Abstract
An increasingly wide range of functions, from repression of NF-κB signaling to protection from apoptosis, is being recognized for tumor necrosis factor α–induced protein 3-interacting protein 1 (TNIP1). The authors recently demonstrated TNIP1 interaction with and repression of liganded retinoic acid receptors, distinguishing it from the more typical NCoR and SMRT corepressors, which function only in the absence of ligand. To improve their understanding of TNIP1’s roles in physiologic and pathologic events, the authors examined its distribution in normal and malignant human tissues and cultured cells. They found cytoplasmic and nuclear TNIP1 in normal skin keratinocytes as it colocalized with retinoic acid receptor α, one of the nuclear receptors it corepresses. Nuclear and cytoplasmic TNIP1 was also found in the malignant keratinocytes of squamous cell carcinomas. Compared to adjacent normal tissues of other organs, TNIP1 staining and distribution varied with increased levels in esophageal cancer and marked decreases in prostate cancer. The varying levels and distribution of TNIP1 in normal and disease state tissues could be expected to affect processes in which TNIP1 is involved, such as NF-κB and nuclear receptor signaling, possibly contributing to the disease course or response to therapies targeting these key players of cell growth and differentiation.
Keywords: squamous cell carcinoma, ABIN-1, epidermis, TNF-α, tissue microarray
The signaling consequences of nuclear factor κB (NF-κB) and nuclear receptor (NR) pathways are often at odds with each other. For instance, ligands of NRs, including retinoic acid receptors (RARs), can antagonize transcriptional activation by NF-κB (Lefebvre 2001) and subsequent inflammatory or hyperproliferative responses (Bollrath and Greten 2009; Mankan et al. 2009; Shishodia and Aggarwal 2004). Contrasting this antagonism are a few cases, exemplified by the hyaluronan synthase 2 promoter (Makkonen et al. 2009; Saavalainen et al. 2007), where activators of either RAR or NF-κB pathways can promote transcription of the same target gene. Thus, it is particularly intriguing that tumor necrosis factor α–induced protein 3–interacting protein 1 (TNIP1) is shared by RAR and NF-κB signaling pathways (Gurevich and Aneskievich 2009; Heyninck et al. 1999). In both instances, TNIP1 contributes to reduced or repressed transcription mediated by NF-κB or RAR.
TNIP1 is a human intracellular protein initially identified as interacting with the HIV proteins nef (Fukushi et al. 1999) and matrix (Gupta et al. 2000). Its endogenous targets include the zinc finger protein A20 (Heyninck et al. 1999; Heyninck et al. 2003), the RARs α and γ (Gurevich and Aneskievich 2009), and peroxisome proliferator-activated receptors (Flores et al., 2011). These distinctly different interaction partners suggest a wide repertoire of TNIP1 functions. TNIP1 enhances CD4 levels, a result inhibited by HIV nef (Fukushi et al. 1999). Consequent to physical association with A20 (also known as TNF-α-induced protein 3, TNFAIP3), TNIP1 decreases cytoplasmic signaling that leads to transcriptional activity via NF-κB (for review, see Verstrepen et al. 2009), earning it the alias of ABIN-1, for the A20-binding inhibitor of NF-κB. We recently reported (Gurevich and Aneskievich 2009) the ligand-dependent physical association of TNIP1 with RAR α and γ and the receptors’ subsequent repression. This repression occurs through mechanisms distinct from the reduction of NF-κB activity. Whether through RAR, NF-κB, or as yet unrecognized targets, the possible roles for TNIP1 at cell and tissue levels are steadily increasing, especially with its recent gene-disease associations in lung cancer and psoriasis (Hosgood et al. 2008; Nair et al. 2009). Furthermore, in experimental models, only a few percent of mice homozygous null for TNIP1 are live born. Death of most embryos occurs at day 18.5 due to extensive liver apoptosis (Oshima et al. 2009), suggesting that in addition to TNIP1’s targeted role of repressing NF-κB and RAR signals, a more global role may be one of protecting cells from apoptosis. Thus, detection of TNIP1 protein-level differences, tissue distribution, and subcellular localization would provide an important context for investigating its control over NF-κB and certain NR signaling pathways.
Here we report a wide distribution of TNIP1 protein, substantiating what was predicted from mRNA studies (Fukushi et al. 1999; Gupta et al. 2000). TNIP1 subcellular localization across different cell types of tissues was mostly cytoplasmic, although on some stratified and simple epithelia, we found nuclear localization. TNIP1 colocalized with RAR-α in human epidermal keratinocytes, a known retinoid-sensitive cell. In several organs, TNIP1 staining in malignant tissue was often altered from its distribution and levels in normal counterparts, with a particularly striking diminution in prostate adenocarcinoma cells. The differing levels and localization of TNIP1 demonstrated by this study can be expected to affect NF-κB and RAR signaling as cells respond to the upstream activators of these important transcription factors.
Materials and Methods
Peptide Selection and Antibody Generation
Amino acid sequence of human TNIP1, GenBank accession number AAH14008, was algorithm examined to determine preferred, potential antigenic peptides and negate off-target matches. The two selected peptides encompassed amino acids 1–14 and 552–562. Peptides were conjugated to keyhole limpet hemocyanin and coinjected into rabbits (Affinity Bioreagents; Golden, CO). Serum was collected three weeks after the last immunization, and antibody was purified using a peptide affinity column that targeted both peptides.
