Abstract
Storage of tissue slides has been claimed to induce dramatically reduced antigen detection particularly for immunohistochemistry (IHC). With tissue microarrays, the necessity to serially cut blocks in order to obtain as much material as possible is obvious. The presumed adverse effect of storage might hamper such an approach. The authors designed an experimental setting consisting of four different storage conditions with storage time of tissue slides of up to 1 year. Detection of proteins, DNA, and mRNA was performed using IHC and in situ hybridization techniques. Slight but significant changes in IHC occurred over time. The most important factor is the primary antibody used: four showed no significant changes, whereas limited decreases in 8 antibodies could be detected by image analysis. Whether the antigen was nuclear or cytoplasmic/membranous did not matter. No major differences between different storage conditions could be shown, but storage at 4C was overall the best procedure. Furthermore, gene copy number aberrations, chromosomal translocations, and the presence of mRNA could be detected on slides stored up to 1 year. In conclusion, in tissues optimally formalin fixed and using modern histological techniques, only minute changes in tissue antigenicity are induced by long-term storage.
Keywords: immunohistochemistry, heat-induced antigen retrieval, antigenicity, formalin-fixed paraffin-embedded, FISH, CISH, tissue microarray
Formalin-fixed and paraffin-embedded tissue is an invaluable resource in research. Formaldehyde fixation has been the method of choice for tissue preservation in pathology for more than a century. Beside tissue structure, the cellular contents of proteins and nucleic acids are preserved by the cross-linking between and within proteins in the tissue (Fox et al. 1985). This gives a tissue sample that is resilient to long-term storage, usually at ambient room temperature. Even after decades of storage of the tissue samples, protein and nucleic acids are preserved within the paraffin block (Shibata et al. 1988; Litlekalsoy et al. 2007). In the clinical setting, tissue blocks are often stored after initial diagnostic testing, constituting a unique biobank for retrospective studies.
Tissue microarray (TMA) allows you to put many tissues from a number of previously prepared blocks (donor blocks) into a new block (recipient block) (Kononen et al. 1998). This technique makes it possible, in an easy and cost-effective way, to study many different markers at the RNA, DNA, and protein levels on a large number of different tissue samples. Because each TMA block constitutes up to a thousand different tissue samples, repetitive new sectioning of the recipient block is likely to result in loss of tissue material (i.e., due to facing the block in the microtome again). Furthermore, the uneven thickness of the different cylinders constituting the TMA block makes it more sensitive to trimming than a block constituting of only a few tissue pieces. Thus, for the TMA technique to be optimally used, the common way to section is by serial sectioning (Kononen et al. 1998; Kallioniemi et al. 2001), with the aim to get as many sections as possible from the block.
Although sustained antigenicity for proteins and DNA in intact tissue blocks has been shown (Manne et al. 1997; Litlekalsoy et al. 2007), it has been reported that sections stored on slides suffer from loss of antigenicity (Jacobs et al. 1996; Fergenbaum et al. 2004; DiVito et al. 2004). A number of studies have shown that the possibility to use these stored slides is limited because, at least for some antigens (e.g., p53), the antigenicity of the tissue section decreases over time (Prioleau and Schnitt 1995; Jacobs et al. 1996; DiVito et al. 2004). However, there are some inconsistencies in these reports, where some say that even after 1 week of storage the antigens are lost forever (Jacobs et al. 1996) and others state that these effects occur only after storage for several months (Bertheau et al. 1998) or even years (Shin et al. 1997). Speculations about what causes the antigens to deteriorate have included storage temperature, environment, and time, but to date no solid explanation has been established.
The technique of heat-induced antigen retrieval (AR) began in 1991 when Shi et al. (1991) started to boil the sections in solutions containing metal ions and obtained results from antigen antibody reactions that before were negative. Today there is no standardized method of antigen retrieval, but from the earliest published works the development of antigen retrieval techniques has moved from metal ion solutions to buffers of different pH, where different antigens prefer different pH and boiling in microwaves, water-baths, or pressure cookers, all depending on preference and accessibility of equipment (Shi et al. 2007; Yamashita 2007; D’Amico et al. 2009).
One way of overcoming storage-associated loss of antigenicity may be to subject the sections to optimal antigen retrieval, using heat as a way of making epitopes in the tissue accessible to the antibodies. The mode of antigen retrieval in the above-mentioned studies of the storage effect of antigenicity has not always been comparable, and some studies were published before the antigen retrieval era.
Another important factor that determines the sensitivity of the reaction is the detection system. Also in this field development has been rapid, and a number of highly sensitive systems have been introduced during recent years (Skaland et al. 2010). Thus, there are several factors that motivate a reevaluation of earlier findings of decreased and even loss of antigenicity in stored slides, which may hamper an optimal use of valuable TMAs.
As stated, TMAs may also be used for studies of DNA and RNA in archival tissues. Even though DNA is known to be stable in many circumstances (Ferrer et al. 2007), there are few studies concerning DNA preservation on stored sections for a defined time (Selvarajan et al. 2002; Ferrer et al. 2007). That mRNA is degraded over time on stored slides has been described before (Lisowski et al. 2001).
The aim of the present study was to evaluate, using adequately formalin-fixed paraffin-embedded tissues and up-to date antigen retrieval techniques and sensitive amplification system, factors that may render it possible to use even long-term stored slides for protein, DNA, and RNA in situ demonstration in order to facilitate the handling of valuable TMA materials.
Materials and Methods
TMA Construction
A TMA block was constructed containing normal tissue and malignant tissue (Table 1). Serial 4-µm sections were cut from the TMA blocks and transferred to Capillary Gap Microscope Slides (DAKO, Copenhagen, Denmark) for IHC or onto SuperFrost Plus slides (Menzel GmbH, Braunschweig, Germany) for FISH and CISH analysis. The slides were incubated at 60C for 60 min and then allowed to cool.
Table 1.
Tissue Origins and Number of Cylinder Analyzed for the Specific Antigens
| Malignant Tissues |
Normal Tissues |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostate—Adca |
NSCLC—Adca |
NSCLC—SCC |
Breast—Adca |
Thyroid—PapAdca |
Colon—Adca |
Lgll—B-CLL |
Lgll—MCL |
Tonsil |
Prostate |
Thyroid |
Pancreas |
Lgl |
Colon |
Lung |
||
| Antigens | n | 1 | 5 | 5 | 10 | 5 | 7 | 5 | 2 | 1 | 1 | 2 | 1 | 1 | 1 | 2 |
| Nuclear | ||||||||||||||||
| Ki-67 | 5 | 5 | 8 | 6 | 2 | |||||||||||
| p53 | 5 | 5 | 8 | 6 | 1 | |||||||||||
| TTF-1 | 5 | 3 | 5 | 1 | ||||||||||||
| PR A | 1 | 4 | 1 | 1 | ||||||||||||
| ER α | 1 | 1 | 6 | 1 | 1 | 1 | 1 | |||||||||
| CDX-2 | 1 | 4 | 1 | |||||||||||||
| BCL-1 | 5 | 3 | 2 | 1 | 1 | |||||||||||
| Cytoplasmic | ||||||||||||||||
| CD20 | 1 | 5 | 2 | 1 | 1 | |||||||||||
| CK5 | 1 | 5 | 2 | 1 | 1 | 1 | ||||||||||
| CD5 | 2 | 1 | 1 | 3 | 1 | 1 | ||||||||||
| CK7 | 1 | 5 | 2 | 8 | 5 | 2 | 1 | 1 | ||||||||
| Membranous | ||||||||||||||||
| HER-2 | 1 | 2 | 2 | 8 | 2 | 1 | ||||||||||
Abbreviations: Lgll = lymph node; Adca = adenocarcinoma; SCC = squamous cell carcinoma; PapAdca = papillary adenocarcinoma; B-CLL = B chronic lymphatic leukemia; MCL = mantel cell lymphoma.
The table shows the total number of cylinders of each tissue sample (n), within the TMA block. For each antibody the number of cylinders with adequate material for immunohistochemistry evaluation is given.
Storage of Slides
Sections were divided into 2 batches, 1 for storage without any coating and 1 for storage with a coat of paraffin wax. The slides destined for coating were deparaffinized in xylene, air dried, and dipped in melted paraffin wax to form an even coat on the section. All slides were then stored either at room temperature (RT) or at 4C, for 1 week or 1, 3, 6, or 12 months, thus rendering four experimental settings.
Immunohistochemistry
Slides were deparaffinized in 2 changes of xylene; slides with paraffin coating were heated to 60C before being treated by xylene and then rehydrated to distilled water.
Antigen retrieval was performed by subjecting the slides to microwave treatment in Tris-EDTA buffer pH 9.0 for 30 min at 750 W. Slides were then allowed to cool in the buffer for 30 min. The immunohistochemistry (IHC) was performed according to DAKO EnVision Detection Kit (Dako, Copenhagen, Denmark), with primary antibody incubation for 25 min at room temperature. Applied antibodies were Ki-67 (mib-1), CD20 (L26), cytokeratin 7 (OV-TL12/30), p53 (DO-7) (Dako, Copenhagen, Denmark), CD5 (4C7), CDX2 (AMT28), Cytokeratin 5 (XM26), progesterone receptor (PR) A (16), TTF-1 (SPT24) (Novocastra, Leica, Wetzlar, Germany), BCL-1 (SP4), and estrogen receptor (ER) α (SP1) (NeoMarkers, Thermo Scientific, Waltham, MA). HercepTest was performed according to the manufacturer’s protocol (Dako, Copenhagen, Denmark). All IHC staining was carried out in Dako Tech Mate (Dako, Copenhagen, Denmark).
Negative control slides were prepared by substituting primary antibody for Dako ChemMate antibody diluent (Dako, Copenhagen, Denmark). Positive control sections with adequate tissue recommended by the manufacturer were included.
Evaluation of Staining Intensity
All slides were compared with the original slides stained at day 0; that is, they were sectioned the same day as they were stained.
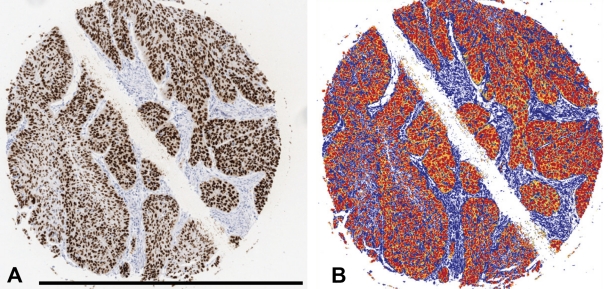
All slides were scanned by Scanscope XT (Aperio, San Diego, CA), at ×20 magnification. Intensity of positive staining was measured using Positive Pixel Count V9.0 included in the Aperio ImageScope (Aperio, San Diego, CA), with standard settings. The grayscale is composed of values ranging from 0 to 255, where 0 is black and 255 is white. For these measurements, positivity was counted if the values were within 0 to 100 in the grayscale. Figure 1 shows image segmentation procedure.
Figure 1.
The picture segmentation demonstrated at cylinder stained for p53 at baseline. Background and counterstaining is blue. Strong positive staining is red (0-100 gray levels) and regarded as true positive. Orange (101-175) and yellow (176-220) were considered negative, mainly constituting cytoplasmic background staining. A-B, bar = 1.0 mm.
FISH
The slides were deparaffinized as above, rinsed in absolute alcohol, and air dried.
The sections were then subjected to treatment according to the manufacturer’s protocol. Slides were hybridized with a probe mix in HYBrite (Vysis, Des Plaines, IL) where denaturation was set at 6 min at 73C and hybridization for 17 hr at 37C. Probe mixes used were PATHYVISION (HER-2/CEP17) and Vysis IGH/CCND1 DF FISH Probe Kit, both from Abbott Molecular, Des Plaines, IL.
Signals were counted in 20 tumor cells at ×60 magnification with oil immersion objective.
Concerning HER-2/CEP17, results were determined either as clusters of HER-2 or, in non-amplified cases, as a ratio between HER-2 and CEP17 signals. In the case of mantel cell lymphoma (MCL), the absence or presence of fusion signal was determined in normal and MCL cases.
CISH on mRNA
Detection of κ and λ light chains was performed according to the manufacturer’s protocol (Histosonda κ light chain and Histosonda λ light chain, Histosonda, Lugo, Spain). Slides were incubated with the probe at 62C for 1 hr. For visualization of the probe, the DAKO EnVision Detection Kit (Dako, Copenhagen, Denmark) was used.
Statistics
The influence of storage time for the four different storage conditions was analyzed for each antibody with 1-way ANOVA statistics using STATISTIX v.8 (Analytical Software, Tallahassee, FL). Interassay variation using three parallel stainings for CD5, CD7, ERα, and PR A was analyzed in the same way.
Data for a number of antibodies with more extensive data sets (Ki-67, p53, HercepTest and cytokeratin 7) were analyzed using a mixed-models method, which is an extension of the linear regression models and especially developed for longitudinal measurements allowing for simultaneous testing of two or more factors and their interactions. The analyses were performed using the procedure MIXED in SAS, version 9.2 (Cary, NC).
Ethics
In accordance with the Swedish biobank act (SFS2002:297), all tissues were made completely anonymous during the preparation of the TMA blocks, and no clinical data were known to the investigators.
Results
Immunohistochemistry
Interassay variation
To evaluate the influence of interassay variation as a cofactor in possible time-dependent effects, an experiment with three parallel stainings for CD5, CK7, ERα, and PR A was performed. The staining intensity was determined and the ranges of means were CD5 70.5-73.9, CK7 52.0-60.3, ERα 66.9-68.9, and PR A 67.7-71.6. For none of the antibodies did any significant difference occur between the different experiments.
Influence of storage time: single-factor analysis
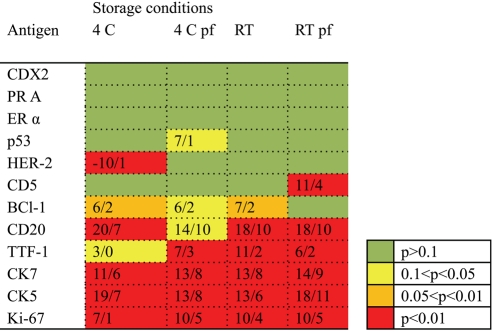
The influence of storage time was analyzed for all antibodies in the study at time 0 (baseline), 1 week, and 1, 3, 6 and 12 months. Within each box, in Fig. 2 the difference in staining intensity for 12 months of storage compared with baseline values is shown. The color of each box indicates the p value in the ANOVA analysis. As shown, data indicate that the main factor explaining the reduced staining intensity over time is the antibody used. However, no obvious difference between different types of antibodies (monoclonal mouse vs. monoclonal rabbit) could be demonstrated. The antigen cellular localizations (nuclear vs. cytoplasmic) did not seem to be of importance, and the low-temperature storage or paraffin coating did not seem to be crucial for adequate IHC detection.
Figure 2.
Figure 2 shows the results of the ANOVA analyses for each antibody (row) and storage condition (column). The color of each box indicates the level of significance in the ANOVA analysis of the whole time series of observations (baseline, 1 week, and 1, 3, 6 and 12 months). No statistical difference over time is thus indicated by a green box. The numbers within the box indicate the difference in gray value (n=256 gray levels) concerning the maximal difference at any time point (left) and the average of all observations (right) versus the baseline value. 4 = 4C, pf = paraffin-coating, RT = room temperature.
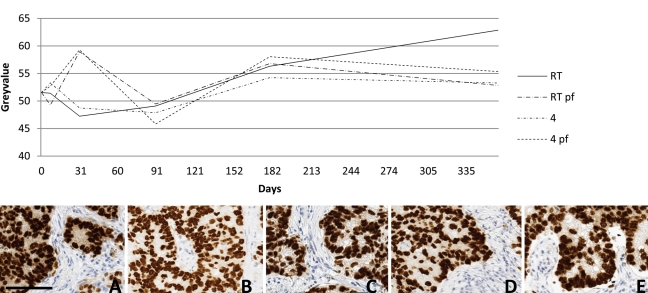
In Fig. 3, pictures for TTF-1 at baseline and after 1 year of storage are shown for the four different storage conditions. No obvious semiquantitative differences occurred. The histogram demonstrates the gray-level determination for the whole time series, demonstrating a slight but significant decrease over time with all storage conditions except 4C.
Figure 3.
The series of pictures show immunohistochemical staining results of the same cylinder at baseline (A) and after 12 months of storage at RT (B), at RT with a coating of paraffin (C), at 4C (D), and at 4C with paraffin coating (E). The graph shows the overall mean gray value for the different storing times at different storing conditions. Diaminobenzidine was used as chromogen showing brown color. 4 = 4C, pf = paraffin-coating, RT = room temperature. A-E, bar 100 µm.
Multifactor analysis
Extensive data sets for four antibodies (p53, Ki-67, CK7, and HercepTest) allowed for a statistical model to handle a multifactorial analysis including interactions between the studied variables.
Three factors were studied in this setting: time of storage (baseline, 1 week, 1, 3, 6, and 12 months), temperature of storage (RT or 4C), and paraffin coating of stored slides (yes/no). Thus, for each storage length four differently handled slides were stained and evaluated for each antibody. Baseline values were established without any previous coating or storage before staining. A positive difference compared with baseline values indicates a decreased intensity in the staining.
p53
p53 were evaluated at all time points (n=6) and different storage conditions. Whereas storage temperature seemed to be of no importance, paraffin coating of the slides significantly decreased staining intensity, especially when considering the time factor (p<0.01). Although statistically significant, differences were limited—7 gray value units, taking the 256 levels in the intensity scale in account.
Ki-67
Ki-67 was evaluated at five time points, baseline and 1, 3, 6, and 12 months of storage (1-week evaluation was excluded because high background staining). A decreased staining intensity occurred over time regardless of storage conditions (temperature, paraffin coating) (p<0.0001). Furthermore, RT storage versus 4C independent of other factors (time, paraffin coating) decreased staining intensity (1.5 U, p<0.001), as did the presence of paraffin coating (1.5 U, p<0.001). However, influence of storage conditions was limited compared with the time factor.
Cytokeratin 7
Cytokeratin 7 staining after storage at RT showed significantly decreased staining intensity compared with 4C storage (1.1 U, p=0.027), furthermore interacting in a time-dependent manner (p<0.0001). The negative impact of paraffin coating was also significant (1.5 U, p=0.001), also interacting with increased storage time (p<0.0001).
HER-2
HER-2 IHC unexpectedly showed a time-dependent increased staining intensity (p<0.0001), due to an extreme value (10 U stronger than baseline) at 12 months for 4C storage. However, as previously noted, paraffin coating decreased staining intensity (p=0.0003) regardless of storage temperature. On average, paraffin coating decreased staining intensity with 3.3 U compared with non-coated slides.
FISH
Two types of FISH analysis were performed. In a HER-2/CEP 17 setting, the ability to detect a HER-2 amplification by means of a cluster detection was unaffected over time and regardless of storage condition. In the non-amplified cases, HER-2/CEP17 ratio was evaluated without any changes over time or storage conditions, baseline value 1.06, and cumulated data, regardless of storage conditions over time 0.99 to 1.12 and for different storage conditions over time 1.04 to 1.07.
Furthermore, the ability to detect a translocation using a two-color fusion signal setting was analyzed in cases of MCL. At all time points and settings, a translocation pattern was observed in the MCL cases whereas a normal pattern was visualized in the normal lymphoid tissue included in the TMA.
CISH
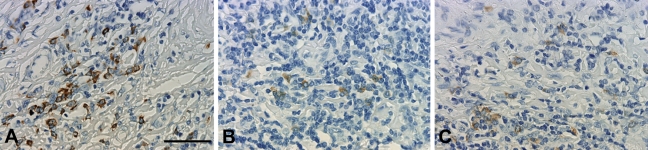
CISH for κ light chain is shown in Fig. 4, showing detection in slides at baseline and after 1 year of storage. In this experiment, κ and λ light chain mRNA were visualized by CISH. Positive signals could be detected in plasma cells regardless of storage conditions and time.
Figure 4.
mRNA detection of κ light chain in freshly cut tonsil tissue (A) versus slides stored for 12 months at RT without a paraffin coating (B) and stored for 12 months at 4C with a paraffin coating (C). A-C, bar 0.1 mm.
Discussion
Our study applied up-to date antigen retrieval methods, with standardized adequate formalin fixation procedures, modern antibodies, and highly sensitive detection systems as well as image analysis, to reevaluate earlier findings (Jacobs et al. 1996; DiVito et al. 2004) of decreased or even obliterated antigenicity in slides shortly after sectioning. If this still is the fact, it would have obvious implications for the possibility to perform immunohistochemical studies and share valuable tissue material in a multicenter setting. The introduction of TMA technique has further emphasized these problems because loss of tissue due to facing of the block is critical. In short, our results were more encouraging than earlier reports, a finding to which a number of different factors may contribute.
Adequate fixation is a well-known prerequisite (Shi and Taylor 2010) for optimal staining results and morphology as well as preservation of DNA and RNA targets (Shi and Taylor 2010). For IHC to give accurate results, the tissue has to be properly fixed (Werner et al. 2000; Buesa 2008). If the tissue is underfixed (i.e., not left long enough in the fixative), the IHC reaction will be poor at the center of the specimen (Shi and Taylor 2010). Another concern has been about overfixation, that is, leaving the specimen for too long in the fixative. Conflicting results have been found (Wester et al. 2000; De Marzo et al. 2002; Shi et al. 2007) regarding whether overfixation hampers IHC. In our experimental setting, clinical material has been used in the construction of the TMA block. For each tissue, fixation time is known and documented within a range of 18 to 48 hr. Fixation time was not explicitly documented in the above-mentioned earlier reports. Usually formalin is used, but Jacobs et al. (1996) used an ethanol-based fixation. Also, a postfixation step was used in another report (Bertheau et al. 1998). Our data were generated in a setting using standardized and well-established techniques for tissue fixation and handling (i.e., formalin fixation and overnight dehydration procedures). Thus, our encouraging results concerning epitope preservation and demonstration in situ may be influenced by shorter fixation times and also by other fixation methods introduced in pathology practice in order to shorten processing and thereby reporting times. These factors must be taken into consideration when biobanking material for future clinical and research use.
Since the introduction of heat-induced antigen retrieval, a number of techniques have been used including water-bath, pressure cookers, and microwave ovens. These methods are different techniques to distribute the heat component of the antigen retrieval process and may vary from setting to setting. Concerning HercepTest, the manufacturer has required a water-bath procedure in order to get an interinstitutional reproducibility that ensures a quality yielding FDA approval. Regarding the other experiments, we have used our in-house procedure using a microwave oven. Compared with previous report, our AR times are prolonged according to recent recommendations (www.nordiqc.org). Thus, AR time seems to be a factor to overcome the previously reported dramatic declines in immunogenicity over time.
Another factor in the AR procedure that has developed over the years is the use of different AR solutions, including the use of different pHs. All the antibodies used have been previously optimized (Shi and Taylor 2010) in-house at pH 9. However, other antibodies require other AR solutions. Comparing the present technique with the earlier reports highlights the importance of the AR solution, which in previous reports mainly used citrate buffer at pH 6.
Finally, different detection techniques have been used. In a recent report (Skaland et al. 2010), modern high-sensitivity systems were compared. That study confirms that the Envision system used in our study is one of the most sensitive systems presently used and is significantly more sensitive than the older detection systems used in earlier studies. The importance of the detection system, as well as the AR procedure, for preserved antigenicity is thus obvious in our study compared with older studies (Fergenbaum et al. 2004; Mirlacher et al. 2004).
Paraffin coating has been introduced (Blind et al. 2008) as a method to reduce oxidation of slides. This, as well as other methods, has previously been described as a technique to overcome storage-induced decline in antigenicity (DiVito et al. 2004). The influences of storage temperature, inert milieu (nitric oxide-storage), and paraffin coating of slides were evaluated. The best results were achieved using storage at 4C, whereas paraffin coating did not add any further protection to degradation over time. On the contrary, a very minor adverse effect was observed.
A difference compared with the earlier studies is the quantitative evaluation of the staining intensity in our study. Even though we report differences in staining over time compared with baseline data, we must bear in mind that these differences are minute in a quantitative aspect, with differences usually less than 10 of 256 U in the intensity scale, barely visible to the naked eye. In an image analysis-based setting, slight differences in intensity should not be of importance using optimal image segmentation. Furthermore, the interassay variations as well as the limitation of enzyme-based methods are limiting factors in a strictly quantitative approach.
The in situ detection of RNA in plasma cells even after 1 year of storage is interesting and shows that the tissue section provides a protective milieu even for the mRNA molecules. FISH-based DNA detection was also consistent over time concerning numerical analysis of DNA locuses including fluorescence low-intensity centromere probes as well as the detection of translocations such as in MCL.
In conclusion, our results show that an up-to-date antigen retrieval procedure allows stored slides to be used for immunohistochemical studies. A prerequisite may be adequate preanalytical fixation, but the previously reported time- and storage-dependent effect seems to be reversible by present immunohistochemical methods. Furthermore, RNA can still be detected but seems to be affected by storage whereas DNA targets are stable. Thus, the limited and most valuable TMA material presently extensively used in pathological studies may easily be stored and shared between institutions using modern methods in molecular pathology.
Acknowledgments
We are grateful to Professor Lennart Bodin, Department of Biostatistics, for statistical advice and for performing the mixed-model analysis, and Ms. Aleksandra Kolaric for performing the FISH experiments.
References
- Bertheau P, Cazals-Hatem D, Meignin V, de Roquancourt A, Verola O, Lesourd A, Sene C, Brocheriou C, Janin A. 1998. Variability of immunohistochemical reactivity on stored paraffin slides. J Clin Pathol. 51:370–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blind C, Koepenik A, Pacyna-Gengelbach M, Fernahl G, Deutschmann N, Dietel M, Krenn V, Petersen I. 2008. Antigenicity testing by immunohistochemistry after tissue oxidation. J Clin Pathol. 61:79–83 [DOI] [PubMed] [Google Scholar]
- Buesa RJ. 2008. Histology without formalin? Ann Diagn Pathol. 12:387–396 [DOI] [PubMed] [Google Scholar]
- D’Amico F, Skarmoutsou E, Stivala F. 2009. State of the art in antigen retrieval for immunohistochemistry. J Immunol Methods. 341:1–18 [DOI] [PubMed] [Google Scholar]
- De Marzo AM, Fedor HH, Gage WR, Rubin MA. 2002. Inadequate formalin fixation decreases reliability of p27 immunohistochemical staining: probing optimal fixation time using high-density tissue microarrays. Hum Pathol. 33:756–760 [DOI] [PubMed] [Google Scholar]
- DiVito KA, Charette LA, Rimm DL, Camp RL. 2004. Long-term preservation of antigenicity on tissue microarrays. Lab Invest. 84:1071–1078 [DOI] [PubMed] [Google Scholar]
- Fergenbaum JH, Garcia-Closas M, Hewitt SM, Lissowska J, Sakoda LC, Sherman ME. 2004. Loss of antigenicity in stored sections of breast cancer tissue microarrays. Cancer Epidemiol Biomarkers Prev. 13:667–672 [PubMed] [Google Scholar]
- Ferrer I, Armstrong J, Capellari S, Parchi P, Arzberger T, Bell J, Budka H, Strobel T, Giaccone G, Rossi G, et al. 2007. Effects of formalin fixation, paraffin embedding, and time of storage on DNA preservation in brain tissue: a BrainNet Europe study. Brain Pathol. 17:297–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox CH, Johnson FB, Whiting J, Roller PP. 1985. Formaldehyde fixation. J Histochem. Cytochem. 33:845–853 [DOI] [PubMed] [Google Scholar]
- Jacobs TW, Prioleau JE, Stillman IE, Schnitt SJ. 1996. Loss of tumor marker-immunostaining intensity on stored paraffin slides of breast cancer. J Natl Cancer Inst. 88:1054–1059 [DOI] [PubMed] [Google Scholar]
- Kallioniemi OP, Wagner U, Kononen J, Sauter G. 2001. Tissue microarray technology for high-throughput molecular profiling of cancer. Hum Mol Genet. 10:657–662 [DOI] [PubMed] [Google Scholar]
- Kononen J, Bubendorf L, Kallioniemi A, Barlund M, Schraml P, Leighton S, Torhorst J, Mihatsch MJ, Sauter G, Kallioniemi OP. 1998. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat. Med. 4:844–847 [DOI] [PubMed] [Google Scholar]
- Lisowski AR, English ML, Opsahl AC, Bunch RT, Blomme EA. 2001. Effect of the storage period of paraffin sections on the detection of mRNAs by in situ hybridization. J Histochem Cytochem. 49:927–928 [DOI] [PubMed] [Google Scholar]
- Litlekalsoy J, Vatne V, Hostmark JG, Laerum OD. 2007. Immunohistochemical markers in urinary bladder carcinomas from paraffin-embedded archival tissue after storage for 5-70 years. BJU Int. 99:1013–1019 [DOI] [PubMed] [Google Scholar]
- Manne U, Myers RB, Srivastava S, Grizzle WE. 1997. Re: loss of tumor marker-immunostaining intensity on stored paraffin slides of breast cancer. J Natl Cancer Inst. 89:585–586 [DOI] [PubMed] [Google Scholar]
- Mirlacher M, Kasper M, Storz M, Knecht Y, Durmuller U, Simon R, Mihatsch MJ, Sauter G. 2004. Influence of slide aging on results of translational research studies using immunohistochemistry. Mod Pathol. 17:1414–1420 [DOI] [PubMed] [Google Scholar]
- Prioleau J, Schnitt SJ. 1995. p53 antigen loss in stored paraffin slides. N Engl J Med. 332:1521–1522 [DOI] [PubMed] [Google Scholar]
- Selvarajan S, Bay BH, Choo A, Chuah KL, Sivaswaren CR, Tien SL, Wong CY, Tan PH. 2002. Effect of fixation period on HER2/neu gene amplification detected by fluorescence in situ hybridization in invasive breast carcinoma. J Histochem Cytochem. 50:1693–1696 [DOI] [PubMed] [Google Scholar]
- Shi S-R, Taylor CR. editors. 2010. Antigen retrieval immunohistochemistry based research and diagnostics. Hoboken, NJ: John Wiley [Google Scholar]
- Shi SR, Key ME, Kalra KL. 1991. Antigen retrieval in formalin-fixed, paraffin-embedded tissues: an enhancement method for immunohistochemical staining based on microwave oven heating of tissue sections. J Histochem Cytochem. 39:741–748 [DOI] [PubMed] [Google Scholar]
- Shi SR, Liu C, Taylor CR. 2007. Standardization of immunohistochemistry for formalin-fixed, paraffin-embedded tissue sections based on the antigen-retrieval technique: from experiments to hypothesis. J Histochem Cytochem. 55:105–109 [DOI] [PubMed] [Google Scholar]
- Shibata D, Martin WJ, Arnheim N. 1988. Analysis of DNA sequences in forty-year-old paraffin-embedded thin-tissue sections: a bridge between molecular biology and classical histology. Cancer Res. 48:4564–4566 [PubMed] [Google Scholar]
- Shin HJ, Kalapurakal SK, Lee JJ, Ro JY, Hong WK, Lee JS. 1997. Comparison of p53 immunoreactivity in fresh-cut versus stored slides with and without microwave heating. Mod Pathol. 10:224–230 [PubMed] [Google Scholar]
- Skaland I, Nordhus M, Gudlaugsson E, Klos J, Kjellevold KH, Janssen EA, Baak JP. 2010. Evaluation of 5 different labeled polymer immunohistochemical detection systems. Appl Immunohistochem Mol Morphol. 18:90–96 [DOI] [PubMed] [Google Scholar]
- Werner M, Chott A, Fabiano A, Battifora H. 2000. Effect of formalin tissue fixation and processing on immunohistochemistry. Am J Surg Pathol. 24:1016–1019 [DOI] [PubMed] [Google Scholar]
- Wester K, Wahlund E, Sundstrom C, Ranefall P, Bengtsson E, Russell PJ, Ow KT, Malmstrom PU, Busch C. 2000. Paraffin section storage and immunohistochemistry. Effects of time, temperature, fixation, and retrieval protocol with emphasis on p53 protein and MIB1 antigen. Appl Immunohistochem Mol Morphol 8:61–70 [PubMed] [Google Scholar]
- Yamashita S. 2007. Heat-induced antigen retrieval: mechanisms and application to histochemistry. Prog Histochem Cytochem. 41:141–200 [DOI] [PubMed] [Google Scholar]