antigen (HLA)-DR+ antigen-presenting cells (APCs) in human deciduae [14].
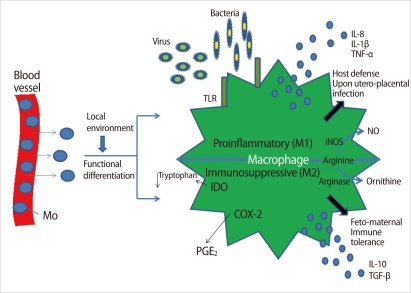
Decidual macrophages activated by pro-inflammatory cytokines and microbial lipopolysaccharide (LPS) are classified as M1-type, which secretes tumor necrosis factor (TNF)-α and interleukin (IL)-12 and participates in the progression of inflammation [14]. M2 polarization induced by glucocorticoids and Th2 cytokines such as IL-4, IL-10, and IL-13 is characterized by enhanced innate immunity receptors (scavenger receptors and macrophage mannose receptors) and upregulation of arginase activity, which counteracts nitric oxide synthesis [14]. In addition, M2 macrophages demonstrate increased secretion of IL-1R antagonist and are essential for tissue remodeling and immune tolerance during pregnancy (Figure 3) [14]. In early pregnancy, pro-angiogenic factors secreted from macrophages prompt vascular remodeling to ensure appropriate utero-placental circulation. In contrast, macrophages accumulated in the low uterine segment participate in cervical ripening during late pregnancy [14]. A balance of M1 and M2 macrophages may contribute to the outcome of pregnancy.
Figure 3.
Molecular mechanisms associated with the two distinctive macrophage phenotypes in the deciduae. Mo, monocyte; TLR, toll-like receptor; iNOS, inducible NO synthase; NO, nitric oxide; IDO, indoleamine dioxygenase; COX2, cyclooxygenase 2; PGE2, prostaglandin E2; IL, interleukin; TNF, tumor necrosis factor (Modified from Nagamatsu T, et al. Am J Reprod Immunol 2010;63:460-71 [14]).
T cells in the endometrium and deciduae
Some authors have reported that the proportion of endometrial lymphocytes was not found to fluctuate during a menstrual cycle [15]. However, this finding is not supported by others, who found a significant decrease of endometrial T cells from 55% to 6.7% between the late proliferative and the late secretory phages [4]. Even though there is a discrepancy regarding the proportion of T cells in the secretory endometrium, functional changes of T cells in the endometrium are evident. The balance between effector T and regulatory T cells may change from the peri-implantation period. This balance is very important for fetomaternal immune tolerance, the essential mechanism for a successful pregnancy.
1. Type1 and Type2 cytokine producing T cells
During pregnancy, type 1 (pro-inflammatory immune reaction mediated by TNF-α, IFN-γ, etc.) and type 2 (anti-inflammatory response characterized by IL-4, IL-10, etc.) immune reactions develop sequentially [16]. The process of embryo implantation and the following invasion into the endometrium is characterized by a pro-inflammatory reaction [16]. The second phase of rapid fetal growth and development represents an anti-inflammatory, Th2 environment. The end stage of pregnancy, parturition, is the phase of inflammation characterized by an influx of immune cells into the myometrium.
Even though the number of T cells present in the decidua decreases during pregnancy as compared to the non-pregnant endometrium, these T cells may affect acceptance of the fetus by producing cytokines [7]. It is well known that a strong Th1 environment is harmful for pregnancy and the Th2 response ameliorates the Th1 response [7]. Type 1 cytokines inhibit trophoblast invasion, stimulate apoptosis of human trophoblast cells, and enhance decidual macrophage activity, all of which result in the production of factors harmful to the embryo. Also, these cytokines negatively influence fetal growth by activation of prothrombinase, generation of thrombin, formation of clots, and production of IL-8, which stimulates granulocyte and endothelial cells to terminate the blood supply to the developing placenta [7].
2. γδ T cells
T cells can be classified by the expression of T cell receptor (TCR): TCR is composed of a combination of α, β, and ζ chains. Most T cells have TCRs with α and β chains. However, some T cells-γδ T cells-do have TCRs made up of γ and δ chains, not of α and β ones. These two T cell subsets seem to have distinct lineages and play different roles.