Cell Protein Preparation and Western Blots
Neonatal human primary keratinocytes were cultured in serum-free KGM-Gold media with Bullet-kit supplements (cells and reagents from Lonza, Walkersville, MD). Other cells were cultured as previously described (Gurevich and Aneskievich 2009) or per vendor (ATCC; Manassas, VA) recommendations. The TNIP1 cDNA used throughout this report is a human lung cDNA originally contained in pOTB7 (ATCC) that we sequenced on both strands and determined to contain a full-length (i.e., 636 amino acids) TNIP1 coding sequence. For expression purposes, the full-length sequence from the pOTB7 construct was cloned (Gurevich and Aneskievich 2009) in-frame into pDBD (pM) or pCMV-HA (both from Clontech Laboratories; Mountain View, CA) to provide fusion-proteins with the Gal4 DNA binding domain (DBD) or hemagglutinin (HA) domains at the amino terminus or pcDNA3.1+ (Invitrogen; Carlsbad, CA) for untagged TNIP1. To induce endogenous TNIP1 expression over basal levels, HeLa cells were seeded, allowed to grow for 24 hr, and treated with TNF-α (50 ng/ml media) for 0, 6, 12, or 24 hr (Tian, Nowak, and Brasier 2005; Tian, Nowak, Jamaluddin, et al. 2005). Treatments were timed so that all the samples were harvested at the same time to have cultures of the same age but differing duration of TNF-α exposure. Nuclear and cytoplasmic fractionation of HaCaT and HeLa cells was performed using the NE-PER kit (Thermo Fisher Scientific; Rockford, IL) according to the manufacturer’s instructions. For TNIP1 knockdown experiments, HaCaT keratinocytes were plated and 24 hr later transfected overnight with 25 nM of individual SMARTpool TNIP1 or non-targeting siRNA (Thermo Fisher Scientific; Lafayette, CO) using DharmaFECT2 (Thermo Fisher Scientific) as per manufacturer instructions. Media were changed the next day and cells collected at the times indicated in RIPA lysis buffer with the point of siRNA addition being time zero. Standard SDS-PAGE, Western transfer onto nitrocellulose membrane (Whatman; Florham Park, NJ), and immunodetection procedures were used (Ausubel et al. 2004). The polyclonal TNIP1 antibody, at a starting concentration of 9.2 µg/µl, was used at 1:1000 dilution, detected with horseradish peroxidase (HRP)–conjugated secondary antibody, and visualized using chemiluminescence reagents (PerkinElmer; Waltham, MA). Other antibodies used were RAR-α polyclonal, mouse Gal4 DBD monoclonal, rabbit Gal4 DBD polyclonal (Santa Cruz Biotechnologies; Santa Cruz, CA), and mouse ABIN-1 monoclonal (Zymed Laboratories; South San Francisco, CA). Even loading was confirmed by cross-reactive bands as done previously (Rasko et al. 2008; Schnepp et al. 2004) or β-actin antibody (Abcam; Cambridge, MA). Quality of cell fractionations was examined by immunoblotting for histone 2A (H2A; Abcam) as a nuclear marker and tubulin as a cytoplasmic marker (Abcam). Signals were digitally captured using a Kodak IS440CF CCD imager (Kodak; Rochester, NY). Molecular weight and densitometry analyses were performed using Kodak Image Station software.
Tissular and Cellular Localization
Slides of individual normal tissues or tissue microarrays of multiple normal tissues, multiple skin cancers, and multiple organ cancers with normal adjacent tissues (US Biomax, Inc.; Rockville, MD) were dewaxed and processed in a graded series of ethanol, water, and PBS washes. Antigen retrieval was performed in 10 mM sodium citrate buffer, pH 6.0, with 0.05% Tween 20, in a boiling water bath for 30 min. Slides were blocked in 5% bovine serum albumin in PBS for 1.5 hr and exposed to primary antibodies overnight. Primary antibodies were anti-TNIP1 at 1:250, anti-RAR-α (Santa Cruz Biotechnologies) at 1:50, anti-keratin AE1/AE3 at 1:1000 (MP Biomedicals; Solon, OH), and anti-keratin 14 at 1:50 (Abcam). Secondary antibodies were Alexa Fluor 488 goat anti-rabbit IgG (Invitrogen) at 1:1000 and Alexa Fluor 555 goat anti-mouse IgG (Invitrogen) at 1:1000. Sections were nuclear counterstained with Draq5 (Biostatus; Leicestershire, UK) and mounted with ProLong Gold Antifade Reagent (Invitrogen). The AE1/AE3 combination of two monoclonal antibodies recognizes almost all cytokeratins, facilitating epithelial cell distinction from mesenchymal and non-epithelial tumors (Tseng et al. 1982). Imaging was performed with a Leica SP2 confocal microscope (University of Connecticut Microscopy Facility). Identical laser and signal capture settings were used across each array. Only minimal fluorescent signal was observed in the absence of primary antibodies. Specificity of TNIP1 detection was confirmed by costaining neonatal primary keratinocytes or individual tissue slides with the polyclonal TNIP1 antibody described here and a mouse monoclonal TNIP1 antibody described above (Zymed). Additional negative control staining was performed using rabbit or mouse anti-Gal4 DBD antibodies as primary antibodies in replacement of TNIP1-directed antibodies. For nuclear retention studies, HaCaT keratinocytes were cultured as described (Boukamp et al. 1988) or in media supplemented with 5 nM leptomycin B (Sigma; St. Louis, MO) for 6 hr. Anti-TNIP1 at 1:250 and mouse anti-keratin 14 (Abcam) at 1:100 were detected with Alexa Fluor 488 or Texas Red secondaries (Invitrogen). Imaging was done with an Olympus DeltaVision Imaging System (Applied Precision; Issaquah, WA).
Results
Antibody Detection of Endogenous and Recombinant TNIP1 Protein
Two peptides with the highest scoring predicted antigenicity (Fig. 1A) were used for antibody production. Preimmune serum, tested against lysates from HaCaT keratinocytes (Boukamp et al. 1988), a non-tumorigenic line of epidermal cells, HeLa cells, an epithelial cervical adenocarcinoma line, and SCC13 (Rheinwald and Beckett 1980), an epidermal squamous cell carcinoma (SCC) line, had no reactivity (not shown). From COS-7 cells (Fig. 1B) transfected with a Gal4-DBD-TNIP1 fusion protein expression plasmid (pDBD-TNIP1), the affinity-purified TNIP1 antibody detected the same band that increased in intensity with an increased amount of transfected plasmid as a monoclonal antibody directed against Gal4-DBD and a commercially available mouse monoclonal against TNIP1 (referred to by the manufacturer with the TNIP1 alias ABIN-1). This signal overlap demonstrated that the antibody we generated recognized TNIP1 protein. TNIP1 siRNA mediated the knockdown of an 85-kD protein in HaCaT keratinocytes, resulting in an 80% decrease in band immunoreactivity as compared to non-targeting siRNA (Fig. 1C). Conversely, immunoreactivity of an endogenous 85-kD band was increased by transient transfection of HaCaT keratinocytes with human TNIP1 cDNA compared to an empty vector control (Fig. 1D). Consistent with TNF-α inducing TNIP1 mRNA (Tian, Nowak, and Brasier 2005; Tian, Nowak, Jamaluddin, et al. 2005), the 85-kD protein increased more than twofold compared to control conditions (Fig. 1E). In addition to HaCaT keratinocytes and HeLa cells, endogenous TNIP1 protein (Fig. 1F) could be detected at varying levels in several epidermal SCC (SCC12B2, 12F2, and 13), oral SCC (SCC4, 9.1, and 25), and cell lines derived from cancers of colorectal (Caco-2 and HT-29), breast (MCF-7), liver (HepG2), or bone tissue (Saos-2) or adenovirus-transformed human embryonic kidney cells (AD293).
Figure 1.
TNIP1 antibody target and reactivity. (A) Immunogen peptides (standard single-letter amino acid abbreviations) derived from human TNIP1 protein sequence. Numbers refer to position in full-length protein. (B) Antibody codetection of Gal4 DBD-TNIP1 fusion protein. COS-7 cells transfected with empty pDBD vector (–) or vector containing TNIP1 cDNA (pDBD-TNIP1) at increasing amounts (0.75, 1.5, or 3.0 µg, indicated by triangle above lanes) per 10-cm plate. Empty pDBD was used to standardize total plasmid amounts per condition. The same membrane was sequentially probed with the indicated antibodies (see Materials and Methods). (C) siRNA knockdown of TNIP1. Lysates, 14 µg per lane, from HaCaT keratinocytes untransfected (Untr) or transfected for 24 hr with non-targeting negative control (Neg Cont) or TNIP1 siRNA were probed with TNIP1 antibody (same as in B, top panel). Protein molecular weight markers (MWMs) indicate the >175- to 40-kD region of the blot. (D) Comigration of endogenous and recombinant human TNIP1. HaCaT keratinocytes transfected with pcDNA3.1 without (–) or with (+) human TNIP1 cDNA insert, 25 µg lysate protein per lane. Area of blot shown is from just above to just below the 100- to 80-kD markers. Loading control (LC) is a cross-reactive band as previously described (Rasko et al. 2008; Schnepp et al. 2004). (E) Endogenous TNIP1 protein increases in HeLa cells following 50 ng/ml tumor necrosis factor α (TNF-α) exposure (times indicated above lanes). TNIP1 protein levels in arbitrary units are relative to band intensity at 0 hr TNF-α treatment; each is normalized to that lane’s corresponding loading control (LC) as in (B). (F) TNIP1 protein in human cell lines. Top immunoblot row loaded at 70 µg per lane; bottom row loaded at 35 µg per lane. Relative loading assessed by Coomassie staining as previously reported (Pearson et al. 2000; Smith et al. 2003) and showing proteins in the 75- to 100-kD range to lessen bias toward any one housekeeping gene that may vary across cell types or culture conditions (Greer et al. 2010). (G) TNIP1 protein detection from fractionated HaCaT keratinocytes and HeLa cells. Cytoplasmic (Cyto) versus nuclear (Nucl) proteins (20 µg per lane) were blotted and sequentially probed with indicated antibodies. H2A, histone 2A. (H) Increased TNIP1 protein production does not decrease retinoic acid receptor α (RAR-α) levels. Immunoblots showing levels of TNIP1 and RAR-α from HeLa cells transfected with either empty expression vector (lanes 1, 3) or one containing the human TNIP1 cDNA (lanes 2, 4) and then collected after 24 hr of exposure either to vehicle control (lanes 1, 2) or 1 µM all-trans retinoic acid (ATRA; lanes 3, 4). LC, loading control as in (B).
TNIP1 subcellular distribution was compared via Western blotting from fractionated HaCaT keratinocytes and HeLa cells using histone 2A (H2A) and tubulin as nuclear and cytoplasmic compartment markers, respectively. Although TNIP1 is predominantly found in the cytoplasmic fraction of both cell lines tested, we were able to detect it in the nuclear fraction as well (Fig. 1G) at a level that exceeds what appears to be trace cytoplasmic carryover based on the faint tubulin band in that fraction.
TNIP1 binds to and is a corepressor of agonist-bound RAR-α (Gurevich and Aneskievich 2009). Because we found no association of TNIP1 with histone deacetylases that could account for decreased RAR-α signaling in that report, TNIP1-instigated degradation of RAR-α remained a formal possibility. As the antibody was capable of detecting HeLa endogenous TNIP1 protein (Fig. 1E, lane 1), we tested if increasing TNIP1 over basal amounts had any deleterious effect on RAR-α protein levels. Recombinant expression of TNIP1 (recTNIP1) produced ~7-fold increase (Fig. 1H, lane 2) in TNIP1 protein over endogenous levels, but there was no significant change in RAR-α protein over empty vector controls. Consistent with previous findings (Zhu et al. 1999), treatment with all-trans retinoic acid (ATRA) induced receptor degradation, as evidenced by ~30% loss in RAR-α protein compared to vehicle control after 24 hr of ATRA exposure (compare vehicle control lanes 1 and 2 against ATRA treatment, lanes 3 and 4). Thus, although ligand-dependent reduction of RAR occurred in these cells, increased expression of TNIP1 did not contribute to loss of receptor protein. These findings agree with our other observations of increased expression of TNIP1 decreasing peroxisome proliferator-activated receptor γ (PPARγ) activity but not receptor protein levels (Flores et al., unpublished data).
Keratinocyte Subcellular Localization of Endogenous TNIP1
Having detected endogenous TNIP1 protein in HaCaT keratinocyte lysates, we examined its subcellular localization by confocal microscopy. Under standard culture conditions, HaCaT keratinocytes (Fig. 2) had TNIP1 localized to both the nucleus and the cytoplasm. To determine what might regulate this distribution, HaCaT keratinocytes were treated for 6 hr with leptomycin B (LMB), an inhibitor of CRM1-dependent nuclear export, resulting in significant nuclear accumulation of TNIP1. In both control and LMB-treated cells, the nuclear localization of TNIP1 was contrasted by staining for keratin 14, a cytoplasmic protein characteristic of early keratinocyte differentiation. Keratin 14 is not known to be affected by LMB treatment and control, and treated cells showed typical cytoskeletal staining.
Figure 2 (on pg 1106).
(A) TNIP1 nuclear and cytoplasmic localization in cultured HaCaT keratinocytes. Standard media (vehicle, top row) or 5 nM leptomycin B (LMB, bottom row) treatment. Deconvolution microscopy images of staining for TNIP1, keratin 14, or nuclei detected with Alexa Fluor 488 (green), Texas Red (red), or DAPI (blue), respectively, are shown individually and merged in the rightmost panel. Bar = 20 µm. (B) TNIP1 colocalization with retinoic acid receptor α (RAR-α) in keratinocyte nuclei in human epidermis. Immunofluorescent microscopy of scalp skin probed for detection of TNIP1 (secondary antibody Alexa Fluor 488, green) and RAR-α (secondary antibody Alexa Fluor 586, red) and costained with DAPI (blue) to mark nuclei with a three-channel merge in the rightmost panel. RAR-α nuclear detection in dermal fibroblasts (arrowheads) as well as nonspecific staining of the stratum corneum was previously noted for antibodies to retinoid receptors (Karlsson et al. 2004). Bar = 20 µm. (C) Colocalization of signal from TNIP1 and ABIN-1 antibodies in cultured cells. Immunofluorescent microscopy of neonatal human primary keratinocytes probed with rabbit anti-TNIP1 (secondary antibody Alexa Fluor 488, green) and mouse monoclonal anti-ABIN-1 (secondary antibody Alexa Fluor 586, red) and costained with DAPI (blue) to mark nuclei with two- and three-channel (red-green and red-green-blue, respectively) merges in panels at right. Bar = 20 µm. (D) Colocalization of signal from TNIP1 and ABIN-1 antibodies in tissues. Immunofluorescent microscopy of human tonsil stratified epithelium (Epith), germinal center (GC), and skeletal muscle (Msl) probed with rabbit anti-TNIP1 (secondary antibody Alexa Fluor 488, green) and mouse monoclonal anti-ABIN-1 (secondary antibody Alexa Fluor 586, red) and costained with DAPI (blue) to mark nuclei with two- and three-channel merges in panels at right. Antibodies against Gal4 DNA binding domain (DBD) protein raised in rabbit (rab DBD) or mouse (mo DBD) were used as negative controls in place of anti-TNIP1 and anti-ABIN-1 antibodies for tonsil epithelium (second row), germinal center, and skeletal muscle (not shown). Bar = 20 µm.
RARs are important regulators of epidermal keratinocyte differentiation, and their activity is repressed by TNIP1 (Gurevich and Aneskievich 2009), prompting us to examine distribution of TNIP1 and this nuclear receptor in human skin. As with the cultured HaCaT keratinocytes, TNIP1 protein was detected in skin keratinocytes in both the cytoplasm and nucleus (Fig. 2B). Localization of the nuclear compartment was determined by costaining with RAR-α, the RAR subtype that exhibits the strongest association with TNIP1 (Gurevich and Aneskievich 2009) and the fluorogen DAPI. The merged images (Fig. 2B) demonstrate colocalization and the opportunity for TNIP1 to coregulate RAR and possibly other nuclear receptors.
Concurrent with our generation of the TNIP1 polyclonal antibody, other antibodies against the protein became commercially available. The mouse monoclonal antibody used in blotting experiments (Fig. 1B), referred to by the manufacturer as anti-ABIN-1, was used together with our rabbit antibody to costain primary epidermal keratinocytes (Fig. 2C). We observed the same staining pattern with both antibodies, with TNIP1 being detected both in the cytoplasm and the nucleus. High levels of TNIP1 mRNA were previously reported in immune system cells and skeletal muscle (Fukushi et al. 1999). As with primary keratinocytes, we observed excellent overlap in staining between our antibody and the monocolonal TNIP1 antibody in both the stratified squamous epithelium and lymphocyte-rich germinal center of the tonsil and also in skeletal muscle. As negative controls, we used rabbit polyclonal or mouse monoclonal primary antibodies against Gal4 DBD protein as an irrelevant target protein in these tissues. Slides exposed to the Gal4 DBD antibodies were processed in parallel with the TNIP1 and ABIN-1 antibody-probed tissues for secondary antibody incubation and fluorescent microscopy. They demonstrated only weak background staining (Fig. 2D, tonsil epithelium, second row). Taken together, these results demonstrate that our affinity-purified rabbit polyclonal antibody effectively targets TNIP1 protein.
Localization within Skin Squamous Cell Carcinomas
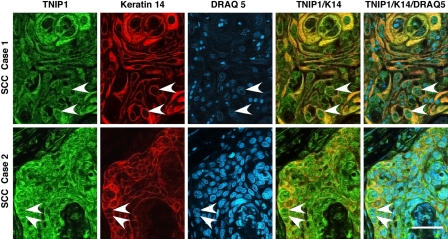
The relatively high levels of TNIP1 protein in cultured SCC malignant keratinocytes (Fig. 1D), as well as the nuclear localization of TNIP1 in keratinocytes of normal epidermis particularly, prompted confocal analysis of TNIP1 on tissue samples of human SCC (Fig. 3). Malignant keratinocytes within the SCCs displayed the TNIP1 cytoplasmic and nuclear staining seen in normal cells. Within the lesion, there was increased TNIP1 intensity in the more flattened, squame-like cells of keratin pearls (Fig. 3, top row). Keratin 14 staining provided cytoplasmic contrast to the frequently nuclear TNIP1 in the merged images.
Figure 3.
TNIP1 distribution in squamous cell carcinomas (SCCs) imaged with confocal microscopy. Top and bottom rows, epidermal SCC. Nuclear TNIP1 is seen throughout SCC (arrowheads in TNIP1, K14, and overlay, for example). Left to right, panels are from anti-TNIP1 (secondary antibody Alexa Fluor 488, green), anti–keratin 14 (secondary antibody Alexa Fluor 586, red), nuclear DRAQ 5 staining (pseudocolor blue), TNIP1/keratin 14 merge, and TNIP1/keratin 14/DRAQ 5 merge. Bar = 50 µm.
TNIP1 Distribution Outside of Skin
To extend study of TNIP1 subcellular localization to other tissues, confocal microscopy was performed on a human multiple-tissue microarray. The AE1/AE3 monoclonal antibody combination was used to detect keratins across the diverse tissues in the array and provide for a cytoplasmic marker (Fig. 4). Skin was present in these arrays, and its epidermal nuclear and cytoplasmic staining for TNIP1 (not shown) confirmed studies in Figure 2B. For other tissues, there was nuclear TNIP1 staining of uterine ectocervical epithelium, although this tended to occur mostly in the upper strata as compared to the epidermis where nuclear staining was evident throughout the layers. Colon was among the more intensely TNIP1-positive tissues (Fig. 4). There was prominent TNIP1 cytoplasmic and nuclear localization. Most cells of pancreatic intercalated ducts and islets of Langerhans were weakly reactive. In contrast, pancreatic acinar cells (exocrine pancreas) were strongly stained. Kidney tubular epithelia cells did stain more intensely than cells within the glomerulus. Although variable in their intensity, granulosa cells of secondary ovarian follicles stained more heavily than nearby thecal cells, moderate staining was spread throughout liver parenchymal cells, and weak, diffuse staining was seen in brain, heart, and lung alveoli (Table 1).
Figure 4.
TNIP1 nuclear and cytoplasmic localization in multiple human organs. Left to right, panels are anti-TNIP1 (secondary antibody Alexa Fluor 488, green), anti-cytokeratin AE1/AE3 (KERATIN, secondary antibody Alexa Fluor 586, red), DRAQ5 staining (NUCLEI, pseudocolor blue), TNIP1/cytokeratin merge (TNIP/KER), and TNIP1/cytokeratin/DRAQ5 merge (TNIP1/KER/NUC). Arrowheads in TNIP1/KER panels for colon, pancreas, and kidney indicate goblet cells, islet of Langerhans, and distal tubule, respectively. Pancreatic islets and kidney distal versus proximal tubules were identified by tissue architecture and differential cytokeratin staining (Tseng et al. 1982). Final magnification for each panel was adjusted for a balance between cellular detail and tissue architecture. Bars = 75 µm for skin, cervix, pancreas, and kidney; 37.5 µm for colon.
Table 1.
TNIP1 Tissue Panel Summary
| Tissue | Regiona | Signal | Comments |
|---|---|---|---|
| Brain | Normal cerebrum | 2+ | Even staining across cerebral neurons; weaker staining in pyramidal neurons |
| Astrocytomaa | 2–3+ | Grainy cytoplasmic staining tended to be more intense than in normal adjacent tissue | |
| Heart | Cardiac muscle | 1+ | Diffuse staining throughout muscle fibers |
| Lung | Alveoli | 1+ | All cells weakly positive with a small percentage, possibly type II cells, more strongly stained |
| Bronchiole | 2–3+ | Epithelial cells intensely stained throughout cytoplasm with weak or no staining in nuclei | |
| SCCa | 3+ | Staining throughout lesion with some more intense staining in histological more differentiated cells | |
| Skin, dermis | Sweat gland | 1–3+ | Staining intensity dependent on region of gland, stronger signal present in ductal epithelia versus excretory gland base |
| Syringocarcinomaa | 1–3+ | Widely variable, mostly cytoplasmic detection dependent on individual tumor | |
| Stomach | Gastric lining | 3+ | Glandular epithelia with moderate cytoplasmic and weak nuclear staining |
| Adenocarcinomaa | 1–2+ | Remaining staining tended to be more associated with cell periphery |
SCC, squamous cell carcinoma. 1–4+: relative ranking of staining intensity where 4+ signal is typical of those tissues shown in figures such as SCC, case 2 in Figure 4. 1+ is signal intensity just over the background seen with secondary antibody alone.
Where available, a malignancy from the listed tissues was also examined.
We extended our study of TNIP1 distribution by examining multiple-organ cancer histological arrays that also had normal adjacent tissues. TNIP1 staining in syringocarcinoma and gastric adenocarcinoma was comparable to glandular cells in normal counterparts (Table 1). As with stratified epithelia of skin and cervix, normal esophagus displayed both cytoplasmic and nuclear TNIP1 (Fig. 5). In esophageal SCC, there was increased cytoplasmic TNIP1 staining coincident with greater histological differentiation. However, these suprabasal cells tended to have less intense nuclear staining for TNIP1 than suprabasal cells of normal esophageal epithelium. Epithelial cells of an infiltrating ductal carcinoma of the breast had reduced nuclear staining compared to normal mammary epithelial cells. One of the most striking differences between normal and malignant cells occurred with prostate adenocarcinoma. Within normal prostate, acinar epithelial cells displayed nuclear TNIP1 staining along with cytoplasmic TNIP1 uniformly overlapping cytokeratin staining. In contrast, prostate adenocarcinoma epithelial cells, as defined by their cytokeratin reactivity, had greatly reduced TNIP1 staining both when compared to uninvolved acinar cells of the same block and normal adjacent tissue of the same patient.
Figure 5.
TNIP1 nuclear and cytoplasmic localization in human epithelial cancers. Panel labeling as in Figure 4. Dashed line in normal esophagus is border of epithelial basal layer and connective tissue. Arrowheads in esophageal squamous cell carcinoma (SCC) indicate keratin pearl to left of panel. Arrowheads in prostate adenocarcinoma indicate epithelial cells (keratin positive) with significantly reduced TNIP1 staining compared to normal adjacent epithelia in the lower left and right of the panel. Bar = 75 µm for all panels.
Discussion
TNIP1 is involved in dampening activity of at least two distinct cell signaling pathways. By targeting cytoplasmic constituents downstream of the TNF-α receptor, TNIP1 can reduce activation of NF-κB (Heyninck et al. 1999; Heyninck et al. 2003; Wullaert et al. 2005) and, through interference with coactivator recruitment, reduce RAR activity (Gurevich and Aneskievich 2009). To complement what is known about the distribution and localization of its known targets, we investigated TNIP1 protein levels and distribution via a novel rabbit polyclonal, peptide-specific, affinity-purified antiserum. TNIP1 protein, at an 85-kD apparent molecular weight, increases to more than twofold by 12 hr after TNF-α addition, as would be expected from TNIP1 mRNA studies reporting that its gene is a mid- to late responder to NF-κB activation (Tian, Nowak, and Brasier 2005; Tian, Nowak, Jamaluddin, et al. 2005). Recombinant expression of TNIP1 in HaCaT keratinocytes resulted in increased intensity of the 85-kD band, whereas siRNA knockdown of TNIP1 decreased it. The broader, slightly slower migrating species observed with TNIP1 overexpression may stem from the accumulation of posttranslationally modified TNIP1. Our bioinformatics analysis of TNIP1 predicts more than 20 potential phosphorylation sites in agreement with phosphorylation, potentially accounting for a second, slightly slower migrating form in lysates of Saos-2 cells overexpressing human TNIP1 (Zhang et al. 2002; Zhang et al. 2008). Phosphorylation within or adjacent to nuclear export signals can affect the ultimate localization of proteins capable of nuclear to cytoplasmic shuttling (Ding et al. 2007). Predicted TNIP1 phosphorylation sites do overlap amino acids within four stretches, matching nuclear export signals. It is important to note here that both nuclear export and localization signals have been functionally recognized on the surface of folded proteins that are not apparent from consecutive linear amino acid sequences (Ayers et al. 2007). In addition, cytoplasmic retention signals, for which there is no clear consensus as for nuclear export or import motifs, are also reported to influence the cytoplasmic-nuclear shuttling of some proteins such as PTEN (Planchon et al. 2008). The antibody’s detection of endogenous TNIP1 will enable studies of cell response to stress or signal activation, which may change phosphorylation level and subcellular localization. Importantly, TNIP1’s translocation between nuclear and cytoplasmic compartments will have an impact on its ability to affect signaling stemming from retinoic acid or TNF-α receptors.
TNIP1 functions as an RAR (Gurevich and Aneskievich 2009) and PPAR (Flores et al., unpublished data) corepressor in the presence of those receptors’ cognate ligands, placing it among corepressors of agonist-bound NRs. TNIP1 colocalized with RAR-α in human scalp epidermis and their presence in the same subcellular compartment provides an in vivo opportunity for this coregulator-receptor interaction to occur. Aberrant levels of PRAME, another of this very small subclass of nuclear receptor corepressors (for review, see Gurevich et al. 2007), have been functionally linked to malignant cell sensitivity to retinoids as chemotherapy agents (Epping and Bernards 2006). As to how TNIP1 may lessen RAR activity, we report here that experimentally increasing TNIP1 protein levels over those naturally found in HeLa cells did not lead to receptor protein reduction, supporting the premise that TNIP1 repression of RAR activity is not an outright receptor degradation effect. This RAR repression along with TNIP1 repression of NF-κB signaling (Verstrepen et al. 2009), through its association with proteins involved in the cytoplasmic activation of that transcription factor (Cohen et al. 2009; Heyninck et al. 2003), emphasizes the importance of documenting TNIP1 levels and distribution.
The studies presented here demonstrate tissue-specific changes in TNIP1 localization for normal versus malignant tissues. In some instances, particularly the cutaneous and esophageal SCC tumors, cytoplasmic staining was notably increased for those keratinocytes. In contrast, the epithelial cells of prostate adenocarcinoma showed significant loss of TNIP1. As additional normal and pathologic tissues are examined, the consequences of alterations in TNIP1 levels will need to be considered against its possible impact on different signaling pathways present in those cells—namely, NF-κB and RAR—and the extent to which those pathways are used. The opportunity for TNIP1 to affect these pathways and, in turn, cell physiology or pathology will clearly depend on its abundance and subcellular localization, be it the nuclear compartment, as seen in normal epidermis and colon, or predominantly cytoplasmic, as exemplified by pancreatic acini.
Acknowledgments
We thank Dr. C. Norris for assistance with confocal microscopy, Dr. A. Pask and C. Bell for use of and assistance with the Olympus DeltaVision platform for deconvolution microscopy, and the journal reviewers and editor for helpful suggestions in improving this report.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the authorship and/or publication of this article.
The author(s) disclosed receipt of the following financial support for the research and/or authorship of this article: This work was funded by grants to BJA from the National Institutes of Health [AR048660] with predoctoral fellowships from Boehringer-Ingelheim Pharmaceuticals and the American Foundation for Pharmaceutical Education to IG for partial stipend support and the Edward A. Khairallah Graduate Fellowship to IG for summer support.
References
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. 2004. Current protocols in molecular biology. New York: John Wiley [Google Scholar]
- Ayers SD, Nedrow KL, Gillilan RE, Noy N. 2007. Continuous nucleocytoplasmic shuttling underlies transcriptional activation of PPARgamma by FABP4. Biochemistry. 46:6744–6752 [DOI] [PubMed] [Google Scholar]
- Bollrath J, Greten FR. 2009. IKK/NF-kappaB and STAT3 pathways: central signalling hubs in inflammation-mediated tumour promotion and metastasis. EMBO Rep. 10:1314–1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boukamp P, Petrussevska RT, Breitkreutz D, Hornung J, Markham A, Fusenig NE. 1988. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol. 106:761–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Ciechanover A, Kravtsova-Ivantsiv Y, Lapid D, Lahav-Baratz S. 2009. ABIN-1 negatively regulates NF-kappaB by inhibiting processing of the p105 precursor. Biochem Biophys Res Commun. 389:205–210 [DOI] [PubMed] [Google Scholar]
- Ding G, Sonoda H, Yu H, Kajimoto T, Goparaju SK, Jahangeer S, Okada T, Nakamura S. 2007. Protein kinase D–mediated phosphorylation and nuclear export of sphingosine kinase 2. J Biol Chem. 282:27493–27502 [DOI] [PubMed] [Google Scholar]
- Epping MT, Bernards R. 2006. A causal role for the human tumor antigen preferentially expressed antigen of melanoma in cancer. Cancer Res. 66:10639–10642 [DOI] [PubMed] [Google Scholar]
- Flores AM, Gurevich I, Zhang C, Ramirez VP, Devens TR, Aneskievich BJ. 2011. TNIP1 is a corepressor of agonist-bound PPARs. Arch Biochem Biophys. Published online ahead of print Sep 22 doi:10.1016/j.abb.2011.08.014. 10.1016/j.abb.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushi M, Dixon J, Kimura T, Tsurutani N, Dixon MJ, Yamamoto N. 1999. Identification and cloning of a novel cellular protein Naf1, Nef-associated factor 1, that increases cell surface CD4 expression. FEBS Lett. 442:83–88 [DOI] [PubMed] [Google Scholar]
- Greer S, Honeywell R, Geletu M, Arulanandam R, Raptis L. 2010. Housekeeping genes; expression levels may change with density of cultured cells. J Immunol Methods. 355:76–79 [DOI] [PubMed] [Google Scholar]
- Gupta K, Ott D, Hope TJ, Siliciano RF, Boeke JD. 2000. A human nuclear shuttling protein that interacts with human immunodeficiency virus type 1 matrix is packaged into virions. J Virol. 74:11811–11824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevich I, Aneskievich BJ. 2009. Liganded RARalpha and RARgamma interact with but are repressed by TNIP1. Biochem Biophys Res Commun. 389:409–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevich I, Flores AM, Aneskievich BJ. 2007. Corepressors of agonist-bound nuclear receptors. Toxicol Appl Pharmacol. 223:288–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyninck K, De Valck D, Vanden Berghe W, Van Criekinge W, Contreras R, Fiers W, Haegeman G, Beyaert R. 1999. The zinc finger protein A20 inhibits TNF-induced NF-kappaB-dependent gene expression by interfering with an RIP- or TRAF2-mediated transactivation signal and directly binds to a novel NF-kappaB-inhibiting protein ABIN. J Cell Biol. 145:1471–1482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyninck K, Kreike MM, Beyaert R. 2003. Structure-function analysis of the A20-binding inhibitor of NF-kappa B activation, ABIN-1. FEBS Lett. 536:135–140 [DOI] [PubMed] [Google Scholar]
- Hosgood HD, III, Menashe I, Shen M, Yeager M, Yuenger J, Rajaraman P, He X, Chatterjee N, Caporaso NE, Zhu Y, et al. 2008. Pathway-based evaluation of 380 candidate genes and lung cancer susceptibility suggests the importance of the cell cycle pathway. Carcinogenesis. 29:1938–1943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson T, Rollman O, Vahlquist A, Torma H. 2004. Immunofluorescence localization of nuclear retinoid receptors in psoriasis versus normal human skin. Acta Derm Venereol. 84:363–369 [DOI] [PubMed] [Google Scholar]
- Lefebvre P. 2001. Molecular basis for designing selective modulators of retinoic acid receptor transcriptional activities. Curr Drug Targets Immune Endocr Metabol Disord. 1:153–164 [PubMed] [Google Scholar]
- Makkonen KM, Pasonen-Seppanen S, Torronen K, Tammi MI, Carlberg C. 2009. Regulation of the hyaluronan synthase 2 gene by convergence in cyclic AMP response element-binding protein and retinoid acid receptor signaling. J Biol Chem. 284:18270–18281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mankan AK, Lawless MW, Gray SG, Kelleher D, McManus R. 2009. NF-kappaB regulation: the nuclear response. J Cell Mol Med. 13:631–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair RP, Duffin KC, Helms C, Ding J, Stuart PE, Goldgar D, Gudjonsson JE, Li Y, Tejasvi T, Feng BJ, et al. 2009. Genome-wide scan reveals association of psoriasis with IL-23 and NF-kappaB pathways. Nat Genet. 41:199–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima S, Turer EE, Callahan JA, Chai S, Advincula R, Barrera J, Shifrin N, Lee B, Yen B, Woo T, et al. 2009. ABIN-1 is a ubiquitin sensor that restricts cell death and sustains embryonic development. Nature. 457:906–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson AS, Spitz FR, Swisher SG, Kataoka M, Sarkiss MG, Meyn RE, McDonnell TJ, Cristiano RJ, Roth JA. 2000. Up-regulation of the proapoptotic mediators Bax and Bak after adenovirus-mediated p53 gene transfer in lung cancer cells. Clin Cancer Res. 6:887–890 [PubMed] [Google Scholar]
- Planchon SM, Waite KA, Eng C. 2008. The nuclear affairs of PTEN. J Cell Sci. 121:249–253 [DOI] [PubMed] [Google Scholar]
- Rasko DA, Moreira CG, de Li R, Reading NC, Ritchie JM, Waldor MK, Williams N, Taussig R, Wei S, Roth M, et al. 2008. Targeting QseC signaling and virulence for antibiotic development. Science. 321:1078–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rheinwald JG, Beckett MA. 1980. Defective terminal differentiation in culture as a consistent and selectable character of malignant human keratinocytes. Cell. 22:629–632 [DOI] [PubMed] [Google Scholar]
- Saavalainen K, Tammi MI, Bowen T, Schmitz ML, Carlberg CM. 2007. Integration of the activation of the human hyaluronan synthase 2 gene promoter by common cofactors of the transcription factors retinoic acid receptor and nuclear factor kappaB. J Biol Chem. 282:11530–11539 [DOI] [PubMed] [Google Scholar]
- Schnepp RW, Hou Z, Wang H, Petersen C, Silva A, Masai H, Hua X. 2004. Functional interaction between tumor suppressor menin and activator of S-phase kinase. Cancer Res. 64:6791–6796 [DOI] [PubMed] [Google Scholar]
- Shishodia S, Aggarwal BB. 2004. Nuclear factor–kappaB activation mediates cellular transformation, proliferation, invasion angiogenesis and metastasis of cancer. Cancer Treat Res. 119:139–173 [DOI] [PubMed] [Google Scholar]
- Smith PD, Crocker SJ, Jackson-Lewis V, Jordan-Sciutto KL, Hayley S, Mount MP, O’Hare MJ, Callaghan S, Slack RS, Przedborski S, et al. 2003. Cyclin-dependent kinase 5 is a mediator of dopaminergic neuron loss in a mouse model of Parkinson’s disease. Proc Natl Acad Sci U S A. 100:13650–13655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian B, Nowak DE, Brasier AR. 2005. A TNF-induced gene expression program under oscillatory NF-kappaB control. BMC Genomics. 6:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian B, Nowak DE, Jamaluddin M, Wang S, Brasier AR. 2005. Identification of direct genomic targets downstream of the nuclear factor–kappaB transcription factor mediating tumor necrosis factor signaling. J Biol Chem. 280:17435–17448 [DOI] [PubMed] [Google Scholar]
- Tseng SC, Jarvinen MJ, Nelson WG, Huang JW, Woodcock-Mitchell J, Sun TT. 1982. Correlation of specific keratins with different types of epithelial differentiation: monoclonal antibody studies. Cell. 30:361–372 [DOI] [PubMed] [Google Scholar]
- Verstrepen L, Carpentier I, Verhelst K, Beyaert R. 2009. ABINs: A20 binding inhibitors of NF-kappa B and apoptosis signaling. Biochem Pharmacol. 78:105–114 [DOI] [PubMed] [Google Scholar]
- Wullaert A, Wielockx B, Van Huffel S, Bogaert V, De Geest B, Papeleu P, Schotte P, El Bakkouri K, Heyninck K, Libert C, et al. 2005. Adenoviral gene transfer of ABIN-1 protects mice from TNF/galactosamine-induced acute liver failure and lethality. Hepatology. 42:381–389 [DOI] [PubMed] [Google Scholar]
- Zhang S, Fukushi M, Hashimoto S, Gao C, Huang L, Fukuyo Y, Nakajima T, Amagasa T, Enomoto S, Koike K, et al. 2002. A new ERK2 binding protein, Naf1, attenuates the EGF/ERK2 nuclear signaling. Biochem Biophys Res Commun. 297:17–23 [DOI] [PubMed] [Google Scholar]
- Zhang S, Mahalingam M, Tsuchida N. 2008. Naf1alpha is phosphorylated in mitotic phase and required to protect cells against apoptosis. Biochem Biophys Res Commun. 367:364–369 [DOI] [PubMed] [Google Scholar]
- Zhu J, Gianni M, Kopf E, Honore N, Chelbi-Alix M, Koken M, Quignon F, Rochette-Egly C, de The H. 1999. Retinoic acid induces proteasome-dependent degradation of retinoic acid receptor alpha (RARalpha) and oncogenic RARalpha fusion proteins. Proc Natl Acad Sci U S A. 96:14807–14812 [DOI] [PMC free article] [PubMed] [Google Scholar]