γδ T cells are commonly distributed in the mucosal organs, such as the uterus, the vagina, the tonsils, and the intestine, and may play a role in bridging the innate and adaptive immunity. Nearly 70% of decidual T cells express γδ TCRs and most γδ T cells are activated [17]. The majority of progesterone receptor (PR)+lymphocytes are γδ TCRs+ and/or CD8+ T cells, and these PR+ lymphocytes in the peripheral blood increase in number during normal pregnancy. PR expression is regulated in a hormone-independent manner and is upregulated by activation of these lymphocytes. Furthermore, lymphocyte immunotherapy for recurrent miscarriages has been shown to induce lymphocyte PRs and has shown that these are related to the success or failure of gestation [17]. It is suggested that γδ T cells can recognize trophoblast-presented antigens [17].
It is reported that progesterone-induced blocking factor (PIBF) is produced by activated lymphocytes and trophoblasts. PIBF downregulates Th1 cytokines, stimulates Th2 cytokines and antibody synthesis, and inhibits NK cell activity and phospholipase A2 in arachidonic acid metabolism [17].
In summary, γδ T cells are likely to be activated by recognition of fetal antigens, and activated γδ T cells produce PIBF under the influence of progesterone. PIBF induces a Th2 dominant microenvironment, inhibits NK cell cytotoxicity, and maintains uterine quiescence by blocking prostaglandin synthesis.
3. Treg cells
There are several types of immune regulatory T lymphocytes: Tr1 cells (IL-10 producing T cells), Th3 cells (TGF-β secreting T cells), TofB cells (regulatory T cells stimulated by B cells), and Foxp3+ Treg cells [18,19]. Among these cells, Foxp3+ Treg cells have been most extensively studied and most of them correspond to CD4+CD25bright T cells.
There are two distinct lineages of Foxp3+ Treg cells. One lineage, nTreg cells, originates from the thymus. The other, iTreg cells, is known to be induced in the periphery by activation of naïve T cells in the presence of TGF-β. Foxp3+ Treg cells can be activated by APCs and play a role in immune regulation against other immune cells such as Th1 and Th2 cells, B cells, and NK cells by expression of TGF-β, IL-10, cytotoxic T-lymphocyte antigen 4 (CTLA-4), etc. [20].
Even though there is no report showing a change in the number of Treg cells in the midluteal phase of the menstrual cycle, Treg cells increased in the peripheral blood during normal pregnancy [21]. In a study in women with recurrent pregnancy loss (RPL), Treg cells decreased in the peripheral blood and in the decidua as compared to those of women underwent elective abortion [22]. These findings indicate that Treg cells may be deeply involved in maternal immune tolerance against a fetus.
Some papers have insisted that estrogen is a key factor in the proliferation of Treg cells in the late follicular phase or during pregnancy [23,24]. According to recent studies, however, expansion of Treg cells during pregnancy is relevant to exposure to paternal antigen or products such as sperm and factors in the seminal fluid, but not to estrogen or progesterone [25-27].
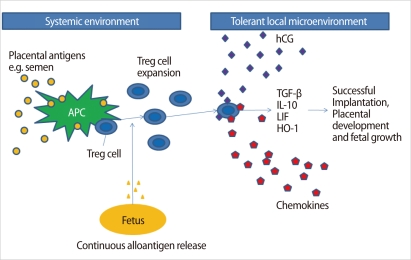
Interestingly, hCG may have a role in the recruitment of Treg cells into the uterus during pregnancy [28]. It remains unclear how Treg cells protect a fetus in the uterus. One explanation is that Treg cells may expand following recognition of paternal antigens, and are trafficked to the fetomaternal interface by hCG and several chemokines, where they help embryo implantation, placentation, and fetal growth by secretion of TGF-β, IL-10, LIF, Heme oxygenase (HO)-1, etc. [28] (Figure 4).
Figure 4.
Possible mechanisms of the origin, expansion, migration, and function of Treg cells during pregnancy. APC, antigen presenting cells; TGF-β, transforming growth factor-β; IL, interleukin; LIF, leukemia inhibitor factor; HO-1, heme-oxygenase-1 (Modified from Leber A, et al. Am J Reprod Immunol 2010;63:445-59 [28]).
4. Th17 cells
Recently, a novel subset of T cells called Th17 cells has been reported to induce experimental autoimmune encephalomyelitis and adjuvant arthritis in a mouse model [29,30]. Th17 cells are directly involved in chronic inflammatory processes by secreting IL-17, which recruits neutrophils to tissue through induction of granulocyte colony-stimulating factor and IL-8 [31]. Th17 cells have a distinct developmental lineage, which is different from Th1 and Th2 cells [29,32]. TGF-β has been suggested as a crucial cytokine for Th17 cell development, in conjunction with IL-6 and IL-21 in human [33-36]. Th17 cells are mainly regulated by Treg cells.
The level of Th17 cells in the endometrium at various points in time during a menstrual cycle remains unexplored. A recent study reported that the proportions of Th17 cells in the peripheral blood and deciduae were higher in pregnant women with unexplained RPL as compared to normal women in early pregnancy [37]. Non-pregnant women with unexplained RPL had a higher Th17 cell level in the circulating blood than did parous controls [38].
B cells in the endometrium and decidua
B cells are very scarce in the human endometrium and decidua [14]. Their role has not yet been explored.
NK cells in the endometrium and decidua
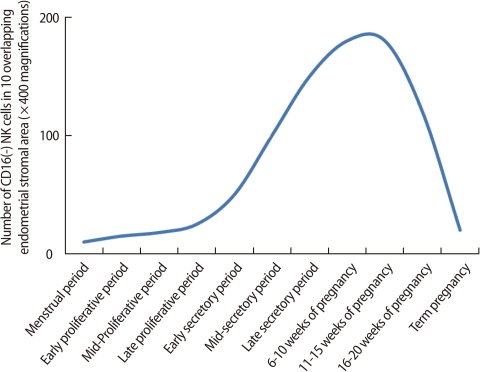
NK cells are one of the key components of the innate immune system and eliminate virus-infected cells and cancer cells by secretion of cytotoxic products such as granzyme and perforin. Endometrial NK cells show different phenotypic characteristics from NK cells in the peripheral blood. Peripheral blood NK cells represent 10-15% of all lymphocytes, and the majority of them express CD56+CD16+, a cytotoxic subset. On the other hand, most endometrial NK cells are CD56+CD16-, a less toxic cell population. During a menstrual cycle, endometrial NK cells increase in number in the secretory phase as compared to the proliferative phase [39]. However, the proportion of human endometrial NK cells remains constant during the cycle [15]. In the late secretory phase and early pregnancy, the percentage of endometrial/decidual NK cells rapidly increases up to 70% of uterine leukocytes [4]. The number of endometrial and decidual NK cells starts to increase in the mid-secretory phase and early pregnancy, reaches a peak at the end of the first trimester, and then decreases as the fetus approaches term (Figure 5) [39]. These findings suggest that uterine NK cells play an important role in the establishment and maintenance of pregnancy.
Figure 5.
Number of NK cells in human endometrium or deciduae. The number of endometrial and decidual NK cells starts to increase in the mid-secretory phase and early pregnancy, reaches a peak at the end of the first trimester, and then decreases as the fetus approaches term (Modified from King A, et al. Dev Immunol 1991;1: 169-90 [39]).
Most endometrial NK cells of non-pregnant women do not express CD16 (a cytotoxicity marker), NKp30, NKp44 (cell activation markers), or L-selectin (an adhesion molecule) [40]. However, they are characterized by expression of other activation markers such as HLA-DR, CD69, NKp46, and NKG2D. NK cells at this stage are functionally less cytotoxic and produce little amount of cytokines. However, secretory endometrial NK cells have the potential to proliferate.
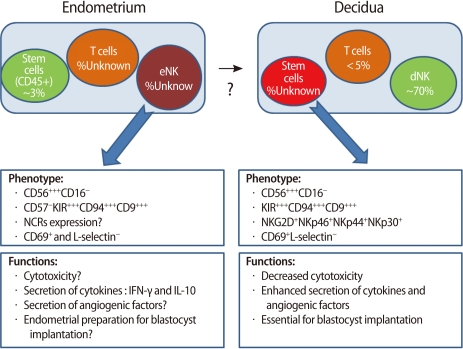
If implantation takes place, endometrial cells secrete IL-15, which causes endometrial NK cells to differentiate into decidual NK cells. Decidual NK cells begin to increase the production of cytokines, growth factors, and angiogenetic factors [41] (Figure 6).
Figure 6.
Cell composition of the endometrium and decidua and the phenotype and functions of endometrial NK (eNK) and decidual NK (dNK) cells (Modified from Manaster I, et al. Placenta 2008;29 Suppl A:S60-6 [41]). NK, natural killer; NCR, natural cytotoxicity receptors; IFN, interferon; IL, interleukin.
Decidual NK cells increase blood flow at the fetomaternal interface by remodeling of spiral arteries and help migration of trophoblasts. Some angiogenetic factors (VEGF, PLGF, Angiopoietin-2, and NKG5) are produced by decidual NK cells. They also secret various cytokines and growth factors such as TNF-α, IL-10, GM-CSF, IL-1β, TGF-β1, CSF-1, LIF, and IFN-γ [42]. Through this mechanism, decidual NK cells seem to contribute to embryo implantation and decidualization of the endometrium (Figure 6) [41].
Several theories have been poposed for the origin of uterine NK cells. Among them, the most popular theory is the trafficking of peripheral blood NK cells. Endometrial chemokines have been suggested to recruit periheral blood CD56brightCD16- NK cells into the uterus. TGF-β may convert CD56+CD16+ NK cells into CD56brightCD16- NK cells. Many chemokines are expressed in the human endometrium (CCL4, CCl5, CCl7, CCL13, CCL19, CCL21, CXCL9, CXCL10, IL-15). The chemokine receptors responding to these include CCR5, CCR7, CXCR3, CXCR4, and IL-15Rα chain [43]. Theories of in situ proliferation of uterine NK cells and of uterine NK cell differentiation from hematopoietic precursors have also been proposed [40].
Conclusion
The human endometrium undergoes significant changes to prepare for implantation of an embryo during the peri-implantation period. The immune cells also alter in number and function systemically and locally in the luteal phase. This modification of the reproductive and immune systems is profoundly influenced by several hormones, especially ovarian steroids.
Recognition of pregnancy is essential for maternal tolerance of a fetus. Although hCG is known as one of the candidates that may be involved in pregnancy recognition, recognition of trophoblast-driven products by immune cells also induces decidual differentiation of the endometrium.
It is evident that an encounter of immune cells with paternal and/or fetal antigens is essential to achieve maternal immune tolerance.
Many innate and adaptive immune cells seem to play a significant role in establishment and maintenance of pregnancy. This preparation of the endometrium and immune system for pregnancy begins before the implantation occurs. As a result, significant biologic and immune modifications have already taken place before the window of implantation. This process is regulated by the complicated and delicate interaction of the endocrine-immune system.
Further studies are warranted to solve the enigma of the establishment of implantation and maintenance of successful pregnancy.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Dey SK, Lim H, Das SK, Reese J, Paria BC, Daikoku T, et al. Molecular cues to implantation. Endocr Rev. 2004;25:341–373. doi: 10.1210/er.2003-0020. [DOI] [PubMed] [Google Scholar]
- 2.Dekel N, Gnainsky Y, Granot I, Mor G. Inflammation and implantation. Am J Reprod Immunol. 2010;63:17–21. doi: 10.1111/j.1600-0897.2009.00792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laird SM, Tuckerman EM, Cork BA, Linjawi S, Blakemore AI, Li TC. A review of immune cells and molecules in women with recurrent miscarriage. Hum Reprod Update. 2003;9:163–174. doi: 10.1093/humupd/dmg013. [DOI] [PubMed] [Google Scholar]
- 4.Flynn L, Byrne B, Carton J, Kelehan P, O'Herlihy C, O'Farrelly C. Menstrual cycle dependent fluctuations in NK and T-lymphocyte subsets from non-pregnant human endometrium. Am J Reprod Immunol. 2000;43:209–217. doi: 10.1111/j.8755-8920.2000.430405.x. [DOI] [PubMed] [Google Scholar]
- 5.Afshar Y, Stanculescu A, Miele L, Fazleabas AT. The role of chorionic gonadotropin and Notch1 in implantation. J Assist Reprod Genet. 2007;24:296–302. doi: 10.1007/s10815-007-9149-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clark DA, Chaouat G, Wong K, Gorczynski RM, Kinsky R. Tolerance mechanisms in pregnancy: a reappraisal of the role of class I paternal MHC antigens. Am J Reprod Immunol. 2010;63:93–103. doi: 10.1111/j.1600-0897.2009.00774.x. [DOI] [PubMed] [Google Scholar]
- 7.Veenstra van Nieuwenhoven AL, Heineman MJ, Faas MM. The immunology of successful pregnancy. Hum Reprod Update. 2003;9:347–357. doi: 10.1093/humupd/dmg026. [DOI] [PubMed] [Google Scholar]
- 8.Coulam CB, Silverfield JC, Kazmar RE, Fathman CG. T-lymphocyte subsets during pregnancy and the menstrual cycle. Am J Reprod Immunol. 1983;4:88–90. doi: 10.1111/j.1600-0897.1983.tb00259.x. [DOI] [PubMed] [Google Scholar]
- 9.Yovel G, Shakhar K, Ben-Eliyahu S. The effects of sex, menstrual cycle, and oral contraceptives on the number and activity of natural killer cells. Gynecol Oncol. 2001;81:254–262. doi: 10.1006/gyno.2001.6153. [DOI] [PubMed] [Google Scholar]
- 10.Lee S, Kim J, Jang B, Hur S, Jung U, Kil K, et al. Fluctuation of peripheral blood T, B, and NK cells during a menstrual cycle of normal healthy women. J Immunol. 2010;185:756–762. doi: 10.4049/jimmunol.0904192. [DOI] [PubMed] [Google Scholar]
- 11.Plaks V, Birnberg T, Berkutzki T, Sela S, BenYashar A, Kalchenko V, et al. Uterine DCs are crucial for decidua formation during embryo implantation in mice. J Clin Invest. 2008;118:3954–3965. doi: 10.1172/JCI36682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pollard JW. Uterine DCs are essential for pregnancy. J Clin Invest. 2008;118:3832–3835. doi: 10.1172/JCI37733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eidukaite A, Tamosiunas V. Endometrial and peritoneal macrophages: expression of activation and adhesion molecules. Am J Reprod Immunol. 2004;52:113–117. doi: 10.1111/j.1600-0897.2004.00201.x. [DOI] [PubMed] [Google Scholar]
- 14.Nagamatsu T, Schust DJ. The contribution of macrophages to normal and pathological pregnancies. Am J Reprod Immunol. 2010;63:460–471. doi: 10.1111/j.1600-0897.2010.00813.x. [DOI] [PubMed] [Google Scholar]
- 15.Manaster I, Mizrahi S, Goldman-Wohl D, Sela HY, Stern-Ginossar N, Lankry D, et al. Endometrial NK cells are special immature cells that await pregnancy. J Immunol. 2008;181:1869–1876. doi: 10.4049/jimmunol.181.3.1869. [DOI] [PubMed] [Google Scholar]
- 16.Mor G, Cardenas I, Abrahams V, Guller S. Inflammation and pregnancy: the role of the immune system at the implantation site. Ann N Y Acad Sci. 2011;1221:80–87. doi: 10.1111/j.1749-6632.2010.05938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Szekeres-Bartho J, Polgar B. PIBF: the double edged sword. Pregnancy and tumor. Am J Reprod Immunol. 2010;64:77–86. doi: 10.1111/j.1600-0897.2010.00833.x. [DOI] [PubMed] [Google Scholar]
- 18.Reichardt P, Dornbach B, Rong S, Beissert S, Gueler F, Loser K, et al. Naive B cells generate regulatory T cells in the presence of a mature immunologic synapse. Blood. 2007;110:1519–1529. doi: 10.1182/blood-2006-10-053793. [DOI] [PubMed] [Google Scholar]
- 19.Tai P, Wang J, Jin H, Song X, Yan J, Kang Y, et al. Induction of regulatory T cells by physiological level estrogen. J Cell Physiol. 2008;214:456–464. doi: 10.1002/jcp.21221. [DOI] [PubMed] [Google Scholar]
- 20.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8:523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Somerset DA, Zheng Y, Kilby MD, Sansom DM, Drayson MT. Normal human pregnancy is associated with an elevation in the immune suppressive CD25+ CD4+ regulatory T-cell subset. Immunology. 2004;112:38–43. doi: 10.1111/j.1365-2567.2004.01869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sasaki Y, Sakai M, Miyazaki S, Higuma S, Shiozaki A, Saito S. Decidual and peripheral blood CD4+CD25+ regulatory T cells in early pregnancy subjects and spontaneous abortion cases. Mol Hum Reprod. 2004;10:347–353. doi: 10.1093/molehr/gah044. [DOI] [PubMed] [Google Scholar]
- 23.Arruvito L, Sanz M, Banham AH, Fainboim L. Expansion of CD4+CD25+and FOXP3+ regulatory T cells during the follicular phase of the menstrual cycle: implications for human reproduction. J Immunol. 2007;178:2572–2578. doi: 10.4049/jimmunol.178.4.2572. [DOI] [PubMed] [Google Scholar]
- 24.Polanczyk MJ, Carson BD, Subramanian S, Afentoulis M, Vandenbark AA, Ziegler SF, et al. Cutting edge: estrogen drives expansion of the CD4+CD25+ regulatory T cell compartment. J Immunol. 2004;173:2227–2230. doi: 10.4049/jimmunol.173.4.2227. [DOI] [PubMed] [Google Scholar]
- 25.Zhao JX, Zeng YY, Liu Y. Fetal alloantigen is responsible for the expansion of the CD4(+)CD25(+) regulatory T cell pool during pregnancy. J Reprod Immunol. 2007;75:71–81. doi: 10.1016/j.jri.2007.06.052. [DOI] [PubMed] [Google Scholar]
- 26.Zenclussen ML, Thuere C, Ahmad N, Wafula PO, Fest S, Teles A, et al. The persistence of paternal antigens in the maternal body is involved in regulatory T-cell expansion and fetal-maternal tolerance in murine pregnancy. Am J Reprod Immunol. 2010;63:200–208. doi: 10.1111/j.1600-0897.2009.00793.x. [DOI] [PubMed] [Google Scholar]
- 27.Robertson SA, Guerin LR, Bromfield JJ, Branson KM, Ahlström AC, Care AS. Seminal fluid drives expansion of the CD4+CD25+ T regulatory cell pool and induces tolerance to paternal alloantigens in mice. Biol Reprod. 2009;80:1036–1045. doi: 10.1095/biolreprod.108.074658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leber A, Teles A, Zenclussen AC. Regulatory T cells and their role in pregnancy. Am J Reprod Immunol. 2010;63:445–459. doi: 10.1111/j.1600-0897.2010.00821.x. [DOI] [PubMed] [Google Scholar]
- 29.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 30.Steinman L. A brief history of T(H)17, the first major revision in the T(H)1/T(H)2 hypothesis of T cell-mediated tissue damage. Nat Med. 2007;13:139–145. doi: 10.1038/nm1551. [DOI] [PubMed] [Google Scholar]
- 31.Kolls JK, Lindén A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 32.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 33.Laurence A, Tato CM, Davidson TS, Kanno Y, Chen Z, Yao Z, et al. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26:371–381. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 34.O'Garra A, Stockinger B, Veldhoen M. Differentiation of human T(H)-17 cells does require TGF-beta. Nat Immunol. 2008;9:588–590. doi: 10.1038/ni0608-588. [DOI] [PubMed] [Google Scholar]
- 35.Yang L, Anderson DE, Baecher-Allan C, Hastings WD, Bettelli E, Oukka M, et al. IL-21 and TGF-beta are required for differentiation of human T(H)17 cells. Nature. 2008;454:350–352. doi: 10.1038/nature07021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manel N, Unutmaz D, Littman DR. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat Immunol. 2008;9:641–649. doi: 10.1038/ni.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang WJ, Hao CF, Yi L, Yin GJ, Bao SH, Qiu LH, et al. Increased prevalence of T helper 17 (Th17) cells in peripheral blood and decidua in unexplained recurrent spontaneous abortion patients. J Reprod Immunol. 2010;84:164–170. doi: 10.1016/j.jri.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 38.Liu YS, Wu L, Tong XH, Wu LM, He GP, Zhou GX, et al. Study on the relationship between th17 cells and unexplained recurrent spontaneous abortion. Am J Reprod Immunol. 2011;65:503–511. doi: 10.1111/j.1600-0897.2010.00921.x. [DOI] [PubMed] [Google Scholar]
- 39.King A, Balendran N, Wooding P, Carter NP, Loke YW. CD3-leukocytes present in the human uterus during early placentation: phenotypic and morphologic characterization of the CD56++ population. Dev Immunol. 1991;1:169–190. doi: 10.1155/1991/83493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Manaster I, Mandelboim O. The unique properties of uterine NK cells. Am J Reprod Immunol. 2010;63:434–444. doi: 10.1111/j.1600-0897.2009.00794.x. [DOI] [PubMed] [Google Scholar]
- 41.Manaster I, Mandelboim O. The unique properties of human NK cells in the uterine mucosa. Placenta. 2008;29(Suppl A):S60–S66. doi: 10.1016/j.placenta.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 42.Hanna J, Goldman-Wohl D, Hamani Y, Avraham I, Greenfield C, Natanson-Yaron S, et al. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat Med. 2006;12:1065–1074. doi: 10.1038/nm1452. [DOI] [PubMed] [Google Scholar]
- 43.Kitaya K, Yamaguchi T, Yasuo T, Okubo T, Honjo H. Post-ovulatory rise of endometrial CD16(-) natural killer cells: in situ proliferation of residual cells or selective recruitment from circulating peripheral blood? J Reprod Immunol. 2007;76:45–53. doi: 10.1016/j.jri.2007.03.010. [DOI] [PubMed] [Google Scholar